Abstract
Glycogen synthase kinase-3 (GSK-3) is a pleiotropic serine/threonine protein kinase found in almost all eukaryotes. It is structurally highly conserved and has been identified as a multifaceted enzyme affecting a wide range of biological functions, including gene expression and cellular processes. There are two closely related isoforms of GSK-3; GSK-3α and GSK-3β. The latter appears to play crucial roles in regulating the pathogenesis of diverse diseases, including neurodegenerative disease. The present review focuses on the involvement of this protein in Parkinson’s disease (PD), a common neurodegenerative disorder characterized by the gradually progressive and selective loss of dopaminergic neurons, and by intracellular inclusions known as Lewy bodies (LBs) expressed in surviving neurons of the substantia nigra (SN). GSK-3β is involved in multiple signaling pathways and has several phosphorylation targets. Numerous apoptotic conditions can be facilitated by the GSK-3β signaling pathways. Studies have shown that GSK-3β inhibition protects the dopaminergic neurons from various stress-induced injuries, indicating the involvement of GSK-3β in PD pathogenesis. However, the underlying mechanisms of the protective effect of GSK-3β inhibition on dopaminergic neurons in PD is not completely understood. Multiple pathological events have been recognized to be responsible for the loss of dopaminergic neurons in PD, including mitochondrial dysfunction, oxidative stress, protein aggregation and neuroinflammation. The present review stresses the regulatory roles of GSK-3β in these events and in dopaminergic neuron degeneration, in an attempt to gain an improved understanding of the underlying mechanisms and to provide a potential effective therapeutic target for PD.
Keywords: Parkinson’s disease, glycogen synthase kinase-3β, regulation
1. Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by the gradually progressive and selective loss of dopaminergic neurons and by intracellular inclusions known as Lewy bodies (LBs) expressed in the surviving neurons of the substantia nigra (SN) (1). The progressive loss of dopaminergic neurons is a complex process, and multiple pathological events, including oxidative stress, mitochondrial dysfunction, protein aggregation and neuroinflammation, are indicated in PD pathogenesis (2,3). Substantial evidence indicates that mitochondrial dysfunction induced by diverse stress conditions plays a crucial role in the pathogenesis of PD (4,5). A central event in the mitochondrial cell death pathway is the formation of a mitochondrial permeability transition pore (mPTP). The production of reactive oxygen species (ROS) triggered by complex I inhibition are believed to be key inducers of mPTP formation (6). Complex I deficiency has been shown to contribute to the dopaminergic cell death in idiopathic PD patients (7,8). The administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone, well-known inhibitors of complex I, induce PD syndrome characterized by the loss of SN neurons in animal models (9–11), supporting the involvement of mitochondrial dysfunction in the pathogenesis of PD. Decreased complex I activity in the mitochondrial respiratory chain leads to excessive ROS production, which contributes to the oxidative damage of cellular macromolecules and the activation of mPTP, ultimately leading to cell death (12,13). Neuroinflammation has increasingly been recognized as a pathological contributor to neurodegenerative diseases (14–16), and particularly as a key promoter to the chronic loss of nigral dopaminergic neurons in PD (17). Postmortem studies revealed activated microglia and accumulation of inflammatory mediators expressed in the SN of PD patients and animal models (16,18,19). Inhibition of the inflammatory response promotes dopaminergic neuron survival in various PD models (20–22), confirming the indicated action of the inflammatory response in neurodegenerative diseases. Several studies have revealed that the inhibition of GSK-3β reduces dopaminergic neuron injury induced by MPTP toxicity, indicating the association of GSK-3β with the pathogenesis of PD (23,24). GSK-3β is a central point in a number of signaling pathways in the pathogenesis of this neurodegenerative disease, affecting multiple pathological events involved in dopaminergic neuron degeneration, thus providing a potential target in the therapeutic management by blocking the pathogenic pathways involved in PD pathogenesis.
2. Properties of GSK-3
GSK-3 is a serine/threonine (Ser/Thr) protein kinase expressed in the cytosol, nucleus and mitochondria of all eukaryotic cells. There are two major GSK-3 protein isoforms (GSK3α and GSK3β) encoded by two highly homologous genes, gsk-3α and gsk-3β (25). This enzyme was originally identified as a regulator of glycogen synthase (26). However, GSK-3 has been recognized as a pleiotropic enzyme, affecting numerous biological functions including gene expression and cellular processes such as cell proliferation, differentiation and apoptosis (27,28). GSK-3 phosphorylates and regulates >50 substrates, which allows this enzyme to modulate a wide range of biological functions (27,28). Dysregulation of GSK-3 is implicated in diverse diseases, including diabetes, ischemia/reperfusion injury, bipolar disorder, cancer and neurodegenerative disease (27,29–32). GSK-3β is a point of convergence for multiple signaling pathways and thus plays a crucial role in regulating the pathogenesis of diverse diseases. Its activity and functions are controlled by phosphorylation at specific sites. Phosphorylation at Ser9 of GSK-3β markedly inhibits its activity (33), whereas phosphorylation at Tyr216 increases its activity. The inactivation of GSK-3β is mainly targeted by the Akt signaling pathway by the phosphorylation of Ser9 of this enzyme. GSK-3β is mainly localized in the cytosol, but lower amounts are expressed in the nucleus and mitochondria (34–37), and its regulatory role in the mitochondrial cell death pathway has been elicited by a variety of stress conditions shown in neuronal cells (38–43). GSK-3β facilitates numerous apoptotic conditions involved in PD pathogenesis, including mitochondrial dysfunction, oxidative stress, protein aggregation and the inflammatory response, by modulating diverse signaling pathways (Fig. 1) (23,24,42). Inhibition of GSK-3β is indicated in the suppression of a number of pathogenic events in PD, thus promoting dopaminergic neuronal survival (23,24,44,45).
Figure 1.
