Abstract
Objective
To provide family physicians with updated, practical, evidence-based information about mild head injury (MHI) and concussion in the pediatric population.
Sources of information
MEDLINE (1950 to February 2013), the Cochrane Database of Systematic Reviews (2005 to 2013), the Cochrane Central Register of Controlled Trials (2005 to 2013), and DARE (2005 to 2013) were searched using terms relevant to concussion and head trauma. Guidelines, position statements, articles, and original research relevant to MHI were selected.
Main message
Trauma is the main cause of death in children older than 1 year of age, and within this group head trauma is the leading cause of disability and death. Nine percent of reported athletic injuries in high school students involve MHI. Family physicians need to take a focused history, perform physical and neurologic examinations, use standardized evaluation instruments (Glasgow Coma Scale; the Sport Concussion Assessment Tool, version 3; the child version of the Sport Concussion Assessment Tool; and the Balance Error Scoring System), instruct parents how to monitor their children, decide when caregivers are not an appropriately responsible resource, follow up with patients promptly, guide a safe return to play and to learning, and decide when neuropsychological testing for longer-term follow-up is required.
Conclusion
A thorough history, physical and neurologic assessment, the use of validated tools to provide an objective framework, and periodic follow-up are the basis of family physician management of pediatric MHI.
Case
Jerry is a 13-year-old boy who hit his head against the cement while skate-boarding with some friends. He was not wearing a helmet and he did not lose consciousness. He felt “dazed” but after resting for a few minutes he felt better and continued skateboarding with his friends. Soon after that, he developed a headache, which persisted after he returned home 20 minutes later. During the next 3 hours he developed nausea, vomited once, and felt fatigued. His parents then brought him to your office for evaluation.
The spectrum of head trauma that family physicians see in their offices usually differs from that seen in the emergency department. Although family doctors provide care in both settings, the objective of this review is to provide practical, current approaches and specific tools for family physicians to use in their office practices to facilitate evidence-based assessment and up-to-date management of mild head injury (MHI) in the pediatric population.
Head injury is common and ranges from concussion to severe head trauma. The management and evaluation vary according to the age of the patient. This article focuses on the school-aged and adolescent population. The understanding of and consensus about concussion is evolving but controversy about definitions persists,1–3 and the terms concussion, mild traumatic brain injury, mild head injury, minor head injury, and minor closed head injury are often used interchangeably. For the purpose of this article, the term mild head injury is used as the more general term. Although there are different definitions for MHI, they share similar characteristics (Table 1).3–5
Table 1.
Definitions of concussion or MHI
| SOURCE | DEFINITION |
|---|---|
| Canadian Academy of Sport and Exercise Medicine, 2010 | “A form of head injury characterized by any alteration in cerebral function and caused by a direct or indirect (rotation) force transmitted to the head. It results in one or more of the following acute signs or symptoms: a brief loss of consciousness, light-headedness, vertigo, cognitive and memory dysfunction, tinnitus, blurred vision, difficulty concentrating, amnesia, headache, nausea, vomiting, photophobia or a balance disturbance. Delayed signs and symptoms may also include sleep irregularities, fatigue, personality changes, and inability to perform usual daily activities, depression or lethargy”4 |
| SCAT3, from the 4th International Conference on Concussion, 2012 | A concussion is a disturbance in brain function caused by a direct or indirect force to the head. It results in various nonspecific signs and symptoms and most often does not involve loss of consciousness. Concussion should be suspected in the presence of any 1 or more of the following: |
| American Academy of Neurology, 2013 | Concussion is a trauma-induced alteration in mental status that might or might not involve loss of consciousness. Confusion and amnesia are the hallmarks of concussion. The confusional episode and amnesia might occur immediately after the blow to the head or several minutes later. Close observation and assessment of the athlete over some period of time is necessary to determine whether evolving neuropathologic change associated with concussion will lead to a confusional state or to the development of memory dysfunction3 |
MHI—mild head injury, SCAT3—Sport Concussion Assessment Tool, version 3.
About one-third of all MHIs annually in the United States occur in the pediatric population (5 to 19 years of age).6,7 Trauma is the main cause of death in children older than 1 year of age, and head trauma is the leading cause of disability and death.8 In the high school population, 9% of athletic injuries involve MHI.9
The mechanism of injury includes direct trauma to the face, head, or neck, or transmitted force from trauma to other parts of the body.1–3,6–11 The reaction to head trauma in the pediatric population differs from that in the adult population. Pathophysiologic changes after MHI are more pronounced in immature brains,12 and in children adverse effects are often detected through worsened academic performance due to impaired cognitive function and behavioural problems.13,14 Compared with adults, pediatric brains have a higher brain water content, the relative size of the head is larger than the rest of the body, the vasculature is more easily disrupted, and the degree of myelination differs.15 Hence, epidural hematoma, subdural hematoma, and second-impact syndrome occur more frequently in children.
