Abstract
Aims/hypothesis
Insulin secretion from pancreatic beta cells and insulin-stimulated glucose uptake into skeletal muscle are processes regulated by similar isoforms of the soluble N-ethylmaleimide-sensitive factor-attachment protein receptor (SNARE) and mammalian homologue of unc-18 (Munc18) protein families. Double C2 domain β (Doc2b), a SNARE- and Munc18-interacting protein, is implicated as a crucial effector of glycaemic control. However, whether Doc2b is naturally limiting for these processes, and whether Doc2b enrichment might exert a beneficial effect upon glycaemia in vivo, remains undetermined.
Methods
Tetracycline-repressible transgenic (Tg) mice engineered to overexpress Doc2b simultaneously in the pancreas, skeletal muscle and adipose tissues were compared with wild-type (Wt) littermate mice regarding glucose and insulin tolerance, islet function in vivo and ex vivo, and skeletal muscle GLUT4 accumulation in transverse tubule/sarcolemmal surface membranes. SNARE complex formation was further assessed using Doc2b overexpressing L6 GLUT4-myc myoblasts to derive mechanisms relatable to physiological in vivo analyses.
Results
Doc2b Tg mice cleared glucose substantially faster than Wt mice, correlated with enhancements in both phases of insulin secretion and peripheral insulin sensitivity. Heightened peripheral insulin sensitivity correlated with elevated insulin-stimulated GLUT4 vesicle accumulation in cell surface membranes of Doc2b Tg mouse skeletal muscle. Mechanistic studies demonstrated Doc2b enrichment to enhance syntaxin-4–SNARE complex formation in skeletal muscle cells.
Conclusions/interpretation
Doc2b is a limiting factor in SNARE exocytosis events pertinent to glycaemic regulation in vivo. Doc2b enrichment may provide a novel means to simultaneously boost islet and skeletal muscle function in vivo in the treatment and/or prevention of diabetes.
Keywords: Doc2b, GLUT4 translocation, Insulin secretion, Insulin sensitivity, Islet, Munc18c, Skeletal muscle, SNARE protein, Type 2 diabetes
Introduction
Insulin secretion and insulin action are critical for normal glucose homeostasis. Defects in both of these processes lead to type 2 diabetes. Glucose-stimulated insulin secretion (GSIS) from islet beta cells and insulin-stimulated glucose uptake into peripheral tissues are mediated by similar exocytotic mechanisms involving a family of soluble N-ethylmaleimide-sensitive factor-attachment protein receptor (SNARE) protein isoforms. SNARE-mediated exocytosis requires the association of two target membrane (t)-SNARE proteins (syntaxins and synaptosome-associated proteins [SNAPs]) with one vesicle/granule membrane (v)-SNARE protein (VAMP) to form a heterotrimeric SNARE core complex, which promotes docking and fusion of vesicles/granules. SNARE protein-mediated GSIS from islet beta cells is biphasic. The two glucose-stimulated phases are proposed to be supported by differing pools of VAMP2-localised granules mobilised to differing SNARE protein isoform binding sites at the cell surface, including syntaxin 1A (Syn1A), syntaxin 3 (Syn3), syntaxin 4 (Syn4) and SNAP25 (or SNAP23) [1–5]. The v-SNARE protein VAMP8 also functions in insulin secretion but is required selectively for glucagon-like peptide (GLP)-1-enhanced GSIS [6]. Similarly, insulin-stimulated glucose uptake into skeletal muscle and adipose cells involves the recruitment of VAMP2-bound vesicles containing the insulin-responsive GLUT4 to the cell surface (as reviewed in [7]), yet involves a smaller subset of SNARE isoforms at the cell surface: Syn4 and SNAP23 [8–10]. Hence, towards achieving normoglycaemia, targeting these Syn4-based mechanisms is desirable because they are limiting for both GSIS and insulin-stimulated glucose uptake [8, 11].
Syntaxin proteins are regulated by specific high-affinity binding partners (mammalian homologue of unc-18 [Munc18] proteins). Three Munc18 isoforms are involved in one or both phases of glucose-stimulated insulin release, Munc18-1, Munc18b and Munc18c [12–14]. Only one isoform, Munc18c, is involved in insulin-stimulated GLUT4 vesicle translocation [15–17]. Munc18c is the only isoform capable of pairing with Syn4 in these cell types [18] and this pairing is altered when Munc18c becomes tyrosine phosphorylated in response to physiologically relevant stimuli [19–22]. However, details regarding the purpose of Munc18c in these complexes remain controversial.
Our earlier studies have suggested that decreased Munc18c–Syn4 binding occurs concomitantly with increased binding of double C2 domain protein β (Doc2b) to Munc18c, and that Doc2b preferentially binds tyrosine-phosphorylated Munc18c [23, 24]. Doc2b is a ubiquitously expressed soluble protein that can localise to the plasma membranes (PM) of beta cells, adipocytes and skeletal muscles [24–27]. Doc2b has also been shown to bind to Syn4 in clonal beta cells and 3T3L1 adipocytes [26, 27] and has been suggested to alter membrane bending via binding to Syn4-based SNARE complexes in vitro [28]. Despite the discrepant mechanistic data, there is cohesive unified support for the role of Doc2b as a potentially limiting factor in GSIS and glucose uptake. Moreover, a possible association between Doc2b deficiency and diabetes in rodents exists [29]. Taken together these data raise the possibility that intentional elevation of Doc2b in vivo could provide a means to simultaneously rescue aberrations in insulin secretion and insulin action.
As a first step towards testing this possibility, we generated regulatable Doc2b transgenic (Tg) mice using a targeting vector known to drive expression selectively in the pancreas, skeletal muscle and adipose tissue [11, 30, 31]. Indeed, elevated levels of Doc2b in these tissues in vivo resulted in improved glucose and insulin tolerance relative to wild-type (Wt) littermate mice. Mechanistically, these improvements were underscored by enhanced Syn4-based SNARE complex formation and exocytosis function in Doc2b Tg islets and skeletal muscle, altogether consistent with the concept that Doc2b is limiting for these processes in vivo. These data suggest that Doc2b enrichment may provide the means to confer superior whole-body glucose homeostasis.