Glycogen synthase kinase-3β (GSK-3β) facilitates the toxic effects of mitochondrial dysfunction, protein aggregation and inflammatory response on dopaminergic neurons. Mitochondrial GSK-3β inhibits complex I activity thus increasing reactive oxygen species (ROS) production. This production of ROS contributes to the oxidative damage of cellular macromolecules, including proteins, lipids and DNA, and facilitates mitochondrial permeability transition pore (mPTP) formation. Cytosolic GSK-3β phosphorylates the α-synuclein and τ proteins, leading to their aggregation, which contributes to cell injury by oxidative stress and the inflammatory response. Activated GSK-3β can also promote the inflammatory response by activating microglia and increasing the production of inflammatory cytokines. In addition, the GSK-3β signal upregulates the levels of Bax and promotes its mitochondrial membrane translocation. Once located in the membrane, this protein increases the mitochondrial membrane permeabilization by sequestering Bcl-2 and oligomerization, finally causing the release of cytochrome c and cell death. Additionally, GSK-3β phosphorylates Mcl-1 on Ser159, resulting in the destabilization of this protein and blockage of the Mcl-1 dependent integrity of the mitochondrial membrane. Cyt-c, cytochrome c; LB, Lewy body; Ser, serine; Bcl, B-cell lymphoma.
3. Regulation of GSK-3β in mitochondrial complex I activity and ROS formation
In eukaryotic cells, mitochondria are key organelles providing essential energy for cell metabolism through adenosine triphosphate (ATP) generation. Complex I is a protein component of the electron transport chain located in the inner part of the mitochondrial membrane and functioning as the effective enzyme of the oxidative phosphorylation system responsible for the generation of cellular ATP. Mitochondrial complex I is the main site of ROS formation as it transfers single electrons to oxygen, thus generating O2− and subsequently H2O2 (46,47). Inhibition of complex I leads to a decrease in ATP levels and excessive production of ROS, the central events of mitochondrial dysfunction that have been indicated in PD pathogenesis (48). The first evidence for the involvement of complex I inhibition in PD was the recognition that MPTP caused a severe and irreversible parkinsonian syndrome in drug abusers (49). MPTP is a lipophilic molecule and can rapidly cross the blood-brain barrier. Once it crosses the barrier, it is oxidized in the brain to its toxic metabolite 1-methyl-4-phenylpyridinium (MPP+) by type B monoamine oxidase (50). MPP+ is then taken up by dopaminergic neurons via a dopamine transporter and accumulates in the mitochondria where it causes excessive ROS formation by inhibiting respiration complex I (51), finally leading to dopaminergic neuron death. Mitochondrial complex I inhibition has also been reported in the SN, platelets and skeletal muscle of idiopathic PD patients (7,8,52). Complex I is generally known to be the primary source of mitochondrial ROS (53–55). Inhibition of mitochondrial complex I elicited by neurotoxins MPP+ and rotenone, well-established dopaminergic cell death inducers in PD, have been shown to increase the production of ROS (5,56). GSK-3β has been shown to be located into the mitochondria, where it is highly activated compared with the cytosolic form (36). Although the significance of the presence of GSK-3β in the mitochondria remains poorly understood, its involvement in mitochondrial dysfunction has been reported (57,58). GSK-3β can regulate cell survival and apoptosis by controlling mitochondrial complex I activity and ROS production (43). GSK-3β regulates oxidative phosphorylation by inhibiting NADH (complex I), which is the main site of ROS formation, whereas this enzyme is implicated in homeostatic redox equilibrium (43). Previous studies have shown that mitochondrial toxins, including rotenone and MPTP treatments, increase GSK3β activity. Inhibition of GSK-3β protects dopaminergic neurons from the toxicity of rotenone and MPTP, indicating the involvement of GSK-3β in the complex I inhibition-induced cell death pathway in PD (23). Studies in the MPTP model of PD also demonstrate that mitochondrial GSK-3β significantly promotes ROS production by further inhibiting complex I, and that this can be reversed by GSK-3β inhibitors (43). Similar studies have indicated that GSK-3β inhibition promotes mitochondrial biogenesis and prevents ROS production during ischemic cerebral damage (59). Although the mechanism underlying the contribution of GSK-3β to the mitochondrial complex I inhibition remains unclear, these reports clearly indicate that GSK-3β inhibition contributes to cell survival induced by mitochondrial complex I inhibition and ROS formation.
4. GSK-3β and mitochondrial intrinsic apoptosis pathway
Mitochondria are integrated in diverse signaling pathways linked to multiple cell processes, including apoptosis. The major property for mitochondria is the maintenance of its membrane potential and the low-conductance state of the mitochondrial permeability transition pore (mPTP) in living cells. mPTP activation is a central event in mitochondria-mediated intrinsic cell apoptosis, which has been implicated in the pathogenesis of PD and several other neurodegenerative disorders (60–62). The mPTP pathway of cell death is mediated by the disruption of the mitochondrial membrane and the release of apoptogenic molecules, which can be regulated by GSK-3β signaling pathways through modulating the opening of the mPTP (63,64). Studies have shown that GSK-3β inactivation protects cardiac cells from ischemia/reperfusion injury through the inhibition of the mPTP opening, indicating its regulatory role in the mitochondrial cell death pathway (65–68). It has also been shown in cell and animal models of PD that GSK-3β inhibition can protect dopaminergic neurons from MPTP toxicity (23,24,42,69). This contribution of GSK-3β to cell death and survival appears to correlate with its ability to control the mitochondrial localization and activation of a number of proteins, particularly B-cell lymphoma 2 (Bcl-2) family proteins, including Bax, Bcl-2 and Mcl-1, considered as central players in mPTP formation (70,71). Generally, Bax is a cytosolic protein that can be translocated to the mitochondrial membrane in response to apoptotic stimuli (72). Once located in the mitochondrial membrane, this protein increases the mitochondrial membrane permeabilization by sequestering Bcl-2 and by oligomerization within the mitochondrial membrane, leading to the release of pro-apoptotic molecules into the cytoplasm (73,74). By contrast, Bcl-2 and Mcl-1 are anti-apoptotic members that preserve mitochondrial membrane integrity, thereby preventing the release of apoptogenic molecules and cell apoptosis (75). GSK-3β activation promotes mitochondria-mediated apoptosis by the upregulation of Bax expression levels (58,76). Treatment with lithium, a pharmacological inhibitor of GSK-3β, could suppress the pro-apoptotic pathway by decreasing the expression levels of Bax, but promote anti-apoptotic signaling through increasing Bcl-2 expression (77–79). In addition, GSK-3β can facilitate the mitochondrial localization of Bax by directly phosphorylating Ser163 of this protein (70). In PD, models reveal that the inhibition of GSK-3β protects dopaminergic cells against neurotoxin-induced damage through attenuating the translocation of Bax to the mitochondria (80–82). Additionally, GSK-3β phosphorylates Mcl-1 on Ser159, resulting in the destabilization of this protein and the blockage of the Mcl-1-dependent integrity of the mitochondrial membrane (71). Overall, GSK-3β may be vital in the regulation of cell death and survival through the modulation of the mitochondrial apoptotic cell death pathway.
