Abstract
Previous studies report functional differences in large conductance Ca2+ activated-K+ channels (BKCa) of smooth muscle cells (VSMC) from rat cerebral and cremaster muscle resistance arteries. The present studies aimed to determine if this complexity in BKCa activity may, in part, be due to splice variants in the pore-forming α-subunit. BKCa variants in the intracellular C terminus of the α-subunit, and their relative expression to total α-subunit, were examined by qPCR. Sequencing of RT-PCR products showed two α-subunit variants, ZERO and STREX, to be identical in cremaster and cerebral arteries. Levels of STREX mRNA expression were, however, significantly higher in cremaster VSMCs (28.9±4.2% of total α-BKCa) compared with cerebral vessels (16.5±0.9%). Further, a low level of BKCa SS4 α-subunit variant was seen in cerebral arteries, while undetectable in cremaster arteries. Protein biotinylation assays, in expression systems and arterial preparations, were used to determine whether differences in splice variant mRNA expression affect surface membrane/cytosolic location of the channel. In AD-293 and CHO-K1 cells, rat STREX was more likely to be located at the plasma membrane compared to ZERO, although the great majority of channel protein was in the membrane in both cases. Co-expression of β1-BKCa subunit with STREX or ZERO did not influence the dominant membrane expression of α-BKCa subunits, whereas in the absence of α-BKCa, a significant proportion of β1-subunit remained cytosolic. Biotinylation assays of cremaster and cerebral arteries showed that differences in STREX/ZERO expression do not alter membrane/cytosolic distribution of the channel under basal conditions. These data, however, revealed that the amount of α-BKCa in cerebral arteries is approximately 20X higher than in cremaster vessels. Thus, the data support the major functional differences in BKCa activity in cremaster, as compared to cerebral VSMCs, being related to total α-BKCa expression, regardless of differences in splice variant expression.
Introduction
Potassium channels play an important role in the regulation of VSMC membrane potential and contractile activity. In particular, large conductance Ca2+-activated, K+ channels (BKCa) are activated in response to membrane depolarization and increases in intracellular Ca2+ to affect membrane hyperpolarization [1], [2]. While BKCa channels are widely expressed in both electrically excitable and non-excitable cells [3], [4], they are relatively abundant in smooth muscle and play a key role in the regulation of vascular tone [5], [6]. Structurally, the functional BKCa channel exists as a tetramer of α-subunits forming the ion channel pore together with tissue specific auxiliary β-subunits (β1–β4) which are typically present in a 1∶1 stoichiometry [7]. The BKCa α-subunit consists of seven transmembrane spanning domains (S0–S6) including the extracellular N-terminus, P-loop between S5 and S6 domains, and a large intracellular C terminus containing a number of regulatory sites including the regulators of conductance for K+ (RCK 1 and 2) and 2–3 Ca2+ binding sites.
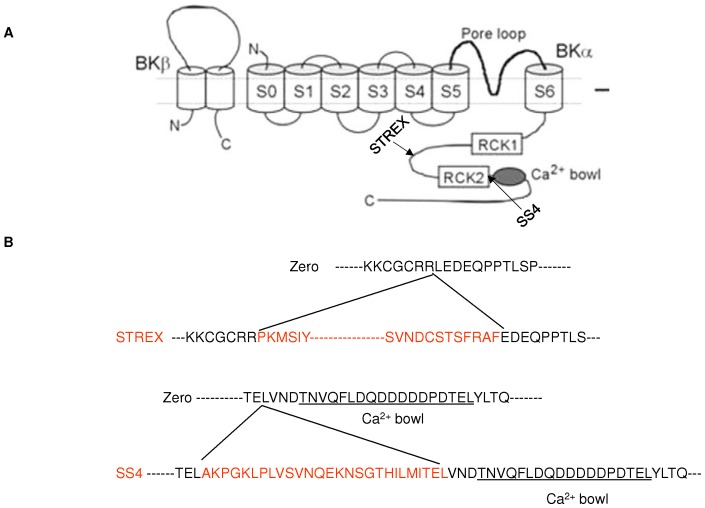
The BKCa α-subunit is encoded by a single gene (KCNMA1) containing 27 distinct exons, in contrast to each β-subunit, which is encoded by four distinct exons [8]. BKCa channels appear to achieve part of their functional diversity through alternative pre-mRNA splicing of the KCNMA1 gene [9], [10]. Up to ten alternative splicing sites have been identified for the vertebrate BKCa α-subunit [11]. Most variation occurs in the intracellular C-terminal part in the linker region between domains RCK1 and RCK2 and upstream of the “calcium bowl” [12]. Alternative splicing can modify the functional properties of BKCa channels, including Ca2+ and voltage sensitivity, cell surface expression, and regulation by diverse intracellular signaling pathways. One of the most thoroughly studied α-BKCa splice variants is the STREX exon (STRess axis regulated EXon), which derives its name from its splicing regulation by stress-axis hormones [13]. It has been shown that the STREX exon (an insertion of 58 amino acids in the C-terminal splice site 2 of the α-subunit protein) confers distinct functional phenotypes onto BKCa channels, such as altered Ca2+ sensitivity and changing responsiveness of channels to cAMP signaling from stimulatory to inhibitory, compared with the ZERO variant, lacking this insert [9], [14]–[17]. It has also been shown that BKCa channels containing the SS4 splice variant (an insertion of a 27 amino acid segment upstream to the C-terminal Ca2+-bowl in splice site 4 of the α-subunit) were activated more rapidly than the ZERO variant in the presence of the same voltage stimulus, and the difference in these activation kinetics was dependent on the concentration of intracellular Ca2+ [18], (also see Figure 1).
Figure 1. Location and amino acid sequences of ZERO and STREX splice variants of BKCa α-subunit.
Schematic diagram illustrating (A) sites of STREX and SS4 splicing variants of α-BKCa and (B) the amino acid sequences of the splicing inserts (Adapted from reference [5]).
Alternatively, it has been reported that intracellular trafficking of α-BKCa may be one of the main post-translational modifications that can regulate the number of ion channels at the cell surface [19]. This mode of regulation can also be modulated by accessory β-subunits. While limited studies have addressed the effects of α-BKCa splice variants on channel trafficking to plasma membrane [20]–[24], there is some discrepancy in the reported findings. For example, Kim et al (2007) reported the expression of two α-BKCa splice variants, termed VEDEC and QEDRL, in chick ciliary ganglion neurons that differ at the extreme C-terminus. Using HEK293T and NG108-15 cells and a cell surface biotinylation assay, QEERL channels showed markedly higher levels of constitutive expression of α-BKCa at the plasma membrane compared with VEDEC channels, which tend to remain in the cytosol [21]. The same group further showed that co-expression of avian β1-subunits with the VEDEC isoform α-BKCa, prevented the inhibitory effect of the VEDEC sequence on cell surface expression [20]. In contrast, studies from Toro and colleagues (2006) showed that co-expression of human β1-subunit with a human pore-forming α-subunit enhanced internalization of the α-subunit [25].
