Abstract
Eggplant (Solanum melongena L.) is one of the solanaceous crops of economic and cultural importance and is widely cultivated in the state of Goa, India. Eggplant cultivation is severely affected by bacterial wilt caused by Ralstonia solanacearum that colonizes the xylem tissue. In this study, 167 bacteria were isolated from the xylem of healthy eggplant, chilli, and Solanum torvum Sw. by vacuum infiltration and maceration. Amplified rDNA restriction analysis (ARDRA) grouped these xylem residing bacteria (XRB) into 38 haplotypes. Twenty-eight strains inhibited growth of R. solanacearum and produced volatile and diffusible antagonistic compounds and plant growth promoting substances in vitro. Antagonistic strains XB86, XB169, XB177, and XB200 recorded a biocontrol efficacy greater than 85% against BW and exhibited 12%–22 % increase in shoot length in eggplant in the greenhouse screening. 16S rRNA based identification revealed the presence of 23 different bacterial genera. XRB with high biocontrol and plant growth promoting activities were identified as strains of Staphylococcus sp., Bacillus sp., Streptomyces sp., Enterobacter sp., and Agrobacterium sp. This study is the first report on identity of bacteria from the xylem of solanaceous crops having traits useful in cultivation of eggplant.
1. Introduction
Ralstonia solanacearum is a vascular wilt pathogen that belongs to the β subdivision of the Proteobacteria [1] and is one of the most destructive plant pathogens causing bacterial wilt (BW) in many crop plants. It has broad host range and infects around 54 plant families and 450 plant species [2]. This pathogen also has a wide geographical distribution ranging from tropical, subtropical, and warm temperate regions of the world [3]. Cultivation of eggplant in the coastal state of Goa, India, is severely affected by BW leading to 30–100% crop loss [4]. The bacterium infects the plant through root cracks at the site of root emergence. Subsequently, the intercellular spaces of the root cortex and vascular parenchyma are colonized. Cell wall degrading exoenzymes disrupt the cell walls and facilitate its entry in the vascular system [5]. Inside the xylem vessels, the bacterial populations rapidly reach very high levels of 1010 cells/cm of stem [6]. High cell density and production of high molecular weight exopolysaccharides by R. solanacearum lead to clogging of xylem vessels, wilting, and eventually death of plant.
Xylem of healthy plants has been reported to be colonized by endophytic xylem residing bacteria (XRB) at low population levels and has been isolated from xylem of various crops, namely, citrus [7], sugar beets [8], maize [9], alfalfa [10], grape [11, 12], and Bermuda grass [13]. Several endophytic bacteria have been reported to originate from the rhizosphere soil, initially entering the host plant during germination and radicle development, through wounds or by colonizing the cracks formed in lateral root junctions when the endodermis and casparian strips are disrupted thus gaining an easy access to the stele [14, 15]. After their initial entry, depending on the endophytic colonization ability, bacteria may remain localized in the roots [16] or colonize intercellular spaces and vascular system [11] and move to the stems [17]. Few endophytes have been reported to be able to migrate to aerial plant parts through the vascular system passively with the transpirational flow or through additional assistance by production of cell wall degrading enzymes [18, 19]. These systemically migrated endophytes have been isolated from leaves [20], inflorescence [21], fruits [22], and seeds [23].
Among the several methods of plant disease management, biocontrol plays an important role particularly in the control of soil borne diseases. Biocontrol agents may be used as an alternative pathogen management strategy or can be combined with other management practices. Biological control not only helps in suppressing the disease and increasing crop yield but also has importance in reducing the environmental pollution due to use of chemical pesticides [24]. Several studies have shown that endophytic bacteria can be used as biocontrol agents against plant pathogens. The capability of colonizing internal host tissues and ability to produce volatile and diffusible substances which inhibit pathogen, induction of systemic resistance in the plant, and directly or indirectly promoting plant growth have made endophytes a valuable tool in agriculture to improve crop performance [18]. Endophytic biocontrol agents isolated from potato [25], tomato, chilli [26] and eggplant [27] have been used for management of BW. However, the wilt prevention ability of xylem residing bacteria of solanaceous crops that share an ecological niche with the BW pathogen has remained unexplored. This study was undertaken to identify and screen bacteria isolated from the xylem of eggplant, chilli, and S. torvum for their biocontrol activities against R. solanacearum and growth promotion abilities in eggplant.
2. Materials and Methods
2.1. Isolation of Xylem Residing Bacteria from Eggplant, Chilli, and S. torvum
2.1.1. Collection of Xylem Sap
Apparently healthy plants were collected from the major vegetable growing locales in North Goa and South Goa districts of the coastal state of Goa, India. Eggplant samples were from two different varieties, namely, BW susceptible and BW resistant variety. Chilli samples were from the locally grown cultivar, which is moderately susceptible to BW. Wild eggplant S. torvum is known to be naturally resistant to BW and was sampled from a field in ICAR Research Complex for Goa, India. Stem pieces of 13–15 cm length were surface sterilized by dipping in 0.1% mercuric chloride for 1 min and rinsed several times in sterile water. Wash water used for rinsing each surface sterilized stem piece was plated onto Tryptic soy agar (TSA) (Hi Media Laboratories, Mumbai) to confirm surface sterilization of each stem piece. Xylem sap was extracted by vacuum infiltration as described earlier [7, 28]. Briefly, after the surface sterilization of stem, one cm piece from each end was discarded. Epidermis and cortex from each end were removed and the vascular cylinder was fitted to sterile glass tubing attached in a rubber cork. To the other end of the stem piece a sterile plastic tubing was attached that could hold at least 500 μL of 1 X phosphate buffered saline (PBS) (NaCl 8 g/L, KCl 0.2 g/L, Na2HPO4·2H2O, 1.44 g/L, and KH2PO4 0.24 g/L, pH 7.4). The cork with plant sample attached was then fitted onto a Buchner flask. For extraction of PBS through the xylem vessels a suction pressure 8 mbar was applied using a diaphragm pump MPC101Z (Ilmvac GmbH, Germany). A total of four successive infiltrations using 500 μL of PBS were performed for each sample. The sap was collected directly in a sterile test tube placed inside the Buchner flask. Alternatively, maceration/trituration was performed for isolation of the XRB from young eggplant and chilli samples which had thin and soft stems. The epidermis and cortex from the surface sterilized stem piece were removed aseptically to expose the vascular bundles. The decorticated pieces were macerated in a sterile mortar and pestle using 2 mL of sterile 1X PBS.
2.1.2. Isolation
One hundred μL of the vacuum in-filtered sap or macerate was plated onto TSA or medium 523 [29]. The plates were incubated at 28 ± 2°C for 5 days. Different colonies from isolation plates were selected based on differences in their shape, color, and texture and purified onto medium 523. Pure cultures of the xylem residing bacteria (XRB) thus obtained were maintained at −80°C, as glycerol stocks for long term, and 4°C for temporary storage.
2.2. Amplified rDNA Restriction Analysis (ARDRA)
ARDRA was performed to determine the genetic diversity of the XRB in the collection. Genomic DNA from the XRB was extracted as described by Wilson [30]. Quality and quantity of the DNA were measured using Nanodrop 1000 (Thermo Scientific, USA). 16S rRNA gene was amplified using universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). Twenty μL reaction mix contained 1X PCR buffer, 0.75 units of Taq DNA polymerase (Sigma Aldrich, USA), 200 μM dNTPs (Sigma Aldrich, USA), 0.5μM each primer (Chromous Biotech, Bangalore, India), and 50 ng/μL of genomic DNA. Amplifications were carried out on Eppendorf Mastercycler Pro Thermal cycler (Eppendorf, Germany). Amplification cycle included a denaturation step of 94°C for 5 min followed by 32 cycles of denaturation at 94°C for 30s, annealing at 55°C for 40s, extension at 72°C for 1 min, and a final extension at 72°C for 10 min. The amplification of the 1500 bp PCR product was determined by electrophoresis on 0.8% agarose gel. Fifteen microliters of the PCR product was digested with one unit of MspI (Thermo Scientific, USA) for 4 h at 37°C. Restriction fragments were separated on a 2% agarose gel in 1X Tris-acetate EDTA buffer containing 0.5 μg/mL ethidium bromide at 60 volts for 2 h. Gel was documented using Alpha Imager (Alpha Innotech Inc., USA). ARDRA restriction fingerprints were compared visually and scored manually as 1 for presence and 0 for absence of fragment, and the binary data was entered in the NT Edit software version 1.1 b (Applied Biostatistics Inc. USA). The similarity matrix derived using the binary data of ARDRA restriction fragment was subjected to cluster analysis using unweighted pair group method for arithmetic average (UPGMA) using Dice coefficient in the NTSYSpc 2.02i software (Applied Biostatistics Inc. USA). Subsequent to analysis several clusters were obtained. Each cluster consisted of XRB having an identical restriction fragment profile. Strains having very unique restriction profile remained separable and independent clusters. Clusters obtained were denoted as haplotypes. Haplotypes were delineated at 80% similarity values of Dice coefficient and numbered as M80-1 to M80-38. Representative strains from each haplotype were identified by 16S rRNA gene sequencing.
