Abstract
Background/Objectives
The elderly are at increased risk for vitamin D deficiency and low vitamin D levels have been related to increased risk of cognitive dysfunction. However, this association has never been investigated in centenarians, who exhibit delayed onset of aging and age-related diseases. We aimed to define vitamin D levels and their association with cognition in subjects with exceptional longevity.
Design/Setting/Participants
A cross-sectional study in Ashkenazi Jewish subjects (n=253) with exceptional longevity, with comparison made to NHANES III participants, age ≥70 years.
Measurements
25-hydroxyvitamin D levels were measured using liquid chromatography/tandem mass spectrometry analysis. Cognitive function was assessed using the Mini-Mental State Examination (MMSE) and clock drawing test (CDT: command and copy).
Results
Median age (IQR) of the Ashkenazi subjects was 97 years (95–104). Age-associated rise in the prevalence of vitamin D insufficiency, defined as serum vitamin D level <30ng/mL, was noted in NHANES III (p=0.001). In the Ashkenazi group with longevity, the rate of vitamin D insufficiency was comparable to the NHANES III participants, who were up to 25 years younger. In the cohort with exceptional longevity, 49% demonstrated cognitive impairment as assessed by the MMSE (median score (IQR) 9.5 (0–24) vs. 29 (18–30) in the group with impaired vs. normal cognition, p<0.001). Vitamin D insufficiency was more prevalent in those with impaired cognition, defined by the MMSE and the CDT: copy, compared to those with normal cognition (71.8% vs. 57.7%, p=0.02 and 84.6% vs. 50.6%, p=0.02, respectively). This association remained significant after multivariable adjustment in logistic regression models, for cognitive assessments made by the MMSE (OR 3.2, 95% CI 1.1–9.29, p=0.03) and by the CDT: copy (OR 8.96, 95% CI 1.08–74.69, p=0.04).
Conclusion
Higher vitamin D levels may be a marker of delayed aging, as they are associated with better cognitive function in people achieving exceptional longevity.
Keywords: vitamin D, exceptional longevity, cognitive function
INTRODUCTION
Aging is a major risk factor for many diseases, including cognitive dysfunction. In cross-sectional studies, vitamin D deficiency in the elderly has been linked to higher odds of cognitive impairment1,2 and prospectively, to greater risk of cognitive decline2,3. The elderly are particularly vulnerable to vitamin D deficiency secondary to physiological changes that affect vitamin D synthesis4. Although epidemiological data suggests that vitamin D may play a role in protecting from cognitive dysfunction in the general elderly population, whether this association exists in populations with exceptional longevity (centenarians), in whom dementia risk is delayed in proportion to their lifespan5, is not determined. Furthermore, it is unknown if centenarians differ in their metabolism of vitamin D and susceptibility to vitamin D deficiency. Thus, we aimed to test the hypothesis that vitamin D insufficiency is associated with cognitive impairment in centenarians using a well-characterized Ashkenazi Jewish cohort with exceptional longevity6. In addition, since we and others have previously demonstrated that centenarians frequently display biological profiles that are similar to younger individuals5,7–9, we compared the vitamin D levels in centenarians to a cross-sectional younger cohort from the general population, using data from the Third National Health and Nutrition Examination Survey (NHANES III). Identification of factors that contribute to delay in age-associated cognitive decline, as observed in centenarians, may lead to novel therapeutic interventions for dementia and other age-related diseases in the general elderly population.
METHODS
Study participants
Ashkenazi Jewish (AJ)individuals, age ninety-five and older (centenarians, n=545), who were living independently at age ninety-five, which was considered a reflection of good health, were recruited for the Longevity Genes Project at Albert Einstein College of Medicine, from the Northeastern United States (US) between 1998 to present, as previously described6. The AJ population is Caucasian, originating from a small founder population and is relatively homogeneous genetically and in its socioeconomic status. Participants’ ages were verified with government issued identification. A single study nurse visited each participant in their residence and performed measurements of weight and height, as well as evaluations of cognitive function and mood. A thorough medical and social history was obtained using a structured questionnaire. A venous blood sample was collected and the processed serum was stored at −80°C. Written informed consent was obtained from the participants or their proxies, in the event that the subject lacked cognitive capacity. The study was approved by the Institutional Review Board at the Albert Einstein College of Medicine.