5. Regulation of GSK-3β in α-synuclein and τ protein expression and aggregation
Protein aggregation and inclusion body formation in selected areas of the neuronal system are pathological hallmarks of neurodegenerative diseases, including PD, in which intracellular inclusions known as LBs are expressed in surviving SN neurons. LBs are composed mainly of the α-synuclein protein, a presynaptic neuronal protein abundantly expressed in the nervous system (83–85). The regulatory role of α-synuclein in the production of dopamine through the interaction with tyrosine hydroxylase has been shown in cultured cells (86,87). TH is the rate-limiting enzyme responsible for the conversion of tyrosine to L-3,4-dihydroxyphenylalanine in the dopamine synthesis pathway (86,88). Overexpression of α-synuclein inhibits TH activity and decreases dopamine biosynthesis, while suppression of α-synuclein expression levels promotes TH activity and consequently increases dopamine production (86,87,89). The toxicity of α-synuclein overexpression and accumulation to the neurons has been established in in vivo and in vitro models (90–93). α-synuclein protein overexpression and aggregation exacerbate the impairment of mitochondrial functions by augmenting oxidative stress (94–97). This protein overexpression can also directly activate microglia via a classical activation pathway, leading to the increase of the inflammatory response by the production and release of proinflammatory mediators (98–100). The actions of α-synuclein in promoting oxidative stress and the inflammatory response may be the underlying mechanism responsible for the toxicity of its overexpression and accumulation to dopaminergic neurons in PD. α-synuclein is a substrate for GSK-3β phosphorylation. GSK-3β inhibition decreases α-synuclein protein expression and prevents cell death in a cellular model of PD, indicating that inhibition of GSK-3β activity may be neuroprotective to dopaminergic neurons by attenuating the toxicity of α-synuclein overexpression (101). τ protein was originally discovered as a key component of intracellular neurofibrillary tangles within the brain of AD patients, however, this protein is also expressed highly in LBs and in the striatum of PD brains, indicating that it contributes to the pathogenesis of PD (102,103). Blockage of τ phosphorylation with special inhibitors prevents the dopaminergic neuronal death of PD models (101). GSK-3β is a main kinase affecting τ function through interfering with τ phosphorylation. Activation of GSK-3β increases τ phosphorylation (104–106), which can be reversed by GSK-3β inhibitors or upstream Akt inhibitors (107,108). Additionally, GSK-3β may also facilitate the aggregation of τ protein and neurodegeneration (109,110). Animal models indicate that the inhibition of GSK-3β promotes neuron survival by reducing τ-induced toxicity (111–113). These findings provide a potential target in the therapeutic management of PD by blocking the pathogenic pathway of protein overexpression and aggregation.
6. GSK-3β and neuroinflammation
The inflammatory response, including a host of cytokines has been shown to be implicated in neuronal degeneration in PD and other neurodegenerative diseases (15,114). The activation of microglia and the upregulation of proinflammatory cytokines are key characters of brain inflammation. Microglia are resident immunocompetent cells in the brain and become activated in response to infection and damage (115). The release of proinflammatory and neurotoxic mediators from activated microglia contributes to progressive neuron damage in neurodenerative conditions (116,117). Studies have shown that microglia are activated regionally in the SN of PD patients and animal models (16,18,19,118), and that the levels of a number of inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-2 and IL-6, are also upregulated in PD (119–122), indicating the involvement of the inflammatory response in PD pathogenesis. The contribution of inflammation-derived oxidative stress and cytokine-dependent toxicity to the nigrostriatal dopaminergic neuron death has also been reported in PD models (117,123,124). Additionally, suppression of the inflammatory response leads to the protection of dopaminergic neurons against neurotoxin-induced cell damage (22,125), which further supports the indication that the inflammatory mechanism is involved in neurodegenerative disease. Microglia can be activated by injured neurons through generating a spectrum of noxious endogenous mediators. Once activated, microglia produce and release multiple proinflammatory factors. This production of proinflammatory factors in turn exacerbates neuron damage by oxidative stress and cytokine toxicity (14,19), leading to further release of noxious endogenous mediators from injured neurons and an everlasting inflammatory response. This positive feedback between activated microglia and damaged neurons contributes to an uncontrolled, prolonged inflammatory process, which is believed to be, at least in part, responsible for the progressive loss of dopaminergic neurons in PD (17,114). Thereby, inhibition of the inflammatory response caused by microglia activation may be beneficial in neurodegenerative conditions. GSK-3β is a point of convergence of a wide range of signaling pathways, and has been recognized as a key regulator of inflammation (126,127). Activation of GSK-3β promotes inflammatory responses by activating microglia and increasing the production of inflammatory cytokines (45,128–129). The signals of GSK-3β can also promote various insult-induced neuronal injuries and the noxious generation of endogenous mediators. Thus, GSK-3β plays a central role in the maintenance of the vicious cycle between activated microglia and damaged neurons responsible for the progressive loss of dopaminergic cell loss in PD (Fig. 2). Inhibition of GSK-3β attenuates the microglia response to inflammatory stimuli and reduces cytokine production, thereby providing protection from inflammation-induced toxicity (45,126). However, the direct substrates of GSK-3β that are involved in inflammation-induced neuron damage remain unclear. TNF-α may be a key downstream signal transducer that is indicated in the proinflammatory effect of GSK-3β in activated microglia-mediated neuroinflammation (130). Within the brain, TNF-α is a mainly proinflammatory cytokine that is released by activated microglia in response to various insults or injury. This production of TNF-α triggers the uncontrolled inflammatory response by further activating microglia (131), which can be blocked by GSK-3β inhibition through modulation of nuclear factor κB and mixed lineage kinase 3/c-Jun N-terminal kinase 3 signaling cascades (130). These findings indicate that GSK-3β activity is critical for neuronal death in response to the neuroinflammation elicited by the microglial activation. Attenuation of the microglia-mediated inflammatory response targeted by GSK-3β inhibition to prevent dopaminergic neuron degeneration in PD requires further investigation.