Most pediatric patients appear to recover promptly, and 80% to 90% of MHI cases resolve within 7 to 10 days.1,6,16,17 In some children and adolescents recovery can be more prolonged,18–20 with 24.5% of 13- to 21-year-olds still having disabling symptoms 1 month after head trauma. In some cases recovery might take up to 3 months,2,21 and 5.9% of those affected remain symptomatic after 6 months.22 For some, symptoms of concussion can persist for up to 1 year.23–26
A key risk factor is a history of previous MHI,27,28 likely owing to lifestyle and risk-taking, and possibly because previous MHI might cause less responsiveness to physiologic neural activation.12,29 Other risk factors are younger age (children and adolescents),18–20 the mechanism and force of the injury, the sport that caused the injury (if the injury is due to a sport) and the position played on the field,1,30 and female sex.31
Sources of information
MEDLINE (1950 to February 2013) and the Cochrane Library, including the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials, and DARE (2005 to 2013), were searched using terms relevant to concussion and head trauma. Guidelines, position statements, articles, and original research relevant to MHI were selected.
The current standards for the initial assessment of a patient with a concussion are based on international conference consensus statements and recommendations from medical academies (level III evidence). For the purpose of this manuscript we will use the clinician level of obligation of recommendation used by the American Academy of Neurology (Table 2).3
Table 2.
Clinician level of obligation for recommendations from the American Academy of neurology: A modified Delphi approach was used to achieve consensus.
| CATEGORY | DEFINITION OR EXAMPLE |
|---|---|
| Modal modifiers used to indicate the final clinician level of obligation | |
| • Level A | “Must” |
| • Level B | “Should” |
| • Level C | “Might” |
| • Level U | No recommendation supported |
| Initial rating of confidence in the evidence for each intervention outcome pair | |
| • High | Requires 2 or more class I studies |
| • Moderate | Requires 1 class I study or 2 or more class II studies |
| • Low | Requires 1 class II study or 2 or more class III studies |
| • Very low | Requires only 1 class III study or 1 or more class IV studies |
| Classification of evidence for therapeutic intervention for risk of bias | |
| • Class I |
|
| • Class II |
|
| • Class III |
|
| • Class IV |
|
Adapted from Giza et al.3
Main message
Assessment and diagnosis
A comprehensive initial assessment should include symptoms (Table 3),1,32,33 details of the mechanism of injury, the timeline of symptoms, and any factor that could affect its presentation or management (Box 1).1,32,34 Child abuse should be ruled out. Standardized tools (Table 4) should be used to assess physical symptoms and cognitive status1,2,10,33,35,36 (level C), and physicians should be instructed in their proper use (level B). A recently developed tool is the Sport Concussion Assessment Tool, version 3 (SCAT3), which is based on consensus reached at the last International Conference on Concussion in Sport.32 A SCAT3 for specific use in 5- to 12-year-old children has also been developed (Table 4).
Table 3.
Signs and symptoms of concussion
| SOURCE | SIGNS AND SYMPTOMS |
|---|---|
| Included in the last consensus statement on concussion32 | |
| • Physical domain | Headache Neck pain “Pressure in head” Nausea and vomiting Dizziness Blurred vision Balance disturbances Sensitivity to light Sensitivity to noise |
| • Cognitive domain | Feeling slowed down Feeling “in a fog” “Don’t feel right” Difficulty concentrating Difficulty remembering Fatigue or low energy Confusion |
| • Emotional domain | Feeling more emotional Irritability Sadness Nervousness or anxiety |
| • Sleep domain | Drowsiness Trouble falling asleep |
| Other signs and symptoms included by other organizations, statements, or tools1,32,33 | Loss of consciousness Seizure or convulsion Amnesia Feeling “dazed or stunned” Visual problems Answering questions slowly Repeating questions Sleeping more than usual Sleeping less than usual |
Box 1. Aggravating factors to be considered at the initial assessment of children with MHI.
The following should be considered at the initial assessment:
|
Table 4.
