Abstract
Although alpine meadows of Tibet are expected to be strongly affected by climatic warming, it remains unclear how soil organic C (SOC), total N (TN), ammonium N (NH4 +-N) , nitrate N (NO3 +-N), and dissolved organic C (DOC) and N (DON) respond to warming. This study aims to investigate the responses of these C and N pools to short-term experimental warming in an alpine meadow of Tibet. A warming experiment using open top chambers was conducted in an alpine meadow at three elevations (i.e., a low (4313 m), mid-(4513 m), and high (4693 m) elevation) in May 2010. Topsoil (0–20 cm depth) samples were collected in July–September 2011. Experimental warming increased soil temperature by ~1–1.4°C but decreased soil moisture by ~0.04 m3 m−3. Experimental warming had little effects on SOC, TN, DOC, and DON, which may be related to lower warming magnitude, the short period of warming treatment, and experimental warming-induced soil drying by decreasing soil microbial activity. Experimental warming decreased significantly inorganic N at the two lower elevations,but had negligible effect at the high elevation. Our findings suggested that the effects of short-term experimental warming on SOC, TN and dissolved organic matter were insignificant, only affecting inorganic forms.
1. Introduction
Soil organic C (SOC) and total N (TN) are very important C and N pools in the terrestrial ecosystems [1, 2]. As the components of labile C and N pools in soils, dissolved organic C (DOC) and N (DON) and soil ammonium and nitrate N (NH4 +-N and NO3 −-N) play crucial roles in the biogeochemistry of C and N and in the nutrient transformation [3–5]. With the context of climatic warming, how SOC, TN, DOC, DON, NH4 +-N, and NO3 −-N respond is vital to global C and N cycling [1, 2]. However, inconsistent results on the responses of these C and N pools to climatic warming have been observed with respect to vegetation types and initial soil characteristics [2, 3, 6–14]. For example, He et al. [2] demonstrated that six-year warming (~1.4°C increase of 10 cm soil temperature) significantly decreased soil C by 129.3 g m−2 in a temperate steppe of Inner Mongolia. In contrast, Li et al. [7] found that two-year warming significantly increased SOC in an alpine meadow (~2.1°C increase of air temperature) but significantly reduced TN in an alpine swamp meadow (~2.3°C increase of air temperature) on the Tibetan Plateau. Hagedorn et al. [13] indicated that one-growing-season warming (~4°C increase of 5 cm soil temperature) did not significantly influence DOC. Song et al. [1] pointed out that six-year warming (~1.2°C increase of 10 cm soil temperature) significantly reduced DOC in a temperate steppe in Inner Mongolia. Biasi et al. [15] indicated that two-year warming (~0.9°C increase of 5 cm soil temperature) did not have obvious effects on DON, NH4 +-N, NO3 −-N, and Nmin in a lichen-rich dwarf shrub tundra in Siberia. Bai et al. [14] stated that experimental warming (~0.6–6.7°C in soil temperature) had a significant positive effect on Nmin but not on TN across all biomes. Therefore, how climatic warming acts on C and N cycling still remains unclear.
More than 70% of the Tibetan Plateau is covered with grasslands [16]. The alpine grasslands of this Plateau are one of the systems most sensitive to global change [17, 18]. In alpine grasslands, understanding the responses of SOC, DOC, TN, DON, NH4 +-N, and NO3 −-N to climatic warming are crucial for predicting future changes in soil fertility and C sequestration. The alpine meadow is one of the most typical grasslands types on the Tibetan Plateau being subjected to climatic warming [19]. Information on how these C and N pools along an elevation gradient respond to climatic warming is scarce on the Tibetan Plateau. Here we set up a warming experiment in an alpine meadow at three elevations (i.e., 4313 m, 4513 m, and 4693 m) on the Northern Tibetan Plateau.
The main objective was to investigate the effects of short-term experimental warming on SOC, TN, DOC, DON, NH4 +-N, and NO3 −-N. Our previous study indicated that short-term experimental warming could not affect soil microbial biomass [20] and soil microbial activity regulated the balances of soil C and N pools in the alpine meadow [21]. We hypothesized that experimental warming may not affect these C and N pools in this study.
2. Materials and Methods
2.1. Study Area, Experimental Design, and Soil Sampling
A detailed description of the study area, the warming experimental design, the measurements of microclimate factors (including soil temperature and soil moisture), and the soil sampling are given in Fu et al. [20, 22].
Briefly, three alpine meadow sites were established at three elevations (i.e., a low (30°30′N, 91°04′E, and 4313 m), mid- (30°31′N, 91°04′E, and 4513 m), and high (30°32′N, 91°03′E, and 4693 m) elevation) at Damxung Grassland Observation Station of Tibet Autonomous Region in China in May 2010.