Methods
Materials
Rabbit anti-Munc18c and rabbit anti-Syn4 antibodies were generated in-house as described [17, 32]. The rabbit anti-Syn4 antibody used for immunoprecipitation was purchased from Chemicon (Temecula, CA, USA). SNAP23 and SNAP25 antibodies were purchased from Affinity Bioreagents (Golden, CO, USA). The Doc2b antibody was purchased from Abcam (Cambridge, MA, USA). The Munc18-1 and VAMP2 antibodies were acquired from Synaptic Systems (Gottingen, Germany). The Syn1A antibody was obtained from Sigma (St Louis, MO, USA). The Akt and phosphoserine (Ser 473)-specific Akt antibodies were purchased from Cell Signaling (Beverley, MA, USA). Goat anti-rabbit–horseradish peroxidase and anti-mouse– horseradish peroxidase secondary antibodies were purchased from Bio-Rad (Hercules, CA, USA). Protein G+ agarose beads and anti-GLUT4 antibody were acquired from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The rat insulin radioimmunoassay kit was acquired from Millipore (Billerica, MA, USA). The enhanced chemiluminescence (ECL) kit was purchased from Amersham Biosciences (Pittsburgh, PA, USA). Humulin R was obtained from Eli Lilly (Indianapolis, IN, USA).
Animals and in vivo procedures
All studies involving mice followed the Guidelines for the Use and Care of Laboratory Animals. The pUC-CombiCMV vector [30] (a kind gift from U. Certa, Roche, Basel, Switzerland) was used to generate tetracycline (tet)-repressible Doc2b-overexpressing Tg mice. The vector contains a tetO minimal promoter to drive the target gene (Doc2b in this case) in one direction, and a CMV promoter to drive the tet-transactivator gene in the other direction (see ESM Methods for details). This vector has been shown to drive expression primarily in the pancreas, fat and skeletal muscle and not in the brain, heart, liver, spleen, kidney or lung [11, 30, 31]. Mice were generated and maintained on the C57BL/6J genetic background; breeders to expand the initial colony were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Female Doc2b Tg and Wt mice (4–6 months old) were fasted for 6 h (08:00–14:00 hours) before intraperitoneal glucose tolerance test (IPGTT) and insulin tolerance test (ITT) analyses as previously described [24] (see ESM Methods). Mouse pancreatic islets were isolated and perifusion performed as previously described [24, 33] (see ESM Methods). Skeletal muscle subcellular fractionation and immunoprecipitation assays were performed as previously described [24, 34] (see ESM Methods).
Cell culture, transient transfection and binding assays
Rat L6 muscle cells stably expressing GLUT4 with an exofacial myc-epitope (a gift from A. Klip, Department of Biochemistry, University of Toronto, Toronto, Canada) were cultured as previously described [35] (see ESM Methods). Myoblasts were electroporated (0.20 kV and 960 μFarad) with 150 μg of green fluorescent protein (GFP) vector or GFP–Doc2b plasmid DNA per 10 cm2 dish. After electroporation, cells were allowed to adhere to plates for 48 h. Cells were pre-incubated in serum-free media for 2 h followed by stimulation for 5 min with 100 nmol/l insulin. Cells were harvested in 1% NP-40 lysis buffer and detergent lysates were used for co-immunoprecipitation or glutathione S transferase (GST)–VAMP2 interaction assays, as described previously [32] (see ESM Methods).
Statistical analysis
All data were evaluated for statistical significance using Student's t test for comparison of two groups; ANOVA was used otherwise. Data are expressed as the average ± SE.
Results
Generation of Doc2b Tg mice
We generated tet-repressible Doc2b Tg mice using a targeting vector previously shown to drive expression almost exclusively in the pancreas, skeletal muscle and adipose tissue [11, 31]. Of the four founder lines, three transmitted the Doc2b gene and one line consistently exhibited an approximately two- to threefold increase in Doc2b protein relative to endogenous expression in Wt littermates in these tissues (Fig. 1a). No alterations in the levels of SNARE proteins, such as Syn4, SNAP23, VAMP2 and Munc18c, were detected. The Doc2b protein level in Tg islets was increased by approximately threefold over that in Wt islets, again with no alteration in the expression of SNARE or Munc18 proteins (Fig. 1b). No transgene expression was detected in heart, liver or spleen (Fig. 1c), or in whole-brain lysate, cerebellum or hypothalamus (Fig. 1d), similar to the findings of other reports using this targeting vector [11, 31]. GLUT4 protein levels in the heart, skeletal muscle and fat of Doc2b Tg mice were similar to those in Wt mice (Fig. 1e).
