Summary
B cell-activating factor belonging to the TNF family (BAFF) exerts its pathogenic role in supporting the survival and proliferation of B cells, regulating class switch recombination as well as the selection of autoreactive B cells. Overexpression of BAFF induces a dramatic expansion of activated B cells, particularly marginal zone B cells, as well as hypergammaglobulinemia, autoantibody production and immune complex deposition. However, in addition to its effect on B cells, recent work has also demonstrated that BAFF can promote T cell activation, proliferation and differentiation. In this review, we have discussed the recent progress on the function and role of BAFF on T cells and T cell-mediated diseases.
Keywords: BAFF, T helper cells, Th17, Tfh cells, Treg cells
1. Induction
In the periphery the B cells maturation depends on the BCR signal, the surrounding stromal micro-environment including appropriate growth factors, and their ability to respond to them. One of the tumor necrosis factor superfamily members, BAFF (B cell-activating factor belonging to the TNF family) (also termed BLyS, zTNF4, TANK, TALL-1 and TNFSF-13b), is an important B-cell survival factor, being primarily expressed by monocytes, macrophages, dendritic cells, neutrophils and mast cells, and functioning to stimulate B cell proliferation, differentiation, and survival [1, 2]. Three BAFF receptors have been identified, including transmembrane activator and calcium-modulating and cyclophilin ligand interactor (TACI, also known as TNFRSF13b), B cell activating factor-receptor (BAFF-R, also known as BR3 and TNFRSF13c) and B cell maturation molecule (BCMA, also known as TNFRSF17). The binding and downstream signaling of BAFF and BAFF receptors are essential for B cells survival and maturation [3]. BAFF-R is the predominant BAFF receptor expressed on peripheral B cells and activated/memory T cells. BCMA and TACI express on a more confined cell subsets. BCMA is mainly expressed on germinal center B cells, plasmblasts and plasma cells whereas TACI is basically expressed by transitional type 2 precursor B cells, marginal zone B cells and activated B cells [4]. BAFF or its receptor BAFF-R deficiency results in immature transitional type-1 stage B cells, and BAFF or BAFF-R knock out mice appeared to have almost none of follicular and marginal zone B cells, although the development of B-1 B cells remains to be unaffected [5, 6]. Furthermore, BAFF plays an important role in regulating class switch recombination as well as in the selection of autoreactive B cells. Blocking BAFF signaling with anti-BAFF-R mAb in vivo also dramatically reduced the follicular and marginal zone B cell numbers [7]. Overexpression of BAFF in mice induces a dramatic expansion of activated B cells, marginal zone B cells and activated T cells, as well as hypergammaglobulinemia, autoantibody production and immune complex deposition [8, 9]. Thus, BAFF and its receptors signaling play an important role in promoting the survival and maintenance of follicular and marginal zone B cells and B cell function.
BAFF also plays a critical role in many autoimmune and other diseases. Increased concentrations of soluble BAFF are found in different pathological conditions, including systemic lupus erythematosus (SLE) and multiple sclerosis (MS), B cell malignancies, and primary Ab deficiencies (PAD) [2, 10, 11]. A direct correlation between serum concentration of BAFF and severity of acute graft-versus-host disease (GVHD) after allogeneic hematopoietic stem transplantation has been identified [12]. Blocking BAFF signaling with TACI-Ig in vitro suppressed spontaneous T cell-dependent B cell anti-dsDNA antibodies production, which is possibly related to the effect on B cell survival [13]. It is helpful to define the mechanisms of BAFF on different immune cells, particularly on B cells [14, 15], however, its function on T cells so far is less studied.
A proliferation-inducing ligand (APRIL), exhibiting structural similarity with BAFF, also plays an important role in the regulation of B-cell survival, differentiation and proliferation [16]. However, BAFF and APRIL display overlapping yet distinct receptor binding specificity. Both BAFF and APRIL bind BCMA (APRIL has higher affinity) although both bind the negative regulator TACI with similar affinity. In addition, BAFF-R exclusively binds BAFF with high affinity [16-18]. Furthermore, APRIL also has the capacity to bind heparin sulfate proteoglycans (HSPGs), which may help to retain to BCMA/TACI affinity [16, 19]. Since T cells only express BAFF-R and hardly bind to APRIL, and only rBAFF induced cytokine secretion by CD4+ and CD8+ T cells in vitro [20, 21], these data implicate that BAFF rather than APRIL could directly affect T cell differentiation and function. In this review, we will focus on the progress of role and function of BAFF in T cells and related diseases (Fig. 1).