Previous electrophysiological studies from our laboratory have demonstrated that BKCa channel activity differs significantly in VSMCs from cremaster muscle arteries compared with cerebral arteries. In particular, our functional data have revealed a decreased Ca2+ sensitivity of cremaster BKCa channels, resulting in more positive levels of Em being required in cremaster VSM cells to generate similar levels of outward K+ conductance [26]. Similarly, Jackson and Blair (1998) described cremaster muscle BKCa channels as being normally ‘silent’, but suggested that their activity could be ‘recruited’ during vasoconstriction [27]. Therefore in this study, we first hypothesized that the functional differences between these two resistance vasculatures may be due, in part, to the expression of splice variants of the BKCa α-subunit. We chose to focus on STREX and SS4 splice variants, as splice sites where they are located (i.e. 2 and 4) contain regulatory phosphorylation, palmitoylation and Ca2+ interaction sites that could functionally impact channel activity. We further hypothesized that an additional influence of α-subunit splice variation may be on the surface membrane location of the channel and whether this could be affected by the accessory β1-subunit.
Material and Methods
Tissue isolation and vessel RNA purification
All experiments and protocols were approved by the Animal Care and Use Committee, University of Missouri, USA. Our studies used male Sprague-Dawley rats weighing between 180–280 g. Rats were anaesthetized with sodium pentobarbital (Nembutal, 100 mg/kg body weight) given by an intraperitoneal injection. Cremaster muscles were surgically removed, as previously described [28], and placed in a cooled (4°C) dissection chamber. Following sacrifice by anesthetic overdose, a craniotomy was performed and the brain was removed intact and similarly placed in a cooled dissection chamber.
First- and second-order arterioles (1A/2A) from cremaster muscle and mid-cerebral arteries were isolated and rapidly subjected to total RNA purification using a Melt Total Nucleic Acid isolation system kit (Life Technologies, Carlsbad, CA, USA) following the manufacturer's instructions. All samples were treated by TURBO DNase digestion (Life Technologies) to minimize contamination with genomic DNA. The concentration and purity of RNA for each sample was determined by UV absorbance using a Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Rockford, IL, USA) and samples were stored at −80°C until conversion to cDNA. Equal amounts of total vessel RNA extract were then reverse-transcribed into a single strand cDNA using a Superscript III First-Strand synthesis system (Life Technologies) according to the manufacturer's instructions.
Real-time quantitative PCR
Real-time PCR was performed in triplicate, in 96-well plates, on cDNAs prepared from each sample (n = 4–5) using KAPA SYBER FAST qPCR Kit Master mix (KAPA Biosystems, Woburn, MA, USA). PCR was performed using a Mastercycler EP Realplex2 (Eppendorf-North America, Westbury, NY, USA). Reaction volume/well contained 20 µl∶10 µl of master mix, 1 µl of each sense and antisense primers (5 µM), 1 µl of cDNA template and the remainder DNase-free water. Primers used in this study were based on previously published papers: ZERO [26] STREX [9], and β-actin [29]. Primers for SS4 variant were designed using Real-Time PCR primer software from Integrated DNA Technologies (IDT). Details of oligo-DNA primers used to amplify BKCa α-subunits (STREX, SS4) and ZERO variants, accession numbers for the template sequences and the expected product sizes are shown in Table 1. ZERO variant primers were designed in regions of transmembrane domains in which no splice variant existed, and its expression was utilized as an indication of the total expressed α-subunit mRNA [9], [13].
Table 1. Sequence of primers used for end-point and real-time PCR.
| Accession number | Primer sequence | Amplicon length | Amplification efficiency | |
| End-point PCR | ||||
| α-BKCa | NM_031828 | F: TACTGCAAGGCCTGTCATGATG | 342 | |
| R: TCATCAGCTTCGGGGATGTGTT | ||||
| Real-time PCR | ||||
| STREX | NM_031828 | F: TTTGATTGCGGACGTTCTGA | 77 | 2.063 |
| R: TCTCTCAAGGGTGTCCACGTTAC | ||||
| SS4 | AF_135265 | F: CAAGTTGCCTTTGGTATCAGTC | 131 | 2.013 |
| R: GCTCTGTGTCAGGGTCATC | ||||
| ZERO | NM_031828 | F:AAACAAGTAATTCCATCAAGCTGGTG | 137 | 2.006 |
| R: CGTAAGTGCCTGGTTGTTTTGG | ||||
| β-actin | NM_031144 | F: CCTCTATGCCAACACAGTGCTGTCT | 128 | 1.993 |
| R: GCTCAGGAGGAGCAATGATCTTGA |
(F: forward primer, R: reverse primer).
Real-time PCR protocols were performed as follows: pre-heating at 95°C for 2 min, 40 cycles of two-step cycling of denaturation at 95°C for 3 sec and annealing/extension steps of 25 sec at 58°C. For each qPCR determination, no enzyme and no template conditions were included to test for contamination of assay reagents. An arbitrary rat mid-cerebral artery cDNA sample was included in each plate to provide a constant calibrator point for all samples. After the final PCR cycle, a melting curve analysis was routinely performed to identify the presence of primer-dimers and to analyze the specificity of the reaction. Data were collected and analyzed using Realplex software (Eppendorf-North America). The amplification efficiencies between targets and housekeeping genes (i.e. β-actin) were initially verified to be approximately equal (Table 1), allowing the comparative threshold (Ct) method for quantification to be used [30]. The relative expression level (R) was calculated with equations as follows: R = 2−ΔΔCt = 2−(ΔCt sample − ΔCt calibrator) for the target genes in each sample set according to the published 2−ΔΔCt method [30]. Changes in mRNA expression levels were calculated from an average of triplicate measurements and are reported as fold changes relative to the ZERO variant, after normalization to β-actin. Data were analyzed using an unpaired student t-test: a statistically significant difference was assumed at P≤0.05.
Cell surface Biotinylation assay on cultured cells
Plasmid constructs containing cDNA for full-length rat BKCa ZERO variant or BKCa STREX variant (gifts from Dr. Michael J. Shipston) were transiently transfected into AD-293 cells (240085, Agilent Technology, Santa Clara, CA, USA) or CHO-K1 cells (CCL-61, ATCC, Manassas, VA, USA) with FuGENE 6 Transfection Reagent (Roche Diagnostics, Indianapolis, IN, USA). A bovine β1-BKCa plasmid DNA was also used in some experiments as its sequence shares high homology (>95%) with rat β1-BKCa channel protein. Cell surface biotinylation assays were performed 24–48 hours post-transfection. In brief, live transfected cells were washed three times with Hanks' buffered salt solution (HBSS) and then incubated on ice for 2 hours in the presence of a freshly prepared 0.5 mg/ml mixture of biotinylation reagents, EZ Link Sulfo-NHS-Lc-Lc-Biotin (21338, Thermo Scientific) and EZ Link Maleimide-PEG-Biotin (21901, Thermo Scientific). Total protein was determined to allow normalization for Avidin pull-down of biotinylated proteins after quenching of biotinylation process by ice-cold 100 mM glycine in HBSS (3x in 1 min interval incubation). Biotinylated cells were homogenized in RIPA buffer plus 1% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA), incubated on ice (30 min) and sonicated for 45 sec. Cellular debris was removed by centrifugation at 6,000 g for 10 min at 4°C. Total protein concentration was determined using the BCA protein assay kit (Thermo Scientific). Equal amounts of total biotinylated cell lysates were subsequently incubated with Monomeric Avidin Agarose (20228, Thermo Scientific) overnight at 4°C, followed by washing with cold HBSS (3x) and one time with water. Finally, the cytosolic fractions of cells transfected with either α-BKCa splice variants or β1-BKCa subunit were separated from biotinylated cell surface proteins by centrifugation (11,000 g/2 min/4°C). The biotinylated membrane proteins were then eluted from the beads by heating at 45°C/15 min in 2× Laemmli protein sample buffer [31]. Isolated cell surface and cytosolic proteins were separated by SDS-PAGE on 4–20% TGX Precast Gels (Bio-Rad, Hercules, CA, USA), transferred onto polyvinylidene difluoride membranes and probed with a mouse monoclonal anti-BKCa channel (clone L6/60, 1∶500, NeuroMab, Davis, CA, USA) or an anti-BKCa β subunit antibody (ab3587, 1∶500, Abcam, Cambridge, MA, USA). Bound antibody was detected using SuperSignal West Dura ECL Chemiluminescent Substrate (34075, Thermo Scientific). Images were collected using a ChemiDoc XRS+ System (Bio-Rad) and analyzed by Image Lab software. Parallel control biotinylation assays were conducted with mock transfected cells and cells with streptavidin beads in the absence of biotin incubation. In mock transfected cells, no bands were detected related to the α-BKCa splice variants (ZERO, STREX) or the β1 subunit (data not shown). These control studies confirmed the absence of endogenous BKCa channels in CHO-K1 cells and the specificity of the antibodies used in this study.