Fig. 1.
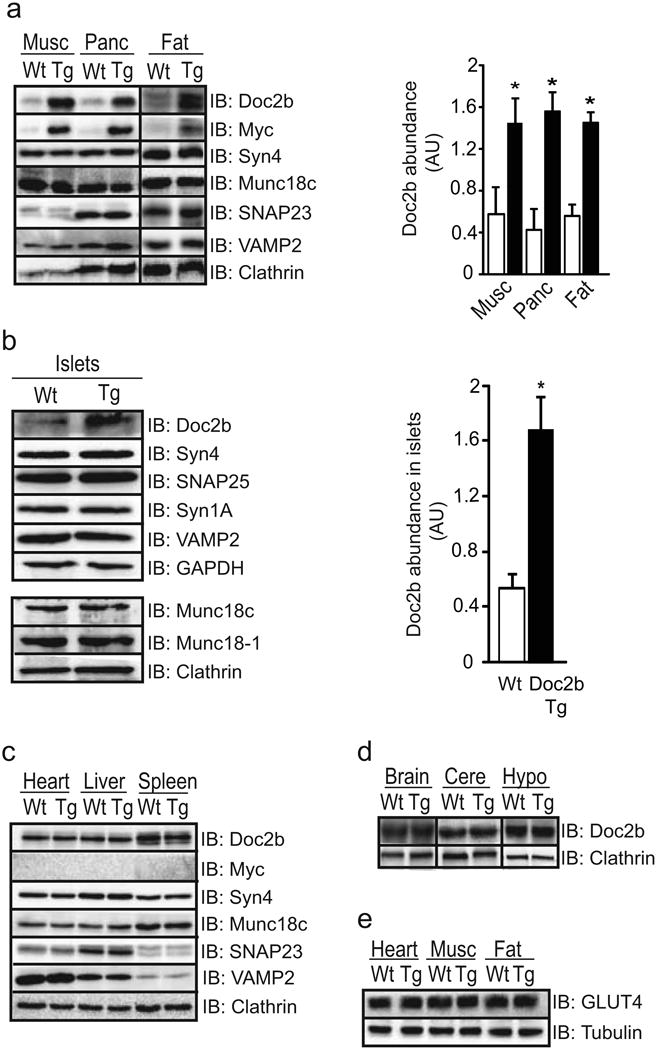
Protein expression in tissues of Doc2b transgenic mice. (a) Gastrocnemius skeletal muscle (Musc), pancreas (Panc) and epigonadal fat were isolated from three to five pairs of Doc2b Tg mice (black bars) and Wt littermates (white bars) and immunoblotted (IB) for detection of SNARE and SNARE accessory proteins. Doc2b abundances were normalised to clathrin to account for minor variations in protein loading; *p<0.05 vs Wt. (b) Isolated islets were assessed for Doc2b and SNARE protein expression as described in (a) above. (c, d) Heart, liver and spleen were similarly assessed for Doc2b levels (c), as was whole-brain lysate, cerebellum (Cere) and hypothalamus (Hypo) (d). (e) GLUT4 protein abundance was assessed in heart, skeletal muscle and fat from mice from panel (a). Data are representative of three to five pairs of mice. AU, arbitrary units
Improved glucose tolerance in Doc2b-enriched mice
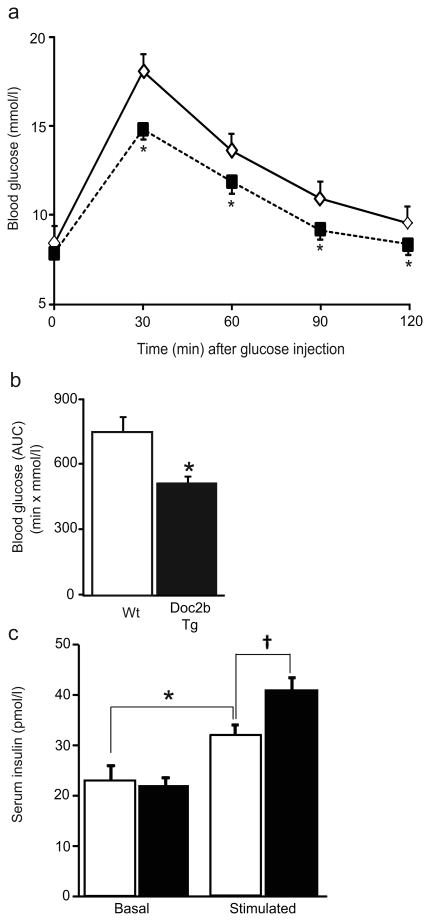
To determine whether Doc2b expression in these select tissues was limiting for glucose homeostasis in vivo, IPGTTs were performed. While fasting glycaemia was unaltered, Doc2b Tg mice showed significantly lower blood glucose levels than Wt mice upon glucose challenge at all time points (Fig. 2a). AUC analysis revealed this to be a 44% improvement in glucose tolerance (Fig. 2b). This improved glucose clearance correlated with a significantly increased serum insulin content in the Doc2b Tg mice within the first 10 min after glucose injection during the IPGTT (Fig. 2c). Body weights and food intake of Doc2b Tg mice were equivalent to those of Wt mice over a 7- to 9-week period (ESM Fig. 1). The lower-expressing transgenic line (< 1.3-fold) was similar to Wt responsiveness in the IPGTT (ESM Fig. 2). These data suggested that an approximately two- to threefold enrichment of Doc2b simultaneously in skeletal muscle, fat and pancreas was sufficient to enhance glucose tolerance, perhaps via heightened insulin release and/or peripheral glucose uptake.
Fig. 2.
Doc2b Tg mice have enhanced glucose tolerance. (a) IPGTT in Doc2b Tg mice (black squares) and Wt littermates (white diamonds) was performed in 4- to 6-month-old female mice fasted for 6 h. (b) AUC data are shown as the average ± SE from seven pairs of mice; *p<0.05, vs Wt. (c) Insulin content present in serum taken before (Basal) and 10 min post injection of glucose (Stimulated) during the IPGTT in Doc2b Tg (black bars) and Wt mice (white bars). Data represent the average ± SE from six pairs of mice; *p<0.05 vs Wt basal; †p<0.05 vs Wt glucose-stimulated
To validate that the enhanced glucose tolerance of the Doc2b Tg mice was due to the presence of the transgene, the same Doc2b Tg and Wt mice examined by IPGTT in Fig. 2a were administered tet-treated drinking water for 1 week to repress the transgene, after which the IPGTT was repeated. The glucose tolerance of the tet-treated Doc2b Tg mice was similar to that of the Wt mice (Fig. 3a, b), as were the Doc2b protein levels (Fig. 3c). These data confirmed that the enhanced glucose-tolerant phenotype of the Doc2b Tg mice corresponded to the expression of the Doc2b transgene.
Fig. 3.
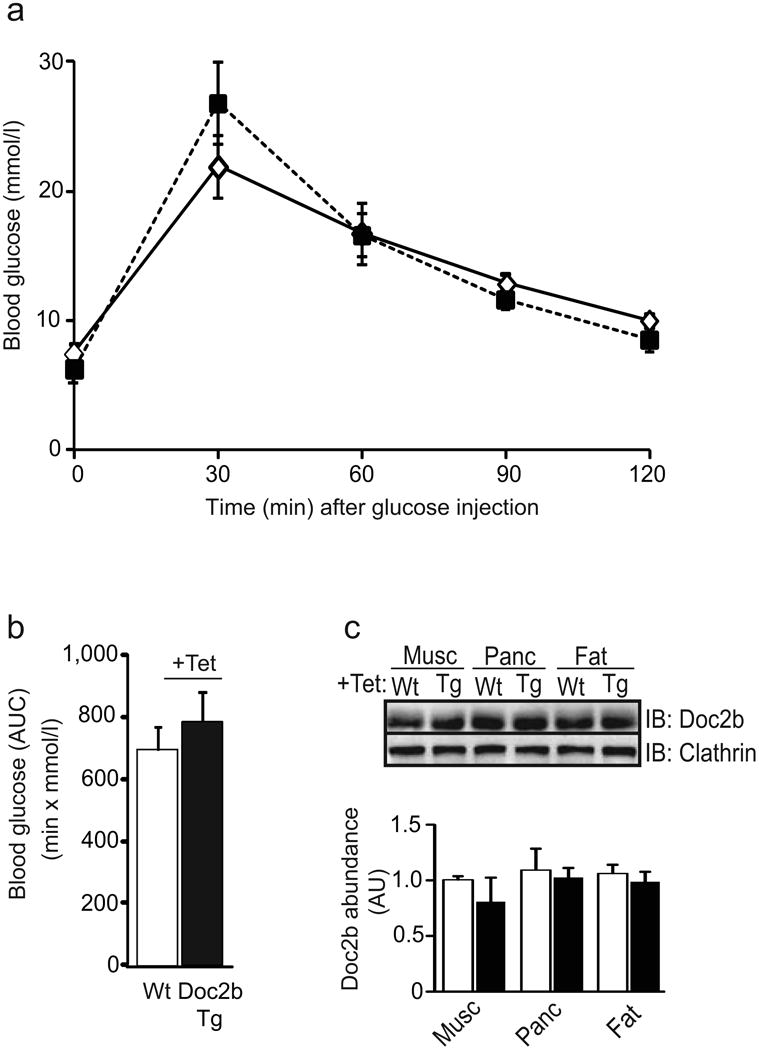
Tet-mediated repression of the Doc2b transgene reduces glucose tolerance compared with that of the Wt mice. (a) Doc2b Tg (black squares) and Wt (white diamonds) female mice assessed in Fig. 2 assays were subsequently administered tet (1 mg/ml) in the drinking water for 1 week and the IPGTT was re-performed. (b) AUC analysis is shown as the average ± SE from seven pairs of mice. (c) Tissue extracts were immunoblotted and quantified as described in Fig. 1 for Doc2b Tg (black bars) and Wt mice (white bars). Data represent the average ± SE of three independent sets of tissues
Doc2b enrichment potentiates biphasic GSIS
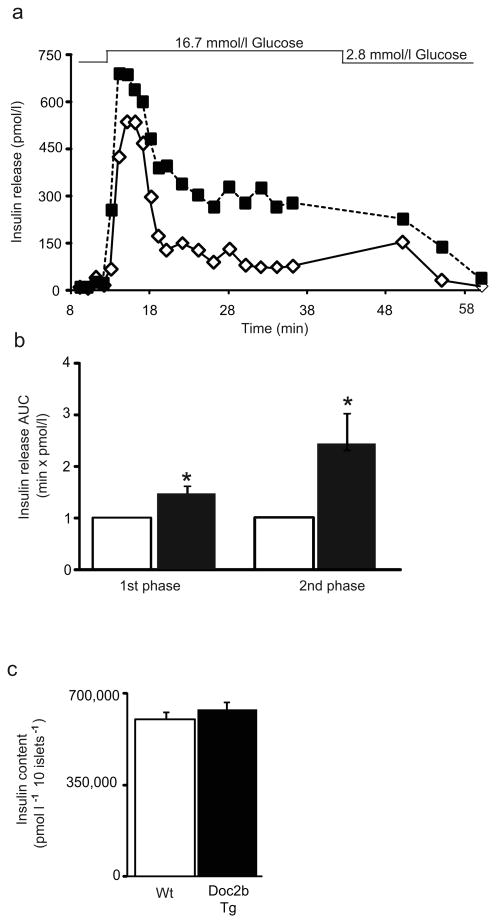