Annual mean air temperature and precipitation is 1.3°C and ~476.8 mm, respectively [20, 21]. The vegetation is Kobresia-dominated alpine meadow and roots are mainly concentrated in the topsoil layer (0–20 cm) [21, 22]. The soil is classified as sandy loam, with pH of 6.0–6.7, organic matter of 0.3–11.2%, and total N of 0.03–0.49% [20, 22].
Open top chambers (OTCs, 3 mm thick polycarbonate) were used to enhance temperature [22, 23]. The bottom and top diameters and the height of OTCs were 1.45 m and 1.00 m and 0.40 m, respectively [20, 22]. For each site, four OTCs and their paired control plots (1 m × 1 m) were randomly established in May 2010. There was ~3 m distance between plots.
Daily mean soil temperature (T s) during the study period of July-September in 2011 inside the OTCs increased by 1.26°C, 0.98°C, and 1.37°C at the low, mid-, and high elevation, respectively, compared to control plots [20]. In contrast, experimental warming decreased daily mean soil moisture (SM) by 0.04 m3 m−3 in all sites [20]. Daily mean T s decreased with increasing elevation from the low to high elevation [20].
We collected topsoil samples (0–20 cm depth) inside each plot using a probe 3.0 cm in diameter on July 7, August 9, and September 10, 2011 [20]. Five soil subsamples were randomly sampled and composited into one soil sample for each plot [20]. Subsamples of the fresh soil were used to measure DOC, DON, NH4 +-N, and NO3 −-N and other subsamples of the fresh soil were air-dried for the measurements of SOC and TN.
2.2. Soil Analysis
A more detailed description of measurements of soil inorganic N (Nmin, i.e., sum of NH4 +-N and NO3 −-N), DON, and DOC can be found in Fu et al. [21]. Briefly, soil inorganic N in 20 g fresh soil sample was extracted with 100 mL K2SO4, filtered through 0.45 μm membrane, and analyzed on a LACHAT Quikchem Automated Ion Analyzer. Dissolved organic C and TN (DTN) in another 20 g fresh soil sample was extracted with 100 mL ultrapure water and filtered through 0.45 membrane. The extractable SOC and TN concentrations in the ultrapure water extracts were measured using a Liqui TOC II elementar analyzer (Elementar Liqui TOC, Elementar Co., Hanau, Germany) and a UV-1700 PharmaSpec visible spectrophotometer (220 nm and 275 nm), respectively. We also analyzed dissolved inorganic N (DIN) in the ultrapure water extracts on a LACHAT Quikchem Automated Ion Analyzer. Then DON was calculated as the difference between DTN and DIN. The potassium dichromate method was used to determine SOC [24]. Soil TN was measured on a CN analyzer (Elementar Variomax CN). Soil microbial biomass (MBC) and N (MBN) data were obtained from Fu et al. [20].
2.3. Statistical Analysis
In order to examine the elevation effect, repeated-measures ANOVA with experimental warming and elevation as the between subject factors and with sampling date as the within subject factor was performed for a specific soil property (i.e., SOC, TN, DOC, DON, ratio of DOC to DON (DOC/DON), NH4 +-N, NO3 −-N, ratio of NH4 +-N to NO3 −-N(NH4 +-N/NO3 −-N), and Nmin). At each site, repeated-measures ANOVA with experimental warming (i.e., OTCs versus control) as the between subject factor and with sampling date as the within subject factor was conducted for each soil property. Single factor linear regressions were performed between soil properties and T s, SM, MBC, and MBN. In addition, multiple stepwise regression analyses were conducted for soil properties to examine the relative importance of T s, SM, MBC, and MBN in affecting the variations of soil properties. All data were examined for normality and homogeneity before analysis and natural logarithm transformations were made if necessary. The level of significance was P < 0.05. All the statistical tests were performed using the SPSS software (version 16.0; SPSS Inc., Chicago, IL).
3. Results
3.1. Effects of Experimental Warming on Soil Properties
Regardless of experimental warming, elevation had significant effects on SOC (F = 183.19, P < 0.001), TN (F = 126.38, P < 0.001), DOC (F = 26.42, P < 0.001), DON (F = 7.08, P < 0.01), NH4 +-N(F = 71.98, P < 0.001), NH4 +-N/NO3 −-N(F = 14.01, P < 0.001), and Nmin(F = 56.29, P < 0.001) across the three sampling dates. In contrast, there were no significant effects of elevation on NO3 −-N and DOC/DON. These C and N pools showed similar seasonal dynamics regardless of experimental warming among the three elevations (Figure 1).
Figure 1.
