Unlike most other broadly disseminated tools of biotechnology, moral, legal, political, and economic factors impact whether and how hES cell lines reach the researchers who need them. On August 9, 2001, President George W. Bush announced that federal funding for hES cell research would be limited to already existing cell lines. That policy constrained academic scientists by hampering their ability to freely create or select research materials (Rao, 2006). Since Bush's announcement, there have been anecdotal reports about how federal and state policies might hinder or aid this promising area of research (Longaker et. Al, 2007). Yet no research has described, when, where and whom these lines go when they leave major repositories, and little empirical evidence examines the effects variation in state level policies might exert on researcher's use of hES cell lines. To address these questions, we use materials transfer agreements (MTAs), to track shipments of 1662 vials of stem cells from two major U.S.-based repositories, WiCell Research Institute (WiCell) and the Harvard Stem Cell Institute (HSCI). We begin by documenting aggregate trends in the global distribution of hES cell lines. Next, we analyze interstate differences in shipment rates by biomedical research capacity, indexed by cumulative NIH funding levels and state-level hES cell policies. We find that predictions about the chilling effect of restrictive federal policies are oversimplified. This report provides the first systematic description of global and national flows of hES cell lines from two major U.S.-based repositories.
Distribution rates and patterns for hES cell lines can serve as a proxy for research activity in this new field. Flows of cell lines can indicate how outside factors help shape the trajectory of biomedical research. In turn, these factors have important consequences for scientific collaboration, policymaking, and the eventual use of stem cell therapies. Furthermore, the lines available from WiCell are approved for use with U.S. federal funds while the lines from HSCI are not, providing an interesting point of comparison.
Many sources of funding also impact research capacity. These include industry-sponsored research, foundations, philanthropy, and state responses such as economic development efforts. Systematic data on such funding sources are difficult to obtain, and thus we use NIH expenditures to ensure the breadth and comparability of our analyses. Though by no means the only measure of a state's research capacity, we treat NIH funding as an important, though rough proxy for state-level biomedical research capacity.
We find evidence of a complex relationship among federal and state policies, state-level research capacity, and demand for cell lines. National proscriptions do not exert equal influence on the behavior of scientists in different states. State attempts to enhance scientific efforts by policy fiat do not uniformly spur demand for lines absent existing research capacity. Past success in NIH funding can make states without supportive policies important sites for stem cell science. In sum, varied state-level polices can shape and underpin national growth in demand for cell lines.
The exchange of research tools between owners and users are often bound by material transfer agreements (MTAs), legal documents that govern the use of reagents and cell lines. We obtained information about MTAs executed between 2000 and 2007 by WiCell and HSCI and document the number of vials in total and from the individual sources over time as well as to what state or nation. Each MTA was categorized by destination and cell line. The resulting data set consisted of 724 MTAs issued by WiCell between 2000 and 2007, and 232 MTAs issued by HSCI between 2004 and 2007. WiCell appears to execute one MTA for every vial it ships, while Harvard issues multiple lines under one agreement. The numbers of vials shipped are 743 and 919, respectively. In total, the data tracks the distribution of 1662 vials of 38 different hES cell lines. The fact that the HSCI has distributed nearly 200 more vials than has WiCell in about half the time might be explained by the fact that until the fall of 2005 the cost for a WiCell line was $5,000, while users of lines from HSCI were charged the cost of shipping.