2.3. Antagonism Towards R. solanacearum
2.3.1. In Vitro Inhibition Bioassay
One hundred and sixty-seven XRB were screened for inhibition of the BW pathogen. R. solanacearum strain Rs-09-100 was isolated from BW infected eggplant cultivated in Goa, India, and was used for screening in vitro and in planta. Rs-09-100 belongs to phylotype I, race 1, and biovar 3 of the R. solanacearum species complex. The strain is pathogenic to eggplant cv. Agassaim and causes 100% wilt within 15 days after inoculation under greenhouse conditions (data not shown). Bioassay was performed by the agar well method as described by Ramesh and Phadke [27]. Briefly, single colony of R. solanacearum and XRB was grown in 5 mL CPG broth (Casein hydrolysate 1.0 g/L, Peptone, 10.0 g/L and Glucose, 5.0 g/L) and King's B broth (Peptone, 20.0 g/L, K2HPO4, 1.5 g/L, MgSO4· 7H2O, 1.5 g/L and Glycerol 10.0 mL/L), respectively, at 28 ± 2°C for 48 h with constant shaking at 140 rpm. One hundred and fifty microliters of R. solanacearum was seeded every 100 mL molten cooled King's B agar, mixed well, and poured into plates. After the plates solidified, three wells were made in each plate by removing a circular agar piece with the help of cork borer (8 mm diameter). Twenty-five μL of culture broth of XRB containing 8.0 Log CFU/mL was added into each of the three wells. All the plates were incubated at 28 ± 2°C for 48 h. Plates were observed for inhibition of R. solanacearum. Zones of inhibition were measured as radius in mm from the edge of the agar well. Strains that were found antagonistic to R. solanacearum were screened for in vitro production of antagonistic compounds and plant growth promoting substances and identified by 16S rRNA gene sequencing.
2.4. Production of Volatile and Diffusible Antagonistic Substances by XRB
2.4.1. Hydrogen Cyanide (HCN) Production
Antagonistic strains were tested for HCN production ability in presence of glycine as described by Saraf et al. [31], a slight modification being the use of broth for the HCN test. Immediately after inoculation of strains in King's B broth containing 4.4 g/L of glycine, sterile filter paper strips dipped in picric acid solution were introduced taking care that the strips did not touch the medium and walls of the tube. The tubes were sealed with parafilm and incubated at 28 ± 2°C for 4 days with constant shaking at 140 rpm. The color change of the filter paper strips from yellow to brick red during incubation indicated the production of HCN.
2.4.2. Ammonia Production
To detect ammonia production, antagonistic XRB were grown in peptone water (peptone 20.0 g/L, NaCl 5.0 g/L) with constant shaking at 140 rpm for 48 h at 28 ± 2°C. Ammonia production was determined using Nessler's reagent as described by Marques et al. [32].
2.4.3. Acetoin Production
Acetoin production by antagonistic isolates was tested in Voges Proskauer broth (peptone 7.0 g/L, K2HPO4 5.0 g/L, dextrose 5.0 g/L pH 7.0). After incubation for 30 h at 28 ± 2°C at 140 rpm, one mL each of 5% α napthol and 40% KOH were added to the culture and mixed well. Appearance of red coloration indicated production of acetoin [33].
2.4.4. Siderophore Production
Antagonistic XRB were tested for siderophore production on a medium containing chrome azurol S (CAS) [34]. Isolates producing orange haloes on the blue green colored medium after incubation at 28 ± 2°C for 48 h were positive for siderophore production.
2.5. Production of Growth Promoting Substances by the Antagonistic XRB
2.5.1. Indole Acetic Acid (IAA) Production
Antagonistic strains were tested for their ability to produce phytohormone IAA in presence of tryptophan as described by Gordon and Paleg [35]. Briefly, strains were grown in nutrient broth amended with 100 mg/L of tryptophan for 30 h at 28 ± 2°C at 140 rpm. The supernatants were obtained by centrifugation at 6200 g for 10 min. One mL of supernatant was mixed with one mL of Salkowsky's reagent (50 mL 35% perchloric acid, 1 mL 0.5 M FeCl3). The mixture was allowed to stand at room temperature for five minutes and the absorbance was read at 530 nm. A standard curve was prepared using analytical grade IAA and the concentrations of IAA in the culture supernatants of XRB were estimated based on the curve.
2.5.2. 1-Aminocyclopropane-1-carboxylate (ACC) Deaminase Activity
Antagonistic strains were tested for their ability to produce enzyme ACC deaminase as per the method described by Godinho et al. [36]. Strains were streaked on Dworkin and Foster's DF salts agar containing 3.0 mM ACC and incubated at 28 ± 2°C for 7 days. Ability of the strains to grow on the medium containing ACC as a sole nitrogen source was indicative of ACC deaminase production.
2.5.3. Phosphate Solubilization
Antagonistic strains were tested for phosphate solubilization by a method described by Godinho et al. [36]. All strains were spot inoculated on Pikovskaya's agar plates (Hi Media Laboratories, Mumbai). Plates were incubated at 28 ± 2°C for 48 h. Transparent zones around the growth of XRB on the opaque white medium were indicative of solubilisation phosphate.
2.6. Greenhouse Experiments
2.6.1. Biocontrol Efficacy (BCE) of Antagonistic XRB
Twenty-eight strains of XRB were selected based on in vitro inhibition of R. solanacearum in the agar well bioassay. Strains were evaluated for controlling BW in seedlings of wilt susceptible eggplant cv. Agassaim under greenhouse conditions. Thirty-day-old seedlings raised in nonsterile soil in greenhouse were transplanted in pots filled with standard nonsterile pot mixture (soil : sand : farmyard manure at 2 : 1 : 1 ratio). Ten mL suspension of antagonistic XRB (8.0 Log CFU/mL) in sterile 1 X PBS was applied per seedling by soil drenching. Each treatment consisted of two replicates with two pots per replication and five seedlings per pot. Twenty days after treatment with the antagonistic XRB the seedlings were challenged by inoculating 10 mL suspension of R. solanacearum strain Rs-09-100 (7.0 Log CFU/mL) by soil drenching. Plants not treated with XRB, but challenged with R. solanacearum, served as control. Plants were maintained with suitable watering and percentage of plants infected by wilt was noted until 25 days after challenging with R. solanacearum. Ability of the XRB to prevent wilt in eggplant was expressed as biocontrol efficacy (BCE) and was determined using the formula BCE = ([percent disease in control] − [percent disease in treatment]/percent disease in control) × 100 [37]. Strains with BCE greater than 25% were evaluated for their effect on growth in eggplant under greenhouse conditions.
2.6.2. Growth Promotion Ability of XRB
Sixteen strains of XRB exhibiting biocontrol efficacies greater than 25% were studied for their effect on growth in eggplant. Ability to increase shoot length in wilt susceptible eggplant cv. Agassaim was used as a measure to evaluate their growth promotion efficacy under greenhouse conditions. Thirty-day-old seedlings raised in nonsterile soil in greenhouse were transplanted in pots filled with standard nonsterile pot mixture (soil : sand : farmyard manure at 2 : 1 : 1 ratio). Ten mL suspension of XRB (8.0 Log CFU/mL) in sterile 1 X PBS was applied per seedling by soil drenching. Each treatment consisted of two replicates with two pots per replication and five seedlings per pot. Plants were maintained with suitable watering and plant height was measured from the soil level to the shoot tip 40 days postinoculation. Ability of antagonistic XRB to increase shoot length in eggplant was expressed as growth promotion efficacy (GPE) using the formula ([shoot length increase in treatment] − [shoot length increase in control]/shoot length increase in control) × 100 [38].