Standardized instruments, online courses, and resources available for assessing or monitoring patients experiencing concussion symptoms
| DOMAIN TO BE ASSESSED OR MONITORED | REQUIRED ASSESSSMENT OR RESOURCE | COMMENTS | AVAILABILITY |
|---|---|---|---|
| For physicians | |||
| • Comprehensive general assessment | SCAT3* | Complete tool developed in 2013; it has detailed instructions for evaluation | www.parachutecanada.org/downloads/resources/SCAT3.pdf |
| • General assessment of children | ChildSCAT3 | Specific version for children 5 to 12 y old | www.parachutecanada.org/downloads/resources/SCAT3-child.pdf |
| • Alternative quick medical assessment and information resource | ACE | Alternative tool for assessment, presented as a physician office version | www.cdc.gov/concussion/headsup/pdf/ACE-a.pdf |
| • Posttraumatic amnesia | A-WPTAS | Picture recognition included | http://onf.org/system/attachments/60/original/Guidelines_for_Mild_Traumatic_brain_Injury_and_Persistent_Symptoms.pdf (page 44) |
| • Static postural stability | BESS | Fully explained BESS test with photos included | www.glata.org/documents/filelibrary/glata_2014_presentations/bESSProtocol_E5D9286115A3C.pdf |
| • Clinical information | ANN | Summary of evidence-based management for clinicians | www.aan.com/uploadedFiles/Website_Library_Assets/Documents/3Practice_Management/5Patient_Resources/1For_your_Patient/6_Sports_Concussion_Toolkit/evaluation.pdf |
| • Tool to identify concussion in children, youth, and adults | Pocket concussion recognition card, 2013 | Summary of evaluative steps based on the consensus statement for concussion in sports | http://links.lww.com/JSM/A32 |
| • Online course | Heads Up for physicians | 5-step course with evaluation included | www.preventingconcussions.org |
| For family members, teachers, and coaches | |||
| • Comprehensive information resource (English and French) | MCH Trauma Concussion Kit | Information brochure and pocket cards; game-specific suggested steps for return to play | www.thechildren.com/health-info/trauma/mch-trauma-concussion-kit |
| • Education | Safety information, teaching activities, and resources | For home, school, or community educational activities | www.parachutecanada.org |
| • Guidelines and definitions | ANN | Summary of evidence-based evaluation and management for patients and families | www.aan.com/Guidelines/Home/GetGuidelineContent/586 |
| • Educational videos for different audiences | Sport Concussion Library | Registration and account creation is required | www.sportconcussionlibrary.com |
| • Online course | Heads Up | 5-step course for the general public | www.cdc.gov/concussion/Headsup/Training/index.html |
ACE—Acute Concussion Evaluation, ANN—American Academy of Neurology, A-WPTAS—Abbreviated Westmead Post Traumatic Amnesia Scale, BESS—Balance Error Scoring System, MCH—Montreal Children’s Hospital, MHI—mild head injury, SCAT3—Sport Concussion Assessment Tool, version 3.
The SCAT3 can be used for specific assessment of MHI symptoms, sideline diagnosis of concussion (Maddocks score), balance assessment (modified BESS test), and evaluation of cognition, memory, concentration, and coordination.
A complete examination includes assessment of the head for signs of trauma, lacerations, abrasions, and skull irregularities that could suggest fractures (eg, depression, determination of skull discontinuity through lacerations). Signs of basilar skull fracture should be sought, including hemotympanum, drainage of fluid or blood from the nose or ears, Battle sign, or “raccoon eyes.”8
A complete neurologic examination should be performed, although focal neurologic signs are frequently not found. Mental status evaluation in the office should start with a Glasgow Coma Scale (GCS) assessment. A balance test should be performed at the initial assessment.1 The combination of these assessment tests increases the accuracy of the diagnosis.37 When cognitive symptoms persist, neuropsychological tests can be used and should be interpreted relative to the preaccident status.1,15
Investigations
Radiographs have low predictive value in patients with no loss of consciousness and no clinical signs of skull fracture. An important concern in the initial evaluation is the possibility of missing an intracranial lesion in any child who has suffered an MHI.8,38,39 For that reason a computed tomography (CT) scan could be requested (level C), but controversy persists about the criteria for choosing one.
The Canadian CATCH (Canadian Assessment of Tomography for Childhood Head Injury) multicentre study enrolled 3866 patients (90.2% had GCS scores of 15, 7.3% had GCS scores of 14, and 2.5% had GCS scores of 13). Their combination of 4 high-risk signs provided a sensitivity of 100% (95% CI 86.2% to 100.0%) and specificity of 70.2% (95% CI 68.6% to 71.6%) for the need for neurologic intervention. Adding the 3 medium-risk signs resulted in a small decrease in sensitivity to 98.1% (95% CI 94.6% to 99.4%) and a 20.1% decrease in specificity to 50.1% (95% CI 48.5% to 51.7%).38 Box 2 and Table 5 present recommendations based on the CATCH study results to help family doctors working in the emergency department or community offices make the decision either to order or forego CT scans in different groups of children.38
Box 2. The CATCH rule.
High risk (neurologic intervention needed)
|
CATCH—Canadian Assessment of Tomography for Childhood Head Injury, CT—computed tomography, GCS—Glasgow Coma Scale.
Data from Osmond et al.38
Table 5.
Performance of the CATCH rule: A) Using the 4 high-risk signs, sensitivity was 100.0% (95% CI 86.2% to 100.0%) and specificity was 70.2% (95% CI 68.6% to 71.6%); 30.2% of patients would undergo CT scanning. B) Using the 4 high-risk and 3 medium-risk signs, sensitivity was 98.1% (95% CI 94.6% to 99.4%) and specificity was 50.1% (95% CI 48.5% to 51.7%); 51.9% of patients would undergo CT scanning.
|
A) RESULT |
NEEDED NEUROLOGIC INTERVENTION | DID NOT NEED NEUROLOGIC INTERVENTION |
| Positive (≥ 1 high-risk factor) | 24 | 1144 |
| Negative (no high-risk factors) | 0 | 2698 |
|
B) RESULT |
NEEDED NEUROLOGIC INTERVENTION | DID NOT NEED NEUROLOGIC INTERVENTION |
|
| ||
| Positive (≥ 1 high-risk factor) | 156 | 1851 |
| Negative (no high-risk factors) | 3 | 1856 |
CATCH—Canadian Assessment of Tomography for Childhood Head Injury, CT—computed tomography.