Cell Surface Biotinylation assay on vessels
Biotinylation of surface proteins in intact cerebral and cremaster arteries was performed to detect the cell surface membrane expression of native α-BKCa channels in these vessel types. To have adequate amounts of total protein, first- and second-order cremaster arterioles from four male Sprague-Dawley rats (180–280 g) were pooled together for each separate experiment. In parallel, the whole Circle of Willis vasculature was isolated from two animals, cleaned of connective tissue and pooled to provide a cerebral artery sample. Arteries were incubated in a freshly prepared 1 mg/ml mixture of Biotin reagents, as above, in whole cell buffer solution (in mM 10 HEPES, 9 Glucose, 6 KCl, 134 NaCl, 2 CaCl2.2H2O, and 1 MgCl2.6H2O) for one hour at room temperature while undergoing constant horizontal shaking. The arteries were then incubated at room temperature with quenching solution of 100 mM glycine in PBS for 15 min to remove any unbound biotin. The biotinylated vessels were homogenized to prepare total protein as previously described [32]. Equal amounts of total protein (∼50–60 µg) were incubated with Monomeric Avidin Agarose. After one hour of avidin incubation at room temperature [33], [34], the non-biotinylated (cytosolic) protein fraction was separated from the biotinylated (cell membrane) protein fraction by centrifugation at 11,000 g/2 min/4°C. Biotinylated surface proteins were eluted from the avidin beads by boiling for 3 min in 2× Laemmli buffer containing β-mercaptoethanol (5% v/v). Western blot analysis of surface and cytosolic proteins was performed using mouse anti α-BKCa channel (1∶500, NeuroMab) or anti-BKCa β subunit (1∶500) primary antibodies. Quantification of cell surface and cytosolic protein bands was analyzed using Image Lab software (Bio-Rad) and are expressed as percentage of total protein.
Results
Identification of α-BKCa splice variants by end-point PCR
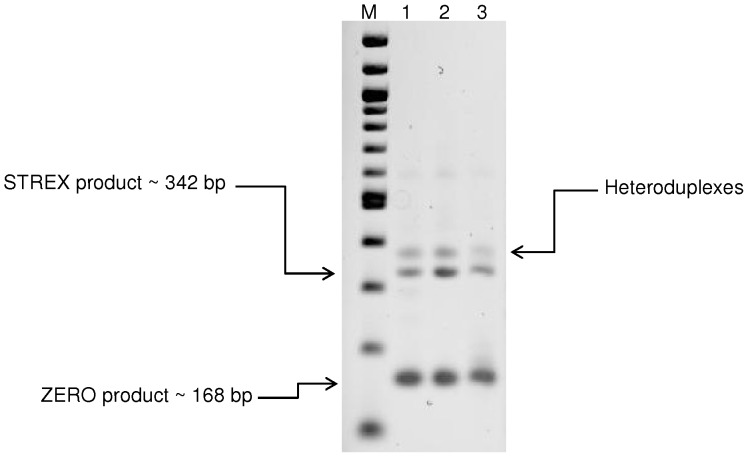
For initial identification of STREX and ZERO variants, end-point PCR was performed using primers designed to amplify the alternative splice site 2 (See Table 1 for details). Testis cDNA was used as a positive control [11]. PCR products of three separate experiments from different experimental animals were analyzed by electrophoresis and subsequently verified by sequencing. As shown in Figure 2, two dominant bands were detected in both vasculatures. The lower band with predicted size of 168 bp was determined to be ZERO variant (α-subunit without splice insert) by direct product sequencing and the upper band (∼342 bp) was confirmed to be STREX variant (α-subunit with the insertion of 174 bp at splice site 2). The third visible band likely constitutes heteroduplexes between sense and antisense strands of STREX and ZERO products consistent with earlier reports [35].
Figure 2. Detection of ZERO and STREX variants in rat cerebral and cremaster arteries.
End-point PCR products generated from cDNA derived from rat total RNA transcripts, with and without the STREX exon. Size markers are shown in the lane marked as M. Additional lanes display PCR products detected in testis (lane 1, included as a positive control), mid-cerebral arteries (lane 2) and cremaster arterioles (lane 3). Results are representative of n = 3 separate experiments.
Quantification of α-BKCa splice variants by qPCR
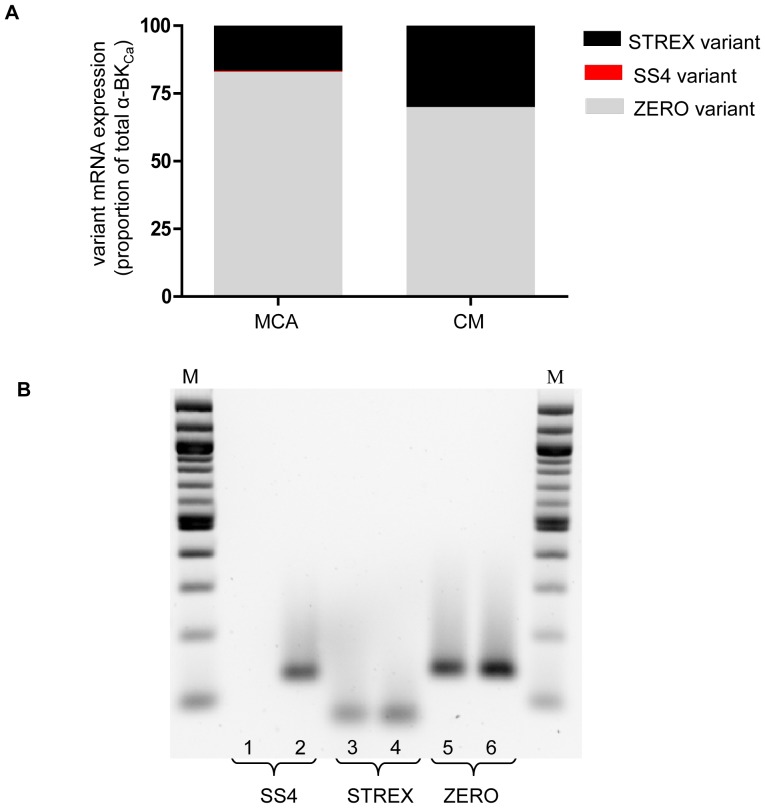
Identification of the SS4 variant was performed by qPCR together with subsequent quantification of expression levels of STREX variant relative to ZERO using a further set of primers (Table 1). As shown in Figure 3A and B, while a very low level of SS4 was detected in mid-cerebral arteries (0.42±0.1% of total α-BKCa), the variant was undetectable in cremaster vessels. A higher level of expression of the STREX variant was detected in cremaster arteries (28.9±4.2% of total α-BKCa) compared to mid-cerebral (16.5±0.9% of total α-BKCa) arteries (P<0.05). Thus, ZERO variant was calculated to be significantly (P<0.05) greater in mid-cerebral (83.1±0.9% of total α-BKCa) compared to cremaster (71.1±4.2%) arteries (Figure 3A).