To determine whether the increased serum insulin content of Doc2b Tg mice was related to increased islet function, we subjected islets to parallel perifusion analyses. Indeed, islets of Doc2b Tg mice exhibited a higher peak of first-phase insulin release as well as sustained elevation of second-phase release (Fig. 4a). AUC analysis of Doc2b Tg secretion revealed a 50% increase over that of Wt islets in the first phase, and a 250% increase in second phase relative to Wt islets (Fig. 4b). Total insulin content was similar between Doc2b Tg and Wt islets (Fig. 4c). Basal insulin secretion was similar between Doc2b Tg and Wt islets (Fig. 4a), consistent with similar fasting serum insulin contents of the mice (Fig. 2c). These data suggest that Doc2b is limiting for each phase of GSIS and that its enrichment could enhance functional GSIS without aberrantly raising basal insulin release.
Fig. 4.
Islets from Doc2b Tg mice exhibit potentiated biphasic insulin release. (a) Islets isolated from Doc2b Tg mice (black squares) and Wt littermates (white diamonds) were perifused in parallel at 2.8 mmol/l glucose for 10 min followed by 16.7 mmol/l glucose for 35 min and then returned to low glucose for 20 min. Eluted fractions were collected and insulin secretion was determined by RIA, as depicted in this representative pair of traces. (b) AUC for first (11–17 min) and second (18–45 min) phases of insulin secretion was quantified in islets, normalised to baseline, from Doc2b Tg (black bars) and Wt (white bars) mice. Data represent the average ± SE from three independent sets of perifused islets; *p<0.05 vs Wt (Wt set equal to 1.0 and Tg normalised thereto for each phase per set). (c) Average insulin content per 10 islets from Doc2b Tg mice and Wt littermates
Doc2b Tg mice have enhanced insulin sensitivity and cell surface GLUT4 accumulation in skeletal muscle
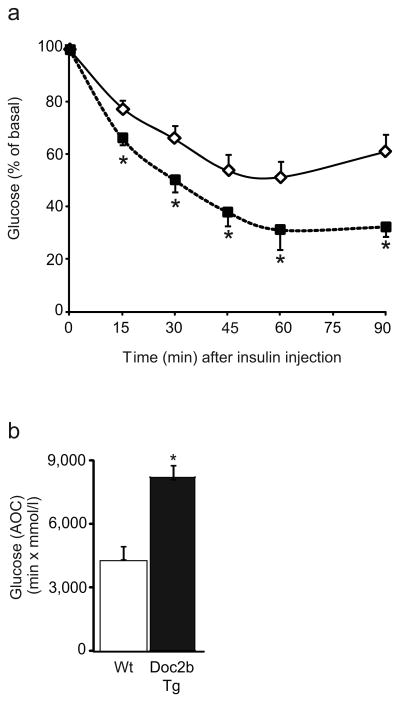
We next assessed whether the beneficial effect of Doc2b enrichment on glucose tolerance was related to improved peripheral insulin sensitivity by performing an ITT. Following insulin injection (0.75 U/kg body weight), blood glucose levels of the littermate Wt mice dropped by ∼45% within 60 min, as is normal for the C57BL/6J strain (Fig. 5a). In Doc2b Tg mice, both the rate and extent of the reduction in glycaemia was significantly enhanced (Fig. 5a); the area over the ITT curve for the Doc2b Tg mice showed this to be an almost twofold improvement in glycaemia compared with Wt mice (Fig. 5b).
Fig. 5.
Doc2b Tg mice exhibit enhanced insulin sensitivity. (a) Insulin tolerance testing of seven pairs of female Doc2b Tg mice (black squares) and Wt littermates (white diamonds) fasted for 6 h. Data are shown as the mean percentage of starting basal blood glucose concentrations ± SE; *p<0.05 vs Wt. (b) Area over the curve (AOC) data are shown as the average ± SE from seven pairs of mice; *p<0.05, vs Wt
The improved insulin sensitivity suggested that Doc2b may be limiting for insulin signalling or insulin-stimulated GLUT4 vesicle translocation to the cell surface membranes in skeletal muscle, given that skeletal muscle accounts for ∼80% of glucose uptake in humans [36]. To test this, transverse tubule/sarcolemmal cell-surface-enriched membrane fractions (referred to as PM) were isolated from saline- or insulin-injected hindlimb skeletal muscle of Doc2b Tg and Wt mice. PM fractions prepared from insulin-injected Wt mice showed the expected ∼1.5-fold increase in GLUT4 accumulation in insulin-stimulated vs unstimulated Wt mouse muscles [34]. Remarkably, Doc2b Tg PM fractions exhibited a ∼2.2-fold increase in insulin-stimulated GLUT4 vesicle accumulation at the PM (Fig. 6a). Since Akt activation in Doc2b Tg skeletal muscle homogenates was similar to that in the homogenates from Wt mice (Fig. 6b), the beneficial action of Doc2b enrichment appeared to lie downstream of Akt activation. Toward this, SNARE and Munc18 abundances were assessed for differences. While Doc2b levels were elevated in the Tg PM muscle fractions compared with Wt, as expected, Syn4 and Munc18c protein levels were similar between Doc2b Tg and Wt muscle PM fractions, under both unstimulated and insulin-stimulated conditions (Fig. 6c). Collectively, these data implicate enhanced insulin-stimulated GLUT4 vesicle accumulation (docking/fusion) at the PM as an underlying cause for the enhanced peripheral insulin sensitivity of the Doc2b Tg mice.