Fig. 1.
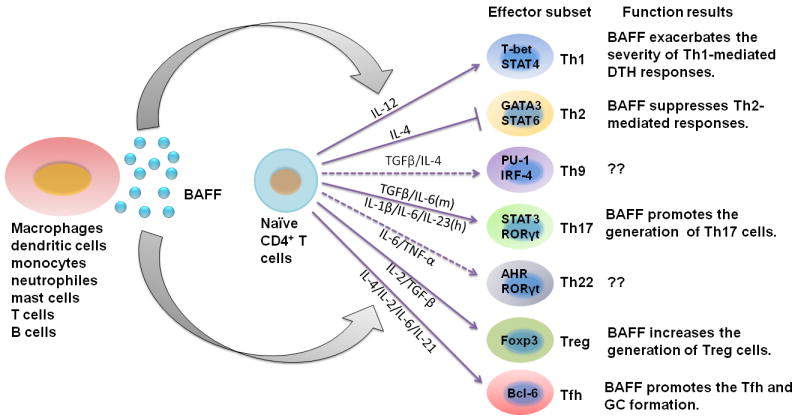
The different function of BAFF on effector T cells. Several kinds of peripheral cells may secrete soluble BAFF as shown in the figure. BAFF then promote or inhibit the differentiation of naive CD4+ T cells to Th1, Th2, Th17, T follicular helper T cells and Treg cells, resulting in corresponding consequences. Blue solid arrows represent stimulatory effect, and broken lines represent suppressive effect of BAFF.
2. Are T cells necessary for BAFF function on B cells?
BAFF transgenic (Tg) mice developed an autoimmune disorder similar to SLE [22]. BAFF-Tg mice show higher frequency of B cells and autoantibody production. Interestingly, in MHC class II-deficient mice which has few CD4+ T cells, overexpression of BAFF did not expand splenic B cells albeit increased the numbers of antibody secreting cells as well as total IgM, IgG autoantibodies [23], indicating that CD4+ T helper cells may play an important role in the expansion of B cells and increased autoantibodies by BAFF overexpression. Blocking BAFF signaling with BAFF-R-Ig or TACI-Ig treatment not only downregulates the B cell responses, but also decreases the frequency of activated and memory T cells [24]. However, BAFF transgenic mice with T cell deficiency still developed autoimmunity like SLE in a T cell-independent but toll-like receptor (TLR) signaling-dependent manner [22], suggesting that BAFF promotes autoimmunity independent upon T cells although T cells are required for BAFF to promote B cell expansion.
3. The differential expression of BAFF on T cell subsets
There are two distinct sources of BAFF in mice. The major one is from stromal cells, which is thought to regulate maturation of the peripheral B cells, and the second source comes from the secretion of myeloid cells during pathological conditions [25, 26]. Although no evidence has showed that mouse T cells express BAFF, a low level of BAFF transcription has been detected in human T cells [27]. CD4+ and CD8+ T cells from peripheral blood of patients with active SLE or salivary glands from primary Sjogren’s syndrome (pSS) patients expressed intracellular BAFF whereas those from normal subjects did not [13, 28]. It has been identified that TCR stimulation with anti-CD3 antibody induced a robust expression of BAFF on T cells of SLE patients but not on T cells from health subject controls [29], implicating that the threshold to induce BAFF in T cells upon TCR stimulation is much lower under pathological conditions. Furthermore, TCR stimulation also triggers BAFF expression in the human T cell line Loucy via the mitogen-activated protein kinase (MAPK) (JNK/p38) cascade signal, and Furin, a membrane-bound protease of the Subtilisin family, is responsible for the cleavage of BAFF to release sBAFF in Loucy cells [29].