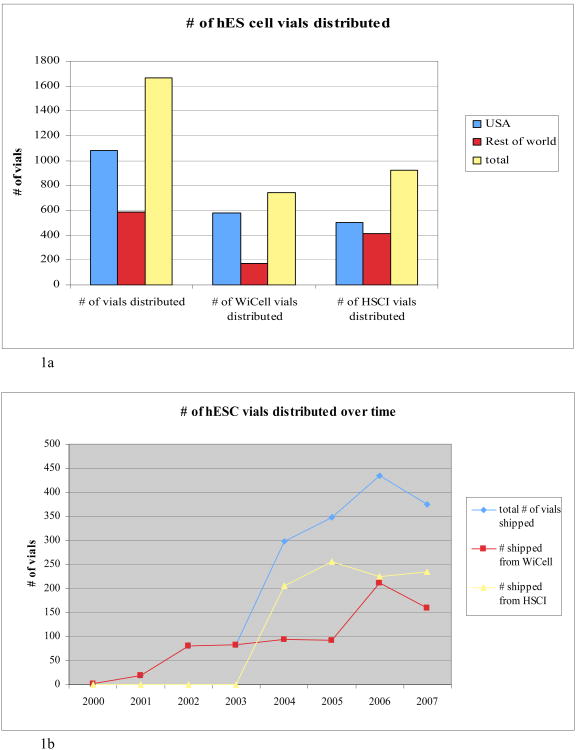
Thirty-six different nations on six continents received at least one vial distributed by WiCell or HSCI. European, North American, and Asian nations are well represented. Shipments also went to Australia, South America, and Africa. Most cell lines shipped within the United States, where scientists have received slightly more than 64% (1077) of the vials. The remaining 585 were distributed to researchers in other nations. HSCI distributed more vials abroad than did WiCell, while WiCell distributed more vials within the U.S. As Figure 1a shows, overall U.S. demand was nearly twice as high as for the rest of the world. Given the research capacity usually associated with the United States as well as the increased state level activity, this finding is not surprising. However, part of the difference may be due to non-US labs with access to non-US lines. Information about which lines are included in our data set can be found in the supplemental materials.
Figure 1.
a) Number of vials of hES cells distributed: the aggregate distribution of vials by WiCell, by HSCI, and in total within the USA, in the rest of the world, and overall; b) Number of vials of hESC distributed over time in total, by WiCell, and by HSCI.
Figure 1b shows that the number of vials distributed increased over time and the distribution pattern follows that characteristic of new technologies: the well-known “S-shaped” curve that tracks market saturation for new technologies (Rogers, 2005). The changes we observe in that curve appear to coincide with particular events. The overall increase during the period 2003-2004 corresponds to HSCI's first shipments in April 2004, and the flattening of the WiCell distribution during this time might be due to HSCI charging only shipping costs for cell lines. That policy made HSCI materials significantly cheaper. In return, WiCell fueled a sharp overall increase in 2005 by dramatically cutting its fees. A $16 million NIH grant to WiCell as the curator of federally approved lines allowed the institute to lower the price of a vial to $500 in October, 2005 (Wisconsin Technology Network). WiCell's lines are eligible for federal funding and the prospect of broader research support combined with a more competitive per vial price may have made them more desirable, flattening demand for HSCI lines. The overall decrease in demand for the period of 2006-2007 may be explained by the fact that more researchers obtained lines from other sources or became efficient at establishing stocks of their own. Thus, our data may under-represent actual use of hES cell lines in both US and other jurisdictions.
Extending our data into 2008 might show even further decrease in demand due to the new and relatively accessible method of deriving induced pluripotent stem (iPS) cells. Some reports indicate that in the first several months they were available, requests for gene delivery vectors for iPS cell protocols was significant: 704 individuals from 142 institutions requested materials from Thomson and colleagues (Yu et al, 2007) and 514 individuals from 113 institutions requested vectors from Yamanaka and colleagues (Takahashi, et al. 2007; Goldman, 2008). Nevertheless, the overall pattern of cell line shipments suggests that hES cell research is alive and growing in the U.S.
The aggregate number of cell line shipments by state is an indicator of research activity, but the date when scientists in a state began work in the field is also important. First mover advantage can be a key to victory in scientific priority races, thus those states that entered the game early may reap the most scientific gains (Merton 1957). The states with earlier shipment dates typically receive more lines overall. Only four states (California, Illinois, Michigan, North Carolina) received at least one line prior to August 9, 2001. California went on to develop the nation's largest stem cell infrastructure, but all four first movers are in the top quartile for total shipments. Forty states and the District of Columbia (henceforth, collectively: states) had received at least one vial by the end of 2007, when our data sets stops.
