Abstract
Cancer is a complex disease caused by genetic and epigenetic abnormalities that affect gene expression. The progression from precursor lesions to invasive cervical cancer is influenced by persistent human papilloma virus (HPV) infection, which induces changes in the host genome and epigenome. Epigenetic alterations, such as aberrant miRNA expression and changes in DNA methylation status, favor the expression of oncogenes and the silencing of tumor-suppressor genes. Given that some miRNA genes can be regulated through epigenetic mechanisms, it has been proposed that alterations in the methylation status of miRNA promoters could be the driving mechanism behind their aberrant expression in cervical cancer. For these reasons, we assessed the relationship among HPV infection, cellular DNA methylation and miRNA expression. We conclude that alterations in the methylation status of protein-coding genes and various miRNA genes are influenced by HPV infection, the viral genotype, the physical state of the viral DNA, and viral oncogenic risk. Furthermore, HPV induces deregulation of miRNA expression, particularly at loci near fragile sites. This deregulation occurs through the E6 and E7 proteins, which target miRNA transcription factors such as p53.
Keywords: miRNAs, methylation, human papilloma virus, cervical cancer
1. Introduction
Cervical cancer is one of the most frequently occurring malignant tumors in women worldwide, with ~470,000 new cases and 233,000 deaths per year (1). Squamous cell cervical carcinoma represents approximately 80% of cases. Cervical cancer develops through well-defined pre-malignant lesions, which are known as cervical intraepithelial neoplasia (CIN), ranging from grades I to III (2). Cervical adenocarcinomas represent 10–20% of cases, but the preceding stages are not well characterized (2). The high-risk HPV (HR-HPV), as well as environmental, immunological, genetic and epigenetic factors, are among the etiological causes contributing to cervical carcinogenesis; progression of precursor lesions to invasive cancer is influenced by HR-HPV infection (3,4). Although the mechanisms by which HR-HPV induces changes to the host’s genome and epigenome are still unknown, it has been established that integration of the viral DNA into the cellular genome causes genetic (deletions, amplifications and DNA rearrangements) and epigenetic (modifications to the DNA methylation status and aberrant miRNA expression) alterations. These result in the silencing of tumor-suppressor genes and the overexpression of oncogenes favoring tumor progression (5–8).
Epigenetic modifications are just as important as genetic modifications in terms of regulating gene expression and controlling disease onset. It has been shown that epigenetic silencing of some miRNA genes is functionally involved in cervical carcinogenesis (2,5,7). The interaction between HR-HPV and miRNAs occurs at different times during carcinogenesis, given that: i) some miRNA loci localize to fragile sites, which are the sites where HR-HPV inserts its DNA; ii) proteins encoded by HR-HPV can influence miRNA expression within the host cell and iii) it has been observed that the E6 and E7 proteins of HR-HPV modulate the expression of DNA methyltransferases, which are enzymes that regulate gene expression by methylating promoter regions (2,5,9,10).
Changes in the expression profile of miRNAs have been reported in cervical cancer cell lines, cervical cancer tissue and precursor lesions (1,11–13). Similarly, studies conducted in cell lines suggest that HPV participates in deregulating miRNA expression by modifying the expression profile of miRNAs associated with the presence of HPV and the viral genotype (1,5,11,14,15). Given that a considerable number of miRNAs are subject to epigenetic regulation, it has been proposed that aberrant methylation of miRNA promoters is one of the mechanisms responsible for deregulated miRNA expression in cervical cancer (2,12,16). Here, we analyzed the influence of methylation on miRNA expression as well as on the expression of proteins that regulate cellular processes and participate in carcinogenesis. We further discuss the likelihood of HPV inducing modifications in the methylation status of miRNA promoters in cervical cancer. Lastly, we also assess the relationship between HR-HPV infection, methylation and miRNA expression.
2. HPV and cancer
HPV is one of the most common sexually transmitted infections worldwide and is associated with a wide spectrum of benign and malignant neoplasias (17). HPV is the second infectious agent implicated in cancer development, after Helicobacter pylori (17). It is estimated that 5.2% of all types of cancer can be attributed to HPV infection; HPV has been associated with 90–93% of anal cancer cases, 12–63% of oropharyngeal cancer cases, 36–40% of penile cancer cases, 40–51% of vulvar cancer cases, 40–64% of vaginal cancer cases and 99.7% of cervical cancer cases (17,18). Approximately 100 HPV subtypes with genetic variations and different oncogenic potentials have been identified and classified into three groups: high-risk (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73 and 82); probable high-risk (types 26, 53 and 66) and low-risk (types 6, 11, 40, 42, 43, 44, 54, 61, 70, 72, 81 and CP6108) (4,19).
3. HPV and cervical cancer
Epidemiological and molecular studies have documented the causal link between HR-HPV infection and cervical cancer (3,18,20). HR-HPV subtypes HPV 16, HPV 18, and HPV 31 have been detected in 99.7% of squamous cell cervical carcinomas and in 94–100% of adenocarcinomas and adenosquamous carcinomas (12). It is estimated that ~11.4% of women worldwide and 9.4% of Mexican women are at risk of contracting an HPV infection at some point during their lives (21). Furthermore, it is estimated that >80% of sexually active women become infected with HPV, and >50% of young women are infected after their first sexual intercourse. Almost 90% of infections are spontaneously eliminated during a 3-year period, with only 10% becoming persistent infections. Among the latter, only 1% develop into cervical cancer (10,21).
Persistent HPV infection is required for normal cells to transform into cancerous cells (21). An important step in malignant progression is the integration of HPV into the host’s genome (9,10,21). It appears that the integration event does not happen randomly. Although almost all chromosomes are susceptible, certain regions of the human genome are favored for viral DNA insertion, such as fragile sites, rupture and translocation points, and transcriptionally active regions (10). HPV integration into the cellular genome has several implications: i) it allows permanent expression of the E6 and E7 oncoproteins, which promote cell transformation and immortalization by inactivating the p53 and Rb tumor-suppressor genes, respectively, as well as other proteins that participate in cell adhesion, apoptosis, cell cycle, DNA repair, cellular metabolism, and signal transduction regulating transcription and translation; ii) viral integration near or within a gene can eventually lead to cell growth and proliferation alterations and iii) viral integration can induce epigenetic modification of viral and cellular genes, which may affect their expression. A series of epigenetic alterations in the cellular and viral genomes can occur during each stage of cervical cancer (7,10,21).
4. DNA methylation in cervical cancer
Regulation of gene expression is a vital process that determines the profile of proteins required to ensure the proper occurrence of processes including development, cellular differentiation, organogenesis, cellular stress response and programmed cell death (22). In normal tissues, epigenetic events such as DNA methylation, histone acetylation and expression of miRNAs, and other small RNAs regulate the expression of genes participating in the activation of differentiation processes as well as cellular functions that contribute to cellular homeostasis (23,24). Twenty-five years ago, it was discovered that epigenetic modifications participate in cancer development, leading to uncontrolled cell proliferation (23). One of the most widely studied epigenetic mechanisms is DNA methylation, a reversible reaction catalyzed by DNA methyltransferase (DNMT) enzymes. DNMT1 is a maintenance methyltransferase that preserves the methylation pattern during each cellular division. DNMT3a and DNMT3b are de novo methyltransferases (25,26). DNMTs add a methyl group onto carbon 5 of cytosine residues adjacent to guanine residues (5′-CpG-3′), which mainly occurs in CpG islands. CpG islands are generally found in the promoter regions of protein-coding genes, and expression is silenced upon their methylation. Non-coding genes, such as miRNAs, are also susceptible to regulation by methylation (25,26).
