Abstract
Schistosomiasis is a highly prevalent neglected tropical disease caused by blood-dwelling helminths of the genus Schistosoma. Praziquantel (PZQ) is the only drug available widely for the treatment of this disease and is administered in racemic form, even though only the (R)-isomer has significant anthelmintic activity. Progress towards the development of a second generation of anthelmintics is hampered by a lack of understanding of the mechanism of action of PZQ. In this letter, we report an efficient protocol for the small-scale separation of enantiomers of 2(hydrolyzed PZQ) using supercritical fluid chromatography (SFC). The enantiopure 2 were then used to develop several molecular probes, which can potentially be used to help identify the protein target of PZQ and study its mode of action.
Keywords: Schistosomiasis, Praziquantel, Supercritical fluid chromatography (SFC), Molecular Probes, Anthelmintic
Schistosomiasis is an intravascular parasitic infection caused by trematode worms of the genus Schistosoma. The disease affects approximately 249 million people including 114 million school-age children, 90% of whom live on the African continent where Schistosoma mansoni and Schistosoma haematobium are the predominant causative agents.1 Praziquantel (PZQ, 1) is the least expensive, easiest to use and most readily available of all current anti-schistosomal drugs.2,3 It is highly effective against all schistosome species that are known to infect humans and is welltolerated, making it suitable for mass treatment campaigns. Although PZQ efficacy is high, reported cure rates range from 60 to 95%.4,5 There are at least two explanations for this phenomenon. First, PZQ does not kill juvenile schistosomes readily between 2 and 5 weeks after infection of the definitive host.6–9 Without rigorous follow-up treatment, this can leave a reservoir of surviving worms to continue the cycle of infection. A second potential problem is the presence of drug resistance traits in laboratory and natural populations of worms.10–14