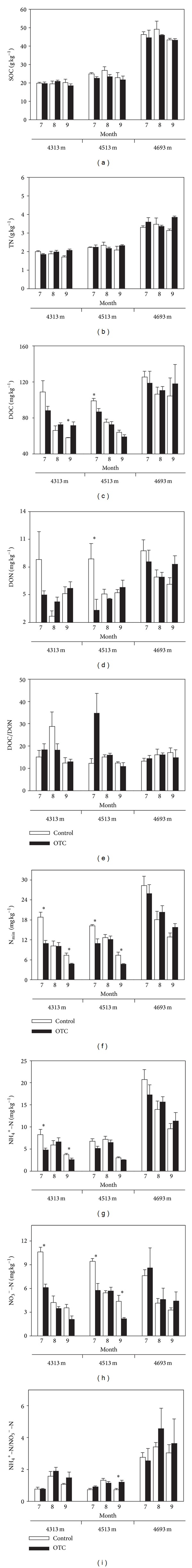
Effects of experimental warming on soil organic C (SOC), total N (TN), dissolved organic C (DOC), dissolved organic N (DON), the ratio of DOC to DON (DOC/DON), soil inorganic N (Nmin), ammonium N (NH4 +-N), nitrate N (NO3 −-N), and the ratio of NH4 +-N to NO3 −-N(NH4 +-N/NO3 −-N) in the three alpine meadow sites located at elevation 4313 m, 4513 m, and 4693 m, respectively (mean ± SE, n = 4). *indicates P < 0.05, while no asterisk indicates not significant.
In line with our initial hypothesis, experimental warming had little effects on SOC, TN, DOC, DON, DOC/DON, and NH4 +-N/NO3 −-N (Table 1). In contrast, the sensitivity of Nmin to experimental warming increased with increasing elevation (Table 1). In detail, experimental warming significantly decreased Nmin by 29.2% and 23.5% at the low and mid-elevation, NO3 −-N by 36.4%, 29.5% at the low and mid-elevation, and NH4 +-N by 16.7% at the mid-elevation across all the three sampling dates, respectively. In contrast, experimental warming had little effects on NO3 −-N and Nmin at the high elevation.
Table 1.
Repeated-measures ANOVA (F values) for the main and interactive effects of experimental warming (W) and sampling date (D) on soil organic C (SOC), total N (TN), dissolved organic C (DOC), N (DON), ammonium N (NH4 +-N), nitrate N (NO3 −-N), the ratio of NH4 +-N to NO3 −-N (NH4 +-N/NO3 −-N), and soil inorganic N (Nmin, i.e., sum of NH4 +-N and NO3 −-N) in an alpine meadow on the Tibetan Plateau at three elevations (n = 4).
| Elevation | Model | SOC | TN | DOC | DON | DOC/DON | NO3 −-N | NH4 +-N | NH4 +-N/NO3 −-N | Nmin |
|---|---|---|---|---|---|---|---|---|---|---|
| 4313 m | W | 0.02 | 1.58 | 0.00 | 0.23 | 0.70 | 39.02** | 4.22 | 1.38 | 26.87** |
| D | 0.31 | 0.26 | 16.70*** | 3.66 | 2.68 | 55.47*** | 10.98** | 10.04** | 28.71*** | |
| W × D | 1.04 | 6.87* | 4.13* | 2.51 | 1.40 | 6.32* | 3.44 | 0.49 | 5.91* | |
|
| ||||||||||
| 4513 m | W | 1.43 | 0.03 | 4.07 | 5.33 | 4.52 | 9.90* | 6.45* | 3.01 | 10.89* |
| D | 2.62 | 0.23 | 94.06*** | 0.99 | 5.23 | 57.26*** | 31.90*** | 13.69*** | 51.19*** | |
| W × D | 0.41 | 3.35 | 2.32 | 6.36* | 6.15* | 11.70** | 0.63 | 8.10** | 4.39* | |
|
| ||||||||||
| 4693 m | W | 0.40 | 2.61 | 0.07 | 0.09 | 0.04 | 0.26 | 0.00 | 0.19 | 0.14 |
| D | 1.96 | 0.12 | 0.67 | 7.80** | 0.81 | 20.22*** | 21.83*** | 3.36 | 29.26*** | |
| W × D | 0.27 | 3.89 | 0.33 | 3.84 | 0.34 | 0.12 | 2.66 | 0.88 | 1.47 | |
*, **, and *** indicate P < 0.05, P < 0.01, and P < 0.001, respectively, while no asterisk indicates not significant.
3.2. Relationships between Soil Properties and Environmental Variables and Soil Microbial Biomass
Soil organic C, TN, DOC, NH4 +-N, NO3 −-N, NH4 +-N/NO3 −-N, and Nmin were significantly and positively correlated with SM (Figure 2). In contrast, SOC, TN, DOC, NH4 +-N, and NH4 +-N/NO3 −-N declined with increasing T s (Table 2). The negative correlations of T s with DON and Nmin were relatively lower (Table 2). Soil organic C, TN, DOC, DON, NH4 +-N, NH4 +-N/NO3 −-N, and Nmin increased significantly with increasing MBC and MBN, while NO3 −–N only increased significantly with increasing MBN (Table 2). Nitrate N was not related to MBC and T s (Table 2), while DON was not correlated with SM (data not shown). In addition, DOC/DON was not correlated with T s, SM, MBC, and MBN (data not shown).