4. BAFF contributes to T cell activation
BAFF co-stimulation promotes T cell activation with cytokine production via BAFF-R in vitro and in vivo [4, 21]. BAFF co-stimulation drives T cell proliferation and up-regulates Bcl-2 expression in activated T cells, suggesting that BAFF might act as a survival factor of T cell activation as of B cells [4, 21]. BAFF-deficient mice have displayed impaired T cell-mediated allograft rejection and prolonged cardiac allograft survival, implicating that BAFF may play a role in co-stimulating T cell activation and allo-proliferation [30]. It has been demonstrated that mouse T cells express the BAFF receptors TACI and BAFF-R, but not BCMA [4, 26, 30]. Moreover, T cells from BAFF-R mutant mice fail to respond to BAFF, indicating that BAFF provides co-stimulatory signals to T cells via BAFF-R other than TACI [4, 30]. Thus, BAFF may regulate T cell immune responses as an autocrine factor [27]. Administration of rBAFF in mice increased CD4+ T lymphocyte including effector T cells and memory T cells, but not CD8+ T cells [31]. Nonetheless, this is not the case in human research. Human T cells do not express TACI or BCMA [4]. In an in vitro assay, recombinant human BAFF (rhBAFF) promoted the survival of human CD8+ T cells and decreased the annexin V percentage of CD8+ cells. Conversely, BAFF did not affect apoptosis of human CD4+ T cells [32]. In addition, blocking the BAFF signal with the decoy receptor TACI-Fc fusion protein promoted the apoptosis of T lymphocytes [33]. Scapini et al [26] has proved that BAFF stimulated T cell activation in a BAFF-R dependent pathway. In autoimmunity disease progression model by Lyn deficiency, dysregulated BAFF production by myeloid cells stimulated T cell activation to secrete IFN-γ which then promotes BAFF production from myeloid cells, this inflammatory loop plays a critical role in the progress of autoimmunity in lyn-/- mice [26]. Collectively, T cells maybe one of the major targets of BAFF-associated autoimmune diseases.
5. Distinct effects of BAFF on Th1/2 responses
Dysregulation of Th1 or Th2 responses may contribute to the pathogenesis of autoimmune and allergic diseases. Th1 responses control intracellular pathogens including bacteria and viruses via the production of IFN-γ, TNF-α and TNF-β, and recruitment of phagocytic leukocytes [34, 35]. It has been reported that IFN-γ and/or TNF-α are involved in the induction of BAFF not only in neutrophils, fibroblast-like synoviocytes (FLS), monocytes and dendritic cells [36, 37], but also in human T cells and T cell line Loucy [29, 38]. In rheumatoid arthritis, the proinflammatory cytokines IFN- γ and TNF-α can induce mesenchymal-derived FLS to express functional BAFF which may prevent apoptosis of B cells in inflammatory microenvironments and increase autoantibody production in vivo [36]. Systemic overexpression of BAFF in BAFF Tg mice exacerbates the severity of Th1-mediated delayed-type hypersensitivity (DTH) responses accompanying with the enhanced T cell expansion and effector function, and BAFF also provides survival signals to the central and effector memory T cells that express BAFF-R [4, 35].
Th2 responses control infections by extracellular parasites, in part through the production of IL-4, IL-5 and IL-13, and recruitment of eosinophils [34, 35]. In a Th2-mediated allergic airways disease model, BAFF overexpression resulted in the suppressed Th2-mediated responses with a markedly reduced Ag-specific T cell proliferation and eosinophil infiltration around airways and pulmonary blood vessels [35]. A possible explanation for the suppression of Th2 effector function in BAFF Tg mice may be due to the increase of local regulatory T (Treg) cells that subsequently inhibit Th2 airway responses, since BAFF Tg mice expressed higher number of Treg cells that possess the ability to home to inflammatory sites [39]. It is arguable since Treg cell also suppress Th1 cells. Besides, the differential effects of BAFF on Th1/Th2 responses may be explained by the B cells. It has been reported that enhanced Th1 responses depend on B cells, whereas BAFF inhibition of Th2 responses is B cell independent [35].