Figure 2.
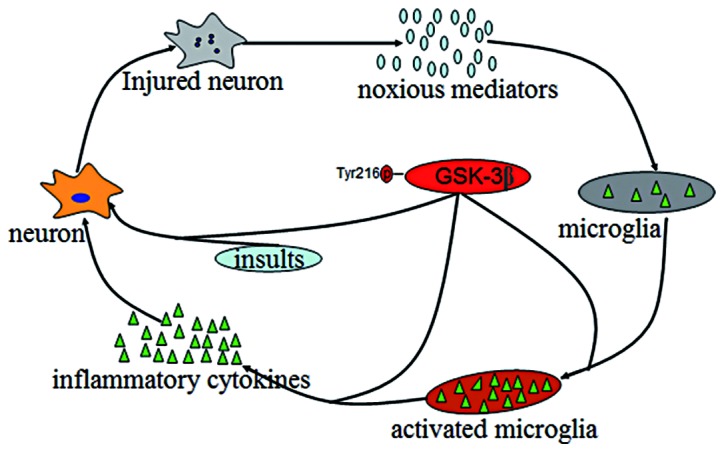
Glycogen synthase kinase-3β (GSK-3β) signaling pathways promote an uncontrolled, prolonged inflammatory and neuron injury process in Parkinson’s disease (PD). GSK-3β can facilitate multiple insult-induced neuronal injuries, thus generating a spectrum of noxious endogenous mediators, which contribute to the activation of microglia. Activated microglia produce and release proinflammatory cytokines, which can be promoted by GSK-3β signaling pathways, resulting in further neuron damage by the inflammatory response through oxidative stress and cytokine toxicity. Thereby GSK-3β plays a central role in the maintenance of the vicious cycle between neuron damage and microglia activation, leading to an uncontrolled, prolonged inflammatory and neuron injury process.
Conclusion
The pathogenesis of PD is a complex process, and multiple pathological events, including oxidative stress, mitochondrial dysfunction, protein aggregation and neuroinflammation, are considered to mediate and drive the gradual loss of dopaminergic neurons in PD (8,132–134). Understanding the intracellular signaling processes that regulate the events involved in the pathogenesis of PD is critical for developing novel therapeutics for PD treatment. GSK-3β is a multifaceted enzyme that has been indicated to be involved in the pathogenesis of neurodegenerative diseases, including PD, by modulating multiple signaling pathways (101,135–137). GSK-3β inhibition protects dopaminergic neurons from various stress-induced injuries in the cell culture and animal models of PD (23,42). The cellular and molecular mechanisms of the protective effects of GSK-3β inhibition on dopaminergic neurons in pathogenic conditions require further elucidation, and may provide a potential efficient target for treating PD by blocking the pathogenic pathway.
Abbreviations
- GSK-3
glycogen synthase kinase-3
- PD
Parkinson’s disease
- LBs
Lewy bodies
- SN
substantia nigra
- mPTP
mitochondrial permeability transition pore
- MPTP
1-methy-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP+
1-methyl-4-phenylpyridinium
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor-α
References
- 1.Forno LS. Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol. 1996;55:259–272. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 2.McNaught KS, Olanow CW. Protein aggregation in the pathogenesis of familial and sporadic Parkinson’s disease. Neurobiol Aging. 2006;27:530–545. doi: 10.1016/j.neurobiolaging.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Vicente M, Talloczy Z, Kaushik S, et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118:777–788. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li DW, Li GR, Lu Y, et al. alpha-lipoic acid protects dopaminergic neurons against MPP+-induced apoptosis by attenuating reactive oxygen species formation. Int J Mol Med. 2013;32:108–114. doi: 10.3892/ijmm.2013.1361. [DOI] [PubMed] [Google Scholar]
- 6.Rasola A, Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 8.Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 9.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 10.Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson’s disease. Neurobiol Dis. 2009;34:279–290. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hantraye P, Brouillet E, Ferrante R, et al. Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat Med. 1996;2:1017–1021. doi: 10.1038/nm0996-1017. [DOI] [PubMed] [Google Scholar]
- 12.Shang T, Kotamraju S, Kalivendi SV, Hillard CJ, Kalyanaraman B. 1-Methyl-4-phenylpyridinium-induced apoptosis in cerebellar granule neurons is mediated by transferrin receptor iron-dependent depletion of tetrahydrobiopterin and neuronal nitric-oxide synthase-derived superoxide. J Biol Chem. 2004;279:19099–19112. doi: 10.1074/jbc.M400101200. [DOI] [PubMed] [Google Scholar]
- 13.Hartley A, Stone JM, Heron C, Cooper JM, Schapira AH. Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: relevance to Parkinson’s disease. J Neurochem. 1994;63:1987–1990. doi: 10.1046/j.1471-4159.1994.63051987.x. [DOI] [PubMed] [Google Scholar]
- 14.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 15.Monahan AJ, Warren M, Carvey PM. Neuroinflammation and peripheral immune infiltration in Parkinson’s disease: an autoimmune hypothesis. Cell Transplant. 2008;17:363–372. [PubMed] [Google Scholar]
- 16.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 17.Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci. 2011;31:1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann Neurol. 2003;54:599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- 19.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 20.Gao HM, Liu B, Zhang W, Hong JS. Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol Sci. 2003;24:395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- 21.Wu DC, Teismann P, Tieu K, et al. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang F, Qian L, Flood PM, Shi JS, Hong JS, Gao HM. Inhibition of IkappaB kinase-beta protects dopamine neurons against lipopolysaccharide-induced neurotoxicity. J Pharmacol Exp Ther. 2010;333:822–833. doi: 10.1124/jpet.110.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Yang Y, Ying C, et al. Inhibition of glycogen synthase kinase-3beta protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007;52:1678–1684. doi: 10.1016/j.neuropharm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 24.King TD, Bijur GN, Jope RS. Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithium. Brain Res. 2001;919:106–114. doi: 10.1016/s0006-8993(01)03005-0. [DOI] [PubMed] [Google Scholar]
