Abstract
Oxidative stress refers to elevated intracellular levels of reactive oxygen species (ROS) that cause damage to lipids, proteins and DNA. Oxidative stress has been linked to a myriad of pathologies. However, elevated ROS are also signaling molecules i.e. redox biology that maintain physiological functions. In this review we discuss the two faces of ROS, redox signaling and oxidative stress, and their contribution to both physiological and pathological conditions. Redox biology refers to low levels of ROS that activate signaling pathways to initiate biological processes while oxidative stress denotes high levels of ROS that incur damage to DNA, protein or lipids. Thus, the response to ROS displays hormesis. The In this review, we argue that redox biology, rather than oxidative stress, underlies physiological and pathological conditions.
Introduction
Reactive oxygen species (ROS) are byproducts of aerobic metabolism. ROS include the superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH·), all of which have inherent chemical properties that confer reactivity to different biological targets. ROS is often associated with the principle of oxidative stress which suggests ROS induce pathology by damaging lipids, proteins, and DNA [1]. However, in the past two decades it has become apparent that ROS also serve as signaling molecules to regulate biological and physiological processes [2]. It appears early in evolution, nature selected for ROS as a signal transduction mechanism to allow for adaptation to changes in environmental nutrients and the oxidative environment [3]. Indeed in prokaryotes, there are well-described mechanisms whereby ROS directly activate transcription factors for adaption to stress [4].
An understood mechanism of redox signaling involves H2O2-mediated oxidation of cysteine residues within proteins [5]. Cysteine residues exist as a thiolate anion (Cys-S-) at physiological pH and are more susceptible to oxidation compared to the protonated cysteine thiol (Cys-SH) [6]. During redox signaling, H2O2 oxidizes the thiolate anion to sulfenic form (Cys-SOH) causing allosteric changes within the protein that alter its function. The sulfenic form can be reduced to thiolate anions by the disulfide reductases, thioredoxin (Trx) and glutaredoxin(Grx), to return the protein function to its original state [7]. Hence, first degree oxidation of cysteine residues within proteins serves as a reversible signal transduction mechanism. It is estimated that thiolate oxidation in living cells occurs in nM range of H2O2 while higher levels of peroxide further oxidize thiolate anions to sulfinic (SO2H) or sulfonic (SO3H) species. Unlike sulfenic modifications, sulfinic and sulfonic can be irreversible alterations and results in permanent protein damage (i.e. oxidative stress). Thus, cells have professional enzymes dedicated to prevent buildup of intracellular H2O2, primarily peroxiredoxins and glutathione peroxidases.
H2O2 is generated from superoxide produced by mitochondria and NADPH oxidases [8, 9]. Superoxide forms from the one-electron reduction of molecular oxygen (O2) and, within the cell, is rapidly converted by superoxide dismutases 1 and 2 (SOD 1 and 2) into H2O2. SOD1 is primarily located in the cytosol and mitochondrial intermembrane space while SOD2 is located in the mitochondrial matrix. SODs prevent accumulation of superoxide that can damage and inactivate proteins containing iron-sulfur clusters [10]. Thus, accumulation of superoxide is more associated with oxidative stress than redox signaling. However, it is important to note that superoxide does not indiscriminately damage proteins. There are a specific set of proteins sensitive to inactivation by superoxide which activate signaling pathways promoting adaptation to elevated superoxide or, alternatively, initiating cell death [11]. This supports our current view of oxidative stress as a combination of cellular damage and stress responsive signaling. A third type of ROS is the extremely reactive hydroxyl radical which indiscriminately oxidizes lipids, proteins, and DNA, resulting in damage or genomic instability [12]. Typically, hydroxyl radicals are generated from H2O2 in the presence of ferrous ions (i.e. the Fenton reaction). Therefore, cells have multiple mechanisms to maintain iron homeostasis to prevent the formation of toxic hydroxyl radicals. It is important to note, that the changes in H2O2 required for signaling do not cause significant changes in intracellular ratio of oxidized glutathione (GSSG)/reduced glutathione (GSH) or NADPH/NADP+ [13]. In fact, large changes in these parameters are usually a sign of oxidative stress causing toxicity rather than signaling associated with redox biology [14].
Aside from the specificity and selectively of ROS on their targets, the compartmentalization of ROS production within cells is an important determinant of whether damage or redox signaling occurs. In order for effective redox signaling, the H2O2dependent oxidation of a given protein is likely to be close to the source of H2O2 production. For example, the protein targets of H2O2 generated from plasma membrane NADPH oxidases are also located on plasma membrane. Mitochondria are known to dynamically move towards their targets thus allowing mitochondrial generated H2O2 to activate signaling pathways [15]. Similarly, superoxide accumulation in mitochondrial matrix has different outcomes than superoxide accumulation in the cytosol. This is in part due to a high content of iron sulfur cluster protein located in the mitochondrial matrix. Indeed, SOD2 knockout mice have a dramatically severe pathological phenotype compared to SOD1 knockout mice. Accordingly, both the type of ROS and it's local concentration collectively determine whether redox signaling or oxidative stress induced damage occurs.
In this review we will discuss the two faces of ROS biology: redox signaling and oxidative stress. We will focus on both physiological and pathological conditions using the examples of (1) normal and cancer cell proliferation, (2) beneficial and pathological inflammation, and (3) the normal and accelerated aging process.
Redox signaling and oxidative stress: Regulation of normal and cancer cell proliferation
Metazoans use growth factors to coordinate mitogenic, survival, and nutrient uptake signals for cell growth and proliferation [16]. Growth factors such as epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) activate the intrinsic tyrosine kinase activity of their receptors (RTK) that leads to the phosphorylation of specific tyrosine residues on the cytoplasmic tails of the receptor [17]. This recruits multiple proteins to the receptor resulting in activation of several key signal transduction pathways, notably PI3K-AKT and RAS-MEK-ERK to promote cell proliferation, nutrient uptake, and cell survival [18]. However, RTKs and PI3K are negatively regulated by protein-tyrosine phosphatases (PTPs) and PTEN, respectively, resulting in dampening of mitogenic signaling [19, 20]. Thus, to sustain signal transduction pathways it is necessary to inactivate these phosphatases.
Initial experiments demonstrated that PDGF and EGF can rapidly and transiently increase ROS generation through NADPH oxidases and that these ROS were required for growth factor induced receptor tyrosine phosphorylation [21]. Subsequently, it was demonstrated that H2O2 produced in response to EGF oxidized the catalytic cysteine of protein-tyrosine phosphatase 1B (PTP1B) to a sulfenic moiety causing inactivation of this phosphatase [22]. PTP1B dephosphorylates tyrosine residues of EGFR [23]. Thus, its inactivation by H2O2 results in increased tyrosine phosphorylation of EGFR and transmission of downstream growth signaling. The oxidized PTP1B is reactivated by thioredoxin, illustrating the reversibility of the redox signal [24]. Intracellular MAP-kinase signaling following PDGF stimulation is also reinforced through oxidation and inactivation of the PDGF-receptor associated phosphatase, SHP-2 [25]. In fact, H2O2 can reversibly oxidize a number of purified PTP family members in vitro resulting in their inactivation [26]. Purified human PTEN phosphatase, a tumor suppressor and negative regulator of phosphoinositide 3-kinase (PI3-K) signaling, is inactivated by H2O2 through oxidation and disulfide bond formation between Cys121 and Cys71, a modification reversed by thioredoxin activity [27]. Increased levels of oxidized PTEN can be measured in cells shortly after stimulation with various growth factors involved in PI3-K activation [28]. Normally, the abundance of peroxiredoxins quickly decreases H2O2 upon growth factor stimulation [29, 30]. However, recent data indicates that local pool of peroxiredoxin I (PRXI) associated with cell membranes is phosphorylated and inactivated upon growth factor stimulation to allow accumulation of local H2O2 and inhibition of phosphatase activity [31]. Since this PRX1 inactivation is localized to membranes it allows cellular PRX1 pools to remain active thus not allowing peroxide build up in the cells. These data support a model where growth factor activation must be accompanied by a localized burst in ROS production at the plasma membrane. ROS then inactivates the action of phosphatases, reinforcing proliferative signaling pathways. Recent studies indicate that in addition to NADPH oxidases, mitochondrial ROS can also cause inactivation of phosphates through oxidation [32].