6. BAFF promotes Th17 cells production in vitro and in vivo
IL-17 is a pro-inflammatory cytokine implicated in autoimmune and inflammatory conditions. Constitutive overexpression of BAFF in BAFF-Tg mice promotes the generation Th17 cell in vitro and in vivo, and aggravates the manifestation of Th17 cell-driven disease, experimental autoimmune encephalomyelitis (EAE) [40]. There were comparable expression of IL-17 in second lymphoid organs in BAFF Tg mice and BAFF−/− mice bearing a BAFF Tg (B6.BAFF−/−.BAFF Tg mice), which express large quantities of soluble BAFF but do not express membrane BAFF on their myeloid-lineage cells, indicating that circulating soluble BAFF rather than membrane BAFF is required for Th17 cell generation [40]. Intra-articular injection of lentivirus expressing shRNA for BAFF gene silencing provided long-term suppression of autoimmune arthritis development, and suppressed generation of plasma cells and Th17 cells, and markedly ameliorated joint pathology [41]. The effect of driving Th17-cell differentiation by BAFF may attribute to the fact that BAFF increased IL-6 receptor expression on CD4+ T cells, and enhanced IL6-IL-6R/p-STAT3 signaling pathway, and promoted the dendritic cell maturation and function [40, 41]. It has been reported that IL-6/IL-6R signal pathway is crucial for Th17 cell differentiation [42-44].
7. The role of BAFF on germinal center (GC) formation and Tfh production
BAFF-Tg mice develop autoimmunity resembling systemic lupus erythematosus (SLE), including hyperplasia of mature B cells, autoantibody production, and deposition in the kidneys [15]. BAFF overexpression rescues self-reactive B cells from peripheral deletion allowing maturation and colonization in the follicular and marginal zone, which then enhances autoantibody production [45]. Follicular and marginal zone B cells are important for the GC formation and antibody production. In the non-diabetic obese NOD mice BAFF neutralization therapy has led to the depletion of follicular and marginal zone B cells, and long-term BAFF neutralization increased the transitional:follicle B cell ratio in the periphery lymphoid organs, indicating an important role of BAFF on GC formation [46]. BAFF is also necessary for the formation of a follicular dendritic cell (FDC) network within GC, due to its help for DC maturation, while BAFF blockade decreased FDC activation and impaired GC function [47]. Furthermore BAFF-deficient mice had an almost complete loss of follicular and marginal zone B lymphocytes and severely impaired humoral responses to T-dependent antigens (Ag) although antigen-specific class switched antibodies were still produced [7, 48, 49]. GC responses and somatic hypermutation are efficiently induced after Ag challenge in BAFF-/- mice despite reduced B cell numbers, however GC dissipated rapidly and did not form a mature follicular dendritic cell reticulum [50, 51]. Therefore, BAFF is required for maintenance, but not initiation, of the GC reaction. Nonetheless, the pathogenic effect of BAFF on the production of autoantibodies was independent of GC formation. BAFF Tg mice with T cell deficiency appeared to be absent of GC, but still produced increased IgG and IgM autoantibodies [22].
Follicular helper T cells (Tfh) have been referred as a cell lineage that provides help for B cells to proliferate and undergo antibody affinity maturation in the GC. Tfh cells are described as a subset of CD4+ T cells expressing the chemokine receptor CXCR5, inducible T cell costimulator (ICOS), PD-1, B and T lymphocyte associated (BTLA), signaling lymphocytic activation molecule (SLAM)-associated protein (SAP), and the transcription factor B cell lymphoma 6 (Bcl-6), and produced the cytokines IL-21 and IL-4 [52, 53]. We have demonstrated that Tfh cells express BAFF-R, but not BCMA or TACI (Chen et al. MS submitted). This suggests that BAFF may play a role in Tfh formation or function. Ou et al [54] have reported that TACI deficient mice showed increased Tfh cells and GC B cells after T cell dependent antigen stimulation, which depends on the expression of inducible costimulator (ICOS) ligand on TACI-deficient B cells. However, the exact effect of BAFF on Tfh cells is not clear, and is difficult to address due to the following reasons: 1) BAFF is secreted not only by follicular dendritic cells but also by T cells. 2) BAFF receptor exists on both T cells and B cells. 3) GC formation and somatic hypermutation depend on Tfh cells, B cells and FDC cells. 4) BAFF-deficient mice are largely deficient of B cells. Our recent data has suggested that the combination of BAFF and B cells is required for Tfh cell differentiation (Chen et al. MS submitted). Therefore it is likely that BAFF plays an essential role in the development of Tfh cells.