Figure 2.
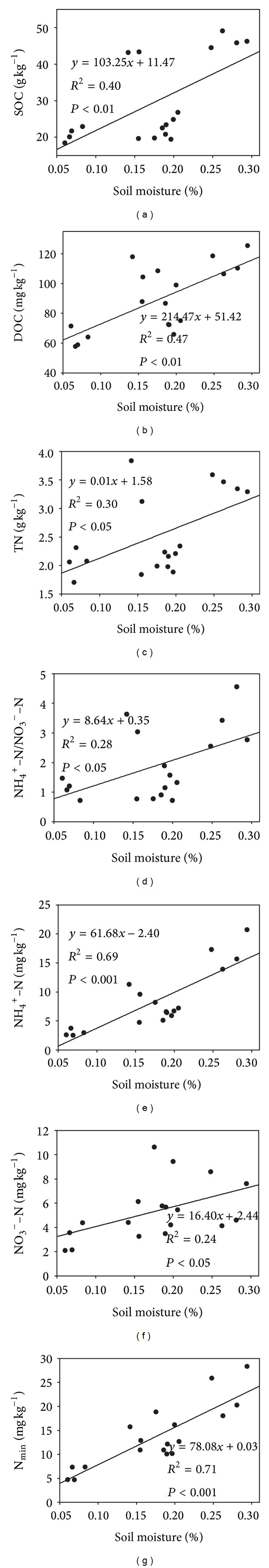
Relationships of soil moisture with soil organic C (SOC), dissolved organic C (DOC), total N (TN), the ratio of NH4 +-N to NO3 −-N(NH4 +-N/NO3 −-N), ammonium N (NH4 +-N), nitrate N (NO3 −-N), and soil inorganic N (Nmin).
Table 2.
Single factor linear regressions between soil properties (soil organic C, SOC; total N, TN; dissolved organic C, DOC; dissolved organic N, DON; nitrate N, NO3 −-N; ammonium N, NH4 +-N; the ratio of NH4 +-N to NO3 −-N, NH4 +-N/NO3 −-N; soil inorganic N, Nmin) and soil temperature (T s), soil microbial biomass C (MBC), and N (MBN) showing regression parameters (slope, constant, R 2, and P). MBC and MBN data were obtained from Fu et al. [20].
| Independent variable |
Regression parameters |
SOC | TN | DOC | DON | NO3 −-N | NH4 +-N | NH4 +-N/NO3 −-N | Nmin |
|---|---|---|---|---|---|---|---|---|---|
| T s | Slope | −5.29 | −0.32 | −7.90 | −0.53 | −0.03 | −1.71 | −0.43 | −1.74 |
| Constant | 100.76 | 6.81 | 195.37 | 13.28 | 5.73 | 31.38 | 7.66 | 37.11 | |
| R 2 | 0.63 | 0.64 | 0.38 | 0.21 | 0.001 | 0.31 | 0.41 | 0.21 | |
| P | <0.001 | <0.001 | <0.01 | 0.057 | 0.93 | <0.05 | <0.01 | 0.056 | |
|
| |||||||||
| MBC | Slope | 0.05 | 0.003 | 0.10 | 0.01 | 0.01 | 0.03 | 0.004 | 0.03 |
| Constant | 6.46 | 1.24 | 46.29 | 2.89 | 3.34 | −3.20 | 0.04 | 0.14 | |
| R 2 | 0.76 | 0.66 | 0.70 | 0.51 | 0.13 | 0.92 | 0.47 | 0.82 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | 0.139 | <0.001 | <0.01 | <0.001 | |
|
| |||||||||
| MBN | Slope | 0.28 | 0.01 | 0.68 | 0.05 | 0.06 | 0.17 | 0.02 | 0.23 |
| Constant | 10.56 | 1.58 | 43.22 | 2.93 | 1.60 | −3.28 | 0.41 | −1.69 | |
| R 2 | 0.43 | 0.30 | 0.68 | 0.42 | 0.39 | 0.79 | 0.25 | 0.88 | |
| P | <0.01 | <0.05 | <0.001 | <0.01 | <0.01 | <0.001 | <0.05 | <0.001 | |
The multiple stepwise regression analyses were listed in Table 3. Both SOC and TN were simultaneously affected by MBC and T s, whereas MBC explained more variation of the two soil properties than T s. Only MBC was included in the multiple regression equations for DOC, DON, and NH4 +-N/NO3 −-N, while only MBN was included in the regression equation for NO3 −-N. Soil microbial biomass C explained the variation of NH4 +-N more than SM. Both MBC and MBN were simultaneously and positively correlated with Nmin. In addition, all the five concerned variables were excluded for DOC/DON.
