Abstract
Background
Despite high hepatitis B virus (HBV) endemicity in various resource-limited settings (RLS), the impact of maternal HIV-HBV coinfection on infant health outcomes has not been defined.
Method
This study determined the seroprevalence of HBV coinfection among HIV-infected pregnant women enrolled in the India six-week extended-dose nevirapine (SWEN) trial. The impact of maternal HIV-HBV coinfection on MTCT of HIV and infant mortality was assessed using univariate and multivariate logistic regression analysis.
Results
Among 689 HIV-infected pregnant Indian women, 32 (4.6%) had HBV coinfection (95% confidence interval [CI] 3.4, 5.3). HBV DNA was detectable in 18 (64%) of 28 HIV-HBV coinfected women; the median HBV viral load was 155 copies/mL (interquartile range [IQR] < 51–6741). Maternal HIV-HBV coinfection did not increase HIV transmission (adjusted odds ratio [aOR] 1.06, 95% CI 0.30, 3.66; p= 0.93). Increased odds of all-cause infant mortality was noted (aOR 3.12, 95% CI 0.67, 14.57; p=0.15), but was not statistically significant.
Conclusion
The prevalence of active maternal HBV co-infection in HIV-infected pregnant women in India was 4.6%. HIV-HBV coinfection was not independently associated with HIV transmission.
Keywords: Hepatitis B virus infection, HIV/AIDS, infant mortality, perinatal infection, India, pregnancy, maternal-to-child transmission
Introduction
Worldwide, approximately 2–4 million HIV-infected persons are coinfected with HBV (defined as HBsAg positive). HIV-HBV coinfection prevalence varies by region, ranging from less than 2% in developed countries to up to 20% in HBV-endemic, resource-limited settings (RLS) such as Asia and Africa. 1,2 HIV-HBV coinfection is associated with low CD4 cell counts and liver disease progression, and MTCT of both HIV and HBV in endemic regions is well documented. However, the impact of maternal HIV-HBV co-infection on maternal and infant health outcomes, including HIV transmission and infant mortality, has not been established in endemic regions.
Studies have associated several maternal HIV coinfections (including cytomegalovirus, hepatitis C virus, active tuberculosis, and malaria) with adverse pregnancy and infant health outcomes, and maternal malaria and tuberculosis coinfections are established risk factors for maternal-to-child transmission (MTCT) of HIV and infant mortality. 3–6 In contrast, data linking maternal HIV-HBV coinfection to adverse pregnancy outcomes such as preterm labor and low birth weight are inconclusive,7–17 and the impact of maternal HIV-HBV coinfection on MTCT of HIV and infant mortality remains unclear.18,19 Definitive studies to establish the role of maternal HIV-HBV coinfection in HBV-endemic RLS are needed and may prioritize changes to local standard of care practices, including routine antepartum screening for HBV in HIV-infected women and timely vaccination/treatment of HIV-HBV co-infected women and their exposed infants.
The India SWEN trial presents the opportunity to assess the prevalence of HBV coinfection among HIV-infected pregnant women in an HBV-endemic region and to evaluate the impact of maternal HIV-HBV coinfection on HIV transmission and infant mortality.
Methods
Study population
The study population comprised 737 mother-infant pairs enrolled in the SWEN India trial, a National Institute of Health (NIH)-funded phase III randomized controlled trial for the prevention of MTCT of HIV in breastfed infants.20 The study assessed the efficacy of six-week extended-dose nevirapine prophylaxis administered to breastfed infants. The primary outcome was HIV transmission at age 6 months, and key secondary outcomes were HIV transmission at age 12 months as well as maternal and infant morbidity and mortality.
The India SWEN trial eligibility criteria and methods have been described in detail elsewhere. 20 In brief, participants were enrolled and followed-up between August 2002 and September 2007. The study site was a large, public teaching hospital (Byramjee-Jeejeebhoy Medical College-Sassoon General Hospital [BJMC-SGH]) that serves an urban population and surrounding semi-urban and rural populations in Pune, Maharashtra. HIV-infected women were enrolled during the 3rd trimester (≥ 32weeks), at delivery or within 24 hours of delivery, and mother-infant pairs were followed prospectively for up to 12 months postpartum. Women who were enrolled antepartum were seen every 4 weeks for scheduled visits until week 36, and then every 2 weeks until delivery. After delivery, on each scheduled visit (weeks 1, 2, 3, 4, 5, 6, 10, 14 and at 6, 9 and 12 months), women and their infants underwent clinical examination and select laboratory investigations.