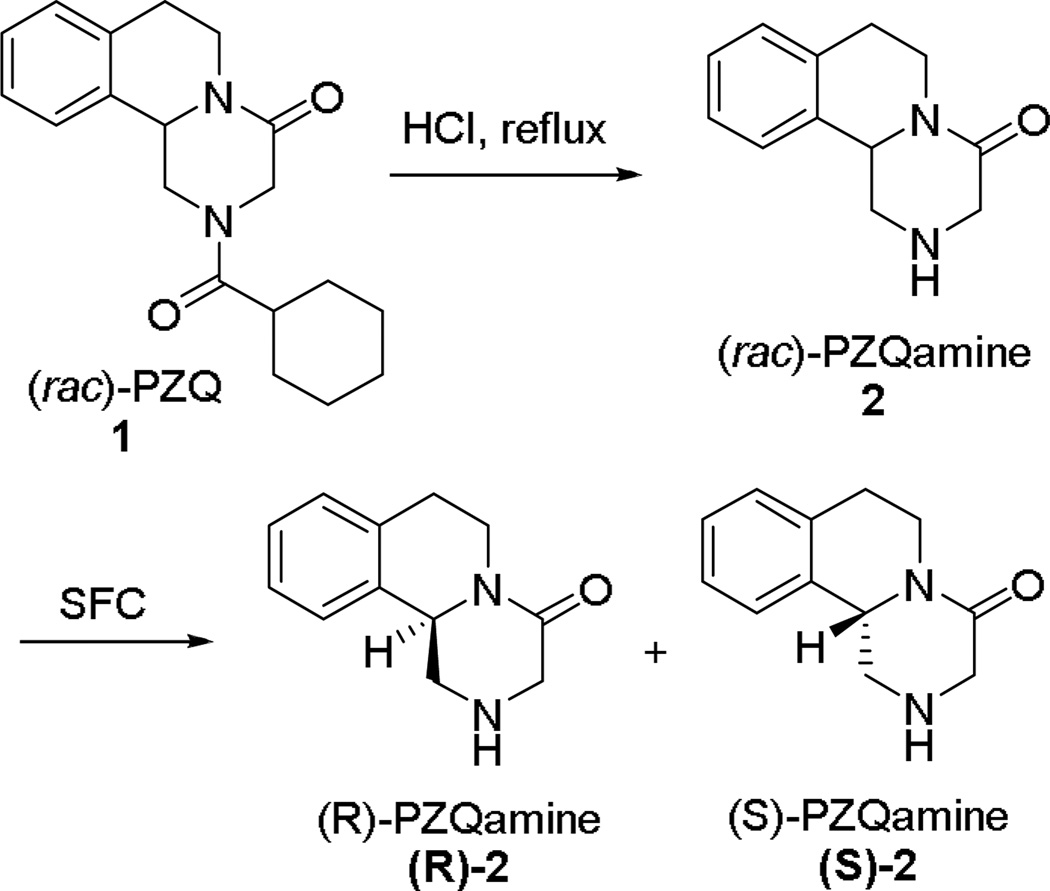
Currently, PZQ (1) is synthesized and employed as a racemic mixture; however, the anthelmintic activity of PZQ is associated mainly with the (R)-stereo isomer.15– 19 The inactive (S)-isomer is responsible for its side effects,18 extremely bitter taste20 and large pill size.21 The classical resolution of the PZQ intermediate 2 has recently been reported and has the potential to lend itself to the preparation of more optically pure versions of the drug on a commercial scale.22 Although the molecular target of PZQ remains unknown, a number of lines of evidence suggests that it disrupts cellular Ca2+ homeostasis by affecting voltage gated Ca2+ channel function.23– 26 The lack of an efficient general small-scale method for developing molecular probes of exceptional high optical purity limits our ability to readily prepare specific molecular probes based on the active and inactive soluble configuration of the PZQ pharmacophore. Molecular probes of this type may lead to an improved understanding of the mode of action of PZQ on schistosomes. Here, we report an efficient method to obtain highly purified samples of each of the two enantiomers of hydrolyzed PZQ (2, see Scheme 1) on gram scales with supercritical fluid chromatography (SFC). Enantiopure (R)-2 and (S)-2 were used to synthesize (R)-PZQ, (S)-PZQ and other PZQ-based molecular probes. These molecular tools can potentially be used to identify the molecular target and site of localization of this drug.
Scheme 1.
Synthesis of racemic 2 and separation of the two enantiomers (R)-2 and (S)-2 using supercritical fluid chromatography (SFC).
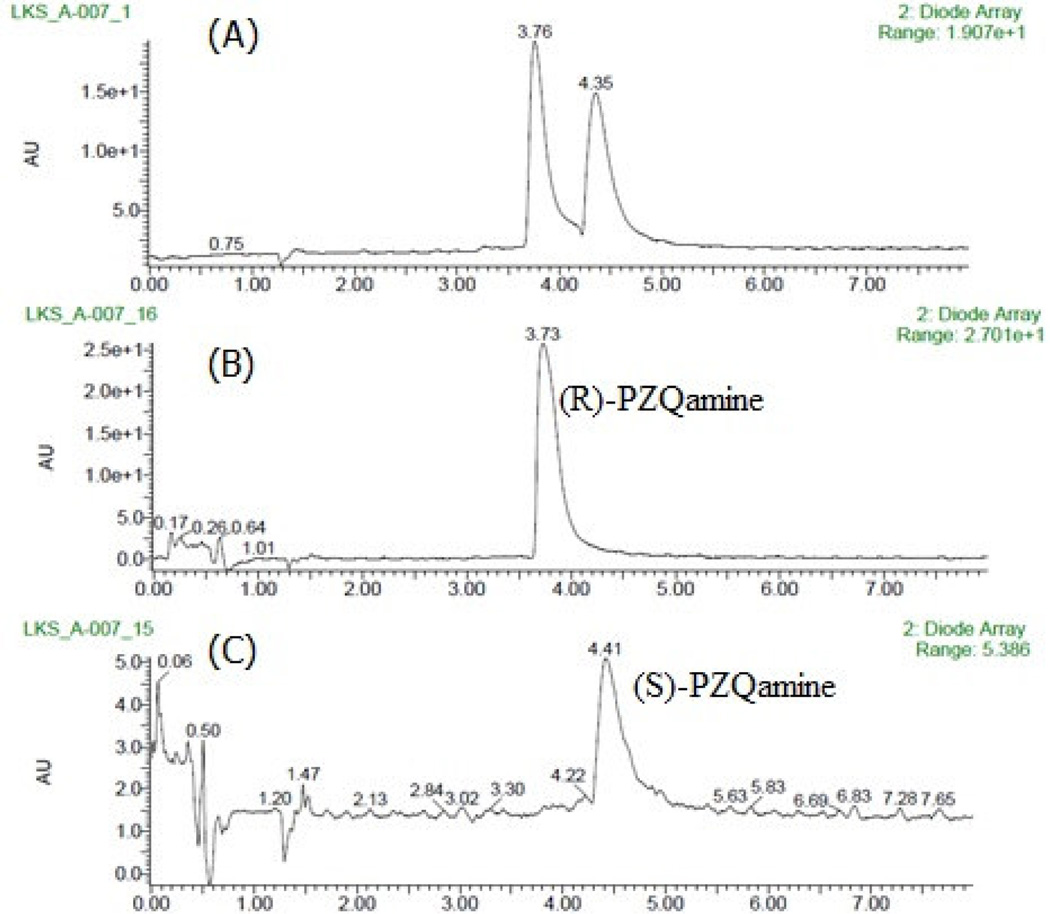
Our synthesis of new molecular probes starts with racemic 2, which is generated from (rac)-PZQ (1) using the precedented hydrolysis of 1 on a 20 g scale under acidic conditions (Scheme 1).22 The reaction provided 2 in 77 % isolated yield. Recently, SFC has gained acceptance as a valuable chromatographic technique because