Table 3.
Multiple stepwise regression analyses between soil properties and environmental variables (soil temperature, T s; soil moisture, SM) and soil microbial biomass (microbial biomass C, MBC; microbial biomass N, MBN) in an alpine meadow on the Tibetan Plateau. MBC and MBN data were obtained from Fu et al. [20].
| Soil properties | Factors | Coefficients | R 2 | P |
|---|---|---|---|---|
| SOC | Constant | 49.31 | 0.003 | |
| MBC | 0.04 | 0.76 | <0.001 | |
| T s | −2.69 | 0.10 | 0.006 | |
|
| ||||
| TN | Constant | 4.28 | 0.001 | |
| MBC | 0.002 | 0.66 | 0.004 | |
| T s | −0.19 | 0.14 | 0.006 | |
|
| ||||
| DOC | Constant | 46.29 | <0.001 | |
| MBC | 0.10 | 0.70 | <0.001 | |
|
| ||||
| DON | Constant | 2.89 | 0.005 | |
| MBC | 0.01 | 0.51 | 0.001 | |
|
| ||||
| NH4 +-N | Constant | −4.57 | <0.001 | |
| MBC | 0.02 | 0.92 | <0.001 | |
| SM | 22.39 | 0.05 | <0.001 | |
|
| ||||
| NO3 −-N | Constant | 1.60 | 0.22 | |
| MBN | 0.06 | 0.39 | 0.005 | |
|
| ||||
| Nmin | Constant | −2.24 | 0.085 | |
| MBN | 0.15 | 0.88 | <0.001 | |
| MBC | 0.01 | 0.05 | 0.005 | |
|
| ||||
| NH4 +-N/NO3 −-N | Constant | 0.95 | 0.036 | |
| MBC | 0.004 | 0.47 | 0.002 | |
4. Discussion
4.1. Effects of Experimental Warming on SOC, TN, DOC, and DON
Recently, some studies showed that short-term (<3 years) experimental warming had little effects on SOC, TN, DOC, and/or DON in a tallgrass prairie with a silt loam soil (~2°C increase of 5 cm soil temperature) in USA [25], in a dragon spruce plantation with a mountain brown soil (~0.6°C increase of 5 cm soil temperature) on the Tibetan Plateau [8], in an alpine treeline with a sandy Ranker and Podzols soil (~4°C increase of 5 cm soil temperature) in Switzerland [13], and in a lichen-rich dwarf shrub tundra with Gleyic Cryosols soils (~0.9°C increase of 5 cm soil temperature) in Siberia [15]. However, other studies with long-term (>3 years) experimental warming indicated that warming significantly increased or decreased SOC, TN, DOC, and/or DON in a temperate steppe with a Calcic Kastanozems soil in Inner Mongolia (~1.4°C increase of 10 cm soil temperature) [2], in an alpine meadow (~3°C increase of 5 cm soil temperature) on the Tibetan Plateau [3], and in a temperate steppe with chestnut soil in Inner Mongolia (~1.2°C increase of 10 cm soil temperature) [1]. Therefore, the insignificant responses of SOC, TN, DOC, and DON to warming (Table 1) may be due to the short period of warming treatment (14–16 months).
A meta-analysis showed that the effects of experimental warming on Nmin, net N mineralization, and nitrification were significantly and positively correlated with raised soil temperature (~0.6–6.7°C for Nmin, ~0.6–5.5°C for net mineralization, and ~1.3–5.5°C for net nitrification) across all biomes [14]. Similarly, we found that experimental warming-induced change of soil temperature tended to be negatively correlated with that of TN (R 2 = 0.43, P = 0.057) and positively correlated with that of MBN (R 2 = 0.43, P = 0.056) [20]. In addition, MBN was significantly correlated with SOC, TN, DOC, and DON (Table 2). Therefore, the negligible responses of soil C and N pools to experimental warming (Table 1) may be also due to lower warming magnitude in this alpine meadow.
Microbial activity regulates the production of dissolved organic matter [5, 8, 26] and experimental warming-induced decline in soil moisture may suppress soil microbial activity [20, 27]. Similarly, we also found that soil C and N pools increased with increasing soil microbial biomass and soil moisture (Figure 2, Table 2). Moreover, short-term experimental warming had little effect on soil microbial biomass in this system [20]. Therefore, the negligible responses of SOC, TN, DOC, and DON to short-term experimental warming may be also related to that of soil microbial biomass [8, 20]. Moreover, experimental warming-induced soil drying may also suppress the production of DOC and DON [8, 20].