A comparison group was selected from NHANES III, a US national survey conducted between 1988 and 1994. For our analysis we selected a non-Hispanic white subgroup of NHANES III, age 70 to 90 years, due to racial differences in sun-mediated skin synthesis of vitamin D precursors. The age for all the NHANES III participants who were older than 90 years was recorded as 90 years to maintain anonymity. Although the oldest NHANES III group may include centenarians, it is highly unlikely, as only about 1/6,000 people reach this age in the general population10.
Cognitive and mood assessment
Cognition was assessed with the Mini-Mental State Examination(MMSE)11 and the clock drawing tests (CDT)12. The MMSE is a test of global cognitive function. It was scored out of 30 points for participants without major visual impairment, and a score of ≥25 represented normal cognition13. For participants with severe visual impairment a Blind MMSE14,15 was utilized, which excluded items that required image processing. The Blind MMSE was scored out of a maximum 22 points14,15 and a score of ≥16 represented normal cognition. The cognitive domain of executive function was evaluated using the command CDT and the visuospatial construction was assessed by the copy CDT16. Both CDT were scored out of 10 points, with a score of <7 indicating cognitive impairment for the command CDT12 and ascore of <8 interpreted as cognitive impairment for the copy CDT12. Depressive mood was evaluated with a fifteen item short form of the Geriatric Depression Scale, with a score of ≥6 suggesting depression17.
Laboratory measures
Serum 25-hydroxyvitamin D (25(OH)D) levels were measured in centenarians’ stored serum samples by a validated liquid chromatography/tandem mass spectrometry (LC/MS/MS) analysis at Montefiore Medical Center (MMC), Bronx, NY. The coefficient of variance of the assay was 5% or better and the limit of quantification was 4 ng/mL. Centenarians’ biochemical and metabolic profiles were assessed based on standard automated methods at the clinical laboratories of MMC. In the NHANES III, the 25(OH)D levels were measured in the nutrition and health laboratory at the National Center for Environmental Health (CDC, Atlanta, GA) by radioimmunoassay (RIA kit; DiaSorin, formerly Incstar Corp., Stillwater, MN)18.
Statistical Analysis
Statistical analysis was done using STATA software, version 12 (StataCorp LP, College Station, TX). Baseline characteristics were compared using bivariate statistics, with non-parametric tests used when appropriate. Normality was assessed by observation of the histograms. The Chi square statistic was used to compare proportions. Using a manual forward building process, we built multivariable logistic regression models to determine the independent association between serum 25(OH)D insufficiency and cognitive impairment, as defined by the MMSE and the CDT scores. Cognitive impairment was modeled as the dependent variable and 25(OH)D, dichotomized as sufficient (≥30ng/mL, reference) or insufficient (<30ng/mL), as the variable of interest. The models were adjusted for potential confounders of vitamin D levels or cognition19, including medical co-morbidities. A medical comorbidity score was a composite sum of comorbidities self-reported on the structured questionnaire and included the following diseases: diabetes mellitus, cancer (excluding a diagnosis of basal or squamous cell carcinoma of the skin), hypertension, heart disease, and astroke. Missing data for medical-comorbidities was imputed as presence of the co-morbid condition, because imputing it as absent or missing did not result in meaningful difference in the final analysis and thus, we also avoided biasing the results away from the null hypothesis. An exception were cases that had data missing for all co-morbidities, in these cases the comorbidities were analyzed as missing. Assumptions of linearity in the logit for continuous variables were evaluated using the Lowess smoother graph, assessment of linearity of odds ratios among the tertiles of a continuous variable, and using fractional polynomials. Variables that did not meet linearity assumptions were categorized. First order multiplicative interactions were assessed between25(OH)D groups and sex, BMI, history of tobacco use and HDL levels, with no interactions identified. Mean serum 25(OH)D levels did not significantly differ between sampling months (p=0.45 for ANOVA) or sampling season(p=0.683 for ANOVA); thus, sampling season was not adjusted for in the final model. Goodness of fit for the model was assessed with the Hosmer and Lameshow goodness of fit test. Results for the logistic regression models are reported as odds ratios (OR) with 95% confidence intervals (CI). A p-value <0.05 is considered statistically significant.