of improved resolution and a shorter analysis time compared to high performance liquid chromatography (HPLC).27 Additionally, SFC generally uses supercritical CO2 in combination with a polar organic solvent (e.g. alcohols) as the mobile phase; making it a greener and cost-effective alternative to HPLC and reverse phase liquid chromatography (RPLC). Given the advantages of SFC, we investigated its ability to separate the enantiomers of 2. The preliminary conditions for separation were determined using the Berger SFC analytical system, ProNTo. Interestingly, a recent study on HPLC analysis of (rac)-2 showed the inefficiency of both Chiralcel AD-H and OD-H columns to separate the enantiomers of 2.22 Our initial study using SFC indicated that the Chiralcel AD-H column worked more efficiently compared to the Chiralcel OD-H column in separating the enantiomers of 2. Figure 1A shows the chromatogram for the separation of (rac)-2 on the chiral AD-H column using 35% methanol as the mobile phase. The retention time was 3.7 ± 0.1 minutes for (R)-2 and 4.3 ± 0.1 minutes for (S)-2.
Figure 1.
SFC analysis of (A) (rac)-2; (B) (R)-2 and (C) (S)-2. Chiralcel ADH column; Solvent system: methanol/supercritical CO2 (35:65).
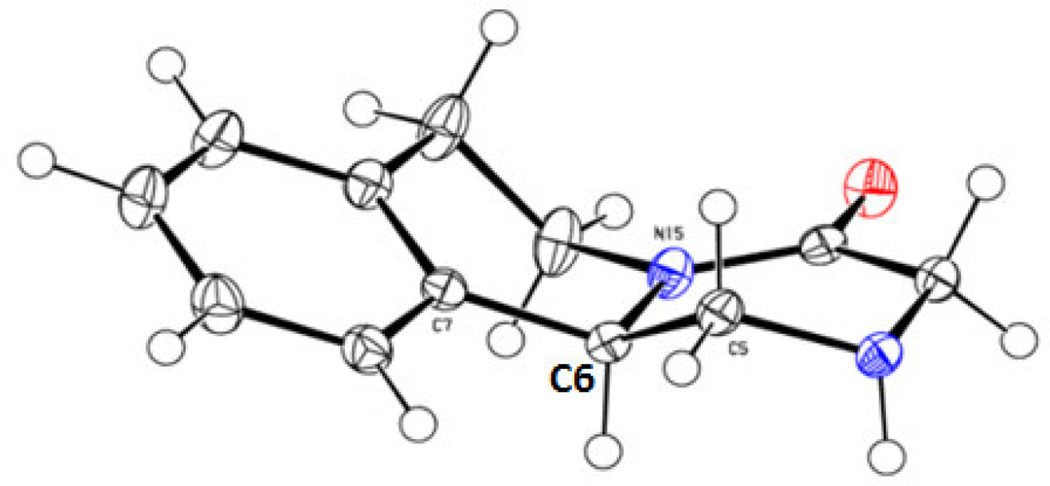
Based on the above data, the following conditions were selected for subsequent work using Berger SFC preparative-scale system: Chiralcel AD-H column, methanol/supercritical CO2 solvent system (35:65), amount per injection 7 mg, total flow 55, cycle time 3 min, injection value duration 45 s and total elution time 7 min. The SFC preparative-scale system worked efficiently to separate the enantiomers of 2 (on a gram scale) as shown by the SFC analysis of (R)-2 and (S)-2 in Figure 1B and 1C respectively. The identity of (R)-2 and (S)-2 was determined by comparing their optical rotation values with the literature.22 In addition to optical rotation, the absolute stereochemistry of (R)-2 was further confirmed by X-ray analysis (CCDC 994262) as shown in Figure 2.