Data from Osmond et al.38
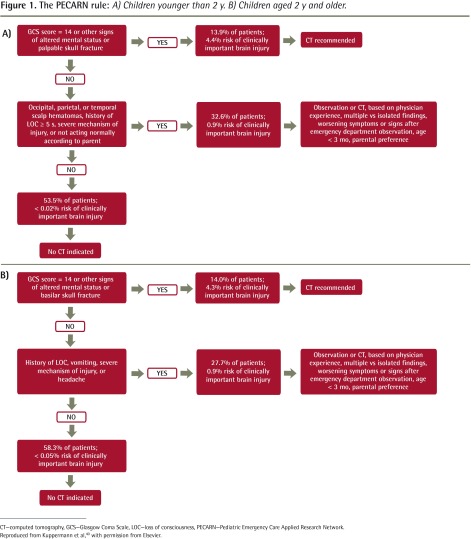
The much larger US PECARN (Pediatric Emergency Care Applied Research Network) multicentre study40 used a similar approach and corroborated the CATCH findings. The PECARN prediction rules are provided in Figure 1.40 The PECARN rules have not yet been applied to the smaller CATCH data set to compare the rules.
Figure 1.
The PECARN rule: A) Children younger than 2 y. B) Children aged 2 y and older.
CT—computed tomography, GCS—Glasgow Coma Scale, LOC—loss of consciousness, PECARN—Pediatric Emergency Care Applied Research Network.
Reproduced from Kuppermann et al, with permission from Elsevier.
Although there is no consensus about the use of neuropsychological testing, one indication could be persistent symptoms preventing return to academic or sport activities,41 or it could be used to determine if the concussion has been resolved (level C).3
Progress with the case
During assessment Jerry describes feeling “in a fog.” He continues to have a headache and is fatigued. Findings of his physical examination and coordination tests are normal, but he scores 13 out of 15 on the memory portion of the standardized assessment of concussion, and results of his balance testing are abnormal. You explain your assessment to Jerry’s parents, provide clear advice about how to observe him during the next 24 hours, emphasize alarm symptoms that should prompt immediate reassessment, and schedule a follow-up visit for the next morning.
Management
Because of the heterogeneity of the causes and presentations of head trauma, it has been difficult to define a standard of acute care for children and adolescents.10,34 The management of concussion is based on the status and progress of the individual patient rather than on grading as was suggested in the past. Once a complete focused history and a physical assessment have been performed, and severe injury or complications have been ruled out, the main strategy of the management of MHI is rest and observation,3,32,42 with a prudent observation period of 24 to 48 hours.34 Any deterioration in clinical status during that time or in the following days should prompt further evaluation.43 Adequate observation includes regular assessment of the patient in the office, clinic, or at home by a person competent to recognize abnormal changes and to make the decision to contact medical personnel when needed. Parents and caregivers should be educated about how to identify improvement, chronicity, or worsening of the condition,35 and various resources are available for that purpose (Table 4).44 Adequate conditions for compliance, such as geographic accessibility, adequate transport, and reliable caregivers or parents, should be assessed. Difficulties complicating parental participation include incompetence, previous neglect of children, intoxication, unavailability, or language barrier. If the conditions for observation are inadequate, the child should be observed in a health care facility or hospital.34
Family physicians should provide education about symptoms, expected timelines and the course of recovery, and advice on how to cope with symptoms, how to access medical services or further support promptly, and how to achieve a gradual return to regular activities.2,33,44–49
There is general agreement that management of MHI includes mental and physical rest.1,11,18,32–34,42,43,50 This is of particular importance when returning to learning activities. The patient should abstain from any activities that include intense mental focus such as reading, use of computers, video games, solving puzzles, texting, watching television, or schoolwork.50 Based on the specific needs of the child, the physician should coordinate the modification of school activities,34,51 such as allowing extra time for completion of tasks or assignments, modification of the learning environment (eg, allow recording of classes, allow somebody else to take notes for the patient), breaking the workload into smaller pieces, rescheduling assignments, modified testing (eg, spoken responses instead of long essays), and changes in the school routine to avoid fatigue (eg, shorter school days, more breaks, days off).
Based on the severity of the case, the physician should lead a team comprising the parents or guardians, school administrators, day-care providers, the school nurse, a psychologist, and anyone else involved with the patient’s case to facilitate the assessment and monitoring of the recovery process.51 This teamwork will help address important factors such as tolerance of academic activities, avoidance of “toughing out” symptoms during study sessions, individualization of management based on specific patient characteristics, modification of tasks, requests for medical reassessment, variation and timing of symptoms during different activities (eg, 15 minutes after concentrating or reading), identification of activities that might pose more difficulty, triggering factors (eg, worsening of symptoms with noise or lighting), monitoring aggravating factors such as attention deficit hyperactivity disorder, and addressing frustration and embarrassment. Tests providing standardized symptom scores are helpful to assess the patient’s progress.1 The child should avoid participation in sports or physical activities, and this should be made clear to parents, coaches, trainers, and teachers.
Once the child who experienced MHI has been assessed in the office, the traditionally advised strategy of keeping the patient awake is not required, as sleep is restorative.1 The child should be allowed to sleep but should be checked periodically for clinical deterioration.8 If there is a concern about the level of consciousness, further assessment including evaluation with neuroimaging should be arranged.