RESULTS
Comparison of 25(OH)D levels in AJ centenarians vs. NHANES III
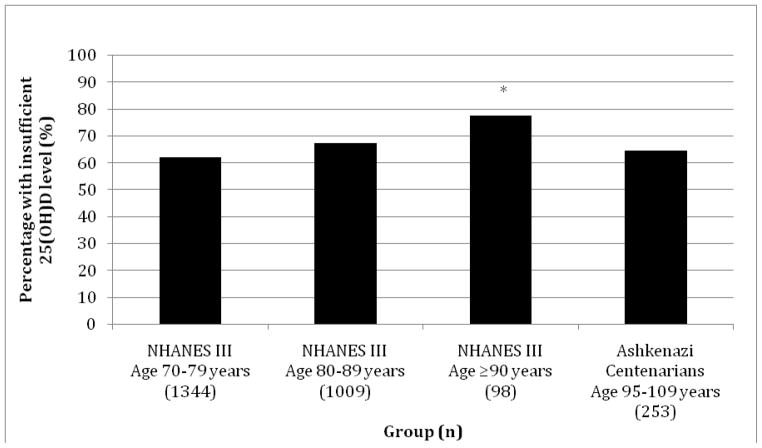
Measures of 25(OH)D levels were determined for 292 AJ participants. MMSE results were available for 253 centenarians, with 13 analyzed using the Blind MMSE (8 had missing MMSE scores, 7 had severe hearing impairment, 4 refused, 20 were unable to complete MMSE secondary to aphasia, physical impairment, or lethargy). Those with missing MMSE results did not significantly differ from the remainder of the cohort in their 25(OH)D level distribution (p=0.13). The selected NHANES III group was comprised of 2,451 individuals (n=1,344 in age group 70–79 years; n=1,009 in age group 80–89 years; and n=98 in age group ≥90 years). The NHANES III cohort demonstrated a significant trend in rising prevalence of 25(OH)D insufficiency with increasing age, p=0.001 (Figure 1). The AJ cohort with longevity had a significantly lower prevalence of 25(OH)D insufficiency compared to the oldest NHANES IIII group (p=0.018, Figure 1).
Figure 1.
Percentage of individuals with insufficient 25(OH)D levels in the NHANES III cohort, subdivided by age, and Ashkenazi Jewish cohort with exceptional longevity. *p=0.001 for comparison between NHANES III Age 70–79 years, NHANES III 80–89 years, and NHANES III Age ≥90 years. p=0.018 for comparison between NHANES III, Age ≥90 years, and Ashkenazi Centenarians.
25(OH)D levels and performance on the MMSE
Forty-nine percent (n=124) of the AJ subjects with longevity demonstrated global cognitive impairment. Detailed subject characteristics are shown in Table 1. Mean ± standard deviation (SD) of 25(OH)D levels were significantly lower in the group with global cognitive impairment compared to the group with normal cognition, 24.6 ± 9.79 ng/mL vs. 27.55 ± 12.09ng/mL, respectively, p=0.03. Of the participants with impaired cognition, 71.8% were found to have insufficient 25(OH)D concentration, compared to 57.7% of subjects with normal cognition, p=0.02. In the logistic regression analysis, insufficient serum 25(OH)D level was significantly associated with greater odds of global cognitive impairment in the unadjusted and multivariable adjusted models (Table 2, Models 1A–3A).
Table 1.
Subject characteristics (n=253) by cognitive function assessed by MMSE.