Figure 2.
X-ray structure of (R)-2 highlighting stereochemistry at C6 (crystallographic numbering). Nitrogen atoms are shown in blue, and oxygen atom is shown in red.
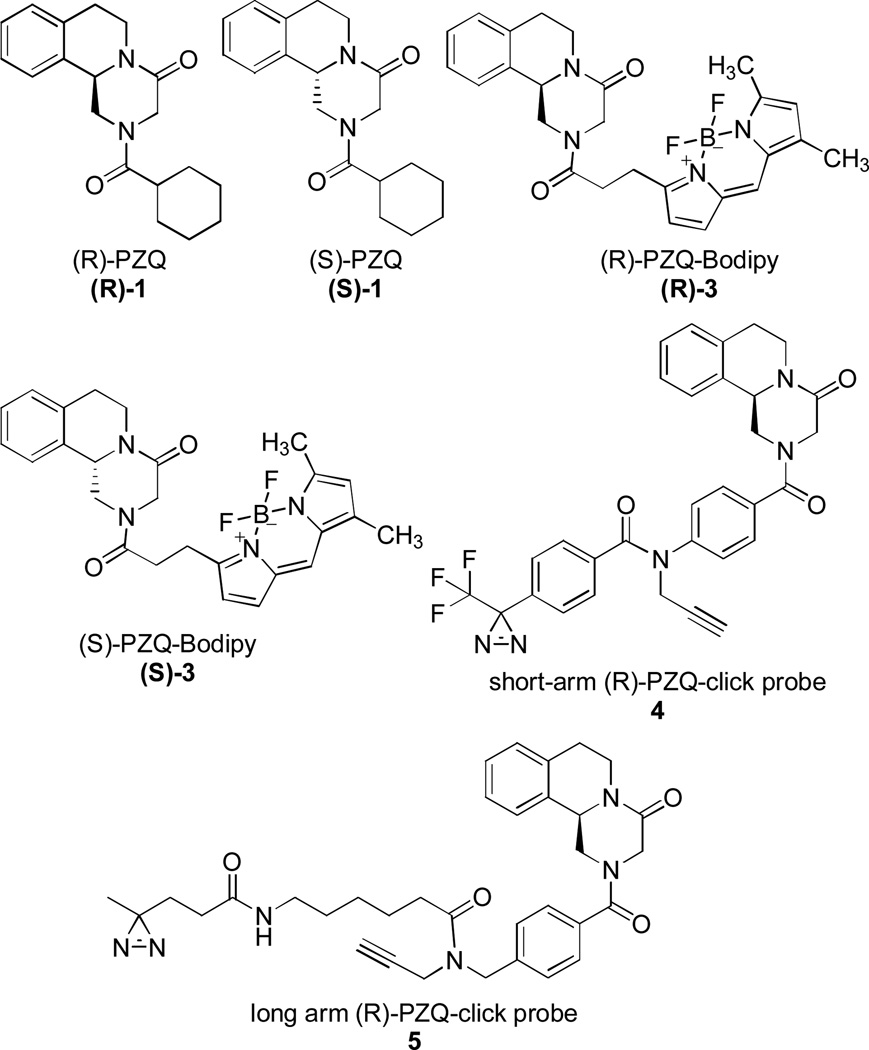
Once the protocol for obtaining the enantiopure (R)-2 and (S)-2 was established, they were then used for the synthesis of several molecular probes (Figure 3). First, (R)-2 and (S)-2 were coupled with cyclohexanoyl chloride under basic conditions that provided enantiopure (R)-PZQ and (S)-PZQ respectively.22 Using a procedure we previously developed for the racemic 2,7 (R)-2 and (S)-2 were used to obtain the optically pure fluorescently labeled probes: (R)- and (S)-PZQ-Bodipy (compounds (R)-3 and (S)-3). Additionally, we designed and synthesized a ‘short-arm’ PZQ molecular probe (compound 4) containing the diazirine functionality for photoaffinity labeling studies, as well as an alkyne moiety making 4 suitable for click chemistry. Using a similar approach, we also prepared another (R)-PZQ-click probe having a longer photoreactive arm (compound 5). Details of the experimental procedures for the synthesis of compounds shown in Figure 3 are in the supplementary information section. While a different method of preparation of PZQ enantiomers has been reported previously,22 to the best of our knowledge the synthesis of molecular probes (R)-3, (S)-3, 4 and 5 in an enantiomerically pure form has not.
Figure 3.
Compounds and probes synthesized from enantiopure (R)-2 and (S)-2.
Next, we evaluated the activity of synthesized compounds against sexually mature male S. mansoni PR-1 worms in vitro. Worms were incubated overnight in the presence of different concentrations of each compound after which they were washed and maintained for 7 days in drug-free media. The