Global DNA hypomethylation in repetitive regions and hypermethylation in CpG island regions of tumor-suppressor gene promoters are DNA modifications that are commonly found early during cancer development (3,27). Alterations to the DNA methylation pattern, which have also been described in cervical cancer, contribute to genomic instability, chromosomal rearrangements, and silencing of coding and non-coding genes, such as miRNAs (2,20,28–30). Silencing of tumor-suppressor genes through DNA hypermethylation has been linked to the development of different types of cancers, including cervical cancer, and is frequently associated with poor clinical results (Table I). However, silencing of tumor suppressor miRNAs through hypermethylation of CpG islands in their promoter regions has also been implicated in carcinogenesis (30,31).
Table I.
Hypermethylated genes associated with cancer development and the biological processes altered during carcinogenesis.
| Hypermethylated genes in cancer | Biological process |
|---|---|
| hMLH1, WRN, BRCA1, MGMT | DNA repair |
| CRBP1, RAR-β2 | Vitamin response |
| NOREIA, RASSF1A | Ras signaling |
| p15INK4b, Rb, P16INK4a, CCNA1, FHIT | Cell cycle |
| P14ARF, p73, HIC-1 | p53 pathway |
| E-cadherin, H-cadherin, FAT, EXT-1, SLIT2, EMP3, CADM1 | Cell adherence and invasion |
| TMS1, WIF-1, SFRP1, hTERT, DcR1, DcR2, DAPK1 | Apoptosis |
| DKK-1, IGFBP-3, APC | Wnt signaling pathway |
| SOCS.1, SOCS-3, SYK | Tyrosine kinase signaling cascade |
| GATA-4, GATA-5, ID4 | Transcription factors |
|
GSTP1, LKB1/STK11, THBS-14, COX-2, SRBC, RIZ1, SLC5 A8, TPEF/HPP1, Laminin, PTEN, CDH1, TSLC1 |
Other pathways |
Bold font, promoters reported to be hypermethylated in cervical cancer.
5. HPV and DNA methylation
It is thought that HR-HPV can induce changes in DNA methylation and histone acetylation and also cause aberrant miRNA expression (6). Little is known concerning the role of oncogenic viruses in the modification of cellular DNA methylation patterns (35). The hepatitis B, hepatitis C, Kaposi’s sarcoma-associated and Epstein-Barr viruses interact with DNMTs, modulating their expression. As a result, viral and cellular genes are trans-activated and trans-repressed, respectively (6,35,36). Although the relationship between HPV and aberrant methylation in cervical cancer is not well understood, some authors have suggested that HPV interferes with the cellular DNA methylation machinery, either to conceal itself or as part of its viral cycle (6,35,37). Some investigators have described that upon HPV 16 infection, cellular DNA undergoes epigenetic alterations induced by the E6 and E7 oncoproteins (35,38,39). It has been proposed that methylation has arisen as a defense mechanism by the host cell to silence viral DNA (6,40,41).
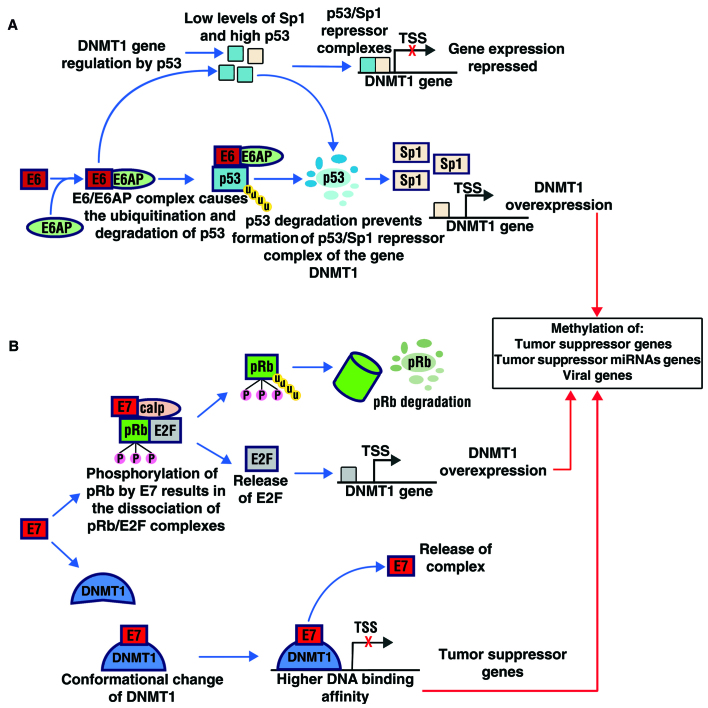
The E6 and E7 oncoproteins of HR-HPV increase the expression and activity of DNMT1 (39). E6 does so by degrading p53 (35,39) (Fig. 1A). In the cervical cancer cell lines SiHa and CaSki, knockdown of E6 is associated with an increase in p53 and a decrease in DNMT1 expression (35,39). In contrast, Lin et al (42) showed that p53 negatively regulates DNMT1 expression both in cell lines and in lung cancer patients. p53 binds to the specificity protein 1 (Sp1) and chromatin-remodeling proteins, and this complex then binds to the promoter region of DNMT1. The formation of the complex inhibits Sp1 from activating the transcription of DNMT1 (42). Normally, Sp1 induces degradation of p53 by MDM2-mediated ubiquitination (Sp1/p53/MDM2 complex) and induces overexpression of DNMT1 (42). Non-small cell lung cancer patients with mutations in p53 and patients with alterations in p53 and/or Sp1 showed hypermethylated promoter regions of tumor-suppressor genes (p=0.003–0.016), most likely due to DNMT1 overexpression (42).
Figure 1.
Regulation of viral and cellular gene methylation by the E6 and E7 oncoproteins of HR-HPV. Integration of viral DNA into the cellular genome causes genetic and epigenetic alterations. The E6 and E7 oncoproteins of HR-HPV increase the expression and activity of DNA methyltransferases, particularly DNMT1. (A) Binding of p53 to Sp1 (p53/Sp1) forms a repressor complex for DNMT1 transcription. Degradation of p53 by E6 avoids the formation of this repressor complex and Sp1 induces the expression and activity of DNMT1. (B) E2F positively regulates the promoter activity of DNMT1. Binding of E7 to pRb (E7/pRb) causes the release of E2F, favoring the expression of DNMT1. Binding of E7 to DNMT1 (E7/DNMT1) induces a conformational change in DNMT1, exposing its DNA binding site and promoting DNA binding; once the E7/DNMT1 complex binds DNA, DNMT1 closes on the DNA and maintains a stable DNMT1/DNA interaction, and E7 dissociates from the complex. Overexpression of DNMT1 results in hypermethylation of tumor-suppressor gene promoters, which leads to cellular transformation and tumorigenesis. HR-HPV, high-risk HPV; DNMT, DNA methyltransferase.
Modulation of DNMT1 expression by E7 (Fig. 1B) can occur in two different ways: i) indirectly, through E7 binding to pRb, which releases the transcription factor E2F; given that conserved E2F-binding sequences exist at the transcription start site for DNMT1, the release of E2F results in the regulation of the DNMT1 promoter activity and ii) directly, by binding of E7 to DNMT1 (38). It has been proposed that the E7/DNMT1 complex induces a conformational change in DNMT1, exposing its active site, promoting DNMT1/DNA binding, and binding to S-adenosyl-L-methionine (AdoMet) (38). Once the E7/DNMT1/DNA complex forms, E7 dissociates from the complex, and DNMT1 closes on the DNA, maintaining a stable DNMT1/DNA interaction (35,40). The increase in DNMT1 activity causes aberrant methylation of the cellular genome, resulting in the silencing of tumor-suppressor genes and favoring cellular transformation (38,40).