| Characteristic | Normal Cognition (n=129) | Impaired Cognition (n=124) | p-value |
|---|---|---|---|
| Demographics | |||
| Female, % | 71.3 | 77.4 | 0.267 |
| Age, years, median (IQRa) | 97 (95–104) | 97 (95–104) | 0.601 |
| Social and Lifestyle characteristics (n) | |||
| Education, years, mean ± SDb | 13.4 ± 3.8(111) | 12 ± 3.7(83) | 0.012 |
| College educated, % | 46.4(51) | 23.1(21) | 0.002 |
| Depression by GDSc, % | 33.7(31) | 50(24) | 0.061 |
| Vitamin D supplementation, % | 49.4(39) | 42.9(18) | 0.495 |
| Lifetime avoidance of sun exposure, % | 46.7(14) | 22.7(5) | 0.077 |
| Tobacco use, ever, % | 41.6(47) | 28.1(27) | 0.042 |
| Alcohol consumption at age 70 years, % | 47.2(51) | 49.4(44) | 0.757 |
| Physical and Cognitive Assessment(n) | |||
| BMId, kg/m2, mean ± SD | 21.61 ± 3.31 (110) | 20.85 ± 3.45 (82) | 0.121 |
| MMSE scoree, median (IQR) | 29 (18–30) | 9.5 (0–24) | <0.001 |
| Medical History, % (n) | |||
| Presence of ≥2 medical comorbiditiesf | 66.4(77) | 47.5(48) | 0.005 |
| Hypertensiong | 62.2 (61) | 46.3(37) | 0.033 |
| Diabetes Mellitush | 12(12) | 7.5(7) | 0.297 |
| Stroke | 17.8(16) | 8.6(7) | 0.08 |
| Myocardial Infarction | 17.4(17) | 7.9(7) | 0.053 |
| Heart diseasei | 51.9(54) | 30.8(28) | 0.003 |
| Cancer | 20.7(23) | 18.3(17) | 0.662 |
| Laboratory measures, mean ± SD (n) | |||
| Vitamin D, ng/mL | 27.55 ± 12.09 (129) | 24.6 ± 9.79 (124) | 0.033 |
| Total Cholesterol, mg/dL | 198.52±40.81 (125) | 192.76±38.94 (124) | 0.256 |
| HDL-Cj level, mg/dL | 57.13±16.42 (125) | 51.44±14.1 (124) | 0.004 |
| LDL-Ck level, mg/dL | 112.19±33.94 (124) | 110.83±34.11(121) | 0.755 |
| Triglyceride level, mg/dL | 141.32±75.55 (124) | 150.4±96.6 (123) | 0.412 |
| TSHl, mIU/L, median [IQR] | 1.92 [0.31–13.9](109) | 1.84 [0.22–6.63] (117) | 0.686 |
IQR: interquartile range
SD: standard deviation
Depression defined as a score of ≥6 on the Geriatric Depression Scale
BMI is calculated based on the formula: current weight in kilograms/maximum reported height in meters2
includes scores for blind MMSE
Medical co-morbidities include a history of heart disease, stroke, diabetes mellitus, hypertension, and cancer
Hypertension is defined as a history of hypertension or taking blood pressure medications
Diabetes Mellitus is defined as a history of diabetes mellitus or taking diabetes medications
Heart Disease is defined as a history of myocardial infarction or taking medications for heart disease
HDL-C: high density lipoprotein cholesterol
LDL-C: low density lipoprotein cholsterol
TSH: thyroid stimulating hormone
Table 2.
Logistic regression models for the odds ratio (OR) of cognitive impairment, as defined by Mini Mental State Exam (MMSE) and the Clock Drawing Tests (CDT: command and copy) in the setting of insufficient (<30ng/mL) vitamin D level
| Model (n) | OR | 95% CI | p-value |
|---|---|---|---|
| A. Cognition evaluated by MMSE | |||
| Model 1A. Unadjusted (253) | 1.89 | 1.12–3.19 | 0.017 |
| Model 2A. Adjusted for age and sex (253) | 2.02 | 1.18–3.45 | 0.01 |
| Model 3A. Fully adjusteda (107) | 3.2 | 1.1–9.29 | 0.033 |
| B. Cognition evaluated by CDT: command | |||
| Model 1B. Unadjusted (127) | 1.5 | 0.72–3.36 | 0.288 |
| Model 2B. Adjustedb (96) | 1.7 | 0.61–4.76 | 0.31 |
| C. Cognition evaluated by CDT: copy | |||
| Model 1C. Unadjusted (92) | 5.3 | 1.11–25.77 | 0.036 |
| Model 2C. Adjustedc (83) | 8.96 | 1.08–74.69 | 0.043 |
Adjusted for age, sex, BMI, education, history of tobacco use, depression, HDL cholesterol levels, and presence of ≥2 medical comorbidities.
Adjusted for age, sex, BMI, education, and the presence of ≥2 medical comorbidities.
Adjusted for education and the presence of ≥2 medical comorbidities.
25(OH)D levels and performance on the CDT
The command CDT was completed by 127 participants, of whom 46 (36%) were considered cognitively impaired. The 25(OH)D levels were significantly lower in participants who did not complete the command CDT compared to those who completed it (23.75±9.52ng/mL vs. 28.16±12ng/mL, p<0.001). Serum 25(OH)D levels did not differ significantly between those with impaired and normal executive function (26.07 ± 11.23ng/mL vs. 29.11 ± 12.21ng/mL, respectively, p=0.17). Median age, sex distribution, history of tobacco use, rates of depression and ≥2 comorbidities, as well as, mean HDL cholesterol levels, BMI, and years of education also did not differ significantly between the two cognitive groups. Executive function was not associated with 25(OH)D insufficiency in any of the logistic regression models, Table 2, Models 1B and 2B.