Figure 3. QPCR of BKCa α-subunit splice variants in rat cerebral and cremaster arteries.
(A) Relative mRNA expression levels for STREX, SS4 and ZERO variants calculated as a percentage of total α-BKCa mRNA detected in mid-cerebral arteries and cremaster arterioles. (B) QPCR products of SS4, STREX and ZERO variants of α-BKCa subunit as separated on a 2% agarose gel. Size markers shown in the lane denoted M. QPCR products generated from cremaster arterioles are depicted in lanes 1, 3, 5 and products from mid-cerebral artery are shown in lanes 2, 4, and 6. Real-time PCR results are shown for n = 4–5 samples of each vascular bed performed in triplicate.
Cell Surface expression of BKCa ZERO or STREX variants in expression systems
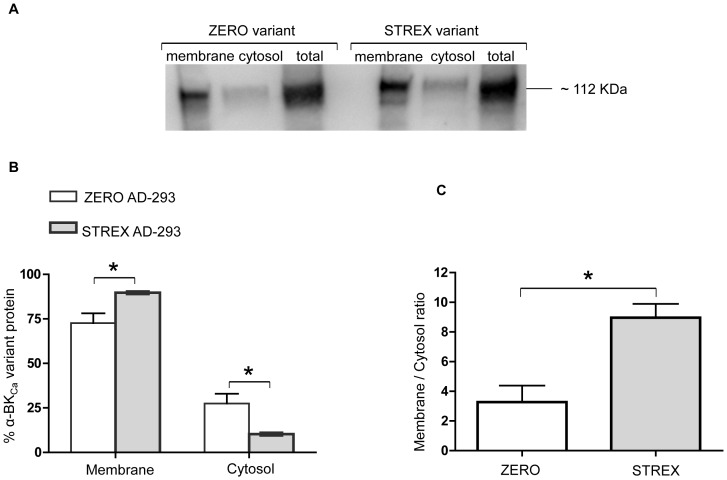
To investigate the cell surface location of α-BKCa splice variants in a cell culture system, equal amounts of full-length cDNAs of rat ZERO or STREX variants were initially transfected into AD-293 cells (Figure 4A). As shown in Figure 4B, although both variants were predominantly targeted to the cell surface, the STREX variant of α-BKCa shows a significantly higher level of cell surface expression (P = 0.02, unpaired t-test) than the ZERO variant. Conversely at the cytosolic level, the ZERO variant shows a significantly higher level of expression as compared with STREX (P = 0.02, unpaired t-test). The ratio of membrane to cytosol expression was also significantly higher for the STREX variant than ZERO (Figure 4C).
Figure 4. The cell surface and cytosolic expression of BKCa α-subunit splice variants in cultured cells.
Using an AD-293 cell culture system, α-BKCa STREX variant shows a significantly higher cell surface expression compared with the ZERO variant. Corresponding changes in the cytosolic fraction are observed. (A) Representative Western blot showing membrane, cytosolic, and total levels of α-BKCa protein in AD-293 cultured cells, transfected with either α-BKCa splice variants ZERO or STREX. (B) Summary of membrane vs cytosolic distribution of transfected ZERO or STREX variants of α-BKCa channel protein (expressed as % of total α-BKCa protein). (C) Membrane to cytosolic expression ratio of ZERO and STREX variants in transfected cultured AD-293 cells. Results are shown for n = 4 separate experiments and are presented as mean ± SEM (*P<0.05, unpaired t-test).
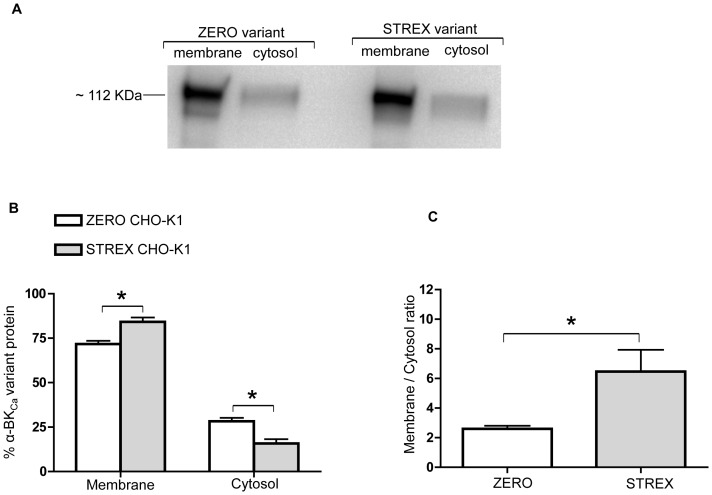
To confirm that this trafficking pattern was not cell dependent, similar experiments were conducted using CHO-K1 cells (Figure 5A). As illustrated in Figure 5B, the STREX variant again shows increased distribution at the cell membrane compared with ZERO (P = 0.002, unpaired t-test). The cytosolic expression of STREX in CHO-K1 cells shows significantly lower expression than the ZERO variant, similar to that observed in the AD-293 cell culture system. As shown in Figure 5C, the ZERO variant shows a significantly lower ratio of membrane to cytosolic expression in CHO-K1 cells. Since these two α-BKCa subunit splice variant exhibit the same expression pattern in AD-293 and CHO-K1 cells, we used CHO-K1 cells as our cell system model for future experiments.
Figure 5. Membrane and cytosolic expression of ZERO and STREX variants in CHO-K1 cells.
In a CHO-K1 cell culture system, the α-BKCa ZERO variant shows significantly lower cell surface expression than the STREX variant. Corresponding changes in the cytosolic fraction are observed. (A) Representative Western blot showing surface and cytosolic levels of α-BKCa protein in CHO-K1 cell culture system, transfected with either α-BKCa splice variants ZERO or STREX. (B) Summary of membrane vs cytosolic expression of transfected ZERO or STREX variants of α-BKCa channel protein (expressed as % of total α-BKCa protein). (C) Membrane to cytosolic expression ratio of ZERO and STREX α-BKCa proteins in transfected cultured CHO-K1 cells. Results are shown for n = 6 separate experiments and are presented as mean ± SEM (*P<0.05, unpaired t-test).