2.7. 16S rRNA Gene Sequencing and Sequence Analysis
Representative strains from each of the 38 ARDRA haplotypes and XRB exhibiting antagonism to R. solanacearum were selected for identification. A total of 55 strains were chosen for identification. Fragments of the 16S rRNA gene of size 1500 bp were amplified as described above in the ARDRA section. Amplicons were purified using GeneJet PCR purification kit (Thermo Scientific, USA) and sequenced using 27F and 1492R primers (Xcelris Labs Pvt. Ltd., India). Partial 16S rRNA gene sequences (about 1200 nt) obtained were matched against the sequences available in the nucleotide database from National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST) using the BLASTn (Basic Local Alignment Search Tool) program.
3. Results
3.1. Isolation of Bacteria from the Xylem of Eggplant, Chilli, and S. torvum
In this study, bacteria could be constantly isolated from the xylem of eggplant, chilli, and S. torvum by vacuum infiltration and maceration techniques. Bacterial counts from each isolation ranged from 10 to 102 CFU/mL of xylem sap or macerate. Colonies appeared between 24 and 120 h of aerobic incubation at 28 ± 2°C. Amongst 167 isolates obtained, 99 were Gram-negative rods (59.28%) and 68 were Gram-positive bacteria (40.72%) comprising of 42 rods (61.76%), 25 cocci (36.76%), and one filamentous actinomycete (Table 1).
Table 1.
Overview of diversity and functionality of XRB obtained from eggplant, chilli, and S. torvum.
| Plant host | Number of samples | Total isolates obtained | Gram-negative | Gram-positive | Haplotypesa obtained | Antagonistic strainsb | Antagonistic strains with BCE > 25% | Biocontrol strains with GPE > 10% | Method of isolation |
|---|---|---|---|---|---|---|---|---|---|
| BW susceptible eggplant | 24 | 89 | 53 | 36 | 31 | 16 | 5 | 2 | VI or M |
| BW resistant eggplant | 12 | 36 | 20 | 16 | 17 | 6 | 6 | 5 | VI or M |
| Chilli (Capsicum annuum) | 7 | 33 | 20 | 13 | 19 | 3 | 2 | 1 | M only |
| Solanum torvum | 2 | 9 | 6 | 3 | 7 | 3 | 3 | 1 | VI only |
|
| |||||||||
| Total | 45 | 167 | 99 | 68 | 28 | 16 | 9 | ||
3.2. ARDRA Analysis
ARDRA generated three to six restriction fragments of the 16S rRNA gene amplified from the XRB. Analysis of ARDRA profiles by UPGMA using Dice's coefficient divided XRB with identical restriction profiles into several groups. At 80% similarity values of Dice coefficient, 167 strains of XRB were grouped into 38 haplotypes. Host based analysis of ARDRA revealed that 89 strains isolated from BW susceptible eggplant were grouped in 31 haplotypes, 36 strains from BW resistant eggplant into 17 haplotypes, 33 strains from chilli into 19 haplotypes, and 9 strains from S. torvum into 7 haplotypes, respectively (Table 1). A detailed representation of the ARDRA based analysis of 167 strains is presented in Table 2. Based on the ARDRA analysis of the collection of XRB, 153 strains were distributed over 24 different haplotypes and 14 XRB strains had unique profiles which formed 14 independent haplotypes with one strain in each. Antagonistic strains (n = 28) were distributed over 14 different ARDRA haplotypes wherein 6 antagonists formed independent haplotypes. Nonantagonistic strains were distributed over 24 haplotypes. Haplotypes M80-9 and M80-15 were shared amongst BW susceptible and resistant eggplant, chilli, and S. torvum. Eleven haplotypes were unique to BW susceptible eggplant. Haplotypes M80-1, M8-12, and M80-38 were unique to BW resistant eggplant whereas haplotypes M80-13, M80-19, and M80-26 comprised of strains isolated from chilli. Haplotype M80-36 had a strain isolated from S. torvum. Other haplotypes were a combination of strains isolated from different plant species. These results indicate that bacterial communities from the xylem of mainly eggplant and chilli cultivated in different locations in Goa comprise of diverse bacteria. However it is observed that each ARDRA group consists of bacteria from different plant species and plants collected from different locales. In addition there are certain XRB unshared between each of the plant species that form unique haplotypes. Moreover, strains with biocontrol ability (BCE > 25%) were restricted to only 8 haplotypes, namely, M80-6, M80-7, M80-10, M80-15, M80-20, M80-29, M80-31, and M80-36 (Table 2). Haplotypes M80-6, M80-7, M80-29, M80-31, M80-36, and M80-38 comprised of XRB with GPE > 10%. Interestingly, the strains with BCE > 25% and GPE > 10% within these haplotypes were from BW resistant eggplant (Table 1).
Table 2.
Haplotypes of XRB based on ARDRA with MspI at 80% similarity, plant host, biocontrol, and growth promotion activities.
| Haplotype numbera | Number of strains in each haplotype | Plant host | Strains selected for identification | Number of biocontrol strains with BCE > 25% | Number of strains with GPE > 10% |
|---|---|---|---|---|---|
| M80-1 | 1 | RE | XB159 | 0 | 0 |
| M80-2 | 1 | SE | XB34 | 0 | 0 |
| M80-3 | 1 | SE | XB66 | 0 | 0 |
| M80-4 | 2 | SE, C | XB40 | 0 | 0 |
| M80-5* | 1 | SE | XB140 | 0 | 0 |
| M80-6* | 10 | SE, RE, C | XB177, XB157, XB93, XB169, XB153, XB170 | 4 | 3 |
| M80-7* | 6 | SE, RE, C | XB1, XB86 | 2 | 2 |
| M80-8 | 4 | SE, RE | XB22, XB190 | 0 | 0 |
| M80-9* | 8 | SE, RE, ST, C | XB99, XB100 | 0 | 0 |
| M80-10* | 10 | SE, RE, C | XB196, XB103 | 1 | 0 |
| M80-11 | 9 | SE, RE, C | XB137 | 0 | 0 |
| M80-12 | 1 | RE | XB158 | 0 | 0 |
| M80-13 | 1 | C | XB87 | 0 | 0 |
| M80-14 | 5 | SE, C | XB88 | 0 | 0 |
| M80-15* | 5 | SE, RE, ST, C | XB70 | 1 | 0 |
| M80-16 | 3 | SE | XB47 | 0 | 0 |
| M80-17 | 2 | SE | XB35 | 0 | 0 |
| M80-18 | 1 | SE | XB41 | 0 | 0 |
| M80-19 | 2 | C | XB94 | 0 | 0 |
| M80-20* | 13 | SE, RE, C | XB8, XB20, XB27 | 2 | 0 |
| M80-21 | 5 | SE, ST, C | XB25 | 0 | 0 |
| M80-22 | 1 | SE | XB53 | 0 | 0 |
| M80-23 | 5 | SE, RE, C | XB98 | 0 | 0 |
| M80-24 | 11 | SE, RE, ST | XB161 | 0 | 0 |
| M80-25 | 4 | SE, RE | XB188 | 0 | 0 |
| M80-26 | 1 | C | XB168 | 0 | 0 |
| M80-27* | 2 | SE, C | XB126, XB7 | 0 | 0 |
| M80-28* | 5 | SE, RE | XB122 | 0 | 0 |
| M80-29* | 9 | SE, RE, C | XB165 | 1 | 1 |
| M80-30 | 15 | SE, RE, C | XB167, XB109 | 0 | 0 |
| M80-31* | 11 | SE, ST, C | XB123, XB203, XB62, XB114, XB202 | 3 | 1 |
| M80-32 | 5 | SE, ST, C | XB92 | 0 | 0 |
| M80-33 | 1 | SE | XB37 | 0 | 0 |
| M80-34 | 1 | SE | XB36 | 0 | 0 |
| M80-35 | 2 | SE | XB64 | 0 | 0 |
| M80-36* | 1 | ST | XB200 | 1 | 1 |
| M80-37* | 1 | SE | XB134 | 0 | 0 |
| M80-38* | 1 | RE | XB197 | 1 | 1 |
aHaplotype based on ARDRA analysis using MspI at 80% similarity values of Dice coefficient.
*Haplotype comprises of bacteria showing antagonistic property in the bioassays.
SE: bacterial wilt susceptible eggplant, RE: bacterial wilt resistant eggplant, ST: Solanum torvum, C: chilli plant.
BCE: biocontrol efficacy, GPE: growth promotion efficacy determined as described in Section 2.