Fig. 6.
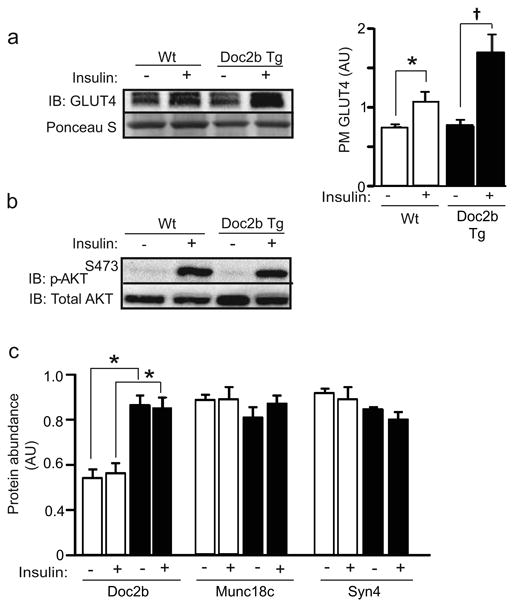
Doc2b Tg mice show increased insulin-stimulated GLUT4 accumulation at the sarcolemma/transverse tubule PMs of skeletal muscle. (a) GLUT4 abundance in the PM fractions was detected by immunoblot (Ponceau S shows protein loading). Quantification of GLUT4 accumulation in PM fractions is shown in the adjacent bar graph where data are shown as the average ± SE for three sets of mice; *p<0.05 vs Wt basal, †p<0.05 vs Tg basal. (b) Whole skeletal muscle detergent homogenates from mice stimulated with insulin were immunoblotted for activated Akt (p-AktS473). Blots were stripped and reprobed for total Akt content. Data are representative of three independent sets of tissue homogenates. (c) PM fractions prepared from Doc2b Tg mice (black bars) and Wt littermates (white bars) from panel (a) were immunoblotted for Doc2b, Munc18c and Syn4. Data are shown as the average ± SE for three sets of mice. While Doc2b was significantly elevated in Tg vs Wt fractions (*p<0.05), no statistical differences in either Syn4 or Munc18c abundances were observed. AU, arbitrary units
Doc2b enrichment promotes SNARE complex formation in skeletal myoblasts
To determine how Doc2b enrichment in skeletal muscle of the Doc2b Tg mice might increase the PM localisation of GLUT4, Munc18c–Syn4 binding and Syn4 activation/SNARE complex formation was studied in L6-GLUT4-myc myoblasts transfected to express exogenous GFP-tagged Doc2b (or GFP vector control). L6-GLUT4-myc skeletal myoblasts are the premiere clonal muscle cell line for recapitulating the events associated with GLUT4 vesicle exocytosis and transfect with ∼30–50% efficiency. Immunoprecipitation of Syn4 from insulin-stimulated GFP–Doc2b-expressing L6 cells showed significantly reduced Munc18c co-precipitation compared with GFP-expressing cells (Fig. 7a). Syn4 accessibility to VAMP2 was examined as an indicator of its ability to form SNARE complexes, given the inability to obtain sufficient PM protein from transfected L6 cells for co-immunoprecipitation analyses. Syn4 accessibility to an exogenous GST–VAMP2 protein linked to sepharose beads from insulin-stimulated GFP–Doc2b-expressing L6 myoblasts was significantly enhanced relative to cells expressing a similar amount of GFP (Fig. 7b). Endogenous Doc2b levels were similar among transfected cells, suggesting that the enrichment in SNARE complex formation was due to the effects of overexpressed Doc2b (ESM Fig. 3). The Doc2b antibody used for this and all Doc2b immunoblotting was confirmed to react specifically with Doc2b (ESM Fig. 4). Together, these results suggest that Doc2b is a limiting factor for Munc18c–Syn4 dissociation in skeletal muscle cells, and that the potentiation of Syn4 accessibility by increased expression of Doc2b may underlie the enhanced GLUT4 accumulation in PM fractions and the insulin sensitivity of the Doc2b Tg mice.
Fig. 7.