Cell Surface location of co-transfected BKCa ZERO or STREX variants with β1-subunit in CHO-K1 cells
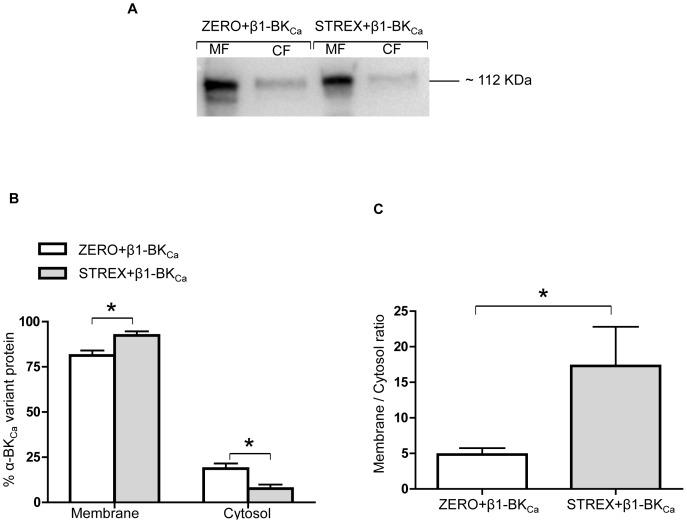
As holo-BKCa channels in resistance arteries contain both α- and β1-BKCa subunits, we hypothesized that co-expression of β1-subunit in CHO-K1 cells may equalize the cell surface levels of the two variants. To investigate the impact of β1-BKCa subunit on cell surface expression of α-BKCa splice variants ZERO and STREX, β1-BKCa subunit was co-transfected with either the ZERO or STREX variant (Figure 6A). In the presence of β1-BKCa subunit, the α-subunit variants continued to be predominantly located in the plasma membrane with the STREX variant present at higher levels than ZERO (Figure 6B). This resulted in the same relative expression pattern (Figure 6C) as seen when the α-BKCa splice variants were expressed without the β1-BKCa subunit (Figure 5C). These data could indicate a dominant cell surface expression pattern of α-BKCa splice variants of ZERO and STREX in cell culture systems.
Figure 6. The effect of β1-BKCa subunit on cell surface expression of α-BKCa splice variants.
Co-transfection of β1-BKCa subunit has little apparent impact on cell surface expression of α-BKCa ZERO or STREX variants. (A) Representative Western blot showing levels of membrane (MF), and cytosolic (CF) fractions of α-BKCa protein in CHO-K1 cultured cells, transfected with either α-BKCa splice variants ZERO or STREX. (B) In the CHO-K1 cell culture system, α-BKCa STREX variant shows dominant cell surface expression when co-transfected with β1-BKCa. Results are shown for n = 5 separate experiments and are presented as mean ± SEM (*P<0.05, unpaired t-test). (C) Membrane to cytosolic ratio for the expression of ZERO or STREX proteins when co-transfected with β1-BKCa in cultured CHO-K1 cells. Results are shown for n = 5 separate experiments and are presented as mean ± SEM (* P = 0.05, unpaired t-test).
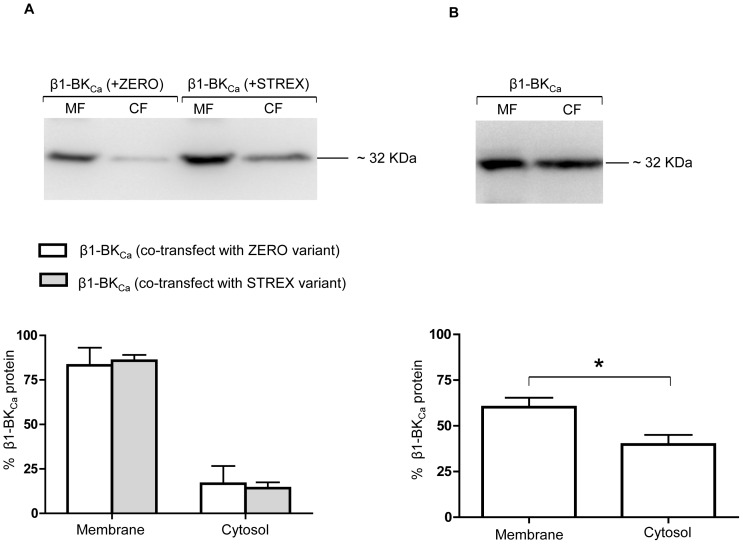
To determine the effect of ZERO or STREX variants on the cellular distribution of β1-BKCa subunits, biotinylated β1-BKCa subunit proteins were also probed in cells co-transfected with β1-BKCa and either the ZERO or STREX variant. As shown in Figure 7A, a high level of cell surface expression of β1-BKCa subunit (84.7±4.4%) was observed when co-transfected with either the ZERO or STREX variant. To assess whether over-expression of β1-BKCa subunit, alone, is sufficient to stimulate its surface trafficking, CHO-K1 cells were transfected with only β1-BKCa cDNA and β1-BKCa subunit surface location assessed by the cell-surface biotinylation assay. Under these conditions, as shown in Figure 7B, the cell surface labeling of β1-BKCa subunit showed a significant decrease (P = 0.01) to approximately 60.2±5.2%, which was accompanied by a significant increase in cytosolic levels of β1-BKCa subunit from approximately 15.3±4.5% to 39.8±5.2%. These data appear to indicate a stimulatory effect of α-BKCa splice variants on the surface trafficking of the regulatory subunit β1-BKCa, while there was no apparent impact on the surface membrane location of either ZERO or STREX by β1-BKCa subunit.
Figure 7. Cell surface expression of β1-BKCa subunit in the presence and absence of α-BKCa splice variants.
Co-transfection of ZERO or STREX variants with β1-BKCa subunit enhances cell surface trafficking of β1-BKCa subunit. The top image in panel (A) represents a Western blot for membrane (MF) and cytosolic (CF) fractions of β1-BKCa protein in CHO-K1 cultured cells, co-transfected with either α-BKCa splice variants ZERO or STREX. The bottom Figure in panel (A) shows the membrane vs cytosolic expression of transfected β1-BKCa channel protein when co-transfected with either α-BKCa splice variants of ZERO or STREX. Results are shown for n = 5 separate experiments. The top image in panel (B) shows a representative Western blot of membrane (MF) and cytosolic (CF) fractions of β1-BKCa protein in CHO-K1 cultured cells, in the absence of either α-BKCa splice variants ZERO or STREX. The bottom Figure in panel (B) shows membrane vs cytosolic expression of β1-BKCa subunit in cultured CHO-K1 cells in the absence of the α-BKCa splice variants ZERO or STREX. Results are shown for n = 6 separate experiments and are presented as mean ± SEM (*P<0.05, unpaired t-test).
Cell surface expression of total α-BKCa protein in cerebral vs cremaster arteries
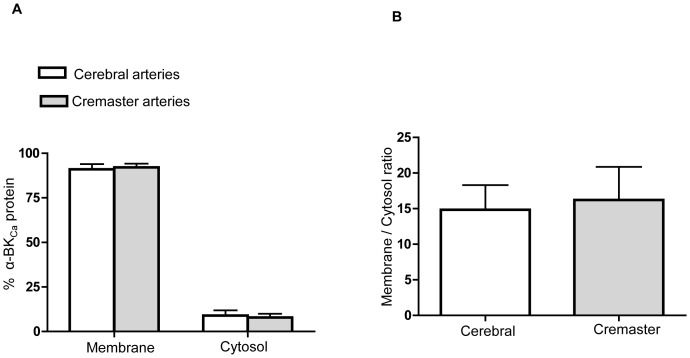
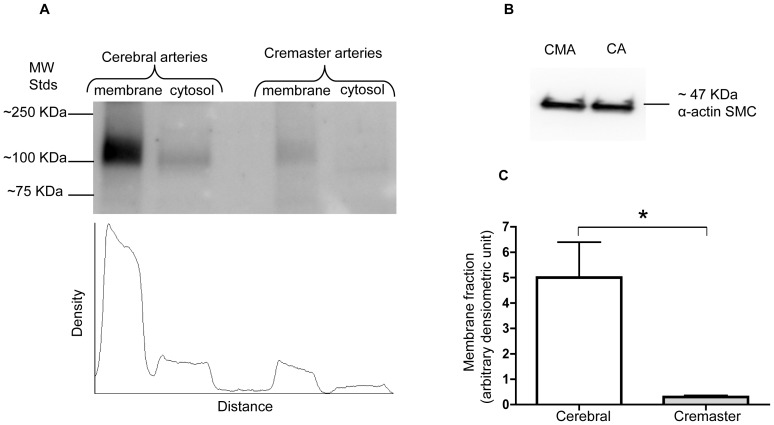
Previous electrophysiological studies from our laboratory have demonstrated that BKCa channel activity differs significantly in VSMCs from cremaster muscle arteries compared with cerebral arteries [26]. Our functional data show a decreased Ca2+ sensitivity of cremaster VSMC BKCa channels compared with those of cerebral arteries, resulting in more positive levels of Em being required for cremaster VSMCs cells to generate similar levels of outward K+ current. As a result, we hypothesized that these functional differences in channel activity could arise from differences in the molecular configuration of the channel in the two VSMC types affecting channel properties such as cell surface trafficking of α-BKCa protein. Although the lack of specific available antibodies to distinguish ZERO from STREX variants is a technical limitation to detect α-BKCa splice variants at the protein level, the biotinylation assay was used to determine the extent of α-BKCa protein at the cell surface compared with the cytosolic fraction. Experiments were performed using homogenates of whole cerebral and cremaster arteries. As shown in Figure 8A and B, in both vessel types more than 90% of total α-BKCa channels were located at the cell membrane. However, when cell surface expression of α-BKCa was normalized to equal amounts of α-smooth muscle actin in each preparation, cerebral arteries show approximately 20 times higher level of total amount of α-BKCa protein at the cell membrane compared with cremaster arteries (Figure 9).