Table 1 categorizes states on three dimensions; 1) the absolute number of hES vials they receive, 2) cumulative NIH obligations to research institutions in the state, and 3) the number of vials received per $10 million dollars of NIH funding. The final measure provides a normalized indication of relative demand intensity across states. In all cases, we treat the top quartile as a cutoff point. We dub states that fall in the top 25% for hES cell shipments as “high vial.” Similarly, we designated states as “high NIH” based on whether they fell above or below the top quartile of cumulative NIH funding and “high normalized” based on whether they fell above or below the top quartile of the normalized data. While not perfect, these binary distinctions allow us to examine contingent differences in state level use of hES cell materials (Additional methodological details can be found in the Online Supplemental Materials).
Because the variation in cell line shipments is significantly smaller than the variation in NIH funding, normalized data are suspect for global comparisons. For example, West Virginia, Nevada, Kentucky and Maine all appear in the top normalized quartile. Their rankings are more attributable to the small amount of NIH funding each receives than actual demand for cell lines. Therefore, focusing on absolute rather than relative numbers of shipments speaks more directly to the interests of scientists and policymakers. We turn to normalized measures only when they distinguish among states that are relative peers in terms of absolute demand and NIH support.
Dramatic interstate variation in demand appears to characterize the overall growth of U.S. stem cell shipments between 2000 and 2007. On average, states received 20.78 vials (+/- 52.7). Ten states have received 20 or more vials of cell lines from either of the two sources since 2000, and four (California, Massachusetts Maryland and New York) have received more than fifty vials each. These states account for 64% of U.S. hES cell shipments and also are the top four recipients of NIH funding (Table 1).
Despite a patchwork of policies, states with a wide range of federally supported biomedical research contribute to the demand for lines. State-level hES cell research policies play a mixed role in this story. In order to examine the relationship between policy and demand for cell lines at the state level, we define states that have passed legal measures that explicitly reduce barriers for researchers and appropriated money to support hES cell research as “first category” states. States that do not meet either of these criteria are defined as “second category” states, which reflect a range of approaches to stem hES cell research. Our binary distinction thus does little to account for the broad spectrum of state policies, ranging from aggressive constitutional action supporting research (California) to legislative silence, thus not deviating from federal policy (Minnesota). More restrictive approaches include Pennsylvania, which has a long-standing law banning research on embryos and South Dakota, which criminalizes hES cell research. In some states, funding and legislative criteria conflict. For example, Wisconsin—the home of WiCell—funds hES cell research, yet passed two bills proscribing it. Neither became law.
While simple, our binary distinction provides some analytic purchase. Table 1 shows eleven first category states. While many of these states are also among the leaders on our measure of research capacity, not all top the charts in NIH funding. Likewise, some first category states (Missouri and New Jersey) are not in the top quartile (greater than 16 vials) in terms of stem cell shipments. In contrast, Ohio, a second category state, has been the destination for relatively few vials. Yet Ohio is among the top quartile for NIH funding. This state has significant research capacity and has done little to expand opportunities for hES research. The point we take from this picture is that local political efforts may smooth entry into this controversial field for researchers under some conditions but other factors are important.
California, Massachusetts, Maryland and New York lead in absolute numbers of hES cell line shipments and all have exceptionally high levels of NIH funding. However, Texas (5th) and Pennsylvania (tied for 6th) exhibit less consistent trends. Both states rank high in terms of research capacity and absolute numbers of cell shipments. But normalized measures reveal a difference as Pennsylvania receives fewer cell lines per $10 million of NIH funding. Neither is a first category state with supportive policy environments. But it does not mean the policy environments are equivalent. Scientists in Texas face no barriers to their research beyond those imposed by federal policy. A longstanding Pennsylvania law prohibits non-therapeutic research on any unborn child—defined as a human organism from fertilization until birth—making it more restrictive than federal policy. (Pennsylvania 1982). The relative difference in shipment rates between Pennsylvania and Texas may reflect the dampening effect more restrictive policies can have on demand for cell lines.
Not surprisingly, of the thirteen states in the top quartile receiving shipments, four first category states (California, Massachusetts, Maryland, and New York) receive the most vials and most NIH funding. Thirty-eight states received fewer than 16 vials, placing them in the bottom three quartiles in terms of shipments. It is interesting to note that four of those states (Iowa, Missouri, New Jersey, and Rhode Island) have taken explicit steps to expand the reach of stem cell science beyond federal restrictions and thus fall under our first category. Two-by-two contingency tables reveal significant associations between supportive policies and high shipment volumes (Fisher's Exact, p=0.003). That difference appears to be driven by second category states that receive few cell lines. The relatively even split between first and second category states in the top quartile of hES cell recipients suggests that extensive research capacity might compensate for lack of policies permitting or encouraging hES cell research.