The copy CDT results were available for 92 participants, with 13 (14%) designated as impaired in visuospatial construction abilities. The 25(OH)D levels were significantly lower in centenarians who did not complete the command CDT (24.22±9.78ng/mL vs. 28.81±12.41ng/mL, p<0.001). In the group with visuospatial construction impairment, there was a trend toward lower mean 25(OH)D levels, as compared to the group with normal cognition (23.49 ± 11ng/mL vs. 29.69 ± 12.47ng/mL, p=0.096). The cognitive groups did not significantly differ in any other characteristics. Of those with normal visuospatial construction abilities, 51% demonstrated insufficient 25(OH)D levels, whereas 85% of those with impaired cognition had insufficient vitamin D concentrations, p=0.02. Serum 25(OH)D insufficiency was found to be independently associated with greater odds of cognitive impairment in the logistic regression models (Table 2, Models 1C and 2C).
DISCUSSION
In this cross-sectional analysis we demonstrated that 25(OH)D insufficiency is independently associated with increased odds of cognitive impairment in a cohort with exceptional longevity. Although this relationship has been previously observed in a number of elderly populations1–3,20, it has not been reported for a group with extended lifespan. Populations with exceptional longevity are unique in that typically they do not manifest the age-associated diseases until much later in life5, as compared to the general population, despite often having similar lifestyle habits as their peers21. Our results suggest that even in this population vitamin D levels may play an important role in cognitive function. Furthermore, the prevalence rates of 25(OH)D insufficiency in individuals with exceptional longevity were similar to a population up to twenty-five years younger and significantly lower compared to the oldest members of the general population, as sampled by the NHANES III. These unique characteristics highlight the delayed aging process exhibited by the centenarians. This is particularly exemplified by the centenarians with normal cognition, as demonstrated by both their cognitive function and high likelihood of having sufficient 25(OH)D levels.
Interestingly, we did not find an association between executive cognitive function and 25(OH)D. This finding is consistent with a study by Slinin et al.2, although several studies did note this association3,22. On the other hand, we did find an association between 25(OH)D and visual-spatial/construction abilities, which has been previously described22; although, not all studies have consistently identifying this relationship3. The discrepancy in the association of 25(OH)D and various measures of cognitive function may result from the differential affect of vitamin D on different areas of the brain. However, our findings may also be limited by insufficient power due to small sample size of individuals completing the CDT and by participation bias, with those having poorer cognition being less likely to participate in the CDT.
Vitamin D is a fat-soluble steroid hormone that operates via a nuclear vitamin D receptor and directly regulates gene transcription. Endogenous vitamin D synthesis requires conversion of 7-dehydrocholesterol to vitamin Din the skin upon exposure to sunlight, hydroxylation to 25(OH)D in the liver, and subsequent hydroxylation to the main active form, 1,25-dihydroxyvitamin D, primarily in the kidneys. The efficiency of these processes and the tissue response to 1,25-dihydroxyvitamin D decrease with age, making the elderly especially vulnerable to vitamin D deficiency4,23. In addition, frailty and immobility may lead to reduced sun exposure, resulting with decreased vitamin D synthesis. However, the fact that the centenarians did not demonstrate significant seasonal differences in 25(OH)D levels suggests that they may possess a unique mechanism, possibly genetically determined, for 25(OH)D homeostasis.
Although the precise mechanism for vitamin D regulation of cognition is not known, evidence is emerging for its action as a neurotrophic24 and anti-inflammatory factor25 in the central nervous system. In addition, vitamin D has been shown to inhibit the up regulation of inducible nitric oxide synthase activity26; which, if left uninhibited, results in production of excessive amounts of nitric oxide during ischemic events and leads to neuronal damage. Thus, vitamin D deficiency may be linked to inflammatory and vascular disease mechanisms underlying neurodegeneration.