During the initial hours after the incident, medications should be avoided that could affect evaluation of cognition (eg, meclizine, benzodiazepines), mask symptoms (eg, antiemetics), or facilitate bleeding (eg, acetylsalicylic acid, nonsteroidal anti-inflammatory drugs).
After the acute phase or as part of postconcussion syndrome management, other management strategies could be included. Headache can be managed with acetaminophen, but if it becomes chronic (more than 6 weeks), traditional multidisciplinary management could be implemented.52 A meta-analysis found no strong evidence to guide treatment of posttraumatic headache, and management is based on treatment by headache category.53 Sleep disturbance is treated with observation and sleep hygiene, but if it is persistent, medication or cognitive therapy can be useful. No medication is advised for daytime somnolence in the acute phase. Mood disorders should be managed without medication, but if they become chronic (more than 6 to 12 weeks) medication or counseling could be used.1 Vestibular therapy is a good treatment for persistent vertigo or dizziness.1 Attention deficits should not be treated with medication, but instead academic demands should be decreased.54,55 A patient who has received medications for concussion-related symptoms should no longer be taking the medication before returning to any contact-sport activity (level B).3
While a young patient is still symptomatic and in the recovery period it is important to ensure that he or she will not experience any further head trauma that could lead to a second-impact syndrome or fatal diffuse cerebral swelling,50 which might involve altered cerebrovascular autoregulation.
The adolescent that is involved in sports should be evaluated and cleared to play by a physician familiar with MHI management (level B).3,4 Return to play should be allowed only when the treating physician issues a medical statement of fitness,1 and an individualized program for gradual return to play should be followed (level C). The different stages of this protocol include rest, light aerobic activity, sport-specific activity, drills without body contact, drills with body contact, and finally return to game play.1,5,11,32,34,50 Recovery should be assessed and if the symptoms reappear at any stage, the patient should return to the previous stage for at least 24 hours. Specific steps should be taken to avoid attempts from coaches, teachers, parents, or anyone else involved to force an earlier return to sport activities. Counseling about past MHI, the risk of future MHI, and the cumulative effect of MHI should be provided, and the patient and relatives should be assessed to determine whether they have an indifferent or competitive attitude that might lead to future concussions.
Progress with the case
The following morning Jerry returns to see you. He has only a mild headache and results of his examination and assessment tests are completely normal. You provide instructions about mental rest (no video games, texting, computer work, or solving puzzles) and a gradual return to school (rescheduling of tests and academic assignments as needed), and explain the protocol to return to sport activities. You provide counseling about the use of helmets in future sport activities and remind Jerry’s parents how to recognize abnormal changes and worsening of his condition. You advise physical rest until his headache resolves, and after that light aerobic activity could be tried. If his symptoms reappear he should return to rest for at least 24 hours, but if he remains asymptomatic the stages of the return-to-activity protocol should be followed. You schedule a follow-up appointment to assess his progress and to guide his return to play.
Conclusion
Assessment and management of MHI continues to evolve, and different medical entities continue to issue statements and guidelines addressing this subject. The SCAT3 and other tools presented in this review are the most up-to-date and relevant examples of this evolution and change in thinking. More large-scale research is needed to clarify the optimal combination of assessment tests (although it is likely that the SCAT3 for sports injury will be the most widely used) and management of MHI. Although the use of imaging studies is still controversial, the decision to request them should be based on adequate clinical suspicion of intracranial lesions, with evidence-based steps followed to guide that decision. The CATCH study offers useful data for an evidence-based decision.
Mental and physical rest and adequate observation are the mainstay of management, and family physicians should guide parents and ensure that this is accomplished, and any deterioration should dictate prompt intervention. Guidance and education to patients, parents, and coaches are important parts of the management that family physicians provide and the educational resources noted in this article are intended to facilitate these tasks. Once return to regular academic or sport activities is deemed appropriate, progressive supervised steps can provide safe re-engagement. The treating doctors should bear in mind that the vulnerability of the pediatric brain results in longer recovery periods and the possibility of more complications in children or adolescents suffering from MHI.
EDITOR’S KEY POINTS
Recent guidelines and international consensus statements recommend and provide diagnostic tools that can help family physicians diagnose, manage, and follow up with children and adolescents with mild head injury.
The Sport Concussion Assessment Tool, version 3, developed based on international consensus, is the most recently developed method of evaluation. It provides a comprehensive and relatively rapid method of assessment. Coordination of adequate follow-up by parents and health care providers is a key element of the management of mild head injury by family physicians.
Footnotes
This article is eligible for Mainpro-M1 credits. To earn credits, go to www.cfp.ca and click on the Mainpro link.
This article has been peer reviewed.
La traduction en français de cet article se trouve à www.cfp.ca dans la table des matières du numéro de juin 2014 à la page e294.
Contributors
Both authors contributed to the literature review and interpretation, and to preparing the manuscript for submission.