4.2. Effects of Experimental Warming on Soil Inorganic N
Bai et al. [14] demonstrated that experimental warming did not significantly increase net N nitrification in grasslands. Similarly, experimental warming did not increase net N mineralization in an alpine meadow on the Tibetan Plateau [28]. In the same alpine meadow as this study, the finding that experimental warming did not increase ecosystem photosynthesis and aboveground plant biomass [22] also indirectly supported that experimental warming may not increase soil N availability because it has been observed that plant productivity is positively correlated with net N mineralization [29]. Therefore, the negligible or negative effect of experimental warming on soil inorganic N (Figure 1, Table 1) may result from the suppression of net N mineralization and nitrification under warming.
The suppression of net N mineralization and nitrification may be owing to decreases in soil moisture and microbial activity because Nmin, NH4 +-N, and NO3 −-N increased significantly with increasing soil moisture and microbial biomass (Figure 2, Table 2). Similarly, the experimental warming-induced significant reductions or insignificant changes of inorganic N (Figure 1, Table 1) were also partly attributed to experimental warming-induced decline in soil microbial biomass [20] and soil drying [10, 29, 30]. This was in line with the finding that the effect of experimental warming on soil moisture was significantly correlated with that on soil nitrification [14]. On the other hand, microbial biomass was more closely related to soil inorganic N than soil moisture (Table 3). This implied that microbial biomass may dominate the variation of soil inorganic N in this study. However, our previous study showed that short-term experimental warming tended to reduce microbial biomass due to soil drying in the same alpine meadow as this study [20]. Therefore, the experimental warming-induced changes of soil inorganic N, net N mineralization, and nitrification may be directly related to that of microbial activity and indirectly related to that of soil moisture.
The different responses of Nmin to experimental warming among the three elevations across the sampling dates could be attributed to several probable underlying mechanisms. First, DON is high-quality N source for N mineralization [8, 31]. This was supported by the positive relationships between DON and Nmin and NH4 +-N and NO3 −-N (Figure 3). DON under warmed plots tended to be decreased by 10.3% at the low elevation and by 28.7% at the mid-elevation but to be increased by 4.4% at the high elevation across all the three sampling dates, compared to control plots. Second, experimental warming-induced different changes in soil microbial biomass N (MBN) among three elevations [20] could partly explain this phenomenon considering that the production of DON and the immobilization of soil inorganic N were regulated by MBN [3, 32, 33]. This viewpoint was confirmed by the positive correlations between MBN and DON, Nmin, NH4 +-N, and NO3 −-N (Table 2). Third, the response of soil N availability to warming could be strongly related to the initial conditions [8, 34]. In our system, Nmin, DON, and microbial biomass at the high elevation were significantly larger compared to the low and mid-elevation, whilst there were insignificant differences between the latter two [20].
Figure 3.
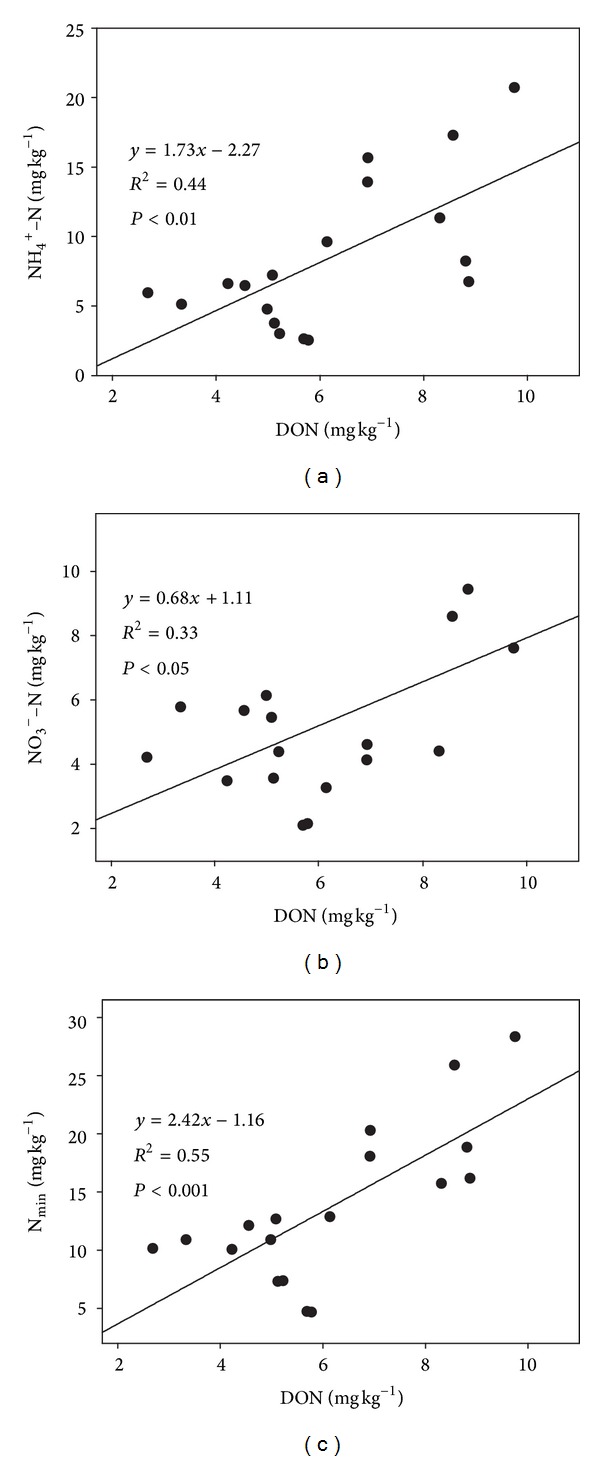
Relationships of dissolved organic N (DON) with ammonium N (NH4 +-N), nitrate N (NO3 −-N), and soil inorganic N (Nmin).