3.3. Antagonism towards R. solanacearum
3.3.1. In Vitro Bioassay and Production of Volatile and Diffusible Antagonistic Compounds
Plate based bioassay was used for rapid screening of antagonism of XRB towards R. solanacearum strain Rs-09-100. Results of the in vitro screening against R. solanacearum revealed that 28 amongst 167 XRB exhibited antagonism towards the pathogen (Table 3). Amongst the antagonists, 16 were strains from BW susceptible eggplant, 6 from BW resistant eggplant, and 3 each from chilli and S. torvum (Table 1). Amongst the 28 antagonists, 7 strains, namely, XB62, XB99, XB100, XB114, XB122, XB196, XB197, and XB202 formed larger inhibition zones against R. solanacearum ranging from 4.0 mm to 8.17 mm (Table 3). The majority of the antagonistic strains (n = 16) produced inhibition zones ranging from 2.0 mm to 3.83 mm. XB27, XB134, XB165, and XB169 formed smaller inhibition zones ranging from 1.5 mm to 2.0 mm. However, 139 strains of XRB did not inhibit R. solanacearum in the bioassay test. Twenty-eight antagonistic XRB were screened for production of volatile inhibitory compounds, namely, acetoin, HCN, ammonia, and diffusible siderophore molecules in vitro (Table 3). Acetoin production was observed in 32.14% of the isolates. Bacterial isolates XB7 and XB122 were found to produce both HCN as well as siderophores. XB62, XB93, and XB170 produced HCN whereas XB114, XB140, and XB203 produced siderophores only. XB93, XB99, XB123, XB134, and XB140 produced ammonia.
Table 3.
In vitro inhibition of R. solanacearum and production of antagonistic compounds and plant growth promoting substances by XRB.
| Strain | Haplotype | Inhibition of R. solanacearum | Volatile and diffusible inhibitory compounds | Plant growth promoting substances | |||||
|---|---|---|---|---|---|---|---|---|---|
| Radius in mm | HCN | Ammonia | Acetoin | Siderophore | Phosphate solubilisation | ACC deaminasea | IAAb μg/mL | ||
| XB1 | M80-7 | 2.83 ± 0.29 | − | − | − | − | − | +++ | 105.00 ± 7.07 |
| XB7 | M80-27 | 3.33 ± 0.58 | + | − | − | + | + | − | 47.73 ± 3.21 |
| XB8 | M80-20 | 3.33 ± 0.29 | − | − | − | − | + | − | 19.09 ± 1.29 |
| XB20 | M80-20 | 3.27 ± 0.40 | − | − | − | − | + | − | 25.45 ± 1.71 |
| XB27 | M80-20 | 1.83 ± 0.29 | − | − | − | − | + | − | 15.91 ± 1.07 |
| XB62 | M80-31 | 8.17 ± 0.76 | + | − | − | − | + | ++++ | 73.18 ± 4.93 |
| XB70 | M80-15 | 3.83 ± 0.58 | − | − | − | − | − | − | 171.82 ± 11.57 |
| XB86 | M80-7 | 2.33 ± 0.76 | − | − | − | − | − | +++ | 66.82 ± 4.50 |
| XB93 | M80-6 | 3.17 ± 0.29 | + | − | + | − | + | − | 57.27 ± 3.86 |
| XB99 | M80-9 | 6.83 ± 0.58 | − | + | + | − | + | − | 321.36 ± 21.64 |
| XB100 | M80-9 | 4.50 ± 0.50 | − | + | + | − | + | − | 238.64 ± 16.07 |
| XB114 | M80-31 | 6.50 ± 0.50 | − | − | − | + | + | − | 89.09 ± 6.00 |
| XB122 | M80-28 | 4.33 ± 0.58 | + | − | − | + | + | − | 66.82 ± 4.50 |
| XB123 | M80-31 | 3.00 ± 0.87 | − | + | + | − | + | − | 416.82 ± 28.07 |
| XB126 | M80-27 | 2.83 ± 0.29 | − | − | + | − | + | − | 60.45 ± 4.07 |
| XB134 | M80-37 | 1.57 ± 0.12 | − | + | + | − | + | − | 645.91 ± 43.50 |
| XB140 | M80-5 | 3.67 ± 0.76 | − | + | − | + | + | ++++ | 50.91 ± 3.43 |
| XB153 | M80-6 | 3.17 ± 0.29 | − | − | + | − | + | − | 155.91 ± 10.50 |
| XB157 | M80-6 | 2.50 ± 1.00 | − | − | + | − | + | − | 184.55 ± 12.43 |
| XB165 | M80-29 | 1.57 ± 0.12 | − | − | − | − | − | + | 190.91 ± 12.86 |
| XB169 | M80-6 | 1.53 ± 0.06 | − | − | − | − | − | − | 15.91 ± 1.07 |
| XB170 | M80-6 | 3.51 ± 0.02 | + | − | − | − | + | − | 28.64 ± 1.93 |
| XB177 | M80-6 | 1.99 ± 0.49 | − | − | − | − | − | − | 76.36 ± 5.14 |
| XB196 | M80-10 | 3.93 ± 0.12 | − | − | − | − | − | − | 35.00 ± 2.36 |
| XB197 | M80-38 | 4.33 ± 0.29 | − | − | + | − | − | − | 41.36 ± 2.79 |
| XB200 | M80-36 | 1.87 ± 0.23 | − | − | − | − | − | ++ | 168.64 ± 11.36 |
| XB202 | M80-31 | 4.67 ± 0.21 | − | − | − | − | + | +++ | 82.73 ± 5.57 |
| XB203 | M80-31 | 3.67 ± 0.29 | − | − | − | − | − | − | 70.00 ± 4.71 |
Inhibition zones are mean of three replications and showing standard deviation. Inhibition zone was measured as radius from the outer edge of well. All the experiments were conducted at 28 ± 2°C. + indicates presence of trait; − indicates absence of trait in plate based assays. aLevels of ACC deaminase activities based on growth on plate based assay denoted as + for less growth, +++ for moderate growth, +++ for luxuriant growth, and ++++ for highly luxuriant growth; bIAA was estimated in culture filtrate and expressed as μg/mL using analytical grade IAA as standard; values indicate mean and standard deviation.
3.4. Production of Plant Growth Promoting Substances by Antagonistic XRB
Results of the screening of antagonistic XRB for in vitro production of several plant growth promoting compounds is presented in Table 3. Majority of the antagonistic strains produced the phytohormone IAA with concentrations ranging from 15.91 μg/mL to 645.91 μg/mL. XB202 was found to be the best ACC deaminase producing strain based on its luxuriant growth on DF salts medium supplemented with 3.0 mM ACC as sole nitrogen source. Other ACC deaminase producing strains include XB1, XB62, XB86, and XB140. Scarce growth of XB165 and XB200 was observed on DF salts medium. 64.28% of the strains produced phosphate solubilizing organic acids as indicated by clear haloes on Pikovskaya's agar plate.
3.5. Greenhouse Experiments
3.5.1. Suppression of Bacterial Wilt by Antagonistic XRB
Ability to suppress BW was assessed as the difference in the percentage of wilt in XRB treated plants with respect to wilt in untreated control and was expressed as the biocontrol efficacy (BCE) of the antagonists. BCE of 16 strains with values ranging from 28.6 to 100% is presented in Figure 1. Plants treated with strains XB86, XB169, and XB177 were free from BW and hence recorded 100% biocontrol efficacy. Treatments with XB170, XB197, XB200, XB202, and XB203 recorded 30 percent or less wilt incidence (70% to 85% BCE). XB1, XB27, XB70, XB93, and XB123 treatments recorded BCE between 42.9 and 57.1%. BCE of 28.6% was recorded in XB20 and XB165 treatments. However, 12 antagonistic XRB recorded BCE of 25% and were least effective in wilt protection. Further it is observed that all the antagonistic strains originating from BW resistant eggplant and S. torvum exhibited BCE greater than 25%. Five antagonistic strains from BW susceptible eggplant and three from chilli were effective in preventing wilt in eggplant (Table 1).
Figure 1.