Competing interests
None declared
References
- 1.Harmon KG, Drezner J, Gammons M, Guskiewicz K, Halstead M, Herring S, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Clin J Sport Med. 2013;23(1):1–18. doi: 10.1097/JSM.0b013e31827f5f93. [DOI] [PubMed] [Google Scholar]
- 2.Guidelines for mild traumatic brain injury and persistent symptoms. Toronto, ON: Ontario Neurotrauma Foundation; 2011. Available from: http://onf.org/system/attachments/60/original/Guidelines_for_Mild_Traumatic_Brain_Injury_and_Persistent_Symptoms.pdf. Accessed 2014 Apr 24. [Google Scholar]
- 3.Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TS, Gioia GA, et al. Evidence-based guideline update: evaluation and management of concussions in sports. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Minneapolis, MN: American Academy of Neurology; 2013. Available from: www.aan.com/uploadedfiles/website_library_assets/documents/3practice_management/5patient_resources/1for_your_patient/6_sports_concussion_toolkit/guidelines.pdf. Accessed 2014 Apr 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delaney J, Frankovich R. Head injuries and concussions in soccer. Ottawa, ON: Canadian Academy of Sport and Exercise Medicine; 2010. Available from: www.sirc.ca/newsletters/august09/S-972359DiscussionPaperHeadInjuries.pdf. Accessed 2014 Apr 21. [DOI] [PubMed] [Google Scholar]
- 5.Guskiewicz KM, Register-Mihalik J, McCrory P, McCrea M, Johnston K, Makdissi M, et al. Evidence-based approach to revising the SCAT2: introducing the SACT3. Br J Sports Med. 2013;47(5):289–93. doi: 10.1136/bjsports-2013-092225. [DOI] [PubMed] [Google Scholar]
- 6.Meehan WP, 3rd, d’Hemecourt P, Comstock RD. High school concussions in the 2008–2009 academic year: mechanism, symptoms and management. Am J Sports Med. 2010;38(12):2405–9. doi: 10.1177/0363546510376737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakhos LL, Lockhart GR, Myers R, Linakis JG. Emergency department visits for concussion in young child athletes. Pediatrics. 2010;126(3):e550–6. doi: 10.1542/peds.2009-3101. [DOI] [PubMed] [Google Scholar]
- 8.Schunk JE, Shutzman SA. Pediatric head injury. Pediatr Rev. 2012;33(9):398–410. doi: 10.1542/pir.33-9-398. [DOI] [PubMed] [Google Scholar]
- 9.Gessel LM, Fields SK, Collins CL, Dick RW, Comstock RD. Concussions among United States high school and collegiate athletes. J Athl Train. 2007;42(4):495–503. [PMC free article] [PubMed] [Google Scholar]
- 10.Gioia GA, Schneider JC, Vaughan CG, Isquith PK. Which symptom assessments and approaches are uniquely appropriate for paediatric concussion? Br J Sports Med. 2009;43(Suppl 1):i13–22. doi: 10.1136/bjsm.2009.058255. [DOI] [PubMed] [Google Scholar]
- 11.McCrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Consensus statement on concussion in sport: the 3rd International Conference on Concussion in Sport Held in Zurich, November 2008. Clin J Sport Med. 2009;19:185–95. doi: 10.1097/JSM.0b013e3181a501db. [DOI] [PubMed] [Google Scholar]
- 12.Shrey DW, Griesbach GS, Giza CC. The pathophysiology of concussions in youth. Phys Med Rehabil Clin N Am. 2011;22(4):577–602. doi: 10.1016/j.pmr.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawley CA. Behaviour and school performance after brain injury. Brain Inj. 2004;18(7):645–59. doi: 10.1080/02699050310001646189. [DOI] [PubMed] [Google Scholar]
- 14.Hawley CA, Ward AB, Magnay AR, Long J. Outcomes following children head injury: a population study. J Neurol Neurosurg Psychiatry. 2004;75(5):737–42. doi: 10.1136/jnnp.2003.020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meehan WP, 3rd, Taylor AM, Proctor M. The pediatric athlete: younger athletes with sport-related concussion. Clin Sports Med. 2011;30(1):133–44. doi: 10.1016/j.csm.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marar M, McIlvain NM, Fields SK, Comstock RD. Epidemiology of concussions among United States high school athletes in 20 sports. Am J Sports Med. 2012;40(4):747–55. doi: 10.1177/0363546511435626. Epub 2012 Jan 27. [DOI] [PubMed] [Google Scholar]
- 17.McCrea M, Barr WB, Guskiewicz K, Randolph C, Marshall SW, Cantu R, et al. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc. 2005;11(1):58–69. doi: 10.1017/S1355617705050083. [DOI] [PubMed] [Google Scholar]
- 18.McCrory P, Johnston K, Meeuwisse W, Aubry M, Cantu R, Dvorak J, et al. Summary and agreement statement of the 2nd International Conference on Concussion in Sport, Prague 2004. Br J Sports Med. 2004;39(4):196–204. doi: 10.1136/bjsm.2005.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuckerman SL, Odom M, Lee YM, Forbes J, Sills AK, Soloman J. Sport-related concussion and age: number of days to neurocognitive baseline. Neurosurgery. 2012;71(2):E558. [Google Scholar]