The role of DNMT1 in cervical carcinogenesis has been reported by Jin-Tao et al (43), who used in vitro and in vivo studies and found that low levels of serum folate and high expression of DNMT1 protein or mRNA were significantly associated with cervical carcinogenesis (p=0.001). Integration of HR-HPV DNA into the host’s genome is an essential step in cervical carcinogenesis, and changes to viral DNA methylation are associated with the oncogenic capacity of HPV (28,29). After its integration into the human genome, the DNA of HPV 16 and HPV 18 is methylated (28,45). However, there are still controversial results regarding the participation of HPV in the aberrant DNA methylation that has been observed in cervical cancer.
Henken et al (28) used primary human foreskin keratinocytes (PHFKs) transfected with HPV 16 and HPV 18 to decipher the most important events in HR-HPV-mediated transformation. The authors used a longitudinal in vitro system utilizing serial passages and found that the transfected keratinocytes gradually developed dysplastic characteristics that were similar to pre-malignant cervical lesions. After immortalization, only the keratinocytes with HPV DNA integrated into their genome accumulated changes in the methylation patterns in the promoter regions of different genes. After transfecting PHFKs with episomal forms of both HPV 16 and HPV 18, Leonard et al (35) and Henken et al (28) observed overexpression of DNMT1 and DNMT3B. They also found changes in the methylation status of different cellular genes that were previously reported to be methylated in cervical cancer, most likely due to an early epigenetic reprogramming induced by HR-HPV. Furthermore, Leonard et al (35) observed differences in the expression topography of DNMTs in relation to the viral genotype. Following transfection with HPV 16, nuclear expression of DNMT1 was restricted to cells in the basal and early differentiation layers and was decreased in more differentiated cells. However, in cells transfected with HPV 18, nuclear DNMT1 expression was observed in the basal and suprabasal layers as well as in the stratum granulosum, with some cells displaying intense DNMT1 staining. Changes in the expression topography of DNMT3B were similar to those observed for DNMT1. However, the global staining intensity was weaker. Moreover, Leonard et al (35) analyzed the whole-genome methylation profile after transfection with the episomal forms of HPV 16 and HPV 18 and found a significant increase in the methylation status of 5,607 and 2,387 genes, respectively. They also found a decrease in the methylation status of 3,568 and 4,160 genes, respectively. Non-overlapping increases and decreases in methylation were found for 2,295 and 1,023 genes, respectively. It is possible that the altered miRNA expression observed in cervical cancer is related to the aberrant methylation of miRNA promoters. Thus, HR-HPV could indirectly induce aberrant miRNA methylation (2,20,46).
6. miRNAs and their deregulation in cancer
miRNAs are small, non-coding RNA molecules ~22–25 nucleotides (nt) in size. They are usually phylogenetically conserved with a tissue- and time-specific expression pattern (47,48). miRNAs have been recognized as epigenetic regulators, controlling gene expression without altering the DNA sequence (49). The expression profile of miRNAs in cell lines and cervical cancer tissues suggests that aberrant miRNA expression contributes to the development of cervical cancer and HR-HPV-induced precursor lesions (1,7,50). Defects in miRNA expression have been associated with: i) genetic alterations, such as deletions, amplifications and point mutations and ii) epigenetic alterations, such as histone modifications and aberrant DNA methylation (25,46,48,52).
Regulation of miRNA expression is important to maintain cellular homeostasis. However, the molecular mechanisms regulating miRNA gene transcription are not well understood to date (31,54). Currently, it is thought that miRNA biogenesis is regulated at several levels: i) at a transcriptional level, which consists of pri-miRNA transcription by RNA polymerase II and III; ii) at a post-transcriptional level, consisting of miRNA maturation, which involves the processing of pri-miRNA to pre-miRNA, export into the cytoplasm, and incorporation into the RISC complex and iii) at a level of miRNA localization within the genome (23,46).
Regulation of miRNA expression at the transcriptional level is one of the most important steps in their biogenesis, and genome localization influences their transcription (23,54,55). miRNA genes are encoded within the genome as a unit or in groups of 2 to 19 miRNAs and can reside within introns or exons of coding genes or in intergenic regions (1,23,46,55,56). The miRNA genes localized to intergenic regions have their own promoter, which allows them to be independently transcribed. miRNAs localized to intragenic regions (in introns or exons) can be transcribed independently from the gene in which they reside, as long as they have their own promoter, or they can be transcribed together with the host gene (1,23,46,55,56).
Identification of transcriptional start sites and regulatory regions is critical to understand the mechanisms and transcription factors that mediate miRNA expression (56). In general, miRNA expression can be regulated by: i) DNA-binding factors, such as c-myc and p53; ii) specific transcription factors, such as myocyte enhancer factor-2 (MEF2), PU.1 and REST and iii) growth factors, such as platelet-derived growth factor (PDGF) and transforming growth factor β (TGF-β), among others (46). Given that miRNA genes are expressed in a tissue- and time-specific manner and their promoters contain characteristics such as CpG islands, TATA boxes, TFIIB recognition elements, and initiators that are similar to the promoters of protein-coding genes, miRNA expression can also be regulated by epigenetic mechanisms, such as nucleosome remodeling and DNA methylation (23,31–33,46,54,57).
Regulation of miRNA expression at a post-transcriptional level is essential for the specificity and function of certain miRNAs in a tissue- and time-specific context. miRNAs that are clustered in groups are individually expressed independently from the other miRNAs in the group (58). This suggests that miRNAs are individually regulated at a post-transcriptional level (58). Their localization within the genome appears to be an important factor for the regulation or deregulation of certain miRNAs, given that several miRNAs have been mapped to or fragile sites, minimal regions of loss of heterozygosity, amplification, common breakpoint regions and transcriptionally active regions that have been linked to cancer in humans (2,23,46,50,54). In cancer tissues, miRNA expression profiling has revealed that their expression is either increased or decreased compared with healthy tissue. They are differentially expressed in different types of tumor, cell lineages and tumor stages (32,59). miRNAs play an important role in cervical carcinogenesis, from HPV infection to cancer progression (Table II) (50).
Table II.
Expression of miRNAs in cervical and uterine cancer.