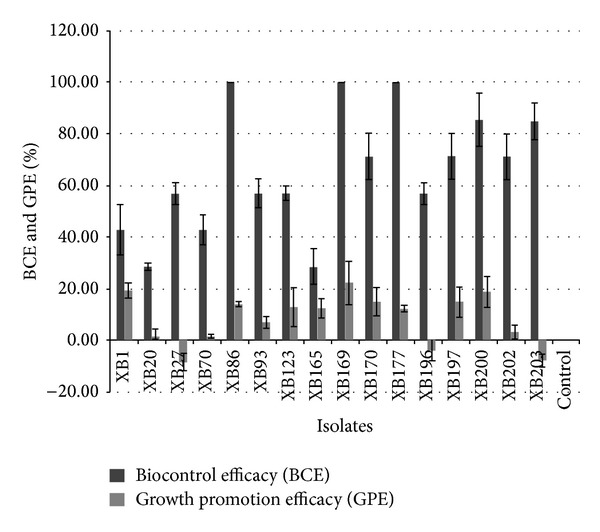
Biocontrol and plant growth promotion efficacies of select XRB in eggplant. BCE: biocontrol efficacy determined 25 days after challenging with R. solanacearum; GPE: growth promotion efficacy determined 40 days after treatment with XRB. Percent BCE and GPE calculated using formulae BCE = ([percent disease in control] − [percent disease in treatment]/percent disease in control) × 100 and GPE = ([shoot length increase in treatment] − [shoot length increase in control]/shoot length increase in control) × 100, respectively. Uninoculated control had BCE and GPE values of 0.00; XB86, XB169, and XB177 had BCE of 100.00% in all the replications. Bars indicate mean values of % BCE and GPE; error bars indicate standard deviation.
3.5.2. Growth Promotion by Antagonistic XRB
Increase in shoot length of eggplant (40 days after treatment) observed in XRB treated plants in relation to untreated control was expressed as growth promotion efficacy (GPE) and is shown in Figure 1. Amongst the 16 strains which were effective in preventing wilt, six strains exhibited the highest increase in shoot length as indicated by their GPE values in the range of 13.9–22.3%. Seven XRB recorded a GPE value ranging from 1.4 to 12.9%. However, strains XB27, XB196, and XB203 stunted shoot growth in eggplant, in comparison to untreated control. When the source of XRB is considered, 55.55% strains that exhibited GPE greater than 10% were isolated from BW resistant eggplant. Whereas, only two antagonists from BW susceptible plant and one each from chilli and S. torvum were able to promote growth in eggplant (Table 1). Strains with GPE > 10% belonged to six different ARDRA haplotypes (Table 2).
3.6. Identification of XRB by 16S rRNA Gene Sequencing
16S rRNA gene sequences of XRB were used to identify the diverse xylem inhabitants and antagonistic strains. Identity of 55 XRB based on 16S rRNA gene sequencing and their GenBank accessions are presented in Table 4. Overall, 23 different genera of bacteria were identified. Major genera identified are Bacillus sp. (11 strains), Enterobacter sp. (6 strains), Microbacterium sp. (5 strains), Staphylococcus sp. (5 strains), Pseudomonas sp. (5 strains), and Agrobacterium sp. (3 strains). Additional genera identified including Micrococcus sp., Sphingomonas sp., Flavobacterium sp., Chryseobacterium sp., Burkholderia sp., and Xenophilus sp. are listed in Table 4. Based on the identification, phylum Proteobacteria consisting of Gram-negative bacteria of subdivisions Alpha Proteobacteria (12.73%), Beta Proteobacteria (3.64%), and Gamma Proteobacteria (25.45%) were predominant (41.81% strains identified), followed by phyla Firmicutes (29.09%), Actinobacteria (25.45%), and Bacteroidetes (3.64%) (Figure 2(a)).
Table 4.
List of XRB identified by partial 16S rRNA gene sequencing their plant host, accession numbers, closest NCBI match, and % similarity.
| Strain | Plant host | Accession number | Closest NCBI match | % similarity |
|---|---|---|---|---|
| XB1* | SE | KF447383 | Agrobacterium tumefaciens | 99 |
| XB7* | SE | KF447384 | Pseudomonas aeruginosa | 99 |
| XB8* | SE | KF447385 | Staphylococcus haemolyticus | 99 |
| XB20* | SE | KF447386 | Staphylococcus haemolyticus | 100 |
| XB22 | SE | KF447387 | Brevibacterium casei | 99 |
| XB25 | C | KF447388 | Enterobacter sp. | 95 |
| XB27* | SE | KF447389 | Staphylococcus haemolyticus | 99 |
| XB34 | SE | KF447390 | Curtobacterium sp. | 99 |
| XB35 | SE | KF447391 | Microbacterium sp. | 99 |
| XB36 | SE | KF447392 | Xenophilus sp. | 99 |
| XB37 | SE | KF447393 | Chryseobacterium sp. | 99 |
| XB40 | SE | KF447394 | Micrococcus luteus | 89 |
| XB41 | SE | KF447395 | Bacillus sp. | 99 |
| XB47 | SE | KF447396 | Bacillus sp. | 99 |
| XB53 | SE | KF447397 | Micrococcus sp. | 99 |
| XB62* | SE | KF447398 | Pseudomonas sp. | 99 |
| XB64 | SE | KF447399 | Bacillus thuringiensis | 100 |
| XB66 | SE | KF447400 | Pectobacterium carotovorum | 99 |
| XB70* | SE | KF447401 | Janibacter melonis | 99 |
| XB86* | C | KF447402 | Agrobacterium tumefaciens | 99 |
| XB87 | C | KF447403 | Bacillus barbaricus | 99 |
| XB88 | C | KF447404 | Bacillus sp. | 99 |
| XB92 | C | KF447405 | Brevundimonas vesicularis | 99 |
| XB93* | C | KF447406 | Bacillus safensis | 100 |
| XB94 | C | KF447407 | Bacillus sp. | 100 |
| XB98 | C | KF447408 | Microbacterium sp. | 100 |
| XB99* | SE | KF447409 | Enterobacter sp. | 99 |
| XB100* | SE | KF447410 | Enterobacter cloacae | 98 |
| XB103 | SE | KF447411 | Brachybacterium phenoliresistens | 99 |
| XB109 | SE | KF447412 | Rhodococcus corynebacterioides | 99 |
| XB114* | SE | KF447413 | Pseudomonas stutzeri | 99 |
| XB122* | SE | KF913446 | Pseudomonas aeruginosa | 100 |
| XB123* | SE | KF447414 | Enterobacter sp. | 99 |
| XB126* | C | KF447415 | Pantoea eucrina | 99 |
| XB134* | SE | KF447416 | Enterobacter sp. | 99 |
| XB137 | SE | KF447417 | Klebsiella sp. | 99 |
| XB140* | SE | KF447418 | Burkholderia sp. | 99 |
| XB153* | SE | KF447419 | Bacillus amyloliquefaciens | 99 |
| XB157* | SE | KF447420 | Bacillus subtilis | 100 |
| XB158 | RE | KF447421 | Bosea sp. | 99 |
| XB159 | RE | KF447422 | Microbacterium xylanilyticum | 99 |
| XB161 | RE | KF447423 | Microbacterium aurum | 99 |
| XB165* | RE | KF447424 | Agrobacterium sp. | 99 |
| XB167 | RE | KF447425 | Microbacterium aurum | 99 |
| XB168 | RE | KF447426 | Bacillus aryabhattai | 100 |
| XB169* | RE | KF447427 | Staphylococcus gallinarum | 99 |
| XB170* | RE | KF447428 | Staphylococcus sp. | 99 |
| XB177* | RE | KF447429 | Bacillus cereus | 99 |
| XB188 | RE | KF447430 | Sphingomonas sp. | 99 |
| XB190 | RE | KF447431 | Brevibacterium casei | 99 |
| XB196* | RE | KF447432 | Enterobacter kobei | 99 |
| XB197* | RE | KF447433 | Sphingomonas sp. | 98 |
| XB200* | ST | KF913447 | Streptomyces sp. | 100 |
| XB202* | ST | KF447434 | Pseudomonas sp. | 99 |
| XB203* | ST | KF447435 | Flavobacterium sp. | 99 |
*Strain is antagonistic to R. solanacearum based on bioassay.
SE: bacterial wilt susceptible eggplant, RE: bacterial wilt resistant eggplant, C: chilli, ST: Solanum torvum.
Figure 2.