Cancer cells “hijack” normal cell machinery by constitutively activating growth factor pathways to sustain cellular growth and proliferation [33]. This allows cancer cells to uptake abundant nutrients, survive stress, and continuously proliferate. Consequently, the hyper-metabolism of cancer cells causes abundant generation of ROS from mitochondria, endoplasmic reticulum, and NADPH oxidases [34]. Initial observations over two decades ago demonstrated that cancer cells generate higher levels of ROS than their non-transformed counterparts [35]. It was assumed that these elevated ROS levels caused genomic instability to promote tumorigenesis [36]. However, chromosomal instability is likely attributable to loss of p53 and other mechanisms that promote aneuploidy. Cancer cells driven by the MYC oncogene demonstrate no detectable increase in chromosomal instability and drive tumorigenesis through ROS dependent increase in signaling pathways [37]. Furthermore, treatment with the antioxidant N-acetyl-cysteine (NAC) or inhibitors of NADPH oxidase prevent mitogenic signaling pathways in oncogenic Kras driven mouse fibroblasts [38]. Human cancer cells driven by oncogenic Kras require mitochondrial ROS for proliferation [39].
Mitochondrial mutations resulting in TCA cycle or electron transport chain dysfunction generate ROS to activate tumorigenic signaling pathways including PI3K and MAPK pathways [40-42]. Another important target of ROS is the transcription factor NF-κ B known to control cell survival of tumor cells [43]. NF-κB was of one of the early transcription factors discovered to be responsive to ROS [44].
The high rate of ROS production is counterbalanced by an equally high rate of antioxidant activity in cancer cells to maintain redox balance [45]. If cancer cells do not control their ROS levels then they are susceptible to oxidative stress-induced cell death [46, 47]. Steady state ROS levels in cancer cells are determined both by rate of ROS production and also rate of ROS scavenging. Thus, at steady state, cancer cells can display either overall increase or decrease in ROS compared to normal cells. Additionally, the signaling pathways responsive to hydrogen peroxide are localized to the sources of ROS generation, allowing activation of these pathways despite the high overall antioxidant activity in cancer cells that protects against oxidative stress-induced cell death.
The major mechanism by which cancer cells increase their antioxidant proteins by is through activating the transcription factor nuclear factor erythriod 2-related factor 2 (NRF2) [48]. Normally NRF2 interacts with Kelch-like ECH-associated protein 1 (KEAP1) thus targeting NRF2 for proteasomal degradation. Elevated ROS oxidizes redox sensitive cysteine residues on KEAP1 resulting in dissociation of KEAP1 from NRF2. Subsequently, NRF2 translocates to the nucleus, heterodimerizes with small protein MAF and binds to antioxidant-responsive elements (AREs) within regulatory region of multiple antioxidant genes. Aside from elevated ROS, signaling pathways such as ERK MAPK and PI3K can activate NRF2. Furthermore, certain tumor cells display mutations of KEAP1 resulting in constitutively activation of NRF2 [49]. The loss of NRF2 in cancer cells increases oxidative stress resulting in diminished tumorigenesis [50]. It is important to note that loss NRF2 diminishes multiple antioxidant defense systems thus making multiple types of ROS (i.e. superoxide, peroxide and hydroxyl radicals) increase at a threshold that invokes damage to cancer cells. However, loss of a specific antioxidant defense system might result in elevation in ROS levels to levels below the threshold that causes damage. In this scenario, the elevated ROS levels hyper-activate signaling pathways to promote tumorigenesis as observed during the loss of peroxiredoxin I, which increases tumorigenesis [51, 52].
If increased ROS levels are essential to promote and reinforce proliferative signals, one could predict tumor suppressors (TSs) could serve as antioxidants, reducing cellular ROS to levels which do not support proliferation. The highly mutated tumor suppressor p53 controls the expression of a variety of antioxidant genes [53]. Tumor formation in p53-/- mouse models can be suppressed through supplementation of NAC in the diet, suggesting a primary tumor suppressive function of p53 in certain cancers is to decrease ROS [54]. Furthermore, a recent study suggested that the major tumor suppressive function of p53 might be through regulating antioxidant and metabolism genes rather than apoptosis and cell cycle arrest [55]. The induction of TIGAR gene is one mechanism that p53 regulates metabolism to control antioxidant function [56]. TIGAR functions as a fructose-2,6-bisphosphatase which lowers fructose-2,6-bisphosphate levels, a positive regulator of phosphofructokinase-1 [57]. This results in a decrease glycolytic flux and shunting of glucose carbons into the pentose phosphate pathway to produce NADPH, which is required to maintain many antioxidant systems. Other tumor suppressor genes such as FOXOs also repress tumorigenesis by inducing antioxidants [58].
While ROS play a key role in maintaining mitogenic signals to drive cancer cell proliferation, they also are integral in adapting to the metabolic stress that occurs when highly proliferative tumors outstrip their blood supply [59]. The resulting tissue hypoxia stabilizes the family of transcription factors termed hypoxia-inducible factors (HIFs) [60]. HIFs are a heterodimer consisting of a constitutively stable subunit, HIFβ, and an oxygen sensitive subunit, HIFα. HIFα is hydroxylated at proline residues by prolyl hydroxylases (PHDs). Hydroxylated proline residues of HIFα protein are recognized by the E3 ubiquitin ligase von Hippel-Landau protein (pVHL), which targets HIFα to the proteasome [61]. Under hypoxia, HIFα is not hydroxylated by PHDs thereby preventing pVHL targeting HIFα to the proteasome. Subsequently, HIFα translocates to the nucleus and dimerize with HIFβ regulating metabolic adaption to hypoxia and pro-angiogenic genes such as VEGF [62]. Hypoxia increases ROS production leading to HIFα stabilization through the inhibition of prolyl hydroxylases (PHDs) [63, 64]. ROS induction of HIFs can promote tumorigenesis of certain cancer cells [37, 65, 66]. Furthermore, the sirtuin protein SIRT3 is a tumor suppressor by upregulating antioxidant defenses to prevent HIF activation [67, 68].