8. Role of BAFF on the regulatory T cells
BAFF has a complex role in the regulation of immune responses. Besides of increased autoantibody production, BAFF-Tg mice showed acceptance of islet allografts and delayed skin graft rejection [39]. The increased Tregs in BAFF-Tg mice may account of this immune tolerance because T effector cells hold normal function in these mice [22]. The phenotype of BAFF-expanded Tregs was CD62LlowCD103highICAM-1high, which was consistent with an ability to home to inflammatory sites and prevent T effector cell responses [39]. It has been shown that a large proportion of CD4+CD25+ regulatory T cells in the mouse expresses BAFF-R [30], but excessive BAFF can not trigger NF-κB2 activation in Tregs [39]. These data imply that there may be other unknown mechanisms involved in the Treg expansion by BAFF.
Our group and others have reported that interleukin-2 (IL-2) and TGF-β play a critical role in the generation, function and stabilization of CD4+Foxp3+ induced Treg cells [55-57]. TGF-β stimulated macrophages to express BAFF via its downstream signals including Smad3 and Smad4 [58, 59]. Reports showed that IL-2 dose-dependently stimulates BAFF synthesis in human T cells in vitro, and an anti-IL-2 antibody neutralized this effect [33, 60]. IL-2 is crucial for Treg cells production and maintenance [61]. It needs further investigation how IL-2 and BAFF work together to promote Treg cells.
Taken together, BAFF plays a dichotomous effect on T cell immune responses. BAFF not only expands CD4+CD62LlowCD44high activated memory T cells and exaggerates delayed-type hypersensitivity (DTH) responses [35, 62], but also increases the number of functional CD4+Foxp3+ regulatory T cells that induces allograft tolerance [39], implicating an immunoregulatory function for BAFF in T cell immunity.
Acknowledgments
This work was supported in part by grants from the NIH (AR059103 and AI084359), ACR Within Our Reach Fund, Arthritis Foundation and Wright Foundation, National Natural Science Foundation of China (No. 81373156); Science and Technology Planning Project of Guangdong Province, China (No. 2010B031600200).
Biography
Song Guo Zheng earned his M.D. at Anhui Medical University, M.S. at Fudan University Shanghai Medical School, and PhD at French National Center for Scientific Research and University of Orleans. He did his postdr studies at UCLA and University of Southern California (USC). He was appointed as Assistant Professor at USC in 2004 and Associate Professor at USC in 2010 and has been a director of Immune Regulation and Tolerance Lab at USC since 2004. He has been appointed as Professor of Medicine (tenured) and Director of Autoimmunity Research Center at Penn State University Hershey College of Medicine since 2013. Dr. Zheng and his colleagues discover TGF-β-induced regulatory T cells and focuses on the development and function of regulatory and effector T cells in autoimmunity.

Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mackay F, Figgett WA, Saulep D, Lepage M, Hibbs ML. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205–25. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- 2.Thangarajh M, Gomes A, Masterman T, Hillert J, Hjelmstrom P. Expression of B-cell-activating factor of the TNF family (BAFF) and its receptors in multiple sclerosis. J Neuroimmunol. 2004;152:183–90. doi: 10.1016/j.jneuroim.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Qin Q, Chang Y, Wang D, Wu Y, Zhang LL, Wei W. TACI-Ig induces immune balance of Th cells in MLN via BLyS/APRIL-receptors signaling in rats with adjuvant-induced arthritis. Int Immunopharmacol. 2011;11:2167–75. doi: 10.1016/j.intimp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Ng LG, Sutherland AP, Newton R, Qian F, Cachero TG, Scott ML, et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. 2004;173:807–17. doi: 10.4049/jimmunol.173.2.807. [DOI] [PubMed] [Google Scholar]
- 5.Swee LK, Tardivel A, Schneider P, Rolink A. Rescue of the mature B cell compartment in BAFF-deficient mice by treatment with recombinant Fc-BAFF. Immunol Lett. 2010;131:40–8. doi: 10.1016/j.imlet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Kalled SL. The role of BAFF in immune function and implications for autoimmunity. Immunol Rev. 2005;204:43–54. doi: 10.1111/j.0105-2896.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 7.Rauch M, Tussiwand R, Bosco N, Rolink AG. Crucial role for BAFF-BAFF-R signaling in the survival and maintenance of mature B cells. PLoS One. 2009;4:e5456. doi: 10.1371/journal.pone.0005456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marston B, Looney RJ. Connective tissue diseases: Translating the effects of BAFF in SLE. Nat Rev Rheumatol. 2010;6:503–4. doi: 10.1038/nrrheum.2010.136. [DOI] [PubMed] [Google Scholar]
- 9.Stohl W, Xu D, Kim KS, Koss MN, Jorgensen TN, Deocharan B, et al. BAFF overexpression and accelerated glomerular disease in mice with an incomplete genetic predisposition to systemic lupus erythematosus. Arthritis Rheum. 2005;52:2080–91. doi: 10.1002/art.21138. [DOI] [PubMed] [Google Scholar]
- 10.Eilertsen GO, Van Ghelue M, Strand H, Nossent JC. Increased levels of BAFF in patients with systemic lupus erythematosus are associated with acute-phase reactants, independent of BAFF genetics: a case-control study. Rheumatology (Oxford) 2011;50:2197–205. doi: 10.1093/rheumatology/ker282. [DOI] [PubMed] [Google Scholar]
- 11.Kreuzaler M, Rauch M, Salzer U, Birmelin J, Rizzi M, Grimbacher B, et al. Soluble BAFF Levels Inversely Correlate with Peripheral B Cell Numbers and the Expression of BAFF Receptors. J Immunol. 2012;188:497–503. doi: 10.4049/jimmunol.1102321. [DOI] [PubMed] [Google Scholar]
- 12.Kim JS, Kim SJ, Cheong JW, Kim Y, Hwang DY, Yoon S, et al. Clinical significance of B cell-activating factor (BAFF) and a proliferation-inducing ligand (APRIL) in acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Korean J Hematol. 2011;46:175–9. doi: 10.5045/kjh.2011.46.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morimoto S, Nakano S, Watanabe T, Tamayama Y, Mitsuo A, Nakiri Y, et al. Expression of B-cell activating factor of the tumour necrosis factor family (BAFF) in T cells in active systemic lupus erythematosus: the role of BAFF in T cell-dependent B cell pathogenic autoantibody production. Rheumatology (Oxford) 2007;46:1083–6. doi: 10.1093/rheumatology/kem097. [DOI] [PubMed] [Google Scholar]
- 14.Youinou P, Pers JO. The late news on baff in autoimmune diseases. Autoimmun Rev. 2010;9:804–6. doi: 10.1016/j.autrev.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher CA, Groom JR, Woehl B, Leung H, Mackay C, Mackay F. Development of autoimmune nephritis in genetically asplenic and splenectomized BAFF transgenic mice. J Autoimmun. 2011;36:125–34. doi: 10.1016/j.jaut.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev. 2011;244:115–33. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day ES, Cachero TG, Qian F, Sun Y, Wen D, Pelletier M, et al. Selectivity of BAFF/BLyS and APRIL for binding to the TNF family receptors BAFFR/BR3 and BCMA. Biochemistry. 2005;44:1919–31. doi: 10.1021/bi048227k. [DOI] [PubMed] [Google Scholar]