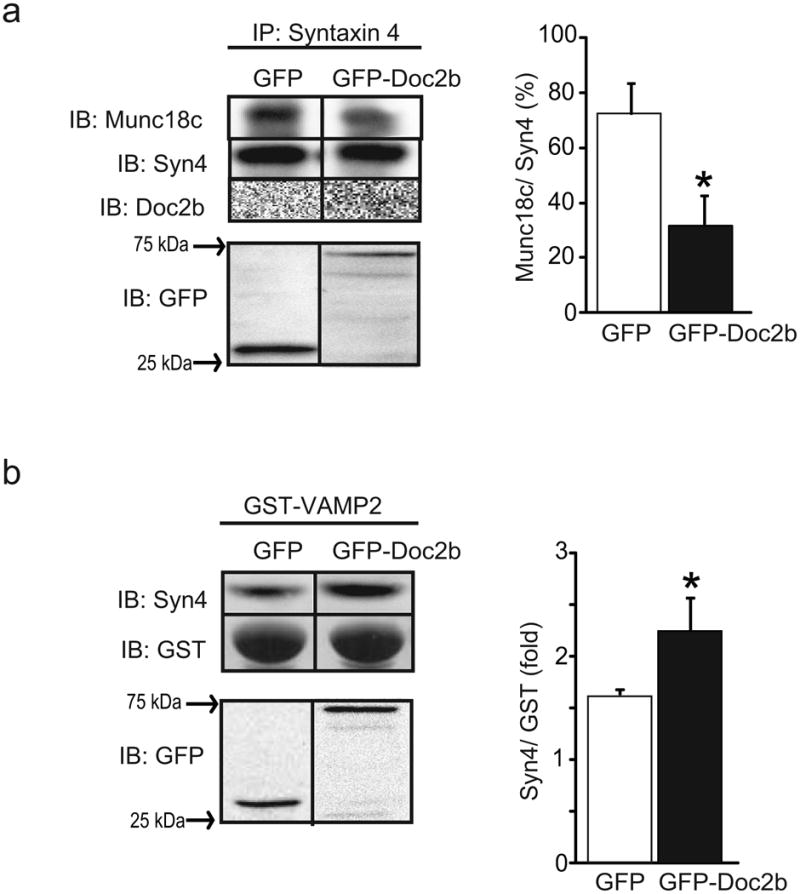
Overexpression of Doc2b coordinately decreases Munc18c–Syn4 binding while increasing Syn4 activation in L6 GLUT4-myc myoblasts. Detergent lysates prepared from L6 GLUT4-myc myoblasts transfected to express GFP-tagged Doc2b or GFP alone and stimulated with insulin for 5 min were used in anti-Syn4 immunoprecipitation reactions and co-precipitated Munc18c or Doc2b proteins were detected by immunoblotting (a), or in GST-VAMP2 interaction assays for detection of the Syn4 present in lysates that is accessible to the exogenous GST–VAMP2 probe (b). Proteins were immunoblotted for Syn4, GST and GFP or GFP–Doc2b (∼75 kDa). Quantification is represented in the adjacent bar graphs. Data are representative of the average ± SE of three independent experiments of the ratio of Munc18c/Syn4 (%), and Syn4/GST–VAMP2 (fold), respectively; *p<0.05 vs GFP
Discussion
In this study, we tested the concept that Doc2b is limiting for glucose homeostasis, and that enriching Doc2b might provide a means to simultaneously improve insulin sensitivity and insulin secretion in an effort to enhance whole-body glucose homeostasis in vivo. Using novel tet-repressible Doc2b Tg mice, we show that increasing the expression of Doc2b by approximately two- to threefold simultaneously in the pancreas, skeletal muscle and adipose tissue conferred enhanced glucose and insulin tolerance in vivo. The enhanced glucose tolerance in the Doc2b Tg mice was found to be mediated by the potentiation of both insulin secretion and peripheral insulin action. Increased serum insulin content in Doc2b Tg mice post glucose injection is likely to be directly related to the improved GSIS in the islet beta cells. In our ex vivo islet studies, the second phase was particularly robust, consistent with clonal cell studies of Doc2b overexpression [25, 27]. It has been suggested that second-phase release of insulin contributes substantially to the responsiveness of peripheral tissues, such as skeletal muscle. Interestingly, Doc2b enrichment also potentiated the first phase of insulin release and, because the first phase rapidly impacts hepatic glucose output [37], it remains possible that the liver glucose output rate was reduced rapidly to contribute to the overall improvement of glucose tolerance. Notably, the Doc2b Tg mice also showed significant enhancement in skeletal muscle insulin sensitivity and increased GLUT4 accumulation at the cell surface. As such, the skeletal muscle would likely require less insulin to accomplish its task, and the need for sustained insulin release during the second phase may be reduced. Collectively, this Doc2b Tg mouse model provides the first proof-of-principle evidence for the potential merit of Doc2b enrichment in glycaemic control. Future studies using skeletal muscle- and islet beta cell-specific Doc2b overexpressing mouse models will facilitate delineation of the relative contribution of Doc2b enrichment in these tissues to maintenance of glucose homeostasis. Further, comparison of such mice against mice overexpressing a dominant-negative Doc2b mutant, such as that referred to as ‘CIM’ [26], would be a useful control for the evaluating the merit of upregulating Doc2b in these tissues.
The positive effect of Doc2b enrichment upon peripheral insulin sensitivity and GLUT4 accumulation at the cell surface of skeletal muscle suggests that endogenous Doc2b in skeletal muscle and/or fat is limiting for insulin-stimulated glucose clearance mechanisms. These in vivo and ex vivo skeletal muscle data validate an earlier report wherein Doc2b overexpression in 3T3-L1 adipocytes enhanced glucose uptake [26]. Our data support an indirect role for Doc2b in its potentiation of skeletal muscle insulin action at the level of SNARE complex formation for the following reasons: (1) Doc2b in skeletal muscle lysates failed to co-precipitate with Syn4 [24] and (2) the overexpression of Doc2b in L6 myoblasts failed to drive an interaction of Doc2b with Syn4. These findings in primary skeletal muscle tissue do not recapitulate the binding interactions derived from in vitro mixing assays using recombinant Doc2b [28]. This discrepancy may suggest that these Doc2b/Munc18c/Syn4 interactions are affected by additional factors such as the particular cellular milieu, post-translational modification of the proteins involved and/or by calcium. For example, insulin triggers the tyrosine phosphorylation of Munc18c in skeletal muscle and adipocytes [20–24, 38], enhancing the affinity of Munc18c for Doc2b by almost twofold while decreasing its affinity for Syn4. These data are congruent with a model whereby Doc2b would seemingly titrate out more Munc18c to make more cellular Syn4 accessible, generating more Syn4-based docking/fusion sites for incoming GLUT4 vesicles. Another possible manner by which Doc2b enrichment potentiates GLUT4 exocytosis is via its recently described role in calcium-induced membrane curvature induction [28]. However, earlier findings suggest that the actions of calcium-activated Doc2b in bona fide skeletal muscle may differ from those seen in vitro, or in neurons and adipocytes [26, 39], and as such remains to be tested. Like GLUT4 vesicle exocytosis, insulin granule exocytosis uses Syn4-based SNARE complexes; Syn4 has an impact on both phases of GSIS [3]. Since second-phase insulin exocytosis requires Munc18c and hinges upon Munc18c's ability to undergo glucose-induced tyrosine phosphorylation, the requirement and the role of Doc2b may be linked indirectly to Syn4 in mechanism(s) similar to that described above for skeletal muscle [19, 21]. Another possibility involves the concept that SNARE complexes regulated by Doc2b could affect different granule pools that may drive biphasic insulin secretion, as seen with Munc18b [12]. Consistent with this, Doc2b can also bind to Munc18-1, albeit through a different C2 domain [40], and Munc18-1 functions in first-phase secretion [13, 41]. Future studies using total internal reflection fluorescence microscopy will be required to distinguish the effects of Doc2b enrichment upon the different pools of insulin granules.
In summary, the data presented show that Doc2b has promise as a new therapeutic target for the management of pre-clinical and frank type 2 diabetes and demonstrate that its use is feasible and safe for betterment of glycaemic control in vivo; the data also provide insight into the mechanisms of Doc2b action, particularly in SNARE-mediated exocytotic events, which may prove valuable in future drug design strategies.