- 20.Sim A, Terryberry-Spohr L, Wilson KR. Prolonged recovery of memory functioning after mild traumatic brain injury in adolescent athletes. J Neurosurg. 2008;108(3):511–6. doi: 10.3171/JNS/2008/108/3/0511. [DOI] [PubMed] [Google Scholar]
- 21.Iverson GL. Outcome from mild traumatic brain injury. Curr Opin Psychiatry. 2005;18(3):301–17. doi: 10.1097/01.yco.0000165601.29047.ae. [DOI] [PubMed] [Google Scholar]
- 22.Pickering A, Grundy K, Clarke A, Townend W. A cohort study of outcomes following head injury among children and young adults in full-time education. Emerg Med J. 2012;29(6):451–4. doi: 10.1136/emj.2010.094755. Epub 2011 May 26. [DOI] [PubMed] [Google Scholar]
- 23.Bohnen N, Van Zutphen W, Twunstra A, Wijnen G, Bongers J, Jolles J. Late outcome of mild head injury: results from a controlled postal survey. Brain Inj. 1994;8(8):701–8. doi: 10.3109/02699059409151024. [DOI] [PubMed] [Google Scholar]
- 24.Emanuelson I, Andersson Holmkvist E, Björklund R, Stålhammar D. Quality of life and postconcussion symptoms in adults after mild traumatic brain injury: a population-based study in western Sweden. Acta Neurol Scand. 2003;108(5):332–8. doi: 10.1034/j.1600-0404.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- 25.Van der Naalt J, van Zomeren AH, Sluiter WJ, Minderhoud JM. One year outcome in mild to moderate head injury: the predictive value of acute injury characteristics related to complains and return to work. J Neurol Neurosurg Psychiatry. 1999;66(2):207–13. doi: 10.1136/jnnp.66.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakola AS, Müller K, Larsen M, Waterloo K, Romner B, Ingebrigtsen T. Five-year outcome after mild head injury: a prospective controlled trial. Acta Neurol Scand. 2007;115(6):398–402. doi: 10.1111/j.1600-0404.2007.00827.x. [DOI] [PubMed] [Google Scholar]
- 27.Emery C, Kang J, Shrier I, Goulet C, Hagel B, Benson B, et al. Risk of injury associated with body-checking experience among youth hockey players. CMAJ. 2011;183(11):1249–56. doi: 10.1503/cmaj.101540. Epub 2011 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz MR, Marshall SW, Mueller FO, Yang J, Weaver NL, Kalsbeek WD, et al. Incidence and risk factors for concussion in high school athletes, North Carolina. Am J Epidemiol. 2004;160(10):937–44. doi: 10.1093/aje/kwh304. [DOI] [PubMed] [Google Scholar]
- 29.Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clin Sports Med. 2011;30(1):33–48. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Boden BP, Kirkendall DT, Garrett WE., Jr Concussion incidence in elite college soccer players. Am J Sports Med. 1998;26(2):238–41. doi: 10.1177/03635465980260021301. [DOI] [PubMed] [Google Scholar]
- 31.Dick RW. Is there a gender difference in concussion incidence and outcomes? Br J Sports Med. 2009;43(Suppl 1):i46–50. doi: 10.1136/bjsm.2009.058172. [DOI] [PubMed] [Google Scholar]
- 32.McCrory P, Meeuwisse W, Aubry M, Cantu B, Dvorak J, Echemendia RJ, et al. Consensus statement on concussion in sport—the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Clin J Sport Med. 2013;23(2):89–117. doi: 10.1097/JSM.0b013e31828b67cf. [DOI] [PubMed] [Google Scholar]
- 33.Marshall S, Bayley M, McCullagh S, Velikonja D, Berrigan L. Clinical practice guidelines for mild traumatic brain injury and persistent symptoms. Can Fam Physician. 2012;58:257–67. (Eng), e128–40 (Fr). [PMC free article] [PubMed] [Google Scholar]
- 34.Committee on Quality Improvement, American Academy of Pediatrics Commission on Clinical Policies and Research The management of minor closed head injury in children. Pediatrics. 1999;104(6):1407–15. [PubMed] [Google Scholar]
- 35.New South Wales Motor Accident Authority . Guidelines for mild traumatic brain injury following closed head injury. Sydney, Australia: New South Wales Motor Accident Authority; 2008. [Google Scholar]
- 36.Greenes DS, Schutzman SA. Clinical significance of scalp abnormalities in asymptomatic head-injured infants. Pediatr Emerg Care. 2001;17(2):88–92. doi: 10.1097/00006565-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Lau BC, Collins NW, Lovell MR. Sensitivity and specificity of sub-acute computerized neurocognitive testing and symptoms evaluation in predicting outcomes after sports-related concussions. Am J Sports Med. 2011;39(6):1209–16. doi: 10.1177/0363546510392016. Epub 2011 Feb 1. [DOI] [PubMed] [Google Scholar]