EC50 for each PZQ molecule at the end of this 7 day period is shown in Table 1. The EC50 of commercially available racemic PZQ (2.5 µM) was similar to that reported by Pica-Mattoccia et al. (1.6 µM).9 The EC50 of (R)-1 was significantly less than that of the racemic PZQ (1) reflecting the increased purity of the active enantiomer. Worm contraction and shortening was observed immediately when either the racemic 1 or (R)-1 was added to the worms. Seven days after the drugs were washed out, worms remained contracted at concentrations as low as 1.0 and 0.3 µM respectively. Only at concentrations >60 µM (S)-1 were schistosomes contracted with little movement at day 7. The EC50 of (S)-1 was 78.1 µM, 50-fold higher than the EC50 for the (R)-1 enantiomer and 31-fold higher than that of the racemic PZQ (1). Andrews (1985) reported that (S)-PZQ was approximately 60 and 100-fold less effective than the racemic PZQ and (R)-PZQ respectively using an in vitro scoring of worm death based on contraction after 10 min and vesiculation after 18 h.28
Table 1.
Summary of in vitro EC50 values for sexually mature S. mansoni PR-1 worms to PZQ molecules and probes.
| Compound | EC50 (µM) a | 95% CIb (µM) |
|---|---|---|
| 1 | 2.5 | 1.9–3.3 |
| (R)-1 | 0.5 | 0.5–0.5 |
| (S)-1 | 78.1 | 72.3–84.4 |
| (R)-3 | 53.3 | 48.6–58.6 |
| (S)-3 | ND | ND |
| 4 | 132.1 | 114–153.1 |
| 5 | >300 | ND |
Worms were exposed overnight to different drug concentrations then cultured in drug-free media for 7 days.
Dose-response curves with the mean and SEM from three replicates were calculated by plotting the percentage worms alive at day 7 against the log of the drug concentration (nM) using GraphPad Prism 6. The R-squared values were always > 0.9.
CI = Confidence Intervals. ND = Not Determined.