Our study used a cross-sectional approach; therefore, we cannot rule out the possibility that vitamin D levels may have fluctuated in individuals throughout their lifetime. Another possibility is that individuals with cognitive impairment may be less likely to go outside due to functional limitations, which may result in lower 25(OH)D levels. In addition, since we relied on self-reported medical and social history, a potential for reporting bias exists, particularly in individuals with cognitive impairment who may not accurately recollect their past. However, given the fact that no significant differences were noted in the reported rates of many lifestyle and disease entities between the cognitive groups, under-reporting only for select factors is unlikely. Furthermore, for a population with exceptional longevity, lifestyle factors may not have as much effect as genetic factors, as previously reported21. Another consideration is that comparison of 25(OH)D levels between studies may be limited by the variations in vitamin D assays used. However, this does not pose a significant problem in this analysis, because the LC/MS/MS and the RIA (DiaSorin) methods used for assaying 25(OH)D demonstrate agreement, with a bias of only 0.64ng/mL27.
This study demonstrates that centenarians are not subjected to the same age-related decline in 25(OH)D level as the general elderly population. However, when 25(OH)D insufficiency occurs in centenarians, it is associated with cognitive impairment, albeit at a much later age than typically observed in the general population. Thus, our results suggest that 25(OH)D sufficiency may be a marker for delayed aging. Since we have previously found similar associations between high HDL cholesterol levels8,19, adiponectin levels7, and health status in our study population, it is possible that these parameters cluster in individuals with longevity and good health; thus, further research is needed to discern between simple associations and direct affect on the aging process. These findings may lead to improved understanding of mechanisms that mediate aging and cognitive decline and may result in interventions that would help maintain cognition for the duration of the lifespan in the general elderly population.
Acknowledgments
We thank Aileen P. McGinn, Ph.D., at the Albert Einstein College of Medicine for her expertise and assistance with the NHANES III database.
Funding Sources: SM is supported by Ellison Medical Foundation/American Federation for Aging Research Postdoctoral Research in Aging Grant. NB is supported by grants from the NIH/NIA: R01 AG 618381, P01 AG 021654, the Einstein Nathan Shock Center P30AG038072, and the Einstein Glenn Center for the Biology of Human Aging. MLM was supported by K23 78774 from the National Institute of Diabetes and Digestive and Kidney Diseases. The project described was supported by the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), through CTSA grant numbers UL1TR000086, TL1RR000087, and KL2TR000088. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Sponsor’s Role: The sponsor had no role in the conduct of the study, interpretation of results or manuscript preparation.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions:
Sofiya Milman contributed to study design, acquisition of data, data analysis, interpretation of analysis and manuscript preparation; Micol Schulder-Katz contributed to study design, acquisition of data and manuscript preparation; Jennifer Deluty contributed to study design, acquisition of data, and manuscript preparation; Molly E. Zimmerman contributed to study design; Jill P. Crandall contributed to interpretation of analysis and manuscript preparation; Nir Barzilai contributed to study design, interpretation of analysis and manuscript preparation; Michal L. Melamed contributed to acquisition of data and manuscript preparation; Gil Atzmon contributed to acquisition of data, data analysis, and manuscript preparation.
References
- 1.Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and cognitive impairment in the elderly U.S. population. J Gerontol A Biol Sci Med Sci. 2011;66:59–65. doi: 10.1093/gerona/glq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slinin Y, Paudel M, Taylor BC, et al. Association between serum 25(OH) vitamin D and the risk of cognitive decline in older women. J Gerontol A Biol Sci Med Sci. 2012;67:1092–1098. doi: 10.1093/gerona/gls075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2:1104–5. doi: 10.1016/s0140-6736(89)91124-0. [DOI] [PubMed] [Google Scholar]
- 5.Andersen SL, Sebastiani P, Dworkis DA, et al. Health span approximates life span among many supercentenarians: Compression of morbidity at the approximate limit of life span. J Gerontol A Biol Sci Med Sci. 2012;67:395–405. doi: 10.1093/gerona/glr223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atzmon G, Schechter C, Greiner W, et al. Clinical phenotype of families with longevity. J Am Geriatr Soc. 2004;52:274–277. doi: 10.1111/j.1532-5415.2004.52068.x. [DOI] [PubMed] [Google Scholar]
- 7.Atzmon G, Pollin TI, Crandall J, et al. Adiponectin levels and genotype: a potential regulator of life span in humans. J Gerontol A Biol Sci Med Sci. 2008;63:447–53. doi: 10.1093/gerona/63.5.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barzilai N, Atzmon G, Schechter C, et al. Unique lipoprotein phenotype and genotype associated with exceptional longevity. Jama. 2003;290:2030–40. doi: 10.1001/jama.290.15.2030. [DOI] [PubMed] [Google Scholar]
- 9.Barzilai N, Gabriely I, Atzmon G, et al. Genetic studies reveal the role of the endocrine and metabolic systems in aging. J Clin Endocrinol Metab. 2010;95:4493–4500. doi: 10.1210/jc.2010-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howden LMMJ. Age and Sex Composition: 2010. 2010 Census Briefs. 2011 Available from http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf.