In summary, we support a model in which tumorigenic cells generate high levels of ROS to activate proximal signaling pathways that promote proliferation, survival and metabolic adaptation (i.e. redox biology). At the same time, cancer cells maintain a high level of antioxidant activity to prevent buildup of ROS to levels that could induce cell death (i.e. oxidative stress) (Figure 2). This presents a conundrum in how to approach ROS therapy in cancer: should treatments focus on lowering ROS levels to prevent signaling or increasing ROS to selectively kill cancer cells? A systematic review of randomized control studies with the antioxidants β-carotene, vitamin A, and vitamin C concluded no significant benefit, and possibly a detrimental effect, of these agents in cancer prevention [69]. However, it is possible that more targeted antioxidants that specifically enrich in cancer cells or prevent localized ROS production from mitochondria and NADPH oxidases may provide a clinical benefit. While randomized control human trials with pro-oxidant cancer therapy have not yet been completed, there is accumulating evidence that raising ROS levels through small molecules can selectively induce cancer cell death by disabling antioxidants [70-72]. There are two caveats to this approach, First, if the ROS levels are not sufficiently raised within the cancer cell then the therapy would simply further activate NF-κB, PI3K, HIFs and MAPKs to promote tumorigenesis. Additionally, agents should disable only the antioxidants utilized by cancer cells compared to normal cells. Since the role of ROS and sensitivity to both oxidants and antioxidants likely differs between cancer types, continuing to test both oxidants and antioxidants in vivo will hopefully yield new agents to add to existing chemotherapy regimens.
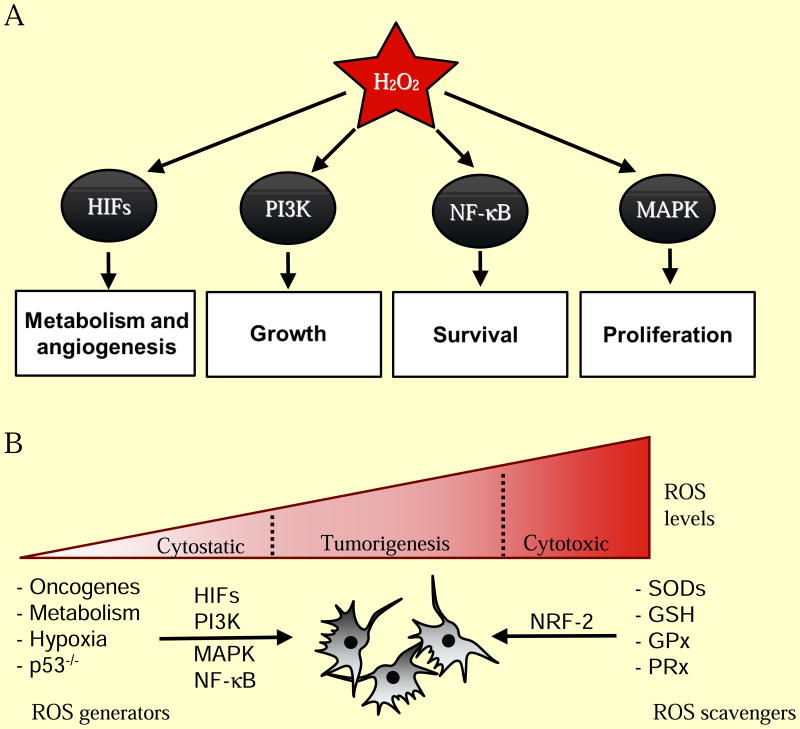
Figure 2. ROS regulation of normal and cancer cell proliferation.
(A) Hydrogen peroxide (H2O2) is required for activation of a number of cellular pathways involved in cellular proliferation. (B) Cancer cells generate higher levels of ROS that are essential for tumorigenesis. Genetic alterations leading to activation of oncogenes (PI3K, MAPK, HIFs, NF-κB) and loss of tumor suppressors (p53) coordinate an elevated redox state. ROS is also generated from increased oxidative metabolism and hypoxia in rapidly expanding tumors. Cancer cells also express elevated levels of cellular antioxidants (SODs, GSH, GPx, PRx) in part through NRF-2 to protect against oxidative stress-induced cell death.
Redox signaling and oxidative stress: Regulation of inflammation
The innate and adaptive immune systems are critical for pathogen-specific defense and immunological memory. Furthermore, the immune system is crucial for tissue repair. However, if immune system either fails to be properly activated or is persistently activated it can contribute to multiple diseases including autoimmunity, cardiovascular disease, and accelerate the normal aging process. In the past two decades, there is substantial evidence that ROS are essential second messengers in innate and adaptive immune cells [73, 74]. Yet, increased levels of ROS within immune cells can result in hyperactivation of inflammatory responses resulting in tissue damage and pathology [75].
The innate immune system responds to microorganism-derived pathogen-associated molecular patterns (PAMPs) and endogenous cell-derived damage-associated molecular patterns (DAMPs) to tissue injury [76]. PAMPs and DMAPs bind to specific receptors including Toll-like receptors (TLR), RIG-I-like receptors (RLRs), and NOD-like receptors (NLRs) to generate cytokines that are essential for fighting against pathogen or repair tissue damage [77-79]. Initial studies implicating ROS in innate immune system came from the observations that LPS activates inflammatory cytokines through the generation of NADPH oxidase and mitochondrial generated ROS [80, 81]. More recent studies have shown that mitochondrial ROS are essential for other TLR-initiated pathways including TLR1, TLR2, and TLR4 and for optimal bactericidal activity of macrophages [82]. The RIG-I-like receptors (RLRs) also signal through mitochondrial ROS [83]. This might not be surprising since the outer mitochondrial membrane serves as a platform for formation of the RLR molecular complex [84]. The NLRP3, a NLR that is a component of the inflammasome, also requires NADPH and mitochondrial ROS for activation [85-89]. Interestingly, patients with tumor necrosis factor receptor-associated periodic syndrome (TRAPS) have heightened responsiveness to LPS due to increased mitochondrial ROS production which promotes inflammation, again suggesting redox biology, and not oxidative stress, is regulating inflammatory diseases [90].
Adaptive immunity involves the expansion of T cells and B cells specific for pathogens via rapid proliferative responses. Initial evidence for redox signaling in the process stems from the observations that treatment of primary T cells with pharmacologic antioxidants inhibits proliferation and production of the cytokine IL-2 following T-cell receptor stimulation in vitro [91]. Antioxidants also diminished the expansion of T cells in vivo [92]. The major initial source of ROS required for T cells activation is mitochondria [93, 94]. Pharmacological or genetic manipulation dampening mitochondrial ROS generation can diminish T cell activation in vitro and in vivo. However, NADPH oxidase can be invoked in response to mitochondrial ROS to further sustain ROS levels to maintain T cell activation [95]. NADPH oxidase and mitochondrial generated ROS have been implicated for B cell activation and proliferation upon stimulation of the B-cell receptor (BCR) [96, 97]. Thus, both TCR and BCR signaling requires ROS generation to mount the proper inflammatory response.
What happens when ROS levels are elevated during immune responses? The simple answer is that this depends on the degree to which ROS levels are elevated beyond what is expected during a normal immune response. A slight elevation could be beneficial under certain conditions or detrimental [98]. For example, uncoupling Protein 2 (UCP2) knockout mice feature higher levels of mitochondrial ROS and possess increased immunity to bacterial pathogens [99]. This suggests a low elevated level of ROS in the immune system might enhance normal immune function. Indeed, mice heterozygous for Mclk1 (a mitochondrial hydroxylase necessary for ubiquinone synthesis) display increased mitochondrial ROS with elevated normal innate and adaptive inflammatory responses to fight pathogens without incurring tissue damage [100]. By contrast, high levels of ROS generation due to loss of NRF2 lead to elevated levels of pro-inflammatory cytokines [101]. NRF2 deficient mice display exacerbated innate immune derived inflammation responses to pathogens resulting in worsened pneumonia and sepsis [102]. Antioxidants improve survival of NRF2 deficient mice in these models of sepsis. Antigen-specific adaptive immunity induced by sensitization to ovalbumin model of asthma is also intensified by Nrf2 deficiency [103]. Thus, slightly elevated ROS levels may enhance immune system function while high levels of ROS could promote a pathological inflammatory response.