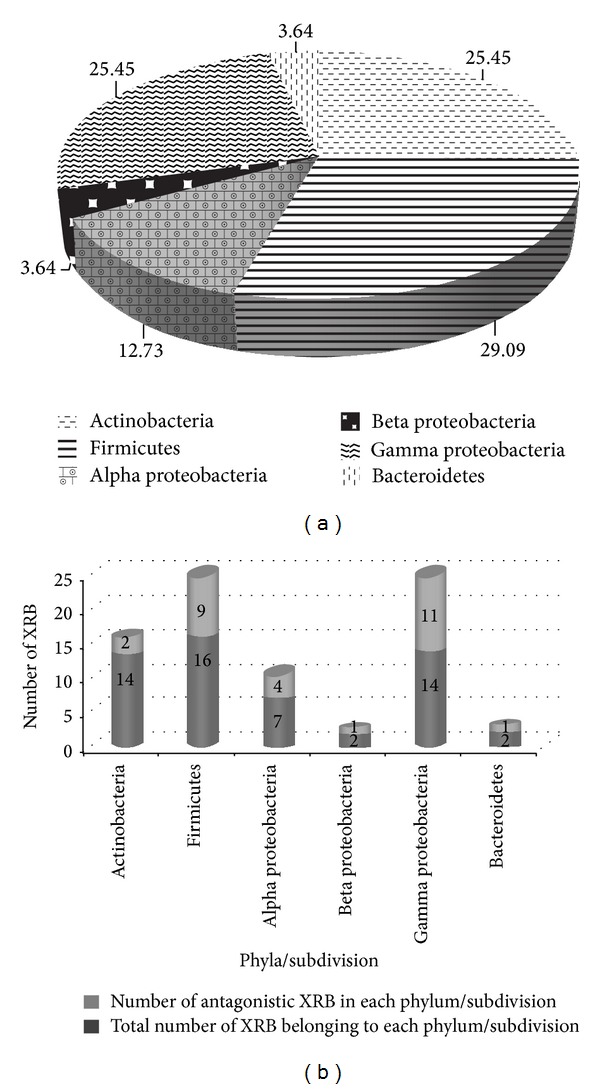
(a) Distribution of XRB (phylum/subdivision level) identified by 16S rRNA gene sequencing. Values indicate percentages of strains belonging to each phyla/subdivision amongst the 55 identified strains. (b) Distribution of antagonistic and nonantagonistic strains of XRB in each phylum/subdivision.
Eleven antagonistic strains identified belonged to Gamma subdivision of Proteobacteria consisting of five strainseach of Enterobacter sp. and fluorescent and nonfluorescent Pseudomonas sp. and one strain of Pantoea eucrina (XB126) (Figure 2(b)). Nine antagonists identified were of phyla Firmicutes consisting of Staphylococcus sp. (5 strains) and Bacillus sp. (4 strains). Agrobacterium strains XB1, XB86, and XB165, Sphingomonas sp. (XB197) of the Alpha Proteobacteria, Streptomyces sp. (XB200), and Janibacter melonis (XB70) of phyla Actinobacteria were found to be antagonistic. Additional antagonistic XRB include Burkholderia sp. (XB140) ofβ Proteobacteria and Flavobacterium sp. (XB203) of phyla Bacteroidetes (Figure 2(b)).
3.7. Statistical Analysis
The statistical analysis of percentage wilts and shoot length of eggplant was performed using Web Agri Statistical Package (WASP) version 2.0 (http://www.icargoa.res.in/wasp2.0/index/php).
4. Discussion
Eggplant and chilli not only are of economic and cultural importance but also are common ingredients in the cuisine throughout India. In the coastal state of Goa, R. solanacearum has been reported to be a destructive pathogen in cultivation of eggplant and chilli [4]. Isolation of biocontrol agents against the BW pathogen has been commonly restricted to endophytic tissue and plant rhizosphere [26, 27]. Studies on xylem colonizing endophytes were undertaken because we speculated existence of interactions between the XRB and vascular wilt pathogen R. solanacearum during xylem colonization. Our study reveals the diversity, biocontrol potential, and identity of endophytic xylem colonizers from solanaceous crops cultivated in Goa, India. A total of 167 bacteria were isolated from the xylem of eggplant, chilli, and S. torvum with Gram-negative bacteria (59.28%) predominating in the collection. Congruent to our observation, Gardner et al. [7] and Bell et al. [11] have earlier reported isolation of more number of Gram-negative rod shaped bacteria from xylem of citrus and grapevine using vacuum infiltration. Scholander pressure bomb was found to be useful in extraction of diverse bacterial genera from xylem tissues in contrast to trituration methods that yielded higher number of Gram-positive rod shaped bacteria [12]. Though, Scholander pressure bomb was not used in this study, a combination of vacuum infiltration [7, 11] and trituration of decorticated stems [10, 12] was employed with an aim to isolate diverse XRB from xylem tissues. This is the first study reporting the use of vacuum infiltration and trituration methods for isolating xylem residing bacteria from eggplant, chilli, and S. torvum.
Traditionally bacteria have been characterized and grouped based on colony morphology and biochemical tests. However, whole genome fingerprinting or PCR-RFLP based methods are rapid tools for determining genetic diversity of bacteria in a given collection. ARDRA which is a type of PCR based RFLP method has been used widely in estimating genetic diversity endophytic bacterial populations and clustering genetically similar strains [39–41]. In addition to earlier reports, our study demonstrates the usefulness of ARDRA as a tool to cluster genetically identical strains of endophytic XRB isolated from eggplant, chilli, and S. torvum. In our study 91.61% strains (n = 153) were grouped in 24 haplotypes by using ARDRA. The majority of these haplotypes represent a combination of XRB isolated from different solanaceous plants from diverse locales. These results indicate that xylem of eggplant, chilli, and S. torvum largely bears similar population of XRB which can efficiently cross colonize eggplant, chilli, or S. torvum. Nevertheless, 8.39% strains had unique ARDRA fingerprint and formed separable haplotypes. This observation leads to a conclusion that a minor population of xylem inhabitants are restricted to a specific plant species and cannot easily cross colonize xylem of other solanaceous plants. Plants are known to selectively support endophytic colonization by specific bacteria [14]. However, factors that determine the selection of xylem colonists or the ability of XRB to colonize eggplant, chilli, and S. torvum in this study remain unknown. Interestingly, the structure of endophytic community in Nicotiana attenuata a member of Solanaceae family is shown to be influenced by soil composition and ethylene homeostasis [40]. Earlier evidence has shown that colonization by endophytic bacteria is also governed by plant genotype as well as root exudates [42].
Only 16.77% XRB out of 167 were antagonistic to R. solanacearum based on in vitro assays. Antagonistic XRB produced volatile and diffusible inhibitory compounds, namely, HCN, ammonia, and acetoin and siderophores. These substances have been long known to be involved in disease suppression and indirect growth promotion in plants [43–46]. These mechanisms possibly played a role in the evident biocontrol effect against BW exhibited by the XRB in the greenhouse screening. Endophytic bacteria have been known to have plant growth promoting traits, namely, production of IAA, ACC deaminase, and phosphate solubilization [25, 33, 36]. These traits were detected in the majority of antagonistic XRB tested in this study and may have resulted in the observed increase in shoot length of eggplant in our greenhouse experiments. In contrast, strains XB20, XB196, and XB203 suppressed growth in eggplant under greenhouse conditions; however no visible symptoms of disease were observed. Vascular plugging and production of certain metabolites toxic to plant cells, but not cell viability, may have resulted in stunted shoot in eggplant [11, 47].
Evaluation of efficacy of antagonistic organisms to suppress the plant diseases under greenhouse conditions is one of the key steps for selecting a potential biocontrol agent for disease management [48]. Endophytic strains from BW susceptible varieties of eggplant have been shown to prevent wilt and promote growth in eggplant earlier [27]. Our greenhouse screening shows that 38.46% of antagonistic XRB with biocontrol efficacies greater than 40% were isolates from BW resistant varieties of eggplant. This raises a question whether the bacteria from resistant varieties are involved in BW resistance and whether BW resistant varieties are able to selectively influence xylem colonization by antagonistic bacteria? Presence of higher number of endophytes with antagonistic abilities was reported in BW resistant varieties of tomato as compared to susceptible varieties, and their role in resistance to BW was proposed [49]. Similar observations on correlation of resistance of potato to soft rot and endophytic bacteria have been reported [50]. Thus BW resistant varieties can be considered a better host for isolating potential biocontrol strains for management of bacterial wilt.