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Rouleau I, Salmon DP, Butters N, et al. Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain Cogn. 1992;18:70–87. doi: 10.1016/0278-2626(92)90112-y. [DOI] [PubMed] [Google Scholar]
- 13.Stuss DT, Meiran N, Guzman DA, et al. Do long tests yield a more accurate diagnosis of dementia than short tests? A comparison of 5 neuropsychological tests. Arch Neurol. 1996;53:1033–1039. doi: 10.1001/archneur.1996.00550100119021. [DOI] [PubMed] [Google Scholar]
- 14.Busse A, Sonntag A, Bischkopf J, et al. Adaptation of dementia screening for vision-impaired older persons: Administration of the Mini-Mental State Examination (MMSE) J Clin Epidemiol. 2002;55:909–915. doi: 10.1016/s0895-4356(02)00449-3. [DOI] [PubMed] [Google Scholar]
- 15.Reischies FM, Geiselmann B. Age-related cognitive decline and vision impairment affecting the detection of dementia syndrome in old age. Br J Psychiatry. 1997;171:449–451. doi: 10.1192/bjp.171.5.449. [DOI] [PubMed] [Google Scholar]
- 16.Pinto E, Peters R. Literature review of the Clock Drawing Test as a tool for cognitive screening. Dement Geriatr Cogn Disord. 2009;27:201–213. doi: 10.1159/000203344. [DOI] [PubMed] [Google Scholar]
- 17.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: A study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 18.Gunter EL, Lewis BL, Koncikowski SM. Laboratory Methods Used for the Third National Health and Nutrition Examination Survey(NHANESIII),1988–1994. Hyattsville, MD: Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 19.Atzmon G, Gabriely I, Greiner W, et al. Plasma HDL levels highly correlate with cognitive function in exceptional longevity. J Gerontol A Biol Sci Med Sci. 2002;57:M712–715. doi: 10.1093/gerona/57.11.m712. [DOI] [PubMed] [Google Scholar]
- 20.Llewellyn DJ, Langa KM, Lang IA. Serum 25-hydroxyvitamin D concentration and cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22:188–195. doi: 10.1177/0891988708327888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajpathak SN, Liu Y, Ben-David O, et al. Lifestyle factors of people with exceptional longevity. J Am Geriatr Soc. 2011;59:1509–1512. doi: 10.1111/j.1532-5415.2011.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menant JC, Close JC, Delbaere K, et al. Relationships between serum vitamin D levels, neuromuscular and neuropsychological function and falls in older men and women. Osteoporos Int. 2012;23:981–989. doi: 10.1007/s00198-011-1637-7. [DOI] [PubMed] [Google Scholar]
- 23.Parfitt AM, Gallagher JC, Heaney RP, et al. Vitamin D and bone health in the elderly. Am J Clin Nutr. 1982;36:1014–1031. doi: 10.1093/ajcn/36.5.1014. [DOI] [PubMed] [Google Scholar]
- 24.Neveu I, Naveilhan P, Jehan F, et al. 1,25-dihydroxyvitamin D3 regulates the synthesis of nerve growth factor in primary cultures of glial cells. Brain Res Mol Brain Res. 1994;24:70–76. doi: 10.1016/0169-328x(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 25.Lin AM, Chen KB, Chao PL. Antioxidative effect of vitamin D3 on zinc-induced oxidative stress in CNS. Ann N Y Acad Sci. 2005;1053:319–329. doi: 10.1196/annals.1344.028. [DOI] [PubMed] [Google Scholar]
- 26.Garcion E, Nataf S, Berod A, et al. 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997;45:255–267. doi: 10.1016/s0169-328x(96)00260-4. [DOI] [PubMed] [Google Scholar]
- 27.van den Ouweland JM, Beijers AM, Demacker PN, et al. Measurement of 25-OH-vitamin D in human serum using liquid chromatography tandem-mass spectrometry with comparison to radioimmunoassay and automated immunoassay. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1163–1168. doi: 10.1016/j.jchromb.2010.03.035. [DOI] [PubMed] [Google Scholar]