The finding that ROS functions both in the normal innate and adaptive immunity presents a challenge as to when antioxidants could be utilized as an immunomodulatory therapy. Clearly antioxidants should not be administered in healthy individuals that have robust antoxidant defense and a healthy immune system, since ROS are intimately tied to optimal pathogen clearance. However, when the immune system becomes dysregulated, as observed in autoimmune disease, antioxidants could be helpful in ameliorating the heightened inflammation response. As with ROS therapy in cancer, there are obstacles that need to be considered when using antioxidants for immunomodulation. For example, the dosing of antioxidants should not be so high as to interfere with normal immune responses. Furthermore, the timing of antioxidants is crucial during the progression of an inflammatory disease. This is certainly the case in critically ill patients in the intensive care unit (ICU). These patients often display signs of elevated ROS and heightened inflammatory responses that result in multi-organ failure and mortality. Even in the cases of an acute infection, it is possible that a pro-inflammatory cytokine storm is primarily responsible for admission to the ICU. Yet, multiple clinical trials have consistently showed no efficacy or an increase in mortality in patients with critical illness in the ICU treated with antioxidants [104]. Reasons for this failure are not fully understood but we speculate that the antioxidants might interfere with the normal responses to pathogens in certain immunosuppressed populations. It is possible that these immunosuppressed patients might even benefit from pro-oxidant therapy to boost their immune system.
Going forward, it will be important to characterize how different inflammatory cells respond to changes in ROS levels. It is now more appreciated that different T cell and macrophage subsets can be pro- or anti-inflammatory. But, it is not fully understood whether these different subsets have differential responses to ROS. Along these lines, it might be beneficial to increase a particular subset of T cells or macrophages by either increasing or decreasing ROS levels to ameliorate immune system pathologies.
Redox signaling and oxidative stress: Regulation of aging
The ability to regenerate tissues as well as to prevent damage to existing tissues are two key determinants of aging. One of the original theories of aging formulated over 50 years ago is Denham Harman's Free Radical Theory of Aging, which proposes ROS contribute to aging through their reactivity towards cellular macromolecules, particularly in the mitochondria [105]. Damaged mitochondria, through inefficient oxidative phosphorylation, produce escalating amounts of ROS inevitably impairing cellular function [106]. However, interventions in reducing ROS levels have had mixed results and it not clear whether ROS induced damage is the underlying cause of aging [107]. On the contrary, recent evidence suggests ROS-signaling is required for maintenance of tissues and increasing ROS can activate cellular stress pathways to dampen tissue degeneration to promote healthy aging [108].
Longevity studies in multiple model organisms have not consistently demonstrated that antioxidants prevent aging. Early studies in Drosophila suggested that increasing SOD and catalase activity in the cytosol extend longevity [109]. However, other investigators could not duplicate these experiments [110]. Furthermore, careful measurements of ROS in Drosophila do not find any correlation between ROS levels and longevity. In mice, overexpression of cytosolic SOD with catalase or mitochondrial SOD also does not increase longevity [111]. By contrast, the overexpression of mitochondrial matrix catalase (CATmm) but not cytosolic or nuclear catalase in mice does extend longevity [112]. The conventional interpretation is that CATmm detoxified mitochondrial matrix hydrogen peroxide to water preventing peroxide induced oxidative damage to mitochondria. An alternative explanation is detoxification of matrix generated hydrogen peroxide prevented leakage of hydrogen peroxide into the cytosol thereby interfering with normal ROS signaling pathways that prevent pathologies such as cancer, a major cause of death in laboratory mice. Since mitochondria are a major source of ROS in the cell, the mitochondrial genome is often thought to be the particularly susceptible to oxidative damage. Yet, deletion of mitochondrial matrix SOD in mice increases mitochondrial DNA damage and increases cancer incidence, but does not accelerate aging [113, 114]. Interestingly, loss of superoxide dismutase enzymes in C. elegans can even extend lifespan [115].
An observation that further questions the Free Radical Theory of Aging is that elevation of ROS through signaling mechanisms can increase longevity from yeast to mice [116]. In yeast, inhibition of TOR (target of rapamycin) or caloric restriction extends chronological lifespan by increasing mitochondrial ROS [117, 118]. In C. elegans, glucose restriction, mitochondrial electron transport mutations, and diminished insulin-growth factor (IGF) signaling extend lifespan by increasing mitochondrial ROS [119-121]. Paraquat, a direct generator of mitochondrial ROS, is sufficient to increase lifespan in C. elegans [122]. Sirtuin dependent extension of lifespan in C. elegans has also been shown to be dependent on an increase in ROS production. This result was unexpected as sirtuin contribution to lifespan was previously thought primarily to be mediated by deactylation of proteins including histones [123]. There is also increasing evidence for a conserved mitochondrial longevity pathway in mammals. Mice heterozygous for MCLK1, a protein required for proper electron transport, have increased mitochondrial ROS [124]. However, these mice feature less oxidative damage to cytosolic proteins and are long-lived, supporting a model where elevated ROS levels are paradoxically protective through induction of stress pathways [125]. A common model of human aging in cell culture is examining replicative senescence. Initial studies to support the free radical theory comes from the observation that hypoxia increase human diploid fibroblasts replicative life span [126]. The original interpretation was that hypoxia decreased ROS resulting in less accumulation of oxidative damage to increase replicative lifespan. However, later studies have demonstrated a paradoxical increase in mitochondrial ROS during hypoxia resulting in activation of HIFs to increase replicative lifespan of human fibroblasts [127]. The long-lived mitochondrial mutants in C. elegans also depend on ROS-dependent activation of HIF for increased lifespan [119]. Beyond HIF, there are likely to be multiple signaling pathways that ROS activates to increase lifespan.
Aging is accelerated when the tissues that are damaged are not repaired [128]. The maintenance of adult tissue and organ systems requires removal of damaged cells and replenishment from undifferentiated stem cell populations. Stem cells have to both self-renew to maintain the stem cell pool and also differentiate to generate specialized tissue. The best-studied example is the hematopoietic stem cell (HSC), which differentiate to provide myeloid and lymphoid progenitors throughout lifespan. An emerging model in HSCs is that generation of low levels of ROS through NADPH oxidases or from mitochondria is required to activate proliferative pathways, serving as a “go” signal to support stem cell proliferation. By contrast, high levels of ROS impair stem cell function by activating signaling pathways that limit self-renewal, but do not necessarily cause cellular damage. For example, HSCs from mice that lack the ataxia telangiectasia mutated (Atm) gene have higher ROS levels that activate p38 MAPK and p16INK4a to reduce HSC repopulating capacity and exhaustion of the stem cell population [129]. Interestingly, the rise in p16INK4a expression increases with age and has been directly shown to limit stem cell renewal and function [130]. The antioxidant NAC rescues defects due to loss of ATM [131]. Other defects caused by loss of ATM include development of thymic lymphoma and innate and adaptive immune dysregulation [132]. These defects are alleviated by the expression of mitochondrial catalase in ATM null mice suggesting that the normal function of ATM might to be to control ROS levels [132]. Indeed, ROS oxidize a specific cysteine residue on ATM to generate disulfide-linked activated ATM dimers that promote anti-oxidant responses by regulating NADPH production from the pentose phosphate pathway (PPP) [134, 134]. ATM also maintains a low level of ROS to maintain stem cell function in part through the Bcl2 protein BID [135].