- 25.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parker PJ, Embi N, Caudwell FB, Cohen P. Glycogen synthase from rabbit skeletal muscle. State of phosphorylation of the seven phosphoserine residues in vivo in the presence and absence of adrenaline. Eur J Biochem. 1982;124:47–55. doi: 10.1111/j.1432-1033.1982.tb05904.x. [DOI] [PubMed] [Google Scholar]
- 27.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Kockeritz L, Doble B, Patel S, Woodgett JR. Glycogen synthase kinase-3 - an overview of an over-achieving protein kinase. Curr Drug Targets. 2006;7:1377–1388. doi: 10.2174/1389450110607011377. [DOI] [PubMed] [Google Scholar]
- 29.Miura T, Tanno M, Sato T. Mitochondrial kinase signalling pathways in myocardial protection from ischaemia/reperfusion-induced necrosis. Cardiovasc Res. 2010;88:7–15. doi: 10.1093/cvr/cvq206. [DOI] [PubMed] [Google Scholar]
- 30.Medina M, Garrido JJ, Wandosell FG. Modulation of GSK-3 as a Therapeutic Strategy on Tau Pathologies. Front Mol Neurosci. 2011;4:24. doi: 10.3389/fnmol.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King MR, Anderson NJ, Guernsey LS, Jolivalt CG. Glycogen synthase kinase-3 inhibition prevents learning deficits in diabetic mice. J Neurosci Res. 2013;91:506–514. doi: 10.1002/jnr.23192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng H, Li W, Wang Y, et al. Glycogen synthase kinase-3 beta regulates Snail and beta-catenin expression during Fas-induced epithelial-mesenchymal transition in gastrointestinal cancer. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Dajani R, Fraser E, Roe SM, et al. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell. 2001;105:721–732. doi: 10.1016/s0092-8674(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 34.Xavier IJ, Mercier PA, McLoughlin CM, Ali A, Woodgett JR, Ovsenek N. Glycogen synthase kinase 3beta negatively regulates both DNA-binding and transcriptional activities of heat shock factor 1. J Biol Chem. 2000;275:29147–29152. doi: 10.1074/jbc.M002169200. [DOI] [PubMed] [Google Scholar]
- 35.Bijur GN, Jope RS. Proapoptotic stimuli induce nuclear accumulation of glycogen synthase kinase-3 beta. J Biol Chem. 2001;276:37436–37442. doi: 10.1074/jbc.M105725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bijur GN, Jope RS. Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria. Neuroreport. 2003;14:2415–2419. doi: 10.1097/00001756-200312190-00025. [DOI] [PubMed] [Google Scholar]
- 37.Hoshi M, Sato M, Kondo S, et al. Different localization of tau protein kinase I/glycogen synthase kinase-3 beta from glycogen synthase kinase-3 alpha in cerebellum mitochondria. J Biochem. 1995;118:683–685. doi: 10.1093/oxfordjournals.jbchem.a124965. [DOI] [PubMed] [Google Scholar]
- 38.Senatorov VV, Ren M, Kanai H, Wei H, Chuang DM. Short-term lithium treatment promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an excitotoxic model of Huntington’s disease. Mol Psychiatry. 2004;9:371–385. doi: 10.1038/sj.mp.4001463. [DOI] [PubMed] [Google Scholar]
- 39.Bijur GN, De Sarno P, Jope RS. Glycogen synthase kinase-3beta facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J Biol Chem. 2000;275:7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- 40.Alvarez AR, Godoy JA, Mullendorff K, Olivares GH, Bronfman M, Inestrosa NC. Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Exp Cell Res. 2004;297:186–196. doi: 10.1016/j.yexcr.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Shang Y, Sun S, Liang H, Liu R. Erythropoietin prevents PC12 cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis via the Akt/GSK-3beta/caspase-3 mediated signaling pathway. Apoptosis. 2007;12:1365–1375. doi: 10.1007/s10495-007-0065-9. [DOI] [PubMed] [Google Scholar]
- 42.Petit-Paitel A, Brau F, Cazareth J, Chabry J. Involvment of cytosolic and mitochondrial GSK-3beta in mitochondrial dysfunction and neuronal cell death of MPTP/MPP-treated neurons. PLoS One. 2009;4:e5491. doi: 10.1371/journal.pone.0005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.King TD, Clodfelder-Miller B, Barksdale KA, Bijur GN. Unregulated mitochondrial GSK3beta activity results in NADH: ubiquinone oxidoreductase deficiency. Neurotox Res. 2008;14:367–382. doi: 10.1007/BF03033861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang WC, Lin YS, Wang CY, et al. Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology. 2009;128:e275–e286. doi: 10.1111/j.1365-2567.2008.02959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuskaitis CJ, Jope RS. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell Signal. 2009;21:264–273. doi: 10.1016/j.cellsig.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+signaling. Am J Physiol Cell Physiol. 2006;291:C1082–C1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 47.Grivennikova VG, Vinogradov AD. Generation of superoxide by the mitochondrial Complex I. Biochim Biophys Acta. 2006;1757:553–561. doi: 10.1016/j.bbabio.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 48.Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 49.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 50.Chiba K, Trevor A, Castagnoli N., Jr Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem Biophys Res Commun. 1984;120:574–578. doi: 10.1016/0006-291x(84)91293-2. [DOI] [PubMed] [Google Scholar]