Supplementary Material
Acknowledgments
We thank the Indiana University School of Medicine Transgenic Animal Core Facility and the Islet Core Facility for generating the Doc2b Tg mice and for isolating islets therefrom, respectively. We are grateful to S. Groffen (Departments of Functional Genomics and Clinical Genetics, VU University, Amsterdam, the Netherlands) for the GFP–Doc2a and –Doc2b plasmid DNAs. We would also like to thank J. Elmendorf (Department of Cellular and Integrative Physiology, Indiana University School of Medicine) for assisting with L6 myoblast methodologies. We would like to thank A. Hernandez (Department of Cellular and Integrative Physiology, Indiana University School of Medicine) for her technical help for generating the Doc2b Tg targeting vector.
Funding: This study was supported by grants from the National Institutes of Health (DK067912 and DK076614 to DCT), the Indiana University School of Medicine Biomedical Research Core Facility grant (to DCT) and the American Heart Association (12PRE11890042 to LR).
Abbreviations
- Doc2b
Double C2 domain β
- GFP
Green fluorescent protein
- GLP
Glucagon-like peptide
- GST
Glutathione S transferase
- GSIS
Glucose-stimulated insulin secretion
- IPGTT
Intraperitoneal glucose tolerance test
- ITT
Insulin tolerance test
- Munc18
Mammalian homologue of unc-18
- PM
Plasma membrane
- SNAP
Synaptosome-associated protein
- SNARE
Soluble N-ethylmaleimide-sensitive factor-attachment protein receptor
- Syn1A
Syntaxin 1A
- Syn4
Syntaxin 4
- tet
Tetracycline
- Tg
Transgenic
- t-SNARE
Target SNARE
- VAMP
Vesicle-associated membrane protein
- v-SNARE
Vesicle SNARE
- Wt
Wild type
Footnotes
Duality of interest: The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement: LR conducted studies, analysed data, contributed to discussion and wrote/reviewed/edited the manuscript. EO conducted studies for the manuscript and contributed to the discussion and reviewed/edited the manuscript. DCT provided input to the study design, contributed to discussion, revised the manuscript critically for important intellectual content, guarantees the content of the manuscript and is responsible for the integrity of the work as a whole. All authors approved the final version to be published.
References
- 1.Ohara-Imaizumi M, Fujiwara T, Nakamichi Y, et al. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. J Cell Biol. 2007;177:695–705. doi: 10.1083/jcb.200608132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu D, Koo E, Kwan E, et al. Syntaxin-3 regulates newcomer insulin granule exocytosis and compound fusion in pancreatic beta cells. Diabetologia. 2013;56:359–369. doi: 10.1007/s00125-012-2757-0. [DOI] [PubMed] [Google Scholar]
- 3.Spurlin BA, Thurmond DC. Syntaxin 4 facilitates biphasic glucose-stimulated insulin secretion from pancreatic beta-cells. Mol Endocrinol. 2006;20:183–193. doi: 10.1210/me.2005-0157. [DOI] [PubMed] [Google Scholar]
- 4.Regazzi R, Wollheim CB, Lang J, et al. VAMP-2 and cellubrevin are expressed in pancreatic beta-cells and are essential for Ca(2+)-but not for GTP gamma S-induced insulin secretion. EMBO J. 1995;14:2723–2730. doi: 10.1002/j.1460-2075.1995.tb07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoul K, Berger A, Niemann H, et al. SNAP-23 is not cleaved by botulinum neurotoxin E and can replace SNAP-25 in the process of insulin secretion. J Biol Chem. 1997;272:33023–33027. doi: 10.1074/jbc.272.52.33023. [DOI] [PubMed] [Google Scholar]
- 6.Zhu D, Zhang Y, Lam PP, et al. Dual role of VAMP8 in regulating insulin exocytosis and islet beta cell growth. Cell Metab. 2012;16:238–249. doi: 10.1016/j.cmet.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Klip A. The many ways to regulate glucose transporter 4. Appl Physiol Nutr Metab. 2009;34:481–487. doi: 10.1139/H09-047. [DOI] [PubMed] [Google Scholar]
- 8.Yang C, Coker KJ, Kim JK, et al. Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J Clin Invest. 2001;107:1311–1318. doi: 10.1172/JCI12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheatham B, Volchuk A, Kahn CR, Wang L, Rhodes CJ, Klip A. Insulin-stimulated translocation of GLUT4 glucose transporters requires SNARE-complex proteins. Proc Natl Acad Sci USA. 1996;93:15169–15173. doi: 10.1073/pnas.93.26.15169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawanishi M, Tamori Y, Okazawa H, Araki S, Shinoda H, Kasuga M. Role of SNAP23 in insulin-induced translocation of GLUT4 in 3T3-L1 adipocytes. Mediation of complex formation between syntaxin4 and VAMP2. J Biol Chem. 2000;275:8240–8247. doi: 10.1074/jbc.275.11.8240. [DOI] [PubMed] [Google Scholar]
- 11.Spurlin BA, Park SY, Nevins AK, Kim JK, Thurmond DC. Syntaxin 4 transgenic mice exhibit enhanced insulin-mediated glucose uptake in skeletal muscle. Diabetes. 2004;53:2223–2231. doi: 10.2337/diabetes.53.9.2223. [DOI] [PubMed] [Google Scholar]
- 12.Lam PP, Ohno M, Dolai S, et al. Munc18b is a major mediator of insulin exocytosis in rat pancreatic beta-cells. Diabetes. 2013;62:2416–2428. doi: 10.2337/db12-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh E, Kalwat MA, Kim MJ, Verhage M, Thurmond DC. Munc18-1 regulates first-phase insulin release by promoting granule docking to multiple syntaxin isoforms. J Biol Chem. 2012;287:25821–25833. doi: 10.1074/jbc.M112.361501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh E, Thurmond DC. Munc18c depletion selectively impairs the sustained phase of insulin release. Diabetes. 2009;58:1165–1174. doi: 10.2337/db08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan AH, Thurmond DC, Yang C, Ceresa BP, Sigmund CD, Pessin JE. Munc18c regulates insulin-stimulated glut4 translocation to the transverse tubules in skeletal muscle. J Biol Chem. 2001;276:4063–4069. doi: 10.1074/jbc.M007419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamori Y, Kawanishi M, Niki T, et al. Inhibition of insulin-induced GLUT4 translocation by Munc18c through interaction with syntaxin4 in 3T3-L1 adipocytes. J Biol Chem. 1998;273:19740–19746. doi: 10.1074/jbc.273.31.19740. [DOI] [PubMed] [Google Scholar]