5. Conclusions
In summary, short-term experimental warming had no obvious effects on topsoil organic C, total N, dissolved organic C, and N pools for the alpine meadow in this study. The insignificant responses of these C and N pools to warming may be due to short-term warming treatment, experiment warming-induced soil drying, and lower warming magnitude. In contrast, the response of soil inorganic N to experimental warming differed among the three elevations, which may be attributed to different response trends of dissolved organic N and microbial biomass and different initial soil inorganic N.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (no. 41171084) and the National Science and Technology Plan Project of China (no. 2011BAC09B03).
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Song B, Niu S, Zhang Z, Yang H, Li L, Wan S. Light and heavy fractions of soil organic matter in response to climate warming and increased precipitation in a temperate steppe. PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0033217.e33217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He NP, Chen Q, Han X, Yu G, Li L. Warming and increased precipitation individually influence soil carbon sequestration of Inner Mongolian grasslands, China. Agriculture Ecosystems & Environment. 2012;158:184–191. [Google Scholar]
- 3.Rui Y, Wang S, Xu Z, et al. Warming and grazing affect soil labile carbon and nitrogen pools differently in an alpine meadow of the Qinghai-Tibet Plateau in China. Journal of Soils and Sediments. 2011;11(6):903–914. [Google Scholar]
- 4.Guicharnaud R, Arnalds O, Paton GI. Short term changes of microbial processes in Icelandic soils to increasing temperatures. Biogeosciences. 2010;7(2):671–682. [Google Scholar]
- 5.Kalbitz K, Solinger S, Park J-H, Michalzik B, Matzner E. Controls on the dynamics dissolved organic matter in soils: a review. Soil Science. 2000;165(4):277–304. [Google Scholar]
- 6.Pendall E, Osanai Y, Williams AL, Hovenden MJ. Soil carbon storage under simulated climate change is mediated by plant functional type. Global Change Biology. 2011;17(1):505–514. [Google Scholar]
- 7.Li N, Wang G, Gao Y, Wang J. Warming effects on plant growth, soil nutrients, microbial biomass and soil enzymes activities of two alpine meadows in Tibetan Plateau. Polish Journal of Ecology. 2011;59(1):25–35. [Google Scholar]
- 8.Xu Z-F, Hu R, Xiong P, Wan C, Cao G, Liu Q. Initial soil responses to experimental warming in two contrasting forest ecosystems, Eastern Tibetan Plateau, China: nutrient availabilities, microbial properties and enzyme activities. Applied Soil Ecology. 2010;46(2):291–299. [Google Scholar]
- 9.Cookson WR, Osman M, Marschner P, et al. Controls on soil nitrogen cycling and microbial community composition across land use and incubation temperature. Soil Biology and Biochemistry. 2007;39(3):744–756. [Google Scholar]
- 10.Allison SD, Treseder KK. Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Global Change Biology. 2008;14(12):2898–2909. [Google Scholar]
- 11.Zhou X, Chen C, Wang Y, et al. Effects of warming and increased precipitation on soil carbon mineralization in an Inner Mongolian grassland after 6 years of treatments. Biology and Fertility of Soils. 2012;48(7):859–866. [Google Scholar]
- 12.Lu M, Zhou X, Yang Q, et al. Responses of ecosystem carbon cycle to experimental warming: a meta-analysis. Ecology. 2013;94(3):726–738. doi: 10.1890/12-0279.1. [DOI] [PubMed] [Google Scholar]
- 13.Hagedorn F, Martin M, Rixen C, et al. Short-term responses of ecosystem carbon fluxes to experimental soil warming at the Swiss alpine treeline. Biogeochemistry. 2010;97(1):7–19. [Google Scholar]
- 14.Bai E, Li S, Xu W, Li W, Dai W, Jiang P. A meta-analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. New Phytologist. 2013;199(2):441–451. doi: 10.1111/nph.12252. [DOI] [PubMed] [Google Scholar]
- 15.Biasi C, Meyer H, Rusalimova O, et al. Initial effects of experimental warming on carbon exchange rates, plant growth and microbial dynamics of a lichen-rich dwarf shrub tundra in Siberia. Plant and Soil. 2008;307(1-2):191–205. [Google Scholar]