| Study groups | miRNA expression | Significance in cervical cancer | Refs. |
|---|---|---|---|
| Cervical tissue and serum from patients with SCCC with LNM and without LNM and samples from healthy patients | Upregulated: miR-1246, miR-20a, miR-2392, miR-3147, miR-3162-5p and miR-4484 | Overexpression of miRNAs in serum can predict gangliar metastasis in patients with early-stage SCCC. | (60) |
| Samples from patients with primary CAC and SCCC | Upregulated: miR-21, miR-27a, miR-34a, miR-155, miR-196a, miR-203 and miR-221 | Differential expression of miRNAs correlates with the histopathological diagnosis of primary CAC and SCCC, independently of clinical stage and HPV infection. | (61) |
| Samples from patients with HSIL and CAC and samples from healthy patients | Upregulated: hsa-miR-9, hsa-miR-15b and hsa-miR-28-5p Downregulated: hsa-miR-100 and hsa-miR-125b |
Altered expression of these five miRNAs, is associated with chromosomal alterations in cervical cancer. | (62) |
| Samples from patients with SCCC (FIGO IB2-IV) and patients with early-stage SCCC (FIGO IB1) | Downregulated: hsa-let-7c, hsa-miR-10b, hsa-miR-100, hsa-miR-125b, hsa-miR-143, hsa-miR-145 and hsa-miR-199a-5p | Decreased expression of let-7c, miR-10b, miR-100, miR-125b, miR-143, miR-145 and miR-199a-5p is associated with advanced-stage SCCC. Decreased expression of let-7c, miR-100, miR-125b, miR-143, miR-145 and miR-199a-5p is associated with LNM and decreased patient survival. Decreased expression of miR-10b and miR-100 is associated with a poor prognosis for SCCC. | (63) |
| Samples from patients with SCCC (CIN2, CIN3) and samples from healthy patients | Upregulated: miR-518a, miR-34b, miR-34c, miR-20b, miR-338, miR-9, miR-512-5p, miR-424, miR-345 and miR-10a Downregulated: miR-193b and miR-203 |
Differential miRNA expression was found in tissues from patients with SCCC and samples from healthy patients. Predictive target analysis revealed that the miRNAs with decreased expression control signaling pathways regulating cell cycle and apoptosis. | (64) |
| Samples from patients with cervical cancer (IB–IIB) and patients with benign gynecological diseases | Upregulated: hsa-miR-15a, hsa-miR-19a, hsa-miR-20b, hsa-miR-21, hsa-miR-141, hsa-miR-106b and miR-hsa-224 Downregulated: hsa-let-7c, hsa-miR-143, hsa-miR-199a-5p, hsa-miR-203 and miR-145 |
hsa-miR-15a, hsa-miR-106b, and hsa-miR-20b regulate a large number of target genes and have strong regulatory effects on the differential expression of genes in cervical cancer. | (65) |
| Samples from patients with cervical cancer, LSIL, HSIL and healthy patients | Upregulated: miR-522*, miR-512-3p, miR-148a, miR-302b, miR-10a, miR-196a and miR-132 Downregulated: miR-26a, miR-143, miR-145, miR-99a, miR-203, miR-513, miR-29a, miR-199a, miR-106a, miR-205, miR-197, miR-16, miR-27a and miR-142-5p |
Different miRNA expression was found between normal cervix, precursor lesions, and cancer tissues. This suggests that deregulated miRNAs play a role in malignant transformation of cervical cells. | (66) |
| Samples from patients with cervical cancer | Upregulated: miR-21, miR-200a and miR-9 Downregulated: miR-203 and miR-218 |
miR-200a affects the metastatic potential of cervical cancer cells by suppressing the expression of genes that are important for cell motility | (67) |
| Samples from patients with cervical cancer and healthy patients | Upregulated: miR-15b, miR-16, miR-146a, miR-155, miR-223, miR-21, miR-205 and let-7f Downregulated: miR-126, miR-424, miR-143, miR-145 |
Functional studies showed that miR-143 and miR-145 suppress cell growth, whereas miR-146a promotes cell proliferation in cervical cancer. | (1) |
| Cervical cancer cell lines CaSki, SiHa, HeLa and C33A. Samples from patients with cervical cancer and healthy patients | Upregulated: miR-182, miR-183 and miR-210 Downregulated: miR-143, miR-145, miR-126, miR-195, miR-218, miR-368 and miR-497 |
miR-218 expression was decreased in HPV-positive cell lines and cervical cancer tissue compared with C33A cells and normal cervix tissue. Expression of the HPV 16 (high-risk) E6 oncoprotein decreases the expression of miR-218 compared with HPV 6 (low-risk). This suggests that some miRNAs are regulated by HPV. | (11) |
| Samples from patients with invasive SCCC and healthy patients | Upregulated: miR-199a, miR-199s, miR-9, miR-199a, miR-199b, miR-145, miR-133a, miR-133b, miR-214 and miR-127 Downregulated: miR-149 and miR-203 |
Overexpression of miR127 is associated with LNM. In vitro, transfection with anti-miR-199a in cervical cancer cell lines inhibits cell growth. | (68) |
| Cervical cancer cell lines SW756, C4I, C33A, CaSki, SiHa and ME-180 as well as samples from patients with benign gynecological pathologies | Upregulated: miR-21 Downregulated: let-7b, let-7c, miR-23b, miR-196b and miR-143 |
Decreased expression of miR-143 and overexpression of miR-21 in cervical cancer samples is reproducible, which highlights the potential value of miRNAs as tumor markers. | (5) |
SCCC, squamous cervical cell carcinoma; CAC, cervical adenocarcinoma; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; LNM, lymph node metastasis; FIGO, classification criteria of the International Federation of Gynecology and Obstetrics.
7. HPV and miRNA promoter methylation in cervical cancer
It is possible that the aberrant methylation of miRNA promoters is responsible for the altered expression of some miRNA genes with tumor-suppressor or oncogenic functions in cancer (Table III). The role of HR-HPV in altering the cellular DNA methylation status is still controversial. However, it is possible that HR-HPV plays a role in the deregulation of miRNA gene methylation in cervical cancer (2,69,70). Although few studies exist regarding DNA methylation in the deregulation of miRNA expression in cancer, it has been proposed that alterations to the methylation status of miRNA genes could explain the deregulation of miRNA expression in cervical cancer (2,69,70).
Table III.
miRNA genes regulated by methylation in certain types of cancer.
| Type of cancer | Methylation status | Refs. |
|---|---|---|
| Colon cancer | Hypermethylated: miR-126, miR-34a, miR-34b/c, miR-1-1, miR-133a-2 and miR-149 | (60,71–73) |
| Gastric cancer | Hypermethylated: miR-433, miR-127, miR-148a, miR-34b, miR-129, miR-9, miR-10b, miR-195 and miR-378 | (74–79) |
| Leukemia | Hypermethylated: miR-663 | (80) |
| Bladder cancer | Hypermethylated: miR-200b, miR-152 and miR-10a | (81) |
| Hepatocellular carcinoma | Hypermethylated: miR-129-2, miR-10a, miR-122 and miR-1-1 | (19,50,82,83) |
| Hypomethylated: miR-191 | (84) | |
| Breast cancer | Hypermethylated: miR-31, miR-130a, let-7a-3/let-7b, miR-155, miR-137, miR-34b/miR-34c, miR-125b and miR-34a | (72,85–87) |
| Prostate cancer | Hypermethylated: miR-205, miR-132 and miR-193b | (88–90) |
| Non-small cell lung cancer | Hypermethylated: miR-9-3, miR-122, miR-124-2, miR-124-3 and miR-34b/c | (91–94) |
| Multiple myeloma | Hypermethylated: miR-203 | (95) |
| Pancreatic cancer | Hypermethylated: miR-132 | (85) |
| Hypomethylated: miR-200a and miR-200b | (14) | |
| Ovarian cancer | Hypermethylated: miR-34a and miR-34b/c | (72) |
In patients and cervical cancer cell lines, it has been observed that silencing of tumor-suppressor miRNAs through aberrant promoter methylation favors cervical carcinogenesis (2,20,69,70). It has been proposed that HR-HPV can lead to modifications in the methylation pattern of miRNA promoters (20,69). Leonard et al (35) reported that changes to cellular DNA methylation associated with HPV 16 and HPV 18 are not randomly distributed but rather cluster in specific chromosomal regions, such as the HR-HPV integration regions and regions of chromosomal loss and gain. After cervical cancer cell lines (HeLa, SiHa, CaSki and C33A) were subjected to treatment with hypomethylating agents, decreased methylation levels were found for certain miRNAs, which resulted in their increased expression and concomitant decreased expression of their target genes (2,69,70). It is possible that the HR-HPV genotypes are involved in the methylation processes of miRNAs in cervical cancer (20). However, in vitro findings suggest that the methylation events take place after cellular immortalization and are not directly related to the presence of HR-HPV (2). It is likely that identifying the methylation status of miRNAs could be useful for the prognosis of precursor lesions and cervical cancer (2).