Consistent with the ATM observation on HSCs is that the loss of FOXOs, Bmi1, or Tsc1 triggered increase in ROS levels in HSCs, which limited HSC repopulating capacity [136-138]. Aside from HSCs, neural stem cells are also sensitive to increase in ROS. The loss of PRDM16, a transcription factor that regulates brown fat, triggers defects in HSCs and neural stem cell function [139]. PRDM16 is highly expressed in HSCs and neural stem cells. However, NAC only rescued neural stem cell defects but not HSCs suggesting that redox biology is context dependent. Thus, ROS levels have to be maintained in a range that allows for stem cells to function properly, and that concentration may differ between tissues.
ROS are also essential for stem cell differentiation. Mouse HSCs deficient in both AKT1 and AKT2 have reduced levels of ROS that impaired differentiation [140]. In Drosophila hematopoietic progenitors, increasing ROS triggers differentiation while decreasing ROS impairs differentiation [141]. Furthermore, human bone marrow mesenchymal stem cells also require ROS for differentiation in to adipocytes and mitochondrial ROS generated from complex I can trigger muscle differentiation [142, 143]. Within the skin epidermis, undifferentiated cells along the basement membrane undergo a regulated transformation into mature apical epidermal cells. Lowering mitochondrial ROS impairs this differentiation process which can surprisingly be restored by supplementing exogenous H2O2 [144]. Similarly, lowering ROS levels decreases the regenerative capacity of neural stem cells and spermatogonial stem cells [145, 146]. However, aberrant elevation of ROS levels impairs cardiac myocyte differentiation [147]. This raises two questions: (1) What levels of ROS are required for stem cell renewal compared to stem cell differentiation? (2) What levels of ROS inhibit stem cell renewal compared to impaired stem cell differentiation? We speculate that quiescent stem cells reside at low levels of ROS and a slight increase in ROS provides the signal for self-renewal and cellular differentiation. ROS levels above those required for self-renewal or differentiation impairs these critical two stem cell properties.
The idea that stem cell and tissue impairment are not a consequence of oxidative stress-induced damage is further supported from experiments in mice harboring mitochondrial mutations. Mice engineered to accumulate mitochondrial DNA mutations due to defective DNA polymerase proofreading prematurely age and display impairment of NSC and HSC function beginning in utero [148]. Remarkably, administration of NAC rescued both NSC and HPC dysfunction, suggesting the genetic mutations caused by the error-prone polymerase were dispensable for self-renewal. Other mutations of mitochondrial DNA, such as A1555G, result in maternally inherited phenotypes by increases in ROS [149]. However, these ROS cause their pathologies through activation of signaling pathways and apoptosis, rather than oxidative damage. Consequently, the stem cell, tissue degeneration, and aging communities have undergone an evolution in the past two decades from viewing ROS as simply toxins that cause cellular damage to molecules that regulate cellular signaling pathways to invoke beneficial or detrimental effects.
Conclusion
In the past two decades, ROS have undergone a shift from being molecules that invoke damage (i.e. oxidative stress) to regulating signaling pathways that impinge on normal physiological and biological responses (i.e. redox biology). The levels and compartmentalization of hydrogen peroxide dictate redox biology while high levels of superoxide or hydroxyl radicals invoke oxidative stress. Redox signaling is required for numerous cellular processes, as indicated by the role of ROS in proper cellular differentiation, tissue regeneration, and prevention of aging. On the other hand, we propose that redox signaling, and not oxidative stress, is also crucial in regulating signaling pathways that control various disease states, including tumorigenesis, automimmunity, and loss of tissue regeneration with age. This conceptual shift makes it difficult to interfere with redox biology by administering antioxidants, which would affect redox biology of both normal and abnormal responses. To date, physical exercise is one strategy that increases ROS resulting in activation of beneficial pathways that diminish cancer, diabetes, and ageing [150]. But, since most of us find it difficult to spend time at the gym, it will be important to identify the molecular effectors of redox biology that keep normal biological and physiological responses functioning from those that are promote human pathologies. This would allow for selective therapies that would alleviate disease, but not interfere with healthy tissue.
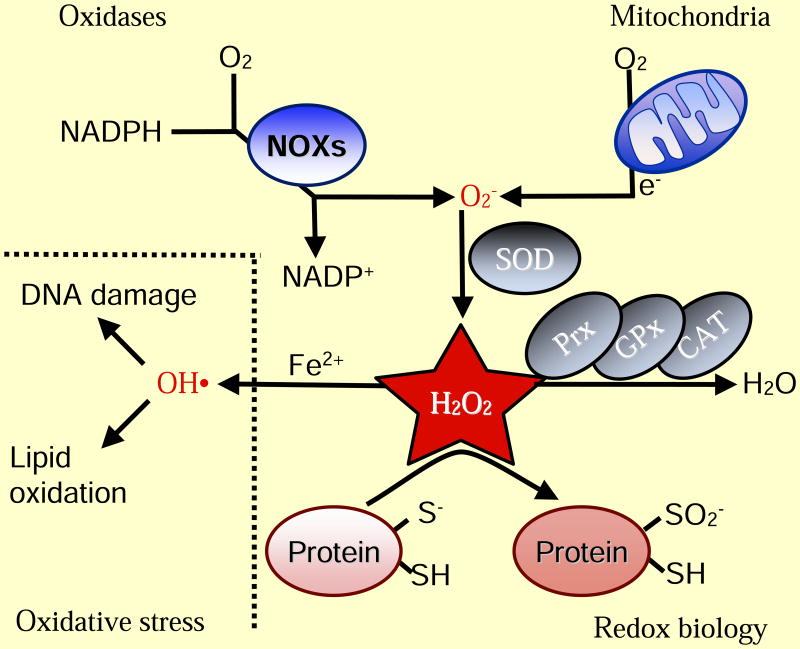
Figure 1. Basics of ROS.
Intracellular superoxide (O2-) is primarily produced from the oxidation of NADPH by oxidase enzymes (NOX) or from electron leak from aerobic respiration in the mitochondria. Superoxide is rapidly converted into hydrogen peroxide (H2O2) by compartment-specific superoxide dismutases (SODs). H2O2 is capable of oxidizing cysteine residues on proteins to initiate redox signaling. Alternatively, H2O2 may be converted to H2O by cellular antioxidant proteins, such as peroxiredoxins (PRx), glutathione peroxidase (GPx), and catalase (CAT). When H2O2 levels increase uncontrollably, hydroxyl radicals (OH·) form through reactions with metal cations (Fe2+) and irreversibly damage cellular macromolecules
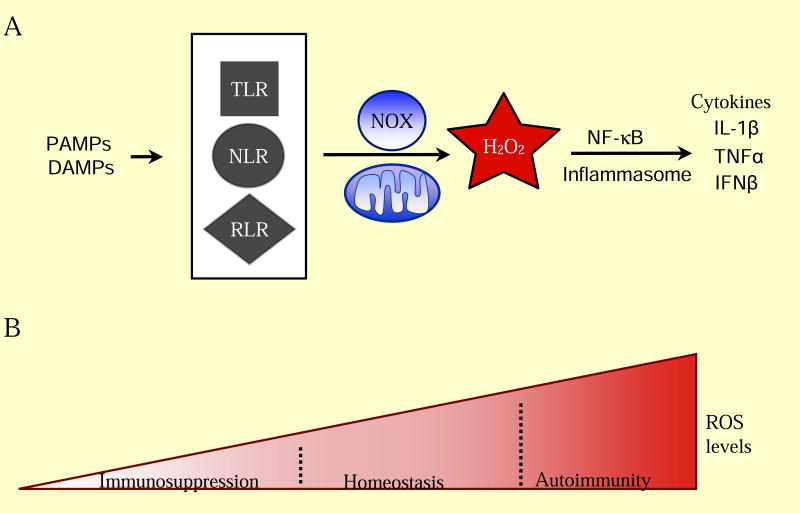
Figure 3. ROS Regulation of Inflammation.