- 16.Zhang L, Guo H, Ji L, et al. Vegetation greenness trend (2000 to 2009) and the climate controls in the Qinghai-Tibetan Plateau. Journal of Applied Remote Sensing. 2013;7(1)073572 [Google Scholar]
- 17.Yu CQ, Zhang Y, Claus H, Zeng R, Zhang X, Wang J. Ecological and environmental issues faced by a developing Tibet. Environmental Science & Technology. 2012;46(4):1979–1980. doi: 10.1021/es2047188. [DOI] [PubMed] [Google Scholar]
- 18.Yao T-D, Xie Z-C, Wu X-L, Thompson LG. Climatic change since Little Ice Age recorded by Dunde Ice Cap. Science in China (Scientia Sinica) Series B. 1991;34(6):760–767. [Google Scholar]
- 19.Cao G, Tang Y, Mo W, Wang Y, Li Y, Zhao X. Grazing intensity alters soil respiration in an alpine meadow on the Tibetan plateau. Soil Biology and Biochemistry. 2004;36(2):237–243. [Google Scholar]
- 20.Fu G, Shen Z, Zhang X, Zhou Y. Response of soil microbial biomass to short-term experimental warming in alpine meadow on the Tibetan Plateau. Applied Soil Ecology. 2012;61:158–160. [Google Scholar]
- 21.Fu G, Zhang X, Yu C, et al. Response of soil respiration to grazing in an alpine meadow at three elevations in Tibet. The Scientific World Journal. 2014;2014:9 pages. doi: 10.1155/2014/265142.265142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu G, Zhang X, Zhang Y, et al. Experimental warming does not enhance gross primary production and above-ground biomass in the alpine meadow of Tibet. Journal of Applied Remote Sensing. 2013;7(1)073505 [Google Scholar]
- 23.Fu G, Shen Z, Zhang X, et al. Response of ecosystem respiration to experimental warming and clipping at daily time scale in an alpine meadow of Tibet. Journal of Mountain Science. 2013;10(3):455–463. [Google Scholar]
- 24.Walkley A, Black IA. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science. 1934;37(1):29–38. [Google Scholar]
- 25.Belay-Tedla A, Zhou X, Su B, Wan S, Luo Y. Labile, recalcitrant, and microbial carbon and nitrogen pools of a tallgrass prairie soil in the US Great Plains subjected to experimental warming and clipping. Soil Biology and Biochemistry. 2009;41(1):110–116. [Google Scholar]
- 26.Christ MJ, David MB. Temperature and moisture effects on the production of dissolved organic carbon in a Spodosol. Soil Biology & Biochemistry. 1996;28(9):1191–1199. [Google Scholar]
- 27.Liu W, Zhang Z, Wan S. Predominant role of water in regulating soil and microbial respiration and their responses to climate change in a semiarid grassland. Global Change Biology. 2009;15(1):184–195. [Google Scholar]
- 28.Wang SP, Duan J, Xu G, et al. Effects of warming and grazing on soil N availability, species composition, and ANPP in an alpine meadow. Ecology. 2012;93(11):2365–2376. doi: 10.1890/11-1408.1. [DOI] [PubMed] [Google Scholar]
- 29.Rustad LE, Campbell JL, Marion GM, et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia. 2001;126(4):543–562. doi: 10.1007/s004420000544. [DOI] [PubMed] [Google Scholar]
- 30.Verburg PSJ, Johnson DW, Schorran DE, Wallace LL, Luo Y, Arnone JA., III Impacts of an anomalously warm year on soil nitrogen availability in experimentally manipulated intact tallgrass prairie ecosystems. Global Change Biology. 2009;15(4):888–900. [Google Scholar]
- 31.Chen CR, Xu ZH. Analysis and behavior of soluble organic nitrogen in forest soils. Journal of Soils and Sediments. 2008;8(6):363–378. [Google Scholar]
- 32.Harris WN, Moretto AS, Distel RA, Boutton TW, Bóo RM. Fire and grazing in grasslands of the Argentine Caldenal: effects on plant and soil carbon and nitrogen. Acta Oecologica. 2007;32(2):207–214. [Google Scholar]
- 33.Dijkstra FA, Blumenthal D, Morgan JA, Pendall E, Carrillo Y, Follett RF. Contrasting effects of elevated CO2 and warming on nitrogen cycling in a semiarid grassland. New Phytologist. 2010;187(2):426–437. doi: 10.1111/j.1469-8137.2010.03293.x. [DOI] [PubMed] [Google Scholar]
- 34.Carrillo Y, Dijkstra FA, Pendall E, Morgan JA, Blumenthal DM. Controls over soil nitrogen pools in a semiarid grassland under elevated CO2 and warming. Ecosystems. 2012;15(5):761–774. [Google Scholar]