No immediate contraction or paralysis was observed when the schistosomes were exposed to (R)-PZQ-Bodipy ((R)-3). After both overnight incubation in the presence of the drug and 7 days after incubation in drug-free media, the worms were contracted at concentrations >40 µM with almost no movement. The EC50 was 53.3 µM and likely reflects the reduced ability of this larger molecule to penetrate the worm tegument. This decrease in activity of (R)-3 compared to (R)-1 may also be attributed to the effect of derivatization at the cyclohexane position.29,30 The EC50 of the (S)-Bodipy was not calculated as this was unlikely to provide informative data due to the relative insolubility of the molecule and the greatly reduced anthelmintic activity associated with (S)-isomers. Probes 4 and 5 were designed to meet all the structural requirements for retention of anthelmintic activity.29,30 The short-arm (R)-PZQ-click probe (4) was only partially soluble at 300 µM in 1 % DMSO. When exposed to the probe, the worms did not immediately contract or shorten. After both overnight incubation and 7 days after the probe was washed out, worms were loosely curled with little movement at concentrations >100 µM. The EC50 was 132.1 µM, approximately 100-fold higher than that of (R)-PZQ and again likely reflects a lack of ability to cross the worm tegument due to the increased molecular weight. The long-arm (R)-PZQ-click probe (5) was also only partially soluble in 1 % DMSO at >300 µM and did not kill schistosomes, even at this relatively high concentration. The longer photoreactive arm increases the hydrophobicity of the probe and its relative insolubility also likely renders it less active and less effective. Thus, while we believe that (R)-1 and 4 may be employed in the identification of PZQ target(s) using live S. mansoni or the S. mansoni proteome, 5 is only likely to be useful with the latter. Further studies using these molecular probes are being pursued in our laboratories.
In summary, this protocol provides an efficient method for rapid small-scale synthesis of compounds and probes derived from enantiopure (R)-2 and (S)-2 by taking advantage of SFC. We have also reported the initial evaluation of the biological activity of these probes on schistosomes. The probes based on enantiopure (R)-2 may have profound advantages in the study of the mechanism of action of PZQ against schistosomes.
Supplementary Material
Acknowledgments
We would like to thank Mr. K.C. Lim, University of California San Francisco, for his assistance with the parasite life-cycle and animal perfusions. We would also like to thank J. Bollinger, St. Jude Children’s Research Hospital for his determination of the single crystal X-ray structures of compounds (R)-2. NIH NIAID grants, R56AI087807 and 1R01AI087807-01A1 and awards from the American Lebanese Syrian Associated Charities (ALSAC) and St Jude Children’s Research Hospital (SJCRH) supported this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at doi:.
References
- 1.WHO Neglected Tropical Diseases Preventative Chemotherapy and Transmission Control (PCT) Databank. [Accessed January 30, 2014]; http://www.who.int/neglected_diseases/preventive_chemotherapy/databank/en/index.html. Statistics for 2012.
- 2.Cioli D, Pica-Mattoccia L, Archer S. Pharmacol.Ther. 1995;68:35. doi: 10.1016/0163-7258(95)00026-7. [DOI] [PubMed] [Google Scholar]
- 3.Hagan P, Appleton CC, Coles GC, Kusel JR, Tchuem-Tchuente LA. Trends Parasitol. 2004;20:92. doi: 10.1016/j.pt.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Gryseels B, Nkulikyinka L, Coosemans MH. Trans. R. Soc. Trop. Med. Hyg. 1987;81:641. doi: 10.1016/0035-9203(87)90439-1. [DOI] [PubMed] [Google Scholar]
- 5.Cioli D, Pica-Mattoccia L. Parasitol. Res. 2003;90:S3. doi: 10.1007/s00436-002-0751-z. [DOI] [PubMed] [Google Scholar]