- 51.Javitch JA, D’Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci USA. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bindoff LA, Birch-Machin M, Cartlidge NE, Parker WD, Jr, Turnbull DM. Mitochondrial function in Parkinson’s disease. Lancet. 1989;2:49. doi: 10.1016/s0140-6736(89)90291-2. [DOI] [PubMed] [Google Scholar]
- 53.Kussmaul L, Hirst J. The mechanism of superoxide production by NADH: ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci USA. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sipos I, Tretter L, Adam-Vizi V. Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J Neurochem. 2003;84:112–118. doi: 10.1046/j.1471-4159.2003.01513.x. [DOI] [PubMed] [Google Scholar]
- 55.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cassarino DS, Fall CP, Swerdlow RH, et al. Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson’s disease. Biochim Biophys Acta. 1997;1362:77–86. doi: 10.1016/s0925-4439(97)00070-7. [DOI] [PubMed] [Google Scholar]
- 57.Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 58.Watcharasit P, Bijur GN, Song L, Zhu J, Chen X, Jope RS. Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J Biol Chem. 2003;278:48872–48879. doi: 10.1074/jbc.M305870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valerio A, Bertolotti P, Delbarba A, et al. Glycogen synthase kinase-3 inhibition reduces ischemic cerebral damage, restores impaired mitochondrial biogenesis and prevents ROS production. J Neurochem. 2011;116:1148–1159. doi: 10.1111/j.1471-4159.2011.07171.x. [DOI] [PubMed] [Google Scholar]
- 60.Vila M, Przedborski S. Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]
- 61.Perier C, Tieu K, Guegan C, et al. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc Natl Acad Sci USA. 2005;102:19126–19131. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roucou X, Martinou JC. Conformational change of Bax: a question of life or death. Cell Death Differ. 2001;8:875–877. doi: 10.1038/sj.cdd.4400910. [DOI] [PubMed] [Google Scholar]
- 63.Obame FN, Plin-Mercier C, Assaly R, et al. Cardioprotective effect of morphine and a blocker of glycogen synthase kinase 3 beta, SB216763 [3-(2,4-dichlorophenyl)-4(1-methyl-1H-indol-3-yl)-1H-pyrrole-2,5-dione], via inhibition of the mitochondrial permeability transition pore. J Pharmacol Exp Ther. 2008;326:252–258. doi: 10.1124/jpet.108.138008. [DOI] [PubMed] [Google Scholar]
- 64.Nishihara M, Miura T, Miki T, et al. Modulation of the mitochondrial permeability transition pore complex in GSK-3beta-mediated myocardial protection. J Mol Cell Cardiol. 2007;43:564–570. doi: 10.1016/j.yjmcc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 65.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3beta. Anesthesiology. 2005;103:987–995. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 66.Park SS, Zhao H, Mueller RA, Xu Z. Bradykinin prevents reperfusion injury by targeting mitochondrial permeability transition pore through glycogen synthase kinase 3beta. J Mol Cell Cardiol. 2006;40:708–716. doi: 10.1016/j.yjmcc.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 67.Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 68.Zhou K, Zhang L, Xi J, Tian W, Xu Z. Ethanol prevents oxidant-induced mitochondrial permeability transition pore opening in cardiac cells. Alcohol Alcohol. 2009;44:20–24. doi: 10.1093/alcalc/agn098. [DOI] [PubMed] [Google Scholar]
- 69.Youdim MB, Arraf Z. Prevention of MPTP (N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium: involvements of Bcl-2 and Bax. Neuropharmacology. 2004;46:1130–1140. doi: 10.1016/j.neuropharm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 70.Linseman DA, Butts BD, Precht TA, et al. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci. 2004;24:9993–10002. doi: 10.1523/JNEUROSCI.2057-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 72.Tsujimoto Y, Shimizu S. VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ. 2000;7:1174–1181. doi: 10.1038/sj.cdd.4400780. [DOI] [PubMed] [Google Scholar]
- 73.Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol. 2001;2:63–67. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- 74.Armstrong JS. Mitochondrial membrane permeabilization: the sine qua non for cell death. Bioessays. 2006;28:253–260. doi: 10.1002/bies.20370. [DOI] [PubMed] [Google Scholar]
- 75.Gollapudi S, McCormick MJ, Gupta S. Changes in mitochondrial membrane potential and mitochondrial mass occur independent of the activation of caspase-8 and caspase-3 during CD95-mediated apoptosis in peripheral blood T cells. Int J Oncol. 2003;22:597–600. [PubMed] [Google Scholar]
- 76.Tan J, Zhuang L, Leong HS, Iyer NG, Liu ET, Yu Q. Pharmacologic modulation of glycogen synthase kinase-3beta promotes p53-dependent apoptosis through a direct Bax-mediated mitochondrial pathway in colorectal cancer cells. Cancer Res. 2005;65:9012–9020. doi: 10.1158/0008-5472.CAN-05-1226. [DOI] [PubMed] [Google Scholar]
- 77.Chen G, Zeng WZ, Yuan PX, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- 78.Kaga S, Zhan L, Altaf E, Maulik N. Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium. J Mol Cell Cardiol. 2006;40:138–147. doi: 10.1016/j.yjmcc.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 79.Chen RW, Chuang DM. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J Biol Chem. 1999;274:6039–6042. doi: 10.1074/jbc.274.10.6039. [DOI] [PubMed] [Google Scholar]
- 80.Ohori K, Miura T, Tanno M, et al. Ser9 phosphorylation of mitochondrial GSK-3beta is a primary mechanism of cardiomyocyte protection by erythropoietin against oxidant-induced apoptosis. Am J Physiol Heart Circ Physiol. 2008;295:H2079–H2086. doi: 10.1152/ajpheart.00092.2008. [DOI] [PubMed] [Google Scholar]