Analysis of the methylation status of the three loci encoding the mature hsa-miR-124 (hsa-miR-124-1/-2/-3) in cervical cancer cell lines by Wilting et al (2) showed that the three promoter regions of hsa-miR-124 were methylated in SiHa and CaSki cells. In contrast, the methylation levels of the three hsa-miR-124 regions, in particular hsa-miR-124-1, were extremely low in HeLa cells compared with those observed in SiHa and CaSki cells. A decrease in methylation levels and overexpression of hsa-miR-124 were observed after SiHa cells were treated with the hypomethylating agent 5-aza-2′-deoxycytidine (5-Aza) (2). Ectopic expression of hsa-miR-124 in SiHa and CaSki cells decreased the proliferation rate and migratory capacity of the cells (2). Yao et al (69) proposed that hypermethylation of miR-432, miR-1286, miR-641, miR-1290, miR-1287 and miR-95 may be related to HR-HPV infection. Following treatment with 5-Aza, miRNAs were only overexpressed in cervical cancer cell lines infected with HR-HPV (CaSki, HeLa and SiHa) and not in a cell line without HPV (C33A). Wilting et al (70) reported that transcriptional repression of hsa-miR-149, hsa-miR-203, hsa-miR-375 and hsa-miR-638 was associated with an increase in methylation levels of these miRNAs. Treatment of the SiHa cell line with the hypomethylating agent 5-Aza induced an increase in the expression of hsa-miR-149, hsa-miR-203 and hsa-miR-375. However, no increased expression was observed for hsa-miR-638. In accordance with these findings, a decrease in the methylation levels of hsa-miR-149, hsa-miR-203 and hsa-miR-375 but not hsa-miR-638 was observed in cells treated with 5-Aza (70). It is likely that high concentrations of the hypomethylating agent are needed to decrease methylation of the hsa-miR-638 promoter region or that more complex epigenetic mechanisms are regulating this locus (70). Ectopic expression of hsa-miR-203 in cervical cancer cell lines decreased their rates of proliferation and anchorage-independent growth (70). The results described by Wilting et al (70) indicate that decreased expression of hsa-miR-149, hsa-miR-203 and hsa-miR-375 in cervical cancer cell lines is associated with the methylation status of their promoter regions.
To investigate the stage in the HR-HPV-mediated transformation process at which hsa-miR-124 becomes methylated, Wilting et al (2) analyzed the three loci encoding the mature form of hsa-miR-124 (hsa-miR-124-1/-2/-3) using a longitudinal panel of human foreskin keratinocytes immortalized with HPV 16 and HPV 18. This panel represented the morphological, genetic and epigenetic aspects of the different transformation stages that are observed in high-grade lesions. These authors found that in the late passages of keratinocytes immortalized with HR-HPV, there was an increase in the methylation levels of the loci encoding hsa-miR-124. Furthermore, this increase was correlated with a decrease in miRNA expression and high levels of IGFBP7, which is considered a potential target gene of hsa-miR-124. Wilting et al (70) reported that methylation of the promoter regions of hsa-miR-149, hsa-miR-203, hsa-miR-375 and hsa-miR-638 was increased in keratinocytes immortalized with HPV 16 and HPV 18 compared with primary keratinocytes. Moreover, these authors found that the increase in hsa-miR-149, hsa-miR-203 and hsa-miR-375 methylation correlated with malignant progression and that expression of these miRNAs can be restored by treatment with 5-Aza.
The findings in samples from patients with precursor lesions and cervical cancer support the in vitro findings. Wilting et al (2) reported that no methylation of hsa-miR-124-1 and hsa-miR-124-2 was found in normal cervical tissue. However, in cervical cancer, 90% of samples showed methylation of these genes. Furthermore, these authors observed that the increase in methylation levels of hsa-miR-124-1 and hsa-miR-124-2 was correlated with reduced expression of hsa-miR-124 (2). However, the authors also found that the methylation status of hsa-miR-124-1 and hsa-miR-124-2 was predictive of high-grade lesions in 43 cervical shave biopsy samples from women who tested positive for HR-HPV. They concluded that silencing of hsa-miR-124 by DNA methylation is functionally implicated in cervical carcinogenesis and can be used as a valuable indicator to improve the timely detection of cervical cancer and high-grade precursor lesions. Botezatu et al (20) assessed the methylation status of CpG islands surrounding the hsa-miR-124a, hsa-miR-34b and hsa-miR-203 genes in cervical cancer and precursor lesions. They further evaluated the relationship between methylation status and the presence of HPV DNA and the viral genotype. They found significant differences in the methylation levels of the miRNA promoter regions in cervical tumor samples compared with control samples. Hypermethylation of miR-124a and miR-203 was also observed in precursor lesion samples compared with control samples. Botezatu et al (20) further observed significant differences in the methylation levels of the miR-124a and miR-203 CpG islands between the HR-HPV group and the low-risk HPV (LR-HPV) group and found a strong association between the methylation process and HR-HPV genotypes. In cervical tumor and precursor lesion samples with LR-HPV genotypes, methylation levels were similar to the ones found in normal samples. This finding strengthens the possibility that the HR-HPV genotypes are involved in miRNA methylation processes (20). Yao et al (69) reported that in primary cervical tumors compared with normal tissue, the expression levels of hsa-miR-432, hsa-miR-1286, hsa-miR-641, hsa-miR-1290, hsa-miR-128 and hsa-miR-95 were inversely correlated with the methylation status found in cervical cancer cell lines treated with 5-Aza. Wilting et al (2) reported significantly increased methylation levels for hsa-miR-149, hsa-miR-203 and hsa-miR-375 in patients with cervical carcinomas. In high-grade lesions, methylation levels were only significantly increased for hsa-miR-203 and hsa-miR-375. Moreover, Wilting et al (70) found an increase in the methylation levels of hsa-miR-203 in cervical samples from women with high-grade lesions who were infected with HPV compared with control samples.
8. Conclusion
The finding that alterations in miRNA expression and methylation of key genes involved in cell cycle regulation are frequent events in the process of carcinogenesis represents a challenge and an incentive for this field of research. Extensive study has been devoted to identifying variations in miRNA expression and the expression of miRNA targets in cervical cancer and its precursor lesions. However, knowledge concerning the role of miRNAs in the carcinogenic process is still in its early stages. Evidence suggests that in cervical cancer, hypermethylation of miRNA promoters contributes to the decreased expression of miRNAs with tumor-suppressor gene functions and favors overexpression of miRNAs with oncogenic functions. Methylation is an important mechanism in the HPV viral cycle. Alterations to the methylation status of cellular DNA are influenced by HPV infection, the viral genotype, the physical state of the viral DNA, and oncogenic risk. The E6 and E7 oncoproteins of HPV 16 induce the overexpression of DNA methyltransferase enzymes, which can catalyze the aberrant methylation of protein-coding and miRNA genes that are susceptible to regulation by methylation. Furthermore, HPV deregulates the expression of miRNAs with loci located at fragile sites through the E6 and E7 oncoproteins. Targets of these proteins include transcription factors of miRNAs, such as p53.
Acknowledgements
The translation of the present study to the English language was financed by the Programa de Fortalecimiento Académico del Posgrado de Alta Calidad, key, I010/455/2013 C-677/2013.