Identification of 55 XRB strains by 16S rRNA gene sequencing revealed the presence of 23 diverse genera of bacteria belonging to 4 phyla of Eubacteria. Strains belonging to phyla Firmicutes, Actinobacteria, and γ subdivision of Proteobacteria were the major xylem colonists identified in this study. Several genera of bacteria belonging to these phyla have also been reported to be present in endophytic tissues and xylem of a variety of other agricultural and horticultural plant species [14, 51]. In addition, the majority of the antagonists identified belonged to Enterobacter sp., Pseudomonas sp., Bacillus sp., and Staphylococcus sp. Congruent to our results, several researchers have reported bacteria isolated from solanaceous crops and belonging to similar genera to be antagonistic to R. solanacearum [26, 51–53]. However, Flavobacterium sp.and Janibacter melonis identified in this study have never been previously reported to be inhibitory to R. solanacearum. Large population of the xylem inhabiting bacterial flora accounting for 83.23% exhibited no antagonism towards R. solanacearum. Nonantagonistic XRB were identified predominantly as Microbacterium sp. Endophytic persistence and nematicidal activities of Microbacterium sp. have been reported [54, 55]. Therefore the collection of XRB isolated in this study can be screened for inhibitory activities against other important agricultural pests.
Though few strains, namely, XB86, XB169, and XB177 exhibited plant beneficial properties in this study their usefulness in plant disease control remains to be seen. XB86 has been identified as Agrobacterium tumefaciens, the crown gall disease pathogen, and its deployment as biocontrol agent is uncertain. XB169 (Staphylococcus gallinarum) and XB177 (Bacillus cereus) are reported as opportunistic animal pathogens and thus unsuitable for field applications. Streptomyces sp. has earlier been reported as antagonistic to R. solanacearum and tested for management of wilt in potato and tomato [56, 57]. XB200 (Streptomyces sp.) is one of the XRB high BCE and GPE; it could be explored further for biocontrol of bacterial wilt after additional characterization and field evaluation.
5. Conclusion
This study is the first report on the identity of novel and diverse XRB colonizing the xylem of eggplant, chilli, and S. torvum. XRB particularly from BW resistant varieties were found to protect eggplant from bacterial wilt and enhanced growth in eggplant in the greenhouse screening. Therefore the repertoire of XRB reported in this study may be useful for cultivation of eggplant in BW affected areas.
Acknowledgments
The authors greatly acknowledge financial support from Indian Council of Agricultural Research (ICAR),New Delhi, India, through “Outreach project on Phytopthora, Fusarium and Ralstonia diseases of horticultural and field crops”- (PhytoFuRa) and the Director ICAR Research Complex for Goa, India, for the facilities.
Conflict of Interests
The authors hereby declare that they have no conflict of interests regarding the publication of this paper.
References
- 1.Yabuuchi E, Kosako Y, Oyaizu H, et al. Proposal of Burkholderia gen. nov. and transfer of seven species of the genus Pseudomonas homology group II to the new genus, with the type species Burkholderia cepacia (Palleroni and Holmes 1981) comb. nov. Microbiology and Immunology. 1992;36(12):1251–1275. doi: 10.1111/j.1348-0421.1992.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 2.Wicker E, Grassart L, Coranson-Beaudu R, et al. Ralstonia solanacearum strains from Martinique (French West Indies) exhibiting a new pathogenic potential. Applied and Environmental Microbiology. 2007;73(21):6790–6801. doi: 10.1128/AEM.00841-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Kanda A, Yano K, et al. Molecular typing of Japanese strains of Ralstonia solanacearum in relation to the ability to induce a hypersensitive reaction in tobacco. Journal of General Plant Pathology. 2009;75(5):369–380. [Google Scholar]
- 4.Ramesh R. Field evaluation of biological control agents for the management of Ralstonia solanacearum in Brinjal. Journal of Mycology and Plant Pathology. 2006;36(2):327–328. [Google Scholar]
- 5.Vasse J, Frey P, Trigalet A. Microscopic studies of intercellular infection and protoxylem invasion of tomato roots by Pseudomonas solanacearum . Molecular Plant-Microbe Interactions. 1995;8(2):241–251. [Google Scholar]
- 6.Genin S, Boucher C. Ralstonia solanacearum: secrets of a major pathogen unveiled by analysis of its genome. Molecular Plant Pathology. 2002;3(3):111–118. doi: 10.1046/j.1364-3703.2002.00102.x. [DOI] [PubMed] [Google Scholar]
- 7.Gardner JM, Feldman AW, Zablotowicz RM. Identity and behavior of xylem-residing bacteria in rough lemon roots of Florida citrus trees. Applied and Environmental Microbiology. 1982;43(6):1335–1342. doi: 10.1128/aem.43.6.1335-1342.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs MJ, Bugbee WM, Gabriellson DA. Enumeration, location, and characterization of endophytic bacteria within sugar beet roots. Canadian Journal of Botany. 1985;63(7):1262–1265. [Google Scholar]
- 9.Patriquin DG, Döbereiner J. Light microscopy observations of tetrazolium-reducing bacteria in the endorhizosphere of maize and other grasses in Brazil. Canadian Journal of Microbiology. 1978;24(6):734–742. doi: 10.1139/m78-122. [DOI] [PubMed] [Google Scholar]
- 10.Gagné S, Richard C, Rousseau H, Antoun H. Xylem-residing bacteria in alfalfa roots. Canadian Journal of Microbiology. 1987;33(11):996–1000. [Google Scholar]
- 11.Bell CR, Dickie GA, Harvey WLG, Chan JWYF. Endophytic bacteria in grapevine. Canadian Journal of Microbiology. 1995;41(1):46–53. [Google Scholar]
- 12.Hallmann J, Kloepper JW, Rodríguez-Kábana R. Application of Scholander pressure bomb to studies on endophytic bacteria of plants. Canadian Journal of Microbiology. 1997;43(5):411–416. [Google Scholar]
- 13.Lampel JS, Canter GL, Dimock MB, et al. Integrative cloning, expression, and stability of the cryIA(c) gene from Bacillus thuringiensis subsp. kurstaki in a recombinant strain of Clavibacter xyli subsp. cynodontis . Applied and Environmental Microbiology. 1994;60(2):501–508. doi: 10.1128/aem.60.2.501-508.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenblueth M, Martínez-Romero E. Bacterial endophytes and their interactions with hosts. Molecular Plant-Microbe Interactions. 2006;19(8):827–837. doi: 10.1094/MPMI-19-0827. [DOI] [PubMed] [Google Scholar]
- 15.Hardoim PR, van Overbeek LS, van Elsas JD. Properties of bacterial endophytes and their proposed role in plant growth. Trends in Microbiology. 2008;16(10):463–471. doi: 10.1016/j.tim.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Aravind R, Kumar A, Eapen SJ, Ramana KV. Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: isolation, identification and evaluation against Phytophthora capsici . Letters in Applied Microbiology. 2009;48(1):58–64. doi: 10.1111/j.1472-765X.2008.02486.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuklinsky-Sobral J, Araújo WL, Mendes R, Geraldi IO, Pizzirani-Kleiner AA, Azevedo JL. Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environmental Microbiology. 2004;6(12):1244–1251. doi: 10.1111/j.1462-2920.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 18.Compant S, Duffy B, Nowak J, Clément C, Barka EA. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Applied and Environmental Microbiology. 2005;71(9):4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorne ET, Young BM, Young GM, et al. The structure of xylem vessels in grapevine (Vitaceae) and a possible passive mechanism for the systemic spread of bacterial disease. American Journal of Botany. 2006;93(4):497–504. doi: 10.3732/ajb.93.4.497. [DOI] [PubMed] [Google Scholar]
- 20.Seo WT, Lim WJ, Kim EJ, Yun HD, Lee YH, Cho KM. Endophytic bacterial diversity in the young radish and their antimicrobial activity against pathogens. Journal of Applied Biological Chemistry. 2010;53(4):493–503. [Google Scholar]
- 21.Compant S, Kaplan H, Sessitsch A, Nowak J, Ait Barka E, Clément C. Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: from the rhizosphere to inflorescence tissues. FEMS Microbiology Ecology. 2005;63(1):84–93. doi: 10.1111/j.1574-6941.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- 22.Miguel PSB, Delvaux JC, de Oliveira MNV, et al. Diversity of endophytic bacteria in the fruits of Coffea canephora . African Journal of Microbiology Research. 2013;7(7):586–594. [Google Scholar]