- 17.Thurmond DC, Ceresa BP, Okada S, Elmendorf JS, Coker K, Pessin JE. Regulation of insulin-stimulated GLUT4 translocation by munc18c in 3T3L1 adipocytes. J Biol Chem. 1998;273:33876–33883. doi: 10.1074/jbc.273.50.33876. [DOI] [PubMed] [Google Scholar]
- 18.Tellam JT, Macaulay SL, McIntosh S, Hewish DR, Ward CW, James de. Characterization of Munc-18c and syntaxin-4 in 3T3-L1 adipocytes. Putative role in insulin-dependent movement of GLUT-4. J Biol Chem. 1997;272:6179–6186. doi: 10.1074/jbc.272.10.6179. [DOI] [PubMed] [Google Scholar]
- 19.Oh E, Thurmond DC. The stimulus-induced tyrosine phosphorylation of Munc18c facilitates vesicle exocytosis. J Biol Chem. 2006;281:17624–17634. doi: 10.1074/jbc.M601581200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umahara M, Okada S, Yamada E, et al. Tyrosine phosphorylation of Munc18c regulates platelet-derived growth factor-stimulated glucose transporter 4 translocation in 3T3L1 adipocytes. Endocrinology. 2008;149:40–49. doi: 10.1210/en.2006-1549. [DOI] [PubMed] [Google Scholar]
- 21.Jewell JL, Oh E, Ramalingam L, et al. Munc18c phosphorylation by the insulin receptor links cell signaling directly to SNARE exocytosis. J Cell Biol. 2011;193:185–199. doi: 10.1083/jcb.201007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakke J, Bettaieb A, Nagata N, Matsuo K, Haj FG. Regulation of the SNARE-interacting protein Munc18c tyrosine phosphorylation in adipocytes by protein-tyrosine phosphatase 1B. Cell Commun Signal. 2013;11:57. doi: 10.1186/1478-811X-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jewell JL, Oh E, Bennett SM, Meroueh SO, Thurmond DC. The tyrosine phosphorylation of Munc18c induces a switch in binding specificity from syntaxin 4 to Doc2beta. J Biol Chem. 2008;283:21734–21746. doi: 10.1074/jbc.M710445200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramalingam L, Oh E, Yoder SM, et al. Doc2b is a key effector of insulin secretion and skeletal muscle insulin sensitivity. Diabetes. 2012;61:2424–2432. doi: 10.2337/db11-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ke B, Oh E, Thurmond DC. Doc2beta is a novel Munc18c-interacting partner and positive effector of syntaxin 4-mediated exocytosis. J Biol Chem. 2007;282:21786–21797. doi: 10.1074/jbc.M701661200. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda N, Emoto M, Nakamori Y, et al. DOC2B: a novel syntaxin-4 binding protein mediating insulin-regulated GLUT4 vesicle fusion in adipocytes. Diabetes. 2009;58:377–384. doi: 10.2337/db08-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazaki M, Emoto M, Fukuda N, et al. DOC2b is a SNARE regulator of glucose-stimulated delayed insulin secretion. Biochem Biophys Res Commun. 2009;384:461–465. doi: 10.1016/j.bbrc.2009.04.133. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Rathore SS, Davis EM, Ouyang Y, Shen J. Doc2b promotes GLUT4 exocytosis by activating the SNARE-mediated fusion reaction in a calcium- and membrane bending-dependent manner. Mol Biol Cell. 2013;24:1176–1184. doi: 10.1091/mbc.E12-11-0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keller MP, Choi Y, Wang P, et al. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome Res. 2008;18:706–716. doi: 10.1101/gr.074914.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultze N, Burki Y, Lang Y, Certa U, Bluethmann H. Efficient control of gene expression by single step integration of the tetracycline system in transgenic mice. Nat Biotechnol. 1996;14:499–503. doi: 10.1038/nbt0496-499. [DOI] [PubMed] [Google Scholar]
- 31.Spurlin BA, Thomas RM, Nevins AK, et al. Insulin resistance in tetracycline-repressible Munc18c transgenic mice. Diabetes. 2003;52:1910–1917. doi: 10.2337/diabetes.52.8.1910. [DOI] [PubMed] [Google Scholar]
- 32.Wiseman DA, Kalwat MA, Thurmond DC. Stimulus-induced S-nitrosylation of syntaxin 4 impacts insulin granule exocytosis. J Biol Chem. 2011;286:16344–16354. doi: 10.1074/jbc.M110.214031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stull ND, Breite A, McCarthy R, Tersey SA, Mirmira RG. Mouse islet of Langerhans isolation using a combination of purified collagenase and neutral protease. J Vis Exp. 2012;67:e4137. doi: 10.3791/4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou M, Sevilla L, Vallega G, et al. Insulin-dependent protein trafficking in skeletal muscle cells. Am J Physiol. 1998;275:E187–E196. doi: 10.1152/ajpendo.1998.275.2.E187. [DOI] [PubMed] [Google Scholar]
- 35.Walker PS, Ramlal T, Sarabia V, et al. Glucose transport activity in L6 muscle cells is regulated by the coordinate control of subcellular glucose transporter distribution, biosynthesis, and mRNA transcription. J Biol Chem. 1990;265:1516–1523. [PubMed] [Google Scholar]
- 36.Ferrannini E, Simonson DC, Katz LD, et al. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism: clinical and experimental. 1988;37:79–85. doi: 10.1016/0026-0495(88)90033-9. [DOI] [PubMed] [Google Scholar]
- 37.Edgerton DS, Lautz M, Scott M, et al. Insulin's direct effects on the liver dominate the control of hepatic glucose production. J Clin Invest. 2006;116:521–527. doi: 10.1172/JCI27073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmelzle K, Kane S, Gridley S, Lienhard GE, White FM. Temporal dynamics of tyrosine phosphorylation in insulin signaling. Diabetes. 2006;55:2171–2179. doi: 10.2337/db06-0148. [DOI] [PubMed] [Google Scholar]
- 39.Groffen AJ, Friedrich R, Brian EC, Ashery U, Verhage M. DOC2A and DOC2B are sensors for neuronal activity with unique calcium-dependent and kinetic properties. J Neurochem. 2006;97:818–833. doi: 10.1111/j.1471-4159.2006.03755.x. [DOI] [PubMed] [Google Scholar]
- 40.Verhage M, de Vries KJ, Roshol H, Burbach JP, Gispen WH, Sudhof TC. DOC2 proteins in rat brain: complementary distribution and proposed function as vesicular adapter proteins in early stages of secretion. Neuron. 1997;18:453–461. doi: 10.1016/s0896-6273(00)81245-3. [DOI] [PubMed] [Google Scholar]
- 41.Mandic SA, Skelin M, Johansson JU, Rupnik MS, Berggren PO, Bark C. Munc18-1 and Munc18-2 proteins modulate beta-cell Ca2+ sensitivity and kinetics of insulin exocytosis differently. J Biol Chem. 2011;286:28026–28040. doi: 10.1074/jbc.M111.235366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.