Figure 8. Membrane and cytosolic expression of α-BKCa subunits in cerebral vs creamster arteries.
α-BKCa channels are predominantly located at the cell surface of VSMCs in both rat cerebral and cremaster arteries under basal conditions. (A) Cell surface α-BKCa proteins were determined using the biotinylation assay from whole cerebral (Circle of Willis pooled from 2 animals in each experiment) and cremaster arteries (first- and second-order cremaster arterioles pooled from 4 animals in each experiment). Results are shown for n = 6 separate experiments for cerebral and n = 5 for cremaster arteries. Results are shown as mean ± SEM. (B) Membrane to cytosolic ratio of α-BKCa channels in cerebral vs cremaster arteries (the corresponding Western blot showing cell surface and cytosolic levels of α-BKCa protein is shown in Figure 9A).
Figure 9. Quantification of cell surface expressed α-BKCa channels in cerebral vs cremaster arteries.
Total amount of membrane α-BKCa channel in cerebral arteries is approximately 20 times higher than that in cremaster arterioles. The Western blot in panel (A) shows a representative experiment depicting the distribution of α-BKCa protein in membrane and cytosolic fractions prepared from cerebral arteries and cremaster arterioles. The scan beneath the blot quantifies the intensity of the α-BKCa protein immunoreactive band in each sample, as detected by densitometry. The Western blot in panel (B) shows a loading control in which 2 µg of total protein for both cerebral arteries (CA) and cremaster arterioles (CMA) was probed with α-actin SMC antibody. Panel (C) shows group data for n = 5 experiments. Results are shown as mean ± SEM (*P<0.05, unpaired t-test).
Discussion
In previous studies, we reported functional differences in BKCa channels in VSMCs between cerebral arteries and those from cremaster muscle [26], [32]. In those studies, we showed that BKCa from cremaster VSMCs exhibit a decreased Ca2+ sensitivity and suggested that this may, in part, be due to a decrease in the amount of the β1 regulatory subunit. Regulation of BKCa is, however, complex involving mechanisms at the levels of expression and post-translational modification, as well as its physical relationship to cellular organelles such as the sarcoplasmic reticulum [5], [6], [8]. On the basis of this, the aims of the present study were to examine whether differences exist in the expression of splice variants of the α-subunit of BKCa and if membrane location of the channel differed between the two vascular beds.
The major findings of the present studies were as follows: Firstly, qPCR studies demonstrate a significantly higher mRNA expression for the BKCa α-subunit STREX splice variant in rat cremaster arteries compared with that in cerebral arteries. Secondly, we detected the predominant cell surface expression of both the α-BKCa splice variants ZERO and STREX in cell expression systems, with no apparent impact of β1-subunit co-expression on the degree of cell surface localization. Thirdly, although the β1-subunit expressed alone is able to reach the cell membrane, a significant proportion remains cytosolic compared with its predominant cell surface localization in the presence of either the ZERO or STREX variant. Finally, cell surface labeling revealed that the vast majority of α-BKCa channel (>90%) in both cremaster and cerebral arteries is located in the cell membrane fraction under basal conditions. However, a major difference between the two vascular beds was that the total amount of plasma membrane α-BKCa is approximately 20 times lower in cremaster arterioles as compared with small cerebral arteries.
Although a single gene, KCNMA1, encodes the pore forming BKCa α-subunit in vertebrates, there is considerable phenotypic diversity of these channels in different tissues. Several factors are known to contribute to this diversity including, alternative splicing [36], the co-expression of regulatory β-subunits [37], [38], and post-translational modifications including protein phosphorylation [39]. Modulation of BKCa channels by a complex network of signal transduction pathways such as reversible protein phosphorylation has been studied extensively [39]–[41]. Importantly, alternate splicing of pre-mRNA leading to channel variants with differing degrees of modulation by reversible protein phosphorylation represents a potential mechanism to generate functional diversity of ion channels. In studies of cloned mouse BKCa variants, expressed in HEK293 cells, Tian et al. (2001) demonstrated that cAMP-dependent protein kinase (PKA)-mediated phosphorylation activates BKCa ZERO variant, but inhibits the STREX variant, which could thus impact channel function including Ca2+ sensitivity [17]. The level of STREX expression also has important modulatory consequences as it has been previously shown that only one subunit within the tetrameric holo-channel needs to be of the STREX type to alter channel function [42]. It has also been shown that protein palmitoylation (a post-translational modification affecting multiple ion channels) can regulate the activity and surface expression of BKCa channel α-subunits in native tissues and cultured cells [19]. Specifically, Tian and colleagues described palmitoylated BKCa channels that include plasma membrane associated STREX variants that are inhibited by PKA-dependent phosphorylation, whereas ZERO channels are activated by PKA [43]. Therefore, the finding of lower expression of the ZERO variant and higher expression of STREX in cremaster arteries compared with mid-cerebral arteries could conceivably relate to functional alterations in BKCa Ca2+ sensitivity, as we have previously observed for cremaster vascular smooth muscle cells [32].
However, in the present studies the cremaster vessels have been shown to express a higher level of STREX as compared to the cerebral arteries which may have been expected to convey a higher Ca2+ sensitivity [9] rather than the decreased Ca2+ sensitivity we previously reported [26]. This apparent discrepancy may relate to a number of factors including the dominant effects of differences in overall expression levels and also that the cremaster vessels were previously shown to have a lower ratio of β1:α subunit. Importantly, the β1 subunit has previously been shown to contribute to the Ca2+ sensitivity of the channel [44]. An additional consideration is that our measurements of STREX and ZERO expression were limited to the mRNA levels. In this previous study, we also reported a decrease in the ratio of BKCa α:β subunits in the crude membrane fraction of cremaster vessels compared with small cerebral arteries [26]. The data from the present study suggest it is unlikely that a lower level of β1-BKCa would negatively influence insertion of the α-subunit into the membrane, as our data in cell expression systems showed similar levels of surface location for α-BKCa in the presence and absence of the β1-subunit. Interestingly, and in contrast, the presence of the α-subunit in the expression system resulted in an increased proportion of β1-subunit being located at the cell surface.