- 81.Ge XH, Zhu GJ, Geng DQ, Zhang ZJ, Liu CF. Erythropoietin attenuates 6-hydroxydopamine-induced apoptosis via glycogen synthase kinase 3b-mediated mitochondrial translocation of Bax in PC12 cells. Neurol Sci. 2012;33:1249–1256. doi: 10.1007/s10072-012-0959-3. [DOI] [PubMed] [Google Scholar]
- 82.Ngok-Ngam P, Watcharasit P, Thiantanawat A, Satayavivad J. Pharmacological inhibition of GSK3 attenuates DNA damage-induced apoptosis via reduction of p53 mitochondrial translocation and Bax oligomerization in neuroblastoma SH-SY5Y cells. Cell Mol Biol Lett. 2013;18:58–74. doi: 10.2478/s11658-012-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363:1783–1793. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 84.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 85.Singleton AB, Farrer M, Johnson J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 86.Liu D, Jin L, Wang H, et al. Silencing alpha-synuclein gene expression enhances tyrosine hydroxylase activity in MN9D cells. Neurochem Res. 2008;33:1401–1409. doi: 10.1007/s11064-008-9599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baptista MJ, O’Farrell C, Daya S, et al. Co-ordinate transcriptional regulation of dopamine synthesis genes by alpha-synuclein in human neuroblastoma cell lines. J Neurochem. 2003;85:957–968. doi: 10.1046/j.1471-4159.2003.01742.x. [DOI] [PubMed] [Google Scholar]
- 88.Perez RG, Waymire JC, Lin E, Liu JJ, Guo F, Zigmond MJ. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu S, Zuo X, Li Y, et al. Inhibition of tyrosine hydroxylase expression in alpha-synuclein-transfected dopaminergic neuronal cells. Neurosci Lett. 2004;367:34–39. doi: 10.1016/j.neulet.2004.05.118. [DOI] [PubMed] [Google Scholar]
- 90.Danzer KM, Haasen D, Karow AR, et al. Different species of alpha-synuclein oligomers induce calcium influx and seeding. J Neurosci. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated alpha-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27:3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Desplats P, Lee HJ, Bae EJ, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Masliah E, Rockenstein E, Veinbergs I, et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 94.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci. 2008;65:1272–1284. doi: 10.1007/s00018-008-7589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int J Biochem Cell Biol. 2009;41:2015–2024. doi: 10.1016/j.biocel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 96.Hsu LJ, Sagara Y, Arroyo A, et al. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol. 2000;157:401–410. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feng LR, Federoff HJ, Vicini S, Maguire-Zeiss KA. Alpha-synuclein mediates alterations in membrane conductance: a potential role for alpha-synuclein oligomers in cell vulnerability. Eur J Neurosci. 2010;32:10–17. doi: 10.1111/j.1460-9568.2010.07266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee EJ, Woo MS, Moon PG, et al. Alpha-synuclein activates microglia by inducing the expressions of matrix metalloproteinases and the subsequent activation of protease-activated receptor-1. J Immunol. 2010;185:615–623. doi: 10.4049/jimmunol.0903480. [DOI] [PubMed] [Google Scholar]
- 99.Su X, Federoff HJ, Maguire-Zeiss KA. Mutant alpha-synuclein overexpression mediates early proinflammatory activity. Neurotox Res. 2009;16:238–254. doi: 10.1007/s12640-009-9053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human alpha-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J Neuropathol Exp Neurol. 2008;67:1149–1158. doi: 10.1097/NEN.0b013e31818e5e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kozikowski AP, Gaisina IN, Petukhov PA, et al. Highly potent and specific GSK-3beta inhibitors that block tau phosphorylation and decrease alpha-synuclein protein expression in a cellular model of Parkinson’s disease. ChemMedChem. 2006;1:256–266. doi: 10.1002/cmdc.200500039. [DOI] [PubMed] [Google Scholar]
- 102.Haggerty T, Credle J, Rodriguez O, et al. Hyperphosphorylated Tau in an alpha-synuclein-overexpressing transgenic model of Parkinson’s disease. Eur J Neurosci. 2011;33:1598–1610. doi: 10.1111/j.1460-9568.2011.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wills J, Jones J, Haggerty T, Duka V, Joyce JN, Sidhu A. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp Neurol. 2010;225:210–218. doi: 10.1016/j.expneurol.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cho JH, Johnson GV. Glycogen synthase kinase 3 beta induces caspase-cleaved tau aggregation in situ. J Biol Chem. 2004;279:54716–54723. doi: 10.1074/jbc.M403364200. [DOI] [PubMed] [Google Scholar]
- 105.Peng JH, Zhang CE, Wei W, Hong XP, Pan XP, Wang JZ. Dehydroevodiamine attenuates tau hyperphosphorylation and spatial memory deficit induced by activation of glycogen synthase kinase-3 in rats. Neuropharmacology. 2007;52:1521–1527. doi: 10.1016/j.neuropharm.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 106.Chun W, Johnson GV. Activation of glycogen synthase kinase 3beta promotes the intermolecular association of tau. The use of fluorescence resonance energy transfer microscopy. J Biol Chem. 2007;282:23410–23417. doi: 10.1074/jbc.M703706200. [DOI] [PubMed] [Google Scholar]