- 38.Osmond MH, Klassen TP, Wells GA, Correll R, Jarvis A, Joubert G, et al. CATCH: a clinical decision rule for the use of computed tomography in children with minor head injury. CMAJ. 2010;182(4):341–8. doi: 10.1503/cmaj.091421. Epub 2010 Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klassen TP, Reed MH, Stiell IG, Nijssen-Jordan C, Tenenbein M, Joubert G, et al. Variation in utilization of computed tomography scanning for the investigation of minor head trauma in children: a Canadian experience. Acad Emerg Med. 2000;7(7):739–44. doi: 10.1111/j.1553-2712.2000.tb02260.x. [DOI] [PubMed] [Google Scholar]
- 40.Kuppermann N, Holmes JF, Dayan PS, Hoyle JD, Jr, Atabaki SM, Holubkov R, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective study. Lancet. 2009;374(9696):1160–70. doi: 10.1016/S0140-6736(09)61558-0. Epub 2009 Sep 14. [DOI] [PubMed] [Google Scholar]
- 41.Van Kampen DA, Lovell MR, Pardini JE, Collins MW, Fu FH. The “value added” of neurocognitive testing after sports-related concussion. Am J Sports Med. 2006;34(10):1630–5. doi: 10.1177/0363546506288677. Epub 2006 Jun 30. [DOI] [PubMed] [Google Scholar]
- 42.Levine Z. Mild traumatic brain injury. Part 2: concussion management. Can Fam Physician. 2010;56:658–62. [PMC free article] [PubMed] [Google Scholar]
- 43.Purcell L, Canadian Paediatric Society Healthy Active Living and Sports Medicine Committee Identification and management of children with sport-related concussion. Paediatr Child Health (Oxford) 2006;11(7):420–8. [Google Scholar]
- 44.Ahmed OH, Sullivan SJ, Schneiders AG, McCrory PR. Concussion information online: evaluation of information quality, content and readability of concussion-related websites. Br J Sports Med. 2012;46(9):675–83. doi: 10.1136/bjsm.2010.081620. Epub 2011 Apr 18. [DOI] [PubMed] [Google Scholar]
- 45.Alla S, Sullivan SJ, McCrory P, Hale L. Spreading the word on sports concussion: citation analysis of summary and agreement, position and consensus statements on sports concussion. Br J Sports Med. 2011;45(2):132–5. doi: 10.1136/bjsm.2010.074088. Epub 2010 Nov 16. [DOI] [PubMed] [Google Scholar]
- 46.New Zealand Guidelines Group . Traumatic brain injury: diagnosis, acute management and rehabilitation. Wellington, NZ: New Zealand Guidelines Group; 2006. [Google Scholar]
- 47.Defense Centre of Excellence for Psychological Health and Traumatic Brain Injury and Veterans Brain Injury Centre . Mild traumatic brain injury pocket guide (CONUS) Washington, DC: Defense Centre of Excellence for Psychological Health and Traumatic Brain Injury and Veterans Brain Injury Centre; 2009. Available from: www.dvbic.org/sites/default/files/DCoE_mTBI-Pocket-Guide.pdf. Accessed 2014 Apr 21. [Google Scholar]
- 48.Department of Veteran Affairs, Department of Defense . Clinical practice guideline for management of concussion/mild traumatic brain injury. Washington, DC: Department of Veteran Affairs, Department of Defense; 2009. Available from: www.dcoe.health.mil/Content/navigation/documents/VA%20DoD%20Management%20of%20Concussion%20mild%20Traumatic%20Brain%20Injury.pdf. Accessed 2014 Apr 21. [Google Scholar]
- 49.Workplace Safety and Insurance Board of Ontario . Program of care for mild traumatic brain injury. Toronto, ON: Workplace Safety and Insurance Board of Ontario; 2006. Available from: www.wsib.on.ca/en/community/WSIB/230/ArticleDetail/24338?vgnextoid=f17de35c819d7210VgnVCM100000449c710aRCRD. Accessed 2014 Apr 21. [Google Scholar]
- 50.Purcell LK, Canadian Paediatric Society Evaluation and management of children and adolescents with sports-related concussions. Paediatr Child Health (Oxford) 2012;17(1):31–2. doi: 10.1093/pch/17.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halstead ME, McAvoy K, Devore CD, Carl R, Lee M, Logan K, et al. Returning to learning following a concussion. Pediatrics. 2013;132(5):948–57. doi: 10.1542/peds.2013-2867. Available from: http://pediatrics.aappublications.org/content/early/2013/10/23/peds.2013-2867. Accessed 2014 Apr 21. [DOI] [PubMed] [Google Scholar]
- 52.Seifert TD, Evans RW. Posttraumatic headache: a review. Curr Pain Headache Rep. 2010;14(4):292–8. doi: 10.1007/s11916-010-0117-7. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe TK, Bell KR, Walker W, Schomer K. Systematic review of interventions for posttraumatic headache. PM R. 2012;4(2):129–40. doi: 10.1016/j.pmrj.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Reddy CC, Collins M, Lovell M, Kontos AP. Efficacy of amantadine treatment on symptoms and neurocognitive performance among adolescents following sports-related concussion. J Head Trauma Rehabil. 2013;28(4):260–5. doi: 10.1097/HTR.0b013e318257fbc6. [DOI] [PubMed] [Google Scholar]
- 55.McGrath N. Supporting the student-athlete’s return to the classroom after a sport-related concussion. J Athl Train. 2010;45(5):492–8. doi: 10.4085/1062-6050-45.5.492. [DOI] [PMC free article] [PubMed] [Google Scholar]