In earlier studies, Jackson and Blair (1998) suggested that BKCa is ‘silent’ in cremaster muscle vasculature under basal conditions, but may be ‘recruited’ under stimulated conditions. Such stimulation was suggested to include vasoconstriction evoked by catecholamines and high tissue PO2 levels [27]. Whether such recruitment involves differences in splice variant expression, translocation of channel subunit proteins to the plasma membrane or post-translational modifications such as phosphorylation has not been fully elucidated. Given the high levels of α-subunit protein found at the membrane in both vessel types, it is unlikely that a simple difference in membrane vs. cytosolic pools explains the differences observed between cremaster and cerebral vessels. This does not, however, exclude the possibility that a dynamic alteration in channel protein trafficking occurs under other conditions.
In the present study, we also found a very low level of expression of the SS4 α-subunit variant relative to the ZERO variant in mid-cerebral, with no detectable expression in cremaster arteries. Although expression of the SS4 variant in vasculature (identified by RT-PCR) has been previously reported in cerebral and coronary arteries [11], its functional importance, particularly in native tissues such as small arteries, is unknown. Similarly, the functional significance of a lack of SS4 variant expression (as we report for cremaster muscle arterioles) is unclear. Using a Xenopus oocytes expression system, previous studies have suggested that ZERO and SS4 variants exhibit identical BKCa channel characteristics, including single-channel conductance and voltage dependent activation [18]. Those authors did, however, show that the activation rates of SS4 channels were more rapid at a similar voltage compared with the ZERO form when [Ca2+]i was higher than 5 µM. In addition to a comparative lack of information as to any functional significance of very low expression levels of the SS4 variant (approximately 0.4% of total α-BKCa mRNA) in cerebral arteries, it should also be considered that non-smooth muscle cell contamination (for example from neurons or adventitial cells) in whole vessel preparations could contribute to this signal.
Apart from differences in splice variant expression, it would be expected that the marked difference in total BKCa channel protein expression would be of functional significance. This is despite the large conductance (approx 240 pS) in VSMCs from both vascular beds. Specifically, an ∼20-fold higher level of α-BKCa protein was detected in cerebral arteries compared with cremaster arteries. Importantly, this would be reflected at the plasma membrane because a similar proportion of total BKCa was surface located in both cerebral and cremaster arteries, as shown by our biotinylation assay. While measurements were performed on homogenates of whole vessels, the majority of signal would be expected to derive from the VSMC layers. Endothelial cells of healthy arteries are thought to be devoid of BKCa channels, although this point has been somewhat controversial [45]. Cellular capacitance measurements performed in our previous studies indicate that VSMC size in the two vessel types is similar, suggesting that functional effects of the expression difference would not be compensated by differences in size alone [26]. The earlier study also demonstrated, in crude membrane fractions, increased α-BKCa protein (approx. 3x) in cerebral vessels as compared to cremaster arterioles. Despite these measurements of marked differences in expression at the protein level actual K+ currents only differed by approximately 2x (at 5 mM Ca2+). Conceivably the seemingly disparate findings may relate to the functional status of the channels including as determined by post-translational modifications such as phosphorylation or possibly the influence of splice variants not directly examined in this study [46]. Another factor perhaps affecting the differences in absolute protein levels between cerebral vessels and cremaster arterioles relates to differences in adventitial structure that we have previously demonstrated [47]. It could be argued that these differences impact the access of the biotinylation reagents. We believe this to be unlikely, however, as the biotinylation reagents are small in regard to molecular weight and it was previously shown that the molecules easily penetrate the vascular wall [33]. Further, while this could theoretically effect the magnitude of the protein expression levels it would not impact the α to β subunit ratios nor the distribution between the plasma membrane and cytosol. As both the cell surface biotinylation approach and measurements performed in crude membrane fractions showed qualitatively similar same results, the conclusion that protein expression levels are greater in cerebral vessels appears robust.
In summary, significant differences exist with respect to the BKCa splice variants expressed in cremaster muscle arterioles compared with small arteries from the cerebral vasculature. Specifically, a higher expression level of the STREX variant of the α-subunit was observed in arterioles from cremaster muscle. While functional significance of this finding is yet to be fully demonstrated, it appears that it does not affect the plasma membrane location of the channels as >95% of α-subunit was found to be at the cell surface in both vessel preparations. In contrast, a marked difference in the detectable expression level was observed, with cerebral arteries expressing α-subunit protein at a level 20 times greater than that of cremaster arterioles.
Acknowledgments
Our especial thanks expressed to Dr. Michael J. Shipston for his generosity to provide DNA materials of BKCa α-subunit splice variants. Thanks are extended to Dr. Michael J. Davis for his constructive criticism of the manuscript prior to its submission.
Funding Statement
This research was supported by the National Institutes of Health (RO1 HL092241: Michael A. Hill and P01 HL095486: Gerald Meininger, P.I.) and the Natural Sciences and Engineering Research Council of Canada (RGPIN/312240: Andrew P. Braun). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Latorre R, Oberhauser A, Labarca P, Alvarez O (1989) Varieties of calcium-activated potassium channels. Annu Rev Physiol 51: 385–399. [DOI] [PubMed] [Google Scholar]
- 2. Marty A (1981) Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature 291: 497–500. [DOI] [PubMed] [Google Scholar]
- 3. Jan LY, Jan YN (1997) Cloned potassium channels from eukaryotes and prokaryotes. Annu Rev Neurosci 20: 91–123. [DOI] [PubMed] [Google Scholar]
- 4. Yan J, Aldrich RW (2010) LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature 466: 513–516. [DOI] [PubMed] [Google Scholar]
- 5. Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP (2010) Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett 584: 2033–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. LeDoux J, Werner ME, Brayden JE, Nelson MT (2006) Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21: 69–78. [DOI] [PubMed] [Google Scholar]
- 7. Wu RS, Marx SO (2010) The BK potassium channel in the vascular smooth muscle and kidney: alpha- and beta-subunits. Kidney Int 78: 963–974. [DOI] [PubMed] [Google Scholar]
- 8. Lee US, Cui J (2010) BK channel activation: structural and functional insights. Trends Neurosci 33: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen L, Tian L, MacDonald SH, McClafferty H, Hammond MS, et al. (2005) Functionally diverse complement of large conductance calcium- and voltage-activated potassium channel (BK) alpha-subunits generated from a single site of splicing. J Biol Chem 280: 33599–33609. [DOI] [PubMed] [Google Scholar]
- 10. Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, et al. (1994) Cloning, expression, and distribution of functionally distinct Ca(2+)-activated K+ channel isoforms from human brain. Neuron 13: 1315–1330. [DOI] [PubMed] [Google Scholar]