- 18.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–9. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 19.Kimberley FC, van Bostelen L, Cameron K, Hardenberg G, Marquart JA, Hahne M, et al. The proteoglycan (heparan sulfate proteoglycan) binding domain of APRIL serves as a platform for ligand multimerization and cross-linking. FASEB J. 2009;23:1584–95. doi: 10.1096/fj.08-124669. [DOI] [PubMed] [Google Scholar]
- 20.Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero TG, et al. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med. 2005;201:1375–83. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huard B, Schneider P, Mauri D, Tschopp J, French LE. T cell costimulation by the TNF ligand BAFF. J Immunol. 2001;167:6225–31. doi: 10.4049/jimmunol.167.11.6225. [DOI] [PubMed] [Google Scholar]
- 22.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–71. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stohl W, Jacob N, Guo S, Morel L. Constitutive overexpression of BAFF in autoimmune-resistant mice drives only some aspects of systemic lupus erythematosus-like autoimmunity. Arthritis Rheum. 2010;62:2432–42. doi: 10.1002/art.27502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn P, Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, et al. Prevention of murine antiphospholipid syndrome by BAFF blockade. Arthritis Rheum. 2008;58:2824–34. doi: 10.1002/art.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–9. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, et al. Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J Exp Med. 2010;207:1757–73. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huard B, Arlettaz L, Ambrose C, Kindler V, Mauri D, Roosnek E, et al. BAFF production by antigen-presenting cells provides T cell co-stimulation. Int Immunol. 2004;16:467–75. doi: 10.1093/intimm/dxh043. [DOI] [PubMed] [Google Scholar]
- 28.Lavie F, Miceli-Richard C, Quillard J, Roux S, Leclerc P, Mariette X. Expression of BAFF (BLyS) in T cells infiltrating labial salivary glands from patients with Sjogren’s syndrome. J Pathol. 2004;202:496–502. doi: 10.1002/path.1533. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimoto K, Takahashi Y, Ogasawara M, Setoyama Y, Suzuki K, Tsuzaka K, et al. Aberrant expression of BAFF in T cells of systemic lupus erythematosus, which is recapitulated by a human T cell line, Loucy. Int Immunol. 2006;18:1189–96. doi: 10.1093/intimm/dxl053. [DOI] [PubMed] [Google Scholar]
- 30.Ye Q, Wang L, Wells AD, Tao R, Han R, Davidson A, et al. BAFF binding to T cell-expressed BAFF-R costimulates T cell proliferation and alloresponses. Eur J Immunol. 2004;34:2750–9. doi: 10.1002/eji.200425198. [DOI] [PubMed] [Google Scholar]
- 31.Shan X, Chen L, Cao M, Xu L, Zhang S. Effects of human soluble BAFF synthesized in Escherichia coli on CD4+ and CD8+ T lymphocytes as well as NK cells in mice. Physiol Res. 2006;55:301–7. doi: 10.33549/physiolres.930816. [DOI] [PubMed] [Google Scholar]
- 32.Zhu XJ, Shi Y, Peng J, Guo CS, Shan NN, Qin P, et al. The effects of BAFF and BAFF-R-Fc fusion protein in immune thrombocytopenia. Blood. 2009;114:5362–7. doi: 10.1182/blood-2009-05-217513. [DOI] [PubMed] [Google Scholar]
- 33.Xu H, He X, Zhu Y, Yan T, Ma H, Zhang X. Abnormally high expression of BAFF on T lymphocytes from lung cancer-associated pleural effusions and its potent anti-tumor effect. Acta Biochim Biophys Sin (Shanghai) 2007;39:964–73. doi: 10.1111/j.1745-7270.2007.00362. [DOI] [PubMed] [Google Scholar]
- 34.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–6. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland AP, Ng LG, Fletcher CA, Shum B, Newton RA, Grey ST, et al. BAFF augments certain Th1-associated inflammatory responses. J Immunol. 2005;174:5537–44. doi: 10.4049/jimmunol.174.9.5537. [DOI] [PubMed] [Google Scholar]
- 36.Ohata J, Zvaifler NJ, Nishio M, Boyle DL, Kalled SL, Carson DA, et al. Fibroblast-like synoviocytes of mesenchymal origin express functional B cell-activating factor of the TNF family in response to proinflammatory cytokines. J Immunol. 2005;174:864–70. doi: 10.4049/jimmunol.174.2.864. [DOI] [PubMed] [Google Scholar]
- 37.Scapini P, Carletto A, Nardelli B, Calzetti F, Roschke V, Merigo F, et al. Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood. 2005;105:830–7. doi: 10.1182/blood-2004-02-0564. [DOI] [PubMed] [Google Scholar]