- 23.López-López A, Rogel MA, Ormeño-Orrillo E, Martínez-Romero J, Martínez-Romero E. Phaseolus vulgaris seed-borne endophytic community with novel bacterial species such as Rhizobium endophyticum sp. nov. . Systematic and Applied Microbiology. 2010;33(6):322–327. doi: 10.1016/j.syapm.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Lwin M, Ranamukhaarachchi SL. Development of biological control of Ralstonia solanacearum through antagonistic microbial populations. International Journal of Agriculture and Biology. 2006;8(5):657–660. [Google Scholar]
- 25.Rasche F, Marco-Noales E, Velvis H, et al. Structural characteristics and plant-beneficial effects of bacteria colonizing the shoots of field grown conventional and genetically modified T4-lysozyme producing potatoes. Plant and Soil. 2006;289(1-2):123–140. [Google Scholar]
- 26.Amaresan N, Jayakumar V, Kumar K, Thajuddin N. Endophytic bacteria from tomato and chilli, their diversity and antagonistic potential against Ralstonia solanacearum . Archives of Phytopathology and Plant Protection. 2012;45(3):344–355. [Google Scholar]
- 27.Ramesh R, Phadke GS. Rhizosphere and endophytic bacteria for the suppression of eggplant wilt caused by Ralstonia solanacearum . Crop Protection. 2012;37:35–41. [Google Scholar]
- 28.French WJ, Christis RG, Stassi DL. Recovery of rickettsialike bacteria by vacuum infiltration of peach tissues affected with phony disease. Phytopathology. 1977;67:945–948. [Google Scholar]
- 29.Viss PR, Brooks EM, Driver JA. A simplified method for the control of bacterial contamination in woody plant tissue culture. In Vitro Cellular and Developmental Biology. 1991;27(1, article 42) [Google Scholar]
- 30.Wilson K. Preparation of genomic DNA from bacteria. In: Ausubel FM, Brent R, Kingston RE, et al., editors. Current Protocols in Molecular Biology. New York, NY, USA: John Wiley & Sons; 1997. pp. 2.4.1–2.4.5. [Google Scholar]
- 31.Saraf M, Thakkar A, Pandya U, Joshi M, Parikh J. Potential of plant growth promoting microorganisms as biofertilizers and biopesticides and it's exploitation in sustainable agriculture. Journal of Microbiology and Biotechnology Research. 2013;3(5):54–62. [Google Scholar]
- 32.Marques APGC, Pires C, Moreira H, Rangel AOSS, Castro PML. Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biology and Biochemistry. 2010;42(8):1229–1235. [Google Scholar]
- 33.Hao X, Xie P, Johnstone L, Miller SJ, Rensing C, Wei G. Genome sequence and mutational analysis of plant-growth-promoting bacterium Agrobacterium tumefaciens CCNWGS0286 isolated from a zinc-lead mine tailing. Applied and Environmental Microbiology. 2012;78(15):5384–5394. doi: 10.1128/AEM.01200-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Analytical Biochemistry. 1987;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 35.Gordon SA, Paleg LG. Quantitative measurement of indole acetic acid. Plant Physiology. 1957;10:37–48. [Google Scholar]
- 36.Godinho A, Ramesh R, Bhosle S. Bacteria from sand dunes of Goa promoting growth in eggplant. World Journal of Agricultural Sciences. 2010;6(5):555–564. [Google Scholar]
- 37.Guo J-H, Qi H-Y, Guo Y-H, et al. Biocontrol of tomato wilt by plant growth-promoting rhizobacteria. Biological Control. 2004;29(1):66–72. [Google Scholar]
- 38.Aliye N, Fininsa C, Hiskias Y. Evaluation of rhizosphere bacterial antagonists for their potential to bioprotect potato (Solanum tuberosum) against bacterial wilt (Ralstonia solanacearum) Biological Control. 2008;47(3):282–288. [Google Scholar]
- 39.Lagacé L, Pitre M, Jacqeus M, Roy D. Identification of the bacterial community of maple sap by using amplified ribosomal DNA (rDNA) restriction analysis and rDNA sequencing. Applied and Environmental Microbiology. 2004;70(4):2052–2060. doi: 10.1128/AEM.70.4.2052-2060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Long HH, Sonntag DG, Schmidt DD, Baldwin IT. The structure of the culturable root bacterial endophyte community of Nicotiana attenuata is organized by soil composition and host plant ethylene production and perception. New Phytologist. 2010;185(2):554–567. doi: 10.1111/j.1469-8137.2009.03079.x. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Fuentes E, Ruíz-Valdiviezo VM, Martínez-Romero E, Gutiérrez-Miceli FA, Dendooven L, Rincón-Rosales R. Bacterial community in the roots and rhizosphere of Hypericum silenoides Juss. 1804. African Journal of Microbiology Research. 2012;6(11):2704–2711. [Google Scholar]
- 42.Fang M, Kremer RJ, Motavalli PP, Davis G. Bacterial diversity in rhizospheres of nontransgenic and transgenic corn. Applied and Environmental Microbiology. 2005;71(7):4132–4136. doi: 10.1128/AEM.71.7.4132-4136.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howell CR, Stiphanovic RD. Production of ammonia by Enterobacter cloaceae and its possible role in biological control of Pythium pre-emergence damping-off by the bacterium. Ecology and Epidemiology. 1988;78(8):1075–1078. [Google Scholar]
- 44.Ramette A, Frapolli M, Défago G, Moënne-Loccoz Y. Phylogeny of HCN synthase-encoding hcnBC genes in biocontrol fluorescent pseudomonads and its relationship with host plant species and HCN synthesis ability. Molecular Plant-Microbe Interactions. 2003;16(6):525–535. doi: 10.1094/MPMI.2003.16.6.525. [DOI] [PubMed] [Google Scholar]
- 45.Loaces I, Ferrando L, Scavino AF. Dynamics, diversity and function of endophytic siderophore-producing bacteria in rice. Microbial Ecology. 2011;61(3):606–618. doi: 10.1007/s00248-010-9780-9. [DOI] [PubMed] [Google Scholar]
- 46.Kim YC, Leveau J, Gardener BBM, Pierson EA, Pierson LS, Ryu C-M. The multifactorial basis for plant health promotion by plant-associated bacteria. Applied and Environmental Microbiology. 2011;77(5):1548–1555. doi: 10.1128/AEM.01867-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardner JM, Chandler JL, Feldman AW. Growth responses and vascular plugging of citrus inoculated with rhizobacteria and xylem-resident bacteria. Plant and Soil. 1985;86(3):333–345. [Google Scholar]
- 48.Pliego C, Ramos C, de Vicente A, Cazorla FM. Screening for candidate bacterial biocontrol agents against soilborne fungal plant pathogens. Plant and Soil. 2011;340(1-2):505–520. [Google Scholar]
- 49.Feng H, Li Y, Liu Q. Endophytic bacterial communities in tomato plants with differential resistance to Ralstonia solanacearum . African Journal of Microbiology Research. 2013;7(15):1311–1318. [Google Scholar]
- 50.Sturz AV, Christie BR, Matheson BG, Arsenault WJ, Buchanan NA. Endophytic bacterial communities in the periderm of potato tubers and their potential to improve resistance to soil-borne plant pathogens. Plant Pathology. 1999;48(3):360–369. [Google Scholar]
- 51.Sturz AV, Christie BR, Nowak J. Bacterial endophytes: potential role in developing sustainable systems of crop production. Critical Reviews in Plant Sciences. 2000;19(1):1–30. [Google Scholar]
- 52.Ramesh R, Joshi AA, Ghanekar MP. Pseudomonads: major antagonistic endophytic bacteria to suppress bacterial wilt pathogen, Ralstonia solanacearum in the eggplant (Solanum melongena L.) World Journal of Microbiology and Biotechnology. 2009;25(1):47–55. [Google Scholar]
- 53.Nawangsih AA, Damayanti I, Wiyono S, Kartika JG. Selection and characterization of endophytic bacteria as biocontrol agents of tomato Bacterial Wilt Disease. Hayati Journal of Biosciences. 2011;18(2):66–70. [Google Scholar]
- 54.Vargas-Ayala R, Rodríguez-Kábana R, Morgan-Jones G, McInroy JA, Kloepper JW. Shifts in soil microflora induced by velvetbean (Mucuna deeringiana) in cropping systems to control root-knot nematodes. Biological Control. 2000;17(1):11–22. [Google Scholar]
- 55.Zinniel DK, Lambrecht P, Harris NB, et al. Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Applied and Environmental Microbiology. 2002;68(5):2198–2208. doi: 10.1128/AEM.68.5.2198-2208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lemessa F, Zeller W. Screening rhizobacteria for biological control of Ralstonia solanacearum in Ethiopia. Biological Control. 2007;42(3):336–344. [Google Scholar]
- 57.El-Albyad MS, El-Sayed MA, El-Shanshoury AR, El-Batanouny NH. Effect of culture conditions on the antimicrobial activities of UV-mutants of Streptomyces corchorusii and S. spiroverticillatus against bean and banana wilt pathogens. Microbiological Research. 1996;151(2):201–211. [Google Scholar]