- 6.Gonnert R, Andrews PZ. Parasitenkd. 1977;52:129. doi: 10.1007/BF00389899. [DOI] [PubMed] [Google Scholar]
- 7.Aragon AD, Imani RA, Blackburn VR, Cupit PM, Melman SD, Goronga T, Webb T, Loker ES, Cunningham C. Mol. Biochem. Parasitol. 164. 2009:57. doi: 10.1016/j.molbiopara.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Exper. Parasitol. 1986;61:294. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- 9.Pica-Mattoccia L, Cioli D. Int. J. Parasitol. 2004;34:527. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Fallon PG, Doenhoff MJ. Am. J. Trop. Med. Hyg. 1994;51:83. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- 11.Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, Day TA, Bennett JL. Am. J. Trop. Med. Hyg. 1999;60:932. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- 12.Alonso D, Munoz J, Gascon J, Valls ME, Corachan M. Am. J. Trop. Med. Hyg. 2006;74:342. [PubMed] [Google Scholar]
- 13.Doenhoff MJ, Kusel JR, Coles GC, Cioli D. T. Roy. Soc. Trop. Med. H. 2002;96:465. doi: 10.1016/s0035-9203(02)90405-0. [DOI] [PubMed] [Google Scholar]
- 14.Melman SD, Steinauer ML, Cunningham C, Kubatko LS, Mwangi IN, Wynn NB, Mutuku MW, Karanja DMS, Colley DG, Black CL, Secor WE, Loker ES. PLoS Negl. Trop. Dis. 2009;3:e504. doi: 10.1371/journal.pntd.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu YH, Qian MX, Wang XG, Jia J, Wang QN, Jiang YF, Wang RQ, Yan SH, Chen BY, Li JS, et al. Chin. Med. J. 1986;99:935. [PubMed] [Google Scholar]
- 16.Tanaka M, Ohmae H, Utsunomiya H, Nara T, Irie Y, Yasuraoka K. Am. J. Trop. Med. Hyg. 1989;41:198. doi: 10.4269/ajtmh.1989.41.198. [DOI] [PubMed] [Google Scholar]
- 17.Xiao SH, Catto BA. J. Infect. Dis. 159. 1989:589. doi: 10.1093/infdis/159.3.589. [DOI] [PubMed] [Google Scholar]
- 18.Wu MH, Wei CC, Xu ZY, Yuan HC, Lian WN, Yang QJ, Chen M, Jiang QW, Wang CZ, Zhang SJ, et al. Am. J. Trop. Med. Hyg. 1991;45:345. doi: 10.4269/ajtmh.1991.45.345. [DOI] [PubMed] [Google Scholar]
- 19.Staudt U, Schmahl G, Blaschke G, Mehlhorn H. Parasitol. Res. 1992;78:392. doi: 10.1007/BF00931694. [DOI] [PubMed] [Google Scholar]
- 20.Meyer T, Sekljic H, Fuchs S, Bothe H, Schollmeyer D, Miculka C. PLoS Negl. Trop. Dis. 2009;3:e357. doi: 10.1371/journal.pntd.0000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming FM, Fenwick A, Tukahebwa EM, Lubanga RG, Namwangye H, Zaramba S, Kabatereine NB. Parasitology. 2009;136:1759. doi: 10.1017/S0031182009990709. [DOI] [PubMed] [Google Scholar]
- 22.Woelfle M, Seerden JP, de Gooijer J, Pouwer K, Olliaro P, Todd MH. PLoS Negl. Trop. Dis. 2011;5:e1260. doi: 10.1371/journal.pntd.0001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg RM. Int. J. Parasitol. 2005;35:1. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Kohn AB, Anderson PA, Roberts-Misterly JM, Greenberg RM. J. Biol. Chem. 2001;276:36873. doi: 10.1074/jbc.C100273200. [DOI] [PubMed] [Google Scholar]
- 25.Nogi T, Zhang D, Chan JD, Marchant JS. PLoS Negl. Trop. Dis. 2009;3:e464. doi: 10.1371/journal.pntd.0000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang D, Chan JD, Nogi T, Marchant JS. J. Neurosci. 2011;31:15983. doi: 10.1523/JNEUROSCI.3029-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JW, Fukusaki E, Bamba T. Bioanalysis. 2012;4:2413. doi: 10.4155/bio.12.198. [DOI] [PubMed] [Google Scholar]
- 28.Andrews P. Pharmacol. Ther. 1985;29:129. doi: 10.1016/0163-7258(85)90020-8. [DOI] [PubMed] [Google Scholar]
- 29.Andrews P, Thomas H, Pohlke P, Seubert J. Med. Res. Rev. 1983;3:147. doi: 10.1002/med.2610030204. [DOI] [PubMed] [Google Scholar]
- 30.Patra M, Ingram K, Pierroz V, Ferrari S, Spingler B, Keiser J, Gasser G. J. Med. Chem. 2012;55:8790. doi: 10.1021/jm301077m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.