- 38.Scapini P, Nardelli B, Nadali G, Calzetti F, Pizzolo G, Montecucco C, et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. J Exp Med. 2003;197:297–302. doi: 10.1084/jem.20021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walters S, Webster KE, Sutherland A, Gardam S, Groom J, Liuwantara D, et al. Increased CD4+Foxp3+ T cells in BAFF-transgenic mice suppress T cell effector responses. J Immunol. 2009;182:793–801. doi: 10.4049/jimmunol.182.2.793. [DOI] [PubMed] [Google Scholar]
- 40.Zhou X, Xia Z, Lan Q, Wang J, Su W, Han YP, et al. BAFF promotes Th17 cells and aggravates experimental autoimmune encephalomyelitis. PLoS One. 2011;6:e23629. doi: 10.1371/journal.pone.0023629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai Kwan Lam Q, King Hung Ko O, Zheng BJ, Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc Natl Acad Sci U S A. 2008;105:14993–8. doi: 10.1073/pnas.0806044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–6. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 43.Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, et al. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010;185:2675–9. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serada S, Fujimoto M, Mihara M, Koike N, Ohsugi Y, Nomura S, et al. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105:9041–6. doi: 10.1073/pnas.0802218105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–98. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Zekavat G, Rostami SY, Badkerhanian A, Parsons RF, Koeberlein B, Yu M, et al. In vivo BLyS/BAFF neutralization ameliorates islet-directed autoimmunity in nonobese diabetic mice. J Immunol. 2008;181:8133–44. doi: 10.4049/jimmunol.181.11.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moisini I, Davidson A. BAFF: a local and systemic target in autoimmune diseases. Clin Exp Immunol. 2009;158:155–63. doi: 10.1111/j.1365-2249.2009.04007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–4. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 49.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 50.Rahman ZS, Rao SP, Kalled SL, Manser T. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med. 2003;198:1157–69. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vora KA, Wang LC, Rao SP, Liu ZY, Majeau GR, Cutler AH, et al. Cutting edge: germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. J Immunol. 2003;171:547–51. doi: 10.4049/jimmunol.171.2.547. [DOI] [PubMed] [Google Scholar]
- 52.Bi E, Ye BH. An expanding job description for bcl6. J Mol Cell Biol. 2010;2:5–7. doi: 10.1093/jmcb/mjp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen M, Guo Z, Ju W, Ryffel B, He X, Zheng SG. The development and function of follicular helper T cells in immune responses. Cell Mol Immunol. 2012 doi: 10.1038/cmi.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ou X, Xu S, Lam KP. Deficiency in TNFRSF13B (TACI) expands T-follicular helper and germinal center B cells via increased ICOS-ligand expression but impairs plasma cell survival. Proc Natl Acad Sci U S A. 2012;109:15401–6. doi: 10.1073/pnas.1200386109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lan Q, Fan H, Quesniaux V, Ryffel B, Liu Z, Zheng SG. Induced Foxp3(+) regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biol. 2012;4:22–8. doi: 10.1093/jmcb/mjr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horwitz DA, Zheng SG, Wang J, Gray JD. Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3+CD4+ Treg. Eur J Immunol. 2008;38:912–5. doi: 10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- 57.Tang Q, Bluestone JA, Kang SM. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4:11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HA, Jeon SH, Seo GY, Park JB, Kim PH. TGF-beta1 and IFN-gamma stimulate mouse macrophages to express BAFF via different signaling pathways. J Leukoc Biol. 2008;83:1431–9. doi: 10.1189/jlb.1007676. [DOI] [PubMed] [Google Scholar]
- 59.Lu L, Wang J, Zhang F, Chai Y, Brand D, Wang X, et al. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suzuki K, Setoyama Y, Yoshimoto K, Tsuzaka K, Abe T, Takeuchi T. Effect of interleukin-2 on synthesis of B cell activating factor belonging to the tumor necrosis factor family (BAFF) in human peripheral blood mononuclear cells. Cytokine. 2008;44:44–8. doi: 10.1016/j.cyto.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–27. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 62.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]