References
- 1.Wang X, Tang S, Le SY, et al. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One. 2008;3:e2557. doi: 10.1371/journal.pone.0002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilting SM, van Boerdonk RA, Henken FE, et al. Methylation-mediated silencing and tumour suppressive function of hsa-miR-124 in cervical cancer. Mol Cancer. 2010;9:167. doi: 10.1186/1476-4598-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang HJ. Aberrant DNA methylation in cervical carcinogenesis. Chin J Cancer. 2013;32:42–48. doi: 10.5732/cjc.012.10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faridi R, Zahra A, Khan K, Idrees M. Oncogenic potential of human papillomavirus (HPV) and its relation with cervical cancer. Virol J. 2011;8:269. doi: 10.1186/1743-422X-8-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lui WO, Pourmand N, Patterson BK, Fire A. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res. 2007;67:6031–6043. doi: 10.1158/0008-5472.CAN-06-0561. [DOI] [PubMed] [Google Scholar]
- 6.Whiteside MA, Siegel EM, Unger ER. Human papillomavirus and molecular considerations for cancer risk. Cancer. 2008;113(Supp 10):2981–2994. doi: 10.1002/cncr.23750. [DOI] [PubMed] [Google Scholar]
- 7.Saavedra KP, Brebi PM, Roa JC. Epigenetic alterations in preneoplastic and neoplastic lesions of the cervix. Clin Epigenetics. 2012;4:13. doi: 10.1186/1868-7083-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechner M, Fenton T, West J, et al. Identification and functional validation of HPV-mediated hypermethylation in head and neck squamous cell carcinoma. Genome Med. 2013;5:15. doi: 10.1186/gm419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaiwongkot A, Vinokurova S, Pientong C, et al. Differential methylation of E2 binding sites in episomal and integrated HPV 16 genomes in preinvasive and invasive cervical lesions. Int J Cancer. 2013;132:2087–2094. doi: 10.1002/ijc.27906. [DOI] [PubMed] [Google Scholar]
- 10.Das P, Thomas A, Mahantshetty U, Shrivastava SK, Deodhar K, Mulherkar R. HPV genotyping and site of viral integration in cervical cancers in Indian women. PLoS One. 2012;7:e41012. doi: 10.1371/journal.pone.0041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez I, Gardiner AS, Board KF, Monzon FA, Edwards RP, Khan SA. Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene. 2008;27:2575–2582. doi: 10.1038/sj.onc.1210919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng ZM, Wang X. Regulation of cellular miRNA expression by human papillomaviruses. Biochim Biophys Acta. 18092011:668–677. doi: 10.1016/j.bbagrm.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao Q, Shen Q, Zhou H, Peng Y, Li J, Lin Z. Aberrant microRNA expression in human cervical carcinomas. Med Oncol. 2012;29:1242–1248. doi: 10.1007/s12032-011-9830-2. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Liu J, Yuan C, Cui B, Zou X, Qiao Y. High-risk human papillomavirus reduces the expression of microRNA-218 in women with cervical intraepithelial neoplasia. J Int Med Res. 2010;38:1730–1736. doi: 10.1177/147323001003800518. [DOI] [PubMed] [Google Scholar]
- 15.Dreher A, Rossing M, Kaczkowski B, Nielsen FC, Norrild B. Differential expression of cellular microRNAs in HPV-11 transfected cells. An analysis by three different array platforms and qRT-PCR. Biochem Biophys Res Commun. 2010;403:357–362. doi: 10.1016/j.bbrc.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278:1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- 17.Colón-López V, Ortiz AP, Palefsky J. Burden of human papillomavirus infection and related comorbidities in men: implications for research, disease prevention and health promotion among Hispanic men. P R Health Sci J. 2010;29:232–240. [PMC free article] [PubMed] [Google Scholar]
- 18.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46:S20–S26. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Lu Q, Ma D, Zhao S. DNA methylation changes in cervical cancers. Methods Mol Biol. 2012;863:155–176. doi: 10.1007/978-1-61779-612-8_9. [DOI] [PubMed] [Google Scholar]
- 20.Botezatu A, Goia-Rusanu CD, Iancu IV, et al. Quantitative analysis of the relationship between microRNA-124a, -34b and -203 gene methylation and cervical oncogenesis. Mol Med Rep. 2011;4:121–128. doi: 10.3892/mmr.2010.394. [DOI] [PubMed] [Google Scholar]
- 21.Sasagawa T, Takagi H, Makinoda S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J Infect Chemother. 2012;18:807–815. doi: 10.1007/s10156-012-0485-5. [DOI] [PubMed] [Google Scholar]
- 22.Correa de Adjounian MF, Adjounian H, Adjounian SH. Silenciamiento de genes mediante RNA interferencia: consideraciones sobre el mecanismo y diseño de los sistemas efectores. AVFT. 2008;27:22–25. [Google Scholar]
- 23.Rouhi A, Mager DL, Humphries RK, Kuchenbauer F. MiRNAs, epigenetics, and cancer. Mamm Genome. 2008;19:517–525. doi: 10.1007/s00335-008-9133-x. [DOI] [PubMed] [Google Scholar]
- 24.Bock C. Epigenetic biomarker development. Epigenomics. 2009;1:99–110. doi: 10.2217/epi.09.6. [DOI] [PubMed] [Google Scholar]
- 25.Yang N, Coukos G, Zhang L. MicroRNA epigenetic alterations in human cancer: one step forward in diagnosis and treatment. Int J Cancer. 2008;122:963–968. doi: 10.1002/ijc.23325. [DOI] [PubMed] [Google Scholar]
- 26.Valeri N, Vannini I, Fanini F, Calore F, Adair B, Fabbri M. Epigenetics, miRNAs, and human cancer: a new chapter in human gene regulation. Mamm Genome. 2009;20:573–580. doi: 10.1007/s00335-009-9206-5. [DOI] [PubMed] [Google Scholar]
- 27.Ehrlich M. DNA hypomethylation in cancer cells. Epigenomics. 2009;1:239–259. doi: 10.2217/epi.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henken FE, Wilting SM, Overmeer RM, et al. Sequential gene promoter methylation during HPV-induced cervical carcinogenesis. Br J Cancer. 2007;97:1457–1464. doi: 10.1038/sj.bjc.6604055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell. 2010;19:698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Serra P, Esteller M. DNA methylation-associated silencing of tumor-suppressor microRNAs in cancer. Oncogene. 2012;31:1609–1622. doi: 10.1038/onc.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito Y, Jones PA. Epigenetic activation of tumor suppressor microRNAs in human cancer cells. Cell Cycle. 2006;5:2220–2222. doi: 10.4161/cc.5.19.3340. [DOI] [PubMed] [Google Scholar]
- 32.Lujambio A, Esteller M. CpG island hypermethylation of tumor suppressor microRNAs in human cancer. Cell Cycle. 2007;6:1455–1459. [PubMed] [Google Scholar]
- 33.Toyota M, Suzuki H, Sasaki Y, et al. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res. 2008;68:4123–4132. doi: 10.1158/0008-5472.CAN-08-0325. [DOI] [PubMed] [Google Scholar]
- 34.Huang YW, Liu JC, Deatherage DE, et al. Epigenetic repression of microRNA-129-2 leads to overexpression of SOX4 oncogene in endometrial cancer. Cancer Res. 2009;69:9038–9046. doi: 10.1158/0008-5472.CAN-09-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leonard SM, Wei W, Collins SI, et al. Oncogenic human papillomavirus imposes an instructive pattern of DNA methylation changes which parallel the natural history of cervical HPV infection in young women. Carcinogenesis. 2012;33:1286–1293. doi: 10.1093/carcin/bgs157. [DOI] [PubMed] [Google Scholar]