(A) Activation of the innate immune system requires ROS signaling. Common features of pathogens and cell damage (PAMPs, DAMPs) activate surveillance receptors (TLR, NLR, RLR) which increase ROS through NAPDH oxidase enzymes and the mitochondria. ROS is required for release of pro-inflammatory cytokines (IL-1β, TNFα, IFNβ) for a proper immune response. (B) Low levels of ROS maintain a healthy immune system. Decreasing ROS levels inhibits activation of proper immune responses, leading to immunosuppression. Elevated ROS levels contribute to autoimmunity through increased release of pro-inflammatory cytokines and proliferation of specific subsets of adaptive immune cells.
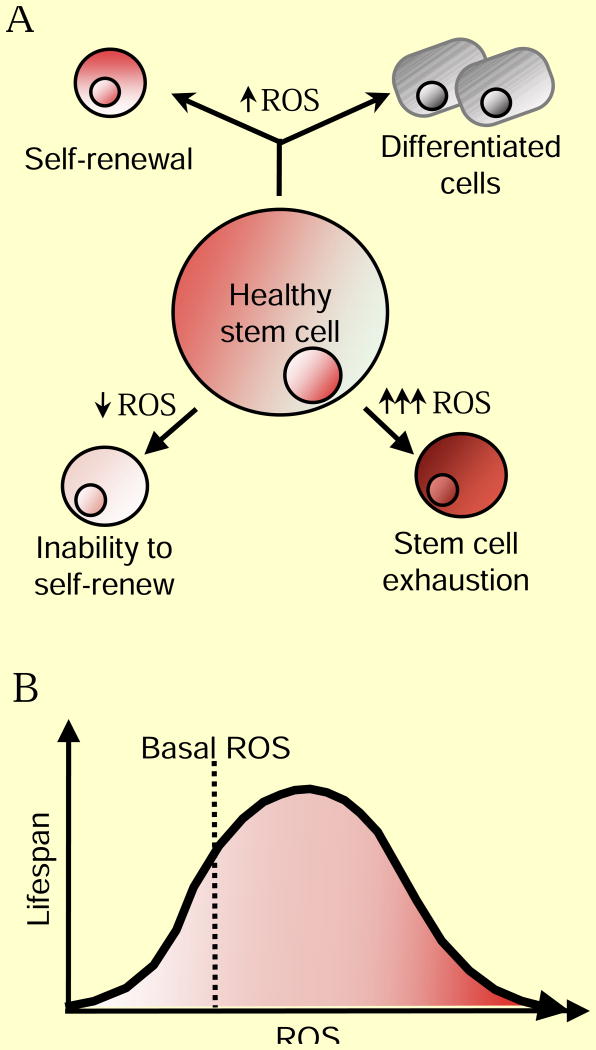
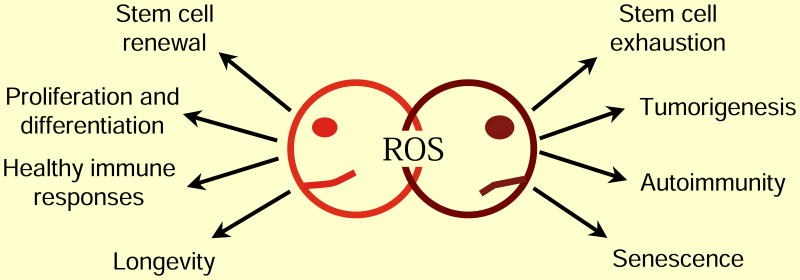
Figure 4. ROS Regulation of Aging.
(A) Moderate ROS levels are required for proper stem cell differentiation and renewal through activation of signaling pathways. On one hand, decreased ROS levels impair stem cell properties, but ROS levels that are too high lead to stem cell exhaustion and premature aging through activation of signaling pathways. (B) Increased ROS are not detrimental to lifespan. Activation of cellular responses due to slight increases in ROS can increase signaling pathways that counter the normal aging process. However, high ROS levels can hyper-activate signaling pathways that promote inflammation, cancer and cell death leading to an accelerated aging phenotype.
Figure 5. Janus of ROS: A Therapeutic Conundrum.
Redox biology encompasses both the physiological and pathological roles of ROS. Determining whether to use prooxidant therapy to promote physiological ROS responses or antioxidant therapy to prevent ROS pathologies remains the central question in redox biology.
Acknowledgments
This work was supported by NIH grants T32-HL76139 (M.S.), 5P01HL071643 (N.S.C. and RO1CA123067 (N.S.C.).
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL, McCord JM, Harman D. Oxygen radicals and human disease. Annals of internal medicine. 1987;107:526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 2.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 4.Kiley PJ, Storz G. Exploiting thiol modifications. PLoS Biol. 2004;2:e400. doi: 10.1371/journal.pbio.0020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee S. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 6.Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci Signal. 2012;5:pe10. doi: 10.1126/scisignal.2002943. [DOI] [PubMed] [Google Scholar]
- 7.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 9.Brand M. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridovich I. Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- 12.Dizdaroglu M, Jaruga P. Mechanisms of free radical-induced damage to DNA. Free Radic Res. 2012;46:382–419. doi: 10.3109/10715762.2011.653969. [DOI] [PubMed] [Google Scholar]
- 13.Morgan B, Sobotta MC, Dick TP. Measuring E (GSH) and H(2)O(2) with roGFP2-based redox probes. Free Radic Biol Med. 2011 doi: 10.1016/j.freeradbiomed.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 14.Murphy MP. Mitochondrial thiols in antioxidant protection and redox signaling: distinct roles for glutathionylation and other thiol modifications. Antioxid Redox Signal. 2012;16:476–495. doi: 10.1089/ars.2011.4289. [DOI] [PubMed] [Google Scholar]
- 15.Al-Mehdi AB, Pastukh VM, Swiger BM, Reed DJ, Patel MR, Bardwell GC, Pastukh VV, Alexeyev MF, Gillespie MN. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci Signal. 2012;5:ra47. doi: 10.1126/scisignal.2002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson CB. Rethinking the regulation of cellular metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:23–29. doi: 10.1101/sqb.2012.76.010496. [DOI] [PubMed] [Google Scholar]
- 17.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 19.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Bio. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 20.Wishart MJ, Dixon JE. PTEN and myotubularin phosphatases: from 3-phosphoinositide dephosphorylation to disease. Trends in Cell Biology. 2002;12:579–585. doi: 10.1016/s0962-8924(02)02412-1. [DOI] [PubMed] [Google Scholar]
- 21.Sundaresan M, Yu Z, Ferrans V, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 22.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. [PubMed] [Google Scholar]
- 23.Adachi M, Fischer EH, Ihle J, Imai K, Jirik F, Neel B, Pawson T, Shen S, Thomas M, Ullrich A, et al. Mammalian SH2-containing protein tyrosine phosphatases. Cell. 1996;85:15. doi: 10.1016/s0092-8674(00)81077-6. [DOI] [PubMed] [Google Scholar]
- 24.Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366–15372. doi: 10.1074/jbc.273.25.15366. [DOI] [PubMed] [Google Scholar]
- 25.Meng T, Fukada T, Tonks N. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–399. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 26.Denu JM, Tanner KG. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry. 1998;37:5633–5642. doi: 10.1021/bi973035t. [DOI] [PubMed] [Google Scholar]
- 27.Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 28.Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi MH, Lee IK, Kim GW, Kim BU, Han YH, Yu DY, Park HS, Kim KY, Lee JS, Choi C, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 30.Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004;101:16419–16424. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 32.Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, Aplin AE, Tai YT, Aguirre-Ghiso J, Flores SC, et al. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem. 2005;280:16916–16924. doi: 10.1074/jbc.M410690200. [DOI] [PubMed] [Google Scholar]
- 33.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 35.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 36.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg F, Hamanaka R, Wheaton W, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu G, Budinger G, Chandel N. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan LB, Martinez-Garcia E, Nguyen H, Mullen AR, Dufour E, Sudarshan S, Licht JD, Deberardinis RJ, Chandel NS. The Proto-oncometabolite Fumarate Binds Glutathione to Amplify ROS-Dependent Signaling. Mol Cell. 2013;51:236–248. doi: 10.1016/j.molcel.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 42.Woo DK, Green PD, Santos JH, D'Souza AD, Walther Z, Martin WD, Christian BE, Chandel NS, Shadel GS. Mitochondrial genome instability and ROS enhance intestinal tumorigenesis in APC(Min/+) mice. Am J Pathol. 2012;180:24–31. doi: 10.1016/j.ajpath.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 44.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nature reviews Drug discovery. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 46.Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by betaphenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Nogueira V, Park Y, Chen C, Xu P, Chen M, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao J, Schulte J, Knight A, Leslie NR, Zagozdzon A, Bronson R, Manevich Y, Beeson C, Neumann CA. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neumann CA, Krause DS, Carman CV, Das S, Dubey DP, Abraham JL, Bronson RT, Fujiwara Y, Orkin SH, Van Etten RA. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 53.