- 11. Poulsen AN, Wulf H, Hay-Schmidt A, Jansen-Olesen I, Olesen J, et al. (2009) Differential expression of BK channel isoforms and beta-subunits in rat neuro-vascular tissues. Biochim Biophys Acta 1788: 380–389. [DOI] [PubMed] [Google Scholar]
- 12. Poulsen AN, Jansen-Olesen I, Olesen J, Klaerke DA (2011) Neuronal fast activating and meningeal silent modulatory BK channel splice variants cloned from rat. Pflugers Arch 461: 65–75. [DOI] [PubMed] [Google Scholar]
- 13. Xie J, McCobb DP (1998) Control of alternative splicing of potassium channels by stress hormones. Science 280: 443–446. [DOI] [PubMed] [Google Scholar]
- 14. MacDonald SH, Ruth P, Knaus HG, Shipston MJ (2006) Increased large conductance calcium-activated potassium (BK) channel expression accompanied by STREX variant downregulation in the developing mouse CNS. BMC Dev Biol 6: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shipston MJ, Duncan RR, Clark AG, Antoni FA, Tian L (1999) Molecular components of large conductance calcium-activated potassium (BK) channels in mouse pituitary corticotropes. Mol Endocrinol 13: 1728–1737. [DOI] [PubMed] [Google Scholar]
- 16. Tian L, Hammond MS, Florance H, Antoni FA, Shipston MJ (2001) Alternative splicing determines sensitivity of murine calcium-activated potassium channels to glucocorticoids. J Physiol 537: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, et al. (2001) Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem 276: 7717–7720. [DOI] [PubMed] [Google Scholar]
- 18. Ha TS, Jeong SY, Cho SW, Jeon H, Roh GS, et al. (2000) Functional characteristics of two BKCa channel variants differentially expressed in rat brain tissues. Eur J Biochem 267: 910–918. [DOI] [PubMed] [Google Scholar]
- 19. Shipston MJ (2013) Regulation of large conductance calcium- and voltage-activated potassium (BK) channels by S-palmitoylation. Biochem Soc Trans 41: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim EY, Zou S, Ridgway LD, Dryer SE (2007) Beta1-subunits increase surface expression of a large-conductance Ca2+-activated K+ channel isoform. J Neurophysiol 97: 3508–3516. [DOI] [PubMed] [Google Scholar]
- 21. Kim EY, Ridgway LD, Zou S, Chiu YH, Dryer SE (2007) Alternatively spliced C-terminal domains regulate the surface expression of large conductance calcium-activated potassium channels. Neuroscience 146: 1652–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiu YH, varez-Baron C, Kim EY, Dryer SE (2010) Dominant-negative regulation of cell surface expression by a pentapeptide motif at the extreme COOH terminus of an Slo1 calcium-activated potassium channel splice variant. Mol Pharmacol 77: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zarei MM, Zhu N, Alioua A, Eghbali M, Stefani E, et al. (2001) A novel MaxiK splice variant exhibits dominant-negative properties for surface expression. J Biol Chem 276: 16232–16239. [DOI] [PubMed] [Google Scholar]
- 24. Zarei MM, Eghbali M, Alioua A, Song M, Knaus HG, et al. (2004) An endoplasmic reticulum trafficking signal prevents surface expression of a voltage- and Ca2+-activated K+ channel splice variant. Proc Natl Acad Sci U S A 101: 10072–10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toro B, Cox N, Wilson RJ, Garrido-Sanabria E, Stefani E, et al. (2006) KCNMB1 regulates surface expression of a voltage and Ca2+-activated K+ channel via endocytic trafficking signals. Neuroscience 142: 661–669. [DOI] [PubMed] [Google Scholar]
- 26. Yang Y, Murphy TV, Ella SR, Grayson TH, Haddock R, et al. (2009) Heterogeneity in function of small artery smooth muscle BKCa: involvement of the beta1-subunit. J Physiol 587: 3025–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jackson WF, Blair KL (1998) Characterization and function of Ca(2+)-activated K+ channels in arteriolar muscle cells. Am J Physiol 274: H27–H34. [DOI] [PubMed] [Google Scholar]
- 28. Meininger GA, Zawieja DC, Falcone JC, Hill MA, Davey JP (1991) Calcium measurement in isolated arterioles during myogenic and agonist stimulation. Am J Physiol 261: H950–H959. [DOI] [PubMed] [Google Scholar]
- 29. Wulf H, Hay-Schmidt A, Poulsen AN, Klaerke DA, Olesen J, et al. (2008) Molecular studies of BKCa channels in intracranial arteries: presence and localization. Cell Tissue Res 334: 359–369. [DOI] [PubMed] [Google Scholar]
- 30. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 31. Chen L, Jeffries O, Rowe IC, Liang Z, Knaus HG, et al. (2010) Membrane trafficking of large conductance calcium-activated potassium channels is regulated by alternative splicing of a transplantable, acidic trafficking motif in the RCK1-RCK2 linker. J Biol Chem 285: 23265–23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang Y, Sohma Y, Nourian Z, Ella SR, Li M, et al. (2013) Mechanisms underlying regional differences in the Ca2+ sensitivity of BK(Ca) current in arteriolar smooth muscle. J Physiol 591: 1277–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bannister JP, Adebiyi A, Zhao G, Narayanan D, Thomas CM, et al. (2009) Smooth muscle cell alpha2delta-1 subunits are essential for vasoregulation by CaV1.2 channels. Circ Res 105: 948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crnich R, Amberg GC, Leo MD, Gonzales AL, Tamkun MM, et al. (2010) Vasoconstriction resulting from dynamic membrane trafficking of TRPM4 in vascular smooth muscle cells. Am J Physiol Cell Physiol 299: C682–C694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mahmoud SF, Bezzerides AL, Riba R, Lai GJ, Lovell PV, et al. (2002) Accurate quantitative RT-PCR for relative expression of Slo splice variants. J Neurosci Methods 115: 189–198. [DOI] [PubMed] [Google Scholar]
- 36. Shipston MJ (2001) Alternative splicing of potassium channels: a dynamic switch of cellular excitability. Trends Cell Biol 11: 353–358. [DOI] [PubMed] [Google Scholar]
- 37. Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW (2000) Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 275: 6453–6461. [DOI] [PubMed] [Google Scholar]
- 38. Wallner M, Meera P, Ottolia M, Kaczorowski GJ, Latorre R, et al. (1995) Characterization of and modulation by a beta-subunit of a human maxi KCa channel cloned from myometrium. Receptors Channels 3: 185–199. [PubMed] [Google Scholar]
- 39. Schubert R, Nelson MT (2001) Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci 22: 505–512. [DOI] [PubMed] [Google Scholar]
- 40. Chung SK, Reinhart PH, Martin BL, Brautigan D, Levitan IB (1991) Protein kinase activity closely associated with a reconstituted calcium-activated potassium channel. Science 253: 560–562. [DOI] [PubMed] [Google Scholar]
- 41. Reinhart PH, Chung S, Martin BL, Brautigan DL, Levitan IB (1991) Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J Neurosci 11: 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tian L, Coghill LS, McClafferty H, MacDonald SH, Antoni FA, et al. (2004) Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A 101: 11897–11902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tian L, Jeffries O, McClafferty H, Molyvdas A, Rowe IC, et al. (2008) Palmitoylation gates phosphorylation-dependent regulation of BK potassium channels. Proc Natl Acad Sci U S A 105: 21006–21011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bao L, Cox DH (2005) Gating and ionic currents reveal how the BKCa channel's Ca2+ sensitivity is enhanced by its beta1 subunit. J Gen Physiol 126: 393–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sandow SL, Grayson TH (2009) Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol 297: H1–H7. [DOI] [PubMed] [Google Scholar]
- 46. Rosati B, McKinnon D (2004) Regulation of ion channel expression. Circ Res 94: 874–883. [DOI] [PubMed] [Google Scholar]
- 47. Clifford PS, Ella SR, Stupica AJ, Nourian Z, Li M, et al. (2011) Spatial distribution and mechanical function of elastin in resistance arteries: a role in bearing longitudinal stress. Arterioscler Thromb Vasc Biol 31: 2889–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]