- 36.Missaoui N, Hmissa S, Dante R, Frappart L. Global DNA methylation in precancerous and cancerous lesions of the uterine cervix. Asian Pac J Cancer Prev. 2010;11:1741–1744. [PubMed] [Google Scholar]
- 37.Kalantari M, Calleja-Macias IE, Tewari D, et al. Conserved methylation patterns of human papillomavirus type 16 DNA in asymptomatic infection and cervical neoplasia. J Virol. 2004;78:12762–12772. doi: 10.1128/JVI.78.23.12762-12772.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgers WA, Blanchon L, Pradhan S, et al. Viral oncoproteins target the DNA methyltransferases. Oncogene. 2007;26:1650–1655. doi: 10.1038/sj.onc.1209950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Au Yeung CL, Tsang WP, Tsang TY, Co NN, Yau PL, Kwok TT. HPV-16 E6 upregulation of DNMT1 through repression of tumor suppressor p53. Oncol Rep. 2010;24:1599–1604. doi: 10.3892/or_00001023. [DOI] [PubMed] [Google Scholar]
- 40.McCabe MT, Davis JN, Day ML. Regulation of DNA methyltransferase 1 by the pRb/E2F1 pathway. Cancer Res. 2005;65:3624–3632. doi: 10.1158/0008-5472.CAN-04-2158. [DOI] [PubMed] [Google Scholar]
- 41.Richards KL, Zhang B, Baggerly KA, et al. Genome-wide hypomethylation in head and neck cancer is more pronounced in HPV-negative tumors and is associated with genomic instability. PLoS One. 2009;4:e4941. doi: 10.1371/journal.pone.0004941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin RK, Wu CY, Chang JW, et al. Dysregulation of p53/Sp1 control leads to DNA methyltransferase-1 overexpression in lung cancer. Cancer Res. 2010;70:5807–5817. doi: 10.1158/0008-5472.CAN-09-4161. [DOI] [PubMed] [Google Scholar]
- 43.Jin-Tao W, Ling D, Shi-Wen J, et al. Folate deficiency and aberrant expression of DNA methyltransferase 1 were associated with cervical cancerization. Curr Pharm Des. 2013 Jul 19; doi: 10.2174/13816128113199990543. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 44.Nambaru L, Meenakumari B, Swaminathan R, Rajkumar T. Prognostic significance of HPV physical status and integration sites in cervical cancer. Asian Pac J Cancer Prev. 2009;10:355–360. [PubMed] [Google Scholar]
- 45.Turan T, Kalantari M, Cuschieri K, Cubie HA, Skomedal H, Bernard HU. High-throughput detection of human papillomavirus-18 L1 gene methylation, a candidate biomarker for the progression of cervical neoplasia. Virology. 2007;361:185–193. doi: 10.1016/j.virol.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis-Dusenbery BN, Hata A. MicroRNA in cancer: the involvement of aberrant microRNA biogenesis regulatory pathways. Genes Cancer. 2010;1:1100–1114. doi: 10.1177/1947601910396213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shivdasani RA. MicroRNAs: regulators of gene expression and cell differentiation. Blood. 2006;108:3646–3653. doi: 10.1182/blood-2006-01-030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho WC. OncomiRs: the discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6:60. doi: 10.1186/1476-4598-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pavicic W, Perkiö E, Kaur S, et al. Altered methylation at microRNA-associated CpG islands in hereditary and sporadic carcinomas: a methylation-specific multiplex ligation-dependent probe amplification (MS-MLPA)-based approach. Mol Med. 2011;17:726–735. doi: 10.2119/molmed.2010.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen Y, Li Y, Ye F, et al. Identification of miR-23a as a novel microRNA normalizer for relative quantification in human uterine cervical tissues. Exp Mol Med. 2011;43:358–366. doi: 10.3858/emm.2011.43.6.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greco D, Kivi N, Qian K, et al. Human papillomavirus 16 E5 modulates the expression of host microRNAs. PLoS One. 2011;6:e21646. doi: 10.1371/journal.pone.0021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.So AY, Jung JW, Lee S, Kim HS, Kang KS. DNA methyltransferase controls stem cell aging by regulating BMI1 and EZH2 through microRNAs. PLoS One. 2011;6:e19503. doi: 10.1371/journal.pone.0019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferreira HJ, Heyn H, Moutinho C, et al. CpG island hypermethylation-associated silencing of small nucleolar RNAs in human cancer. RNA Biol. 2012;9:881–890. doi: 10.4161/rna.19353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hata A, Davis BN. Control of microRNA biogenesis by TGFβ signaling pathway - a novel role of Smads in the nucleus. Cytokine Growth Factor Rev. 2009;20:517–521. doi: 10.1016/j.cytogfr.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fazi F, Nervi C. MicroRNA: basic mechanisms and transcriptional regulatory networks for cell fate determination. Cardiovasc Res. 2008;79:553–561. doi: 10.1093/cvr/cvn151. [DOI] [PubMed] [Google Scholar]
- 56.Wang G, Wang Y, Teng M, Zhang D, Li L, Liu Y. Signal transducers and activators of transcription-1 (STAT1) regulates microRNA transcription in interferon γ-stimulated HeLa cells. PLoS One. 2010;5:e11794. doi: 10.1371/journal.pone.0011794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bandres E, Agirre X, Bitarte N, et al. Epigenetic regulation of microRNA expression in colorectal cancer. Int J Cancer. 2009;125:2737–2743. doi: 10.1002/ijc.24638. [DOI] [PubMed] [Google Scholar]
- 58.Siomi H, Siomi MC. Posttranscriptional regulation of microRNA biogenesis in animals. Mol Cell. 2010;38:323–332. doi: 10.1016/j.molcel.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Kerin MJ. MicroRNAs as novel biomarkers for breast cancer. J Oncol. 2009;2009:950201. doi: 10.1155/2010/950201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, Yao D, Li Y, et al. Serum microRNA expression levels can predict lymph node metastasis in patients with early-stage cervical squamous cell carcinoma. Int J Mol Med. 2013;32:557–567. doi: 10.3892/ijmm.2013.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gocze K, Gombos K, Juhasz K, Kovacs K, Kajtar B, Benczik M, et al. Unique microRNA expression profiles in cervical cancer. Anticancer Res. 2013;33:2561–2567. [PubMed] [Google Scholar]
- 62.Wilting SM, Snijders PJ, Verlaat W, et al. Altered microRNA expression associated with chromosomal changes contributes to cervical carcinogenesis. Oncogene. 2013;32:106–116. doi: 10.1038/onc.2012.20. [DOI] [PubMed] [Google Scholar]
- 63.Huang L, Lin JX, Yu YH, Zhang MY, Wang HY, Zheng M. Downregulation of six microRNAs is associated with advanced stage, lymph node metastasis and poor prognosis in small cell carcinoma of the cervix. PLoS One. 2012;7:e33762. doi: 10.1371/journal.pone.0033762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheung TH, Man KN, Yu MY, et al. Dysregulated microRNAs in the pathogenesis and progression of cervical neoplasm. Cell Cycle. 2012;11:2876–2884. doi: 10.4161/cc.21278. [DOI] [PubMed] [Google Scholar]