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 54.Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li T, Kon N, Jiang L, Tan M, Ludwig T, Zhao Y, Baer R, Gu W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheung EC, Ludwig RL, Vousden KH. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci U S A. 2012;109:20491–20496. doi: 10.1073/pnas.1206530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 58.Dansen TB, Burgering BM. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 2008;18:421–429. doi: 10.1016/j.tcb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 60.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 62.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends in pharmacological sciences. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 64.Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horak P, Crawford AR, Vadysirisack DD, Nash ZM, DeYoung MP, Sgroi D, Ellisen LW. Negative feedback control of HIF-1 through REDD1- regulated ROS suppresses tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:4675–4680. doi: 10.1073/pnas.0907705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma Q, Cavallin LE, Yan B, Zhu S, Duran EM, Wang H, Hale LP, Dong C, Cesarman E, Mesri EA, et al. Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi's sarcoma. Proc Natl Acad Sci U S A. 2009;106:8683–8688. doi: 10.1073/pnas.0812688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bell EL, Emerling BM, Ricoult SJ, Guarente L. SirT3 suppresses hypoxia inducible factor 1 and tumor growth by inhibiting mitochondrial ROS production. Oncogene. 2011;30:2986–2996. doi: 10.1038/onc.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell. 2011;19:416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 70.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Glasauer A, Sena LA, Diebold LP, Mazar AP, Chandel NS. Targeting SOD1 reduces experimental non-small-cell lung cancer. J Clin Invest. 2013 doi: 10.1172/JCI71714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw AT, Winslow MM, Magendantz M, Ouyang C, Dowdle J, Subramanian A, Lewis TA, Maglathin RL, Tolliday N, Jacks T. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc Natl Acad Sci U S A. 2011;108:8773–8778. doi: 10.1073/pnas.1105941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaminski MM, Roth D, Krammer PH, Gulow K. Mitochondria as oxidative signaling organelles in T-cell activation: physiological role and pathological implications. Archivum immunologiae et therapiae experimentalis. 2013;61:367–384. doi: 10.1007/s00005-013-0235-0. [DOI] [PubMed] [Google Scholar]
- 75.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid Redox Signal. 2013 doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–437. doi: 10.1146/annurev.cellbio.21.122303.115827. [DOI] [PubMed] [Google Scholar]
- 78.Takeuchi O, Akira S. Innate immunity to virus infection. Immunological reviews. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chandel N, Trzyna W, McClintock D, Schumacker P. Role of oxidants in NF-kappa B activation and TNF-alpha gene transcription induced by hypoxia and endotoxin. J Immunol. 2000;165:1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- 81.Wang W, Suzuki Y, Tanigaki T, Rank DR, Raffin TA. Effect of the NADPH oxidase inhibitor apocynin on septic lung injury in guinea pigs. Am J Respir Crit Care Med. 1994;150:1449–1452. doi: 10.1164/ajrccm.150.5.7952574. [DOI] [PubMed] [Google Scholar]
- 82.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci U S A. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moore CB, Ting JP. Regulation of mitochondrial antiviral signaling pathways. Immunity. 2008;28:735–739. doi: 10.1016/j.immuni.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 85.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 88.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J Exp Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chaudhri G, Clark IA, Hunt NH, Cowden WB, Ceredig R. Effect of antioxidants on primary alloantigen-induced T cell activation and proliferation. J Immunol. 1986;137:2646–2652. [PubMed] [Google Scholar]
- 92.Laniewski NG, Grayson JM. Antioxidant treatment reduces expansion and contraction of antigen-specific CD8+ T cells during primary but not secondary viral infection. J Virol. 2004;78:11246–11257. doi: 10.1128/JVI.78.20.11246-11257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaminski MM, Sauer SW, Klemke CD, Suss D, Okun JG, Krammer PH, Gulow K. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: mechanism of ciprofloxacinmediated immunosuppression. J Immunol. 2010;184:4827–4841. doi: 10.4049/jimmunol.0901662. [DOI] [PubMed] [Google Scholar]
- 95.Kaminski M, Kiessling M, Suss D, Krammer PH, Gulow K. Novel role for mitochondria: protein kinase Ctheta-dependent oxidative signaling organelles in activation-induced T-cell death. Mol Cell Biol. 2007;27:3625–3639. doi: 10.1128/MCB.02295-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wheeler ML, Defranco AL. Prolonged production of reactive oxygen species in response to B cell receptor stimulation promotes B cell activation and proliferation. J Immunol. 2012;189:4405–4416. doi: 10.4049/jimmunol.1201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca2+ and an oxidant signal. Cell. 2005;121:281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 98.Lang PA, Xu HC, Grusdat M, McIlwain DR, Pandyra AA, Harris IS, Shaabani N, Honke N, Maney SK, Lang E, et al. Reactive oxygen species delay control of lymphocytic choriomeningitis virus. Cell Death Differ. 2013;20:649–658. doi: 10.1038/cdd.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 100.Wang D, Malo D, Hekimi S. Elevated mitochondrial reactive oxygen species generation affects the immune response via hypoxia-inducible factor-1alpha in long-lived Mclk1+/- mouse mutants. J Immunol. 2010;184:582–590. doi: 10.4049/jimmunol.0902352. [DOI] [PubMed] [Google Scholar]
- 101.Kong X, Thimmulappa R, Craciun F, Harvey C, Singh A, Kombairaju P, Reddy SP, Remick D, Biswal S. Enhancing Nrf2 pathway by disruption of Keap1 in myeloid leukocytes protects against sepsis. Am J Respir Crit Care Med. 2011;184:928–938. doi: 10.1164/rccm.201102-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thimmulappa RK, Lee H, Rangasamy T, Reddy SP, Yamamoto M, Kensler TW, Biswal S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J Clin Invest. 2006;116:984–995. doi: 10.1172/JCI25790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202:47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Szakmany T, Hauser B, Radermacher P. N-acetylcysteine for sepsis and systemic inflammatory response in adults. Cochrane Database Syst Rev. 2012;9:CD006616. doi: 10.1002/14651858.CD006616.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of gerontology. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 106.Balaban R, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 107.Van Raamsdonk JM, Hekimi S. Reactive Oxygen Species and Aging in Caenorhabditis elegans: Causal or Casual Relationship? Antioxid Redox Signal. 2010;13:1911–1953. doi: 10.1089/ars.2010.3215. [DOI] [PubMed] [Google Scholar]
- 108.Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 109.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994;263:1128–1130. doi: 10.1126/science.8108730. [DOI] [PubMed] [Google Scholar]