- 107.Greco SJ, Sarkar S, Casadesus G, et al. Leptin inhibits glycogen synthase kinase-3beta to prevent tau phosphorylation in neuronal cells. Neurosci Lett. 2009;455:191–194. doi: 10.1016/j.neulet.2009.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crouch PJ, Hung LW, Adlard PA, et al. Increasing Cu bioavailability inhibits Abeta oligomers and tau phosphorylation. Proc Natl Acad Sci USA. 2009;106:381–386. doi: 10.1073/pnas.0809057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Engel T, Lucas JJ, Gomez-Ramos P, Moran MA, Avila J, Hernandez F. Cooexpression of FTDP-17 tau and GSK-3beta in transgenic mice induce tau polymerization and neurodegeneration. Neurobiol Aging. 2006;27:1258–1268. doi: 10.1016/j.neurobiolaging.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 110.Engel T, Hernandez F, Avila J, Lucas JJ. Full reversal of Alzheimer’s disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. J Neurosci. 2006;26:5083–5090. doi: 10.1523/JNEUROSCI.0604-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Perez M, Hernandez F, Lim F, Diaz-Nido J, Avila J. Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J Alzheimers Dis. 2003;5:301–308. doi: 10.3233/jad-2003-5405. [DOI] [PubMed] [Google Scholar]
- 112.Nakashima H, Ishihara T, Suguimoto P, et al. Chronic lithium treatment decreases tau lesions by promoting ubiquitination in a mouse model of tauopathies. Acta Neuropathol. 2005;110:547–556. doi: 10.1007/s00401-005-1087-4. [DOI] [PubMed] [Google Scholar]
- 113.Engel T, Goni-Oliver P, Lucas JJ, Avila J, Hernandez F. Chronic lithium administration to FTDP-17 tau and GSK-3beta overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. J Neurochem. 2006;99:1445–1455. doi: 10.1111/j.1471-4159.2006.04139.x. [DOI] [PubMed] [Google Scholar]
- 114.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim SU, de Vellis J. Microglia in health and disease. J Neurosci Res. 2005;81:302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
- 116.Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 117.McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 118.Ouchi Y, Yagi S, Yokokura M, Sakamoto M. Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S200–S204. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- 119.Hunot S, Dugas N, Faucheux B, et al. FcepsilonRII/CD23 is expressed in Parkinson’s disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J Neurosci. 1999;19:3440–3447. doi: 10.1523/JNEUROSCI.19-09-03440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- 121.Koziorowski D, Tomasiuk R, Szlufik S, Friedman A. Inflammatory cytokines and NT-proCNP in Parkinson’s disease patients. Cytokine. 2012;60:762–766. doi: 10.1016/j.cyto.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 122.Przedborski S. Inflammation and Parkinson’s disease pathogenesis. Mov Disord. 2010;25(Suppl 1):S55–S57. doi: 10.1002/mds.22638. [DOI] [PubMed] [Google Scholar]
- 123.Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-alpha to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10:391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. J Neural Transm. 2010;117:971–979. doi: 10.1007/s00702-010-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lofrumento DD, Nicolardi G, Cianciulli A, et al. Neuroprotective effects of resveratrol in an MPTP mouse model of Parkinson’s-like disease: Possible role of SOCS-1 in reducing pro-inflammatory responses. Innate Immun. 2013 doi: 10.1177/1753425913488429. [DOI] [PubMed] [Google Scholar]
- 126.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beurel E, Jope RS. Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. J Neuroinflammation. 2009;6:9. doi: 10.1186/1742-2094-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cheng YL, Wang CY, Huang WC, et al. Staphylococcus aureus induces microglial inflammation via a glycogen synthase kinase 3beta-regulated pathway. Infect Immun. 2009;77:4002–4008. doi: 10.1128/IAI.00176-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang MJ, Huang HY, Chen WF, Chang HF, Kuo JS. Glycogen synthase kinase-3beta inactivation inhibits tumor necrosis factor-alpha production in microglia by modulating nuclear factor kappaB and MLK3/JNK signaling cascades. J Neuroinflammation. 2010;7:99. doi: 10.1186/1742-2094-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Colasanti M, Persichini T, Di Pucchio T, Gremo F, Lauro GM. Human ramified microglial cells produce nitric oxide upon Escherichia coli lipopolysaccharide and tumor necrosis factor alpha stimulation. Neurosci Lett. 1995;200:144–146. doi: 10.1016/0304-3940(95)12101-9. [DOI] [PubMed] [Google Scholar]
- 132.Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007;35:1127–1132. doi: 10.1042/BST0351127. [DOI] [PubMed] [Google Scholar]
- 133.Członkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Członkowski A. Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson’s disease mice model. Neurodegeneration. 1996;5:137–143. doi: 10.1006/neur.1996.0020. [DOI] [PubMed] [Google Scholar]
- 134.Przedborski S, Vila M. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model: a tool to explore the pathogenesis of Parkinson’s disease. Ann NY Acad Sci. 2003;991:189–198. [PubMed] [Google Scholar]
- 135.Duka T, Duka V, Joyce JN, Sidhu A. Alpha-Synuclein contributes to GSK-3beta-catalyzed Tau phosphorylation in Parkinson’s disease models. FASEB J. 2009;23:2820–2830. doi: 10.1096/fj.08-120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Watcharasit P, Thiantanawat A, Satayavivad J. GSK3 promotes arsenite-induced apoptosis via facilitation of mitochondria disruption. J Appl Toxicol. 2008;28:466–474. doi: 10.1002/jat.1296. [DOI] [PubMed] [Google Scholar]
- 137.Cookson MR. The biochemistry of Parkinson’s disease. Annu Rev Biochem. 2005;74:29–52. doi: 10.1146/annurev.biochem.74.082803.133400. [DOI] [PubMed] [Google Scholar]