We address the research capacity question by roughly classifying states based on cumulative NIH funding. We consider states in the top quartile for cumulative NIH obligations to have high research capacity. Of the second category states that lack supportive policies, five are both high research capacity and high vial destinations (Michigan, North Carolina, Pennsylvania, Texas, Washington). One high research capacity but second category state (Ohio) received few vials; yet two states with lower research capacity (Virginia, Wisconsin) are top quartile destinations for cell lines. The association between high research capacity and high demand for cell lines is significant (p < .001) for second category states; but not for first category states (p = 0.088). In other words, when it comes to demand for hES cell lines, research capacity trumps supportive state policy.
On the other hand, creating a fertile hES cell research environment is not sufficient to spur demand. Consider New Jersey. The state was the second to pass legislation explicitly making hES cell research legal and the first to appropriate funds for hES cell research but reaches the top quartile on neither absolute nor relative measures of stem cell demand. This might be attributed to its relatively low research capacity, as gauged by NIH funding levels. In contrast, Ohio has high research capacity, yet is neither a first category nor a high vial state. This suggests that infrastructure alone is also insufficient.
Through the end of 2007, we find that U.S.-based hES cell research has been steadily increasing. It is also clear that federal funding restrictions have not uniformly chilled hES research in the U.S. We demonstrate that, at least through formal mechanisms, WiCell distributed more lines within the United States than HSCI. Though pride of place is reversed for non-U.S. cell line distributions. Moreover, over a four-year period HSCI distributed more lines than WiCell did over an eight-year period.
Policy and research capacity also play an important role. Our findings suggest that liberalizing policies regarding stem cell research at the state level does not ensure increased activity in hES cell research. In sum, our analyses imply: (1) that liberalizing policies may not be sufficient to spur hESC cell research in the absence of research funding; (2) that liberal policies may not be necessary to increase hES cell line demand where there biomedical research infrastructure is significant; and (3) that actively restrictive policies may be sufficient to slow the field's growth even when there is a history of significant biomedical research. Even in the wake of a newly supportive federal funding environment, state-level policymakers should note that before embarking on legislative and fundraising campaigns that the complexities of hES cell research do not stop at the laboratory door, but carry across state lines and national borders as researchers endeavor to expand knowledge on one of biology's most promising frontiers.
Supplementary Material
Figure 2.
Table of absolute and normalized hES cell distribution patterns, by state.
Acknowledgments
The authors would like to acknowledge the following individuals for their help with this study: Claire Fitz-Gerald, Douglas Melton, and Brock Reeve of the Harvard Stem Cell Institute; Sue Carlson, Andy Cohn, Beth Donley, and Tammy Torbleau of the WiCell Research Institute; Susan Stayn, Stanford University; and Nitya Thummalachetty, Case Western Reserve University. JOS was supported by NSF Grant Number 0545634, CTS by the Stanford Institute for Stem Cell Biology and Regenerative Medicine, and JBM by NHGRI P50 HG 3389. The project was also supported by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov.
References
- Goldman B. Scientists' enthusiasm grows for induced pluripotent cells. Nature Reports Stem Cells. 2008 http://www.nature.com/stemcells/2008/0805/080501/full/stemcells.2008.67.html.
- Longaker MT, Baker LC, Greely HT. Nat Biotechnol. 2007;25:513. doi: 10.1038/nbt0507-513. [DOI] [PubMed] [Google Scholar]
- Merton RK. American Sociological Review. 1957;22:635–659. [Google Scholar]
- Pennsylvania statute, 1982. 18 Pa. Cons. Stats. Ann. S 3216, 1.
- Rao MS. Stem Cells. 2006;24:1412–1413. doi: 10.1634/stemcells.2006-0279. [DOI] [PubMed] [Google Scholar]
- Rogers E. Diffusion of Innovation. 4th. New York, NY: The Free Pres; 2005. [Google Scholar]
- Takahashi K, Tanabel K, Ohnuki M, et al. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.