- 110.Mockett RJ, Sohal BH, Sohal RS. Expression of multiple copies of mitochondrially targeted catalase or genomic Mn superoxide dismutase transgenes does not extend the life span of Drosophila melanogaster. Free Radic Biol Med. 2010;49:2028–2031. doi: 10.1016/j.freeradbiomed.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perez VI, Van Remmen H, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 113.Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, et al. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y, Ikeno Y, Qi W, Chaudhuri A, Li Y, Bokov A, Thorpe SR, Baynes JW, Epstein C, Richardson A, et al. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64:1212–1220. doi: 10.1093/gerona/glp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Van Raamsdonk JM, Hekimi S. Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000361. doi: 10.1371/journal.pgen.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011;21:569–576. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pan Y, Schroeder EA, Ocampo A, Barrientos A, Shadel GS. Regulation of Yeast Chronological Life Span by TORC1 via Adaptive Mitochondrial ROS Signaling. Cell Metab. 2011;13:668–678. doi: 10.1016/j.cmet.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mesquita A, Weinberger M, Silva A, Sampaio-Marques B, Almeida B, Leao C, Costa V, Rodrigues F, Burhans WC, Ludovico P. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc Natl Acad Sci U S A. 2010;107:15123–15128. doi: 10.1073/pnas.1004432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, Guthke R, Platzer M, Kahn CR, Ristow M. Impaired Insulin/IGF1 Signaling Extends Life Span by Promoting Mitochondrial L-Proline Catabolism to Induce a Transient ROS Signal. Cell Metab. 2012;15:451–465. doi: 10.1016/j.cmet.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 122.Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schmeisser K, Mansfeld J, Kuhlow D, Weimer S, Priebe S, Heiland I, Birringer M, Groth M, Segref A, Kanfi Y, Price NL, Schmeisser S, Schuster S, Pfeiffer AF, Guthke R, Platzer M, Hoppe T, Cohen HY, Zarse K, Sinclair DA, Ristow M. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat Chem Biol. 2013;9:693–700. doi: 10.1038/nchembio.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lapointe J, Stepanyan Z, Bigras E, Hekimi S. Reversal of the mitochondrial phenotype and slow development of oxidative biomarkers of aging in long-lived Mclk1+/- mice. J Biol Chem. 2009;284:20364–20374. doi: 10.1074/jbc.M109.006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu X, Jiang N, Hughes B, Bigras E, Shoubridge E, Hekimi S. Evolutionary conservation of the clk-1-dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 2005;19:2424–2434. doi: 10.1101/gad.1352905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Packer L, Fuehr K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature. 1977;267:423–425. doi: 10.1038/267423a0. [DOI] [PubMed] [Google Scholar]
- 127.Bell EL, Klimova TA, Eisenbart J, Schumacker PT, Chandel NS. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol. 2007;27:5737–5745. doi: 10.1128/MCB.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Signer RA, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12:152–165. doi: 10.1016/j.stem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 130.Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 131.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 132.D'Souza AD, Parish IA, Krause DS, Kaech SM, Shadel GS. Reducing mitochondrial ROS improves disease-related pathology in a mouse model of ataxia-telangiectasia. Mol Ther. 2013;21:42–8. doi: 10.1038/mt.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–21. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 134.Cosentino C, Grieco D, Costanzo V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011;30:546–55. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maryanovich M, Oberkovitz G, Niv H, Vorobiyov L, Zaltsman Y, Brenner O, Lapidot T, Jung S, Gross A. The ATM-BID pathway regulates quiescence and survival of haematopoietic stem cells. Nat Cell Biol. 2012;14:535–541. doi: 10.1038/ncb2468. [DOI] [PubMed] [Google Scholar]
- 136.Liu J, Cao L, Chen J, Song S, Lee IH, Quijano C, Liu H, Keyvanfar K, Chen H, Cao LY, et al. Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature. 2009;459:387–392. doi: 10.1038/nature08040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen C, Liu Y, Liu R, Ikenoue T, Guan K, Zheng P. TSCmTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tothova Z, Kollipara R, Huntly B, Lee B, Castrillon D, Cullen D, McDowell E, Lazo-Kallanian S, Williams I, Sears C, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 139.Chuikov S, Levi BP, Smith ML, Morrison SJ. Prdm16 promotes stem cell maintenance in multiple tissues, partly by regulating oxidative stress. Nat Cell Biol. 2010;12:999–1006. doi: 10.1038/ncb2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Juntilla M, Patil V, Calamito M, Joshi R, Birnbaum M, Koretzky G. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 2010;115:4030–4038. doi: 10.1182/blood-2009-09-241000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. doi: 10.1038/nature08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, Kalyanaraman B, Chandel NS. Mitochondrial Complex III ROS Regulate Adipocyte Differentiation. Cell Metab. 2011;14:537–544. doi: 10.1016/j.cmet.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Malinska D, Kudin AP, Bejtka M, Kunz WS. Changes in mitochondrial reactive oxygen species synthesis during differentiation of skeletal muscle cells. Mitochondrion. 2012;12:144–148. doi: 10.1016/j.mito.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 144.Hamanaka RB, Glasauer A, Hoover P, Yang S, Blatt H, Mullen AR, Getsios S, Gottardi CJ, DeBerardinis RJ, Lavker RM, et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci Signal. 2013;6:ra8. doi: 10.1126/scisignal.2003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Morimoto H, Iwata K, Ogonuki N, Inoue K, Atsuo O, Kanatsu-Shinohara M, Morimoto T, Yabe-Nishimura C, Shinohara T. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell. 2013;12:774–786. doi: 10.1016/j.stem.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 146.Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hom JR, Quintanilla RA, Hoffman DL, de Mesy Bentley KL, Molkentin JD, Sheu SS, Porter GA., Jr The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev Cell. 2011;21:469–478. doi: 10.1016/j.devcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ahlqvist KJ, Hamalainen RH, Yatsuga S, Uutela M, Terzioglu M, Gotz A, Forsstrom S, Salven P, Angers-Loustau A, Kopra OH, et al. Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell Metab. 2012;15:100–109. doi: 10.1016/j.cmet.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 149.Raimundo N, Song L, Shutt TE, McKay SE, Cotney J, Guan MX, Gilliland TC, Hohuan D, Santos-Sacchi J, Shadel GS. Mitochondrial stress engages E2F1 apoptotic signaling to cause deafness. Cell. 2012;148:716–726. doi: 10.1016/j.cell.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–70. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]