- 65.Ma D, Zhang YY, Guo YL, Li ZJ, Geng L. Profiling of microRNA-mRNA reveals roles of microRNAs in cervical cancer. Chin Med J. 2012;125:4270–4276. [PubMed] [Google Scholar]
- 66.Pereira PM, Marques JP, Soares AR, Carreto L, Santos MA. MicroRNA expression variability in human cervical tissues. PLoS One. 2010;5:e11780. doi: 10.1371/journal.pone.0011780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu X, Schwarz JK, Lewis JS, Jr, et al. A microRNA expression signature for cervical cancer prognosis. Cancer Res. 2010;70:1441–1448. doi: 10.1158/0008-5472.CAN-09-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JW, Choi CH, Choi JJ, et al. Altered microRNA expression in cervical carcinomas. Clin Cancer Res. 2008;14:2535–2542. doi: 10.1158/1078-0432.CCR-07-1231. [DOI] [PubMed] [Google Scholar]
- 69.Yao T, Rao Q, Liu L, et al. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in cervical cancer. Virol J. 2013;10:175. doi: 10.1186/1743-422X-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilting SM, Verlaat W, Jaspers A, et al. Methylation-mediated transcriptional repression of microRNAs during cervical carcinogenesis. Epigenetics. 2013;8:220–228. doi: 10.4161/epi.23605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalimutho M, Di Cecilia S, Del Vecchio Blanco G, et al. Epigenetically silenced miR-34b/c as a novel faecal-based screening marker for colorectal cancer. Br J Cancer. 2011;104:1770–1778. doi: 10.1038/bjc.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vogt M, Munding J, Grüner M, et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch. 2011;458:313–322. doi: 10.1007/s00428-010-1030-5. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Wang X, Xu B, et al. Epigenetic silencing of miR-126 contributes to tumor invasion and angiogenesis in colorectal cancer. Oncol Rep. 2013;30:1976–1984. doi: 10.3892/or.2013.2633. [DOI] [PubMed] [Google Scholar]
- 74.Zhu A, Xia J, Zuo J, et al. MicroRNA-148a is silenced by hypermethylation and interacts with DNA methyltransferase 1 in gastric cancer. Med Oncol. 2012;29:2701–2709. doi: 10.1007/s12032-011-0134-3. [DOI] [PubMed] [Google Scholar]
- 75.Tsai KW, Wu CW, Hu LY, et al. Epigenetic regulation of miR-34b and miR-129 expression in gastric cancer. Int J Cancer. 2011;129:2600–2610. doi: 10.1002/ijc.25919. [DOI] [PubMed] [Google Scholar]
- 76.Tsai KW, Liao YL, Wu CW, et al. Aberrant hypermethylation of miR-9 genes in gastric cancer. Epigenetics. 2011;6:1189–1197. doi: 10.4161/epi.6.10.16535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim K, Lee HC, Park JL, et al. Epigenetic regulation of microRNA-10b and targeting of oncogenic MAPRE1 in gastric cancer. Epigenetics. 2011;6:740–751. doi: 10.4161/epi.6.6.15874. [DOI] [PubMed] [Google Scholar]
- 78.Guo LH, Li H, Wang F, Yu J, He JS. The tumor suppressor roles of miR-433 and miR-127 in gastric cancer. Int J Mol Sci. 2013;14:14171–14184. doi: 10.3390/ijms140714171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng H, Guo Y, Song H, et al. MicroRNA-195 and microRNA-378 mediate tumor growth suppression by epigenetical regulation in gastric cancer. Gene. 2013;518:351–359. doi: 10.1016/j.gene.2012.12.103. [DOI] [PubMed] [Google Scholar]
- 80.Yan-Fang T, Jian N, Jun L, et al. The promoter of miR-663 is hypermethylated in Chinese pediatric acute myeloid leukemia (AML) BMC Med Genet. 2013;14:74. doi: 10.1186/1471-2350-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Köhler CU, Bryk O, Meier S, et al. Analyses in human urothelial cells identify methylation of miR-152, miR-200b and miR-10a genes as candidate bladder cancer biomarkers. Biochem Biophys Res Commun. 2013;438:48–53. doi: 10.1016/j.bbrc.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 82.Datta J, Kutay H, Nasser MW, et al. Methylation mediated silencing of microRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer Res. 2008;68:5049–5058. doi: 10.1158/0008-5472.CAN-07-6655. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Jung CJ, Iyengar S, Blahnik KR, et al. Epigenetic modulation of miR-122 facilitates human embryonic stem cell self-renewal and hepatocellular carcinoma proliferation. PLoS One. 2011;6:e27740. doi: 10.1371/journal.pone.0027740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He Y, Cui Y, Wang W, et al. Hypomethylation of the hsa-miR-191 locus causes high expression of hsa-mir-191 and promotes the epithelial-to-mesenchymal transition in hepatocellular carcinoma. Neoplasia. 2011;13:841–853. doi: 10.1593/neo.11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y, Yan LX, Wu QN, et al. miR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res. 2011;71:3552–3562. doi: 10.1158/0008-5472.CAN-10-2435. [DOI] [PubMed] [Google Scholar]
- 86.Augoff K, McCue B, Plow EF, Sossey-Alaoui K. miR-31 and its host gene lncRNA LOC554202 are regulated by promoter hypermethylation in triple-negative breast cancer. Mol Cancer. 2012;11:5. doi: 10.1186/1476-4598-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vrba L, Muñoz-Rodríguez JL, Stampfer MR, Futscher BW. miRNA gene promoters are frequent targets of aberrant DNA methylation in human breast cancer. PLoS One. 2013;8:e54398. doi: 10.1371/journal.pone.0054398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rauhala HE, Jalava SE, Isotalo J, et al. miR-193b is an epigenetically regulated putative tumor suppressor in prostate cancer. Int J Cancer. 2010;127:1363–1372. doi: 10.1002/ijc.25162. [DOI] [PubMed] [Google Scholar]
- 89.Hulf T, Sibbritt T, Wiklund ED, et al. Epigenetic-induced repression of microRNA-205 is associated with MED1 activation and a poorer prognosis in localized prostate cancer. Oncogene. 2013;32:2891–2899. doi: 10.1038/onc.2012.300. [DOI] [PubMed] [Google Scholar]
- 90.Formosa A, Lena AM, Markert EK, et al. DNA methylation silences miR-132 in prostate cancer. Oncogene. 2013;32:127–134. doi: 10.1038/onc.2012.14. [DOI] [PubMed] [Google Scholar]
- 91.Heller G, Weinzierl M, Noll C, et al. Genome-wide miRNA expression profiling identifies miR-9-3 and miR-193a as targets for DNA methylation in non-small cell lung cancers. Clin Cancer Res. 2012;18:1619–1629. doi: 10.1158/1078-0432.CCR-11-2450. [DOI] [PubMed] [Google Scholar]
- 92.Incoronato M, Urso L, Portela A, et al. Epigenetic regulation of miR-212 expression in lung cancer. PLoS One. 2011;6:e27722. doi: 10.1371/journal.pone.0027722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kitano K, Watanabe K, Emoto N, et al. CpG island methylation of microRNAs is associated with tumor size and recurrence of non-small-cell lung cancer. Cancer Sci. 2011;102:2126–2131. doi: 10.1111/j.1349-7006.2011.02101.x. [DOI] [PubMed] [Google Scholar]
- 94.Wang Z, Chen Z, Gao Y, et al. DNA hypermethylation of microRNA-34b/c has prognostic value for stage I non-small cell lung cancer. Cancer Biol Ther. 2011;11:490–496. doi: 10.4161/cbt.11.5.14550. [DOI] [PubMed] [Google Scholar]
- 95.Wong KY1, Liang R, So CC, Jin DY, Costello JF, Chim CS. Epigenetic silencing of MIR203 in multiple myeloma. Br J Haematol. 2011;154:569–578. doi: 10.1111/j.1365-2141.2011.08782.x. [DOI] [PubMed] [Google Scholar]