Most women underwent serum HBsAg testing either at enrollment or within 6 weeks of enrollment. A subset of 140 enrolled women was not screened for HBsAg during the study period; HBsAg testing was performed retrospectively using stored sera collected during the peripartum period. HBV vaccination was not the standard of care in India and was not routinely provided. However, when antepartum maternal HBV co-infection was detected, a few infants born to these mothers were offered both hepatitis B immunoglobulin and the HBV vaccination series immediately after birth.
Infants underwent HIV-1 DNA polymerase chain reaction (PCR) testing within 48 hours of birth and at all scheduled visits, except for the week-3 and week-5 visits. HIV infection was confirmed by quantitative HIV-1 viral load (defined as HIV-1 viral load > 5000 copies/mL) and was externally quality assured as described elsewhere.20 Maternal highly active antiretroviral therapy (HAART) was not readily available during the study period. Only a few women, who were able to pay out-of-pocket, received HAART.
Assessment of HIV-HBV coinfection and definitions
HBV co-infection was defined as a positive serum HBsAg test. Serum samples tested prospectively during the study period used ELISA (Surase Kits, Taiwan). 24,25 Retrospective HBsAg testing was performed on stored sera using Murex kits (Murex biotech, United Kingdom). HBV viral load was assessed retrospectively by Abbott Real Time RNA PCR m2000RT using available, stored maternal samples (+/− 6 months of delivery). HIV transmission was defined as HIV infection positive at age 12 months, and infant mortality was defined as all-cause mortality within the first year of life.
Statistical analysis
Maternal and infant covariates were summarized and compared between HIV-HBV coinfected and HIV mono-infected women. Skewed continuous variables were summarized using medians and interquartile ranges (IQR) and were compared using a Mann-Whitney test. Non-skewed continuous variables were summarized using means and standard deviations (SD) and were compared using an unpaired t-test. Categorical variables were summarized using frequencies and were compared using a χ2 or Fisher’s exact test when applicable.
Univariate and multivariate logistic regression models were used to examine the effect of the primary risk factor, maternal HIV-HBV co-infection, on HIV transmission at 12 months and infant mortality at 12 months. Multivariate models were fitted to estimate an odds ratio that adjusted for covariates with p-values less than 0.1 in univariate analysis and covariates known to be associated with HIV transmission and infant mortality. Continuous variables were categorized in terms of clinically meaningful groups. P-values less than 0.05 were considered statistically significant. All statistical analyses were conducted using STATA software (version 9.1).
Results
Study population characteristics
Among 737 HIV-infected women enrolled in the India SWEN trial, 689 (93%) underwent serologic testing for HBsAg and were included in the analysis. Overall, in the modified intention-to-treat (ITT) population, the median maternal age was 23 years (IQR 21–25), the median maternal CD4 cell count at delivery was 450 cells/μL (IQR 306–643), the median maternal HIV-1viral load at delivery was 3.7 log copies/mL (IQR 2.9– 4.5), the median breastfeeding duration was 3.4 months (IQR 3.2–6.0), and 66 (9%) women received lamivudine-containing HAART at some point during the study period.
Of these 689 HIV-infected women, 32 (4.6%, CI 3.4, 5.3) were HBsAg positive; 549 (79%) samples were tested during the study period, and 140 (21%) samples were tested retrospectively. Most maternal characteristics were similar in HIV-HBV co-infected and HIV mono-infected women (Table 1). However, compared to HIV mono-infected women, HIV-HBV coinfected women had a higher (but not statistically significant) median CD4 cell count at delivery (505 vs. 449 cells/μL, p=0.46), were less likely to have received HAART (0% vs. 9%, p=0.06), were more likely to have received intrapartum nevirapine (91% vs. 66%, p=0.003), and had higher alanine aminotransferase concentrations at enrollment (17.5 vs. 14.4 U/mL, p=0.02). Infant characteristics were similar in the two study groups (Table 1).
Table 1.
Study Population Characteristics by HIV-Coinfection Status (N=689)a
| Maternal Characteristics | Overall (N=689) | HIV-HBV coinfected (N=32) | HIV mono-infected (N=657) | P-value |
|---|---|---|---|---|
| Median age, years (IQR) | 23(21–25) | 22(21–25) | 23(21–25) | 0.30 |
| Education less than primary, n (%) | 415 (60) | 20 (63) | 395 (60) | 0.78 |
| Median CD4 cell countb, cells/μL (IQR) | 450 (306–643) | 505 (382–693) | 449 (306–640) | 0.46 |
| Median HIV-1 viral loadb, log copies/mL (IQR) | 3.7 (2.9–4.5) | 3.4 (2.6–4.4) | 3.7 (2.9–4.5) | 0.47 |
| HAARTc, n (%) | 66 (9) | 0 (0) | 66 (10) | 0.06 |
| Antepartum nevirapine, n (%) | 249 (35) | 15 (47) | 234 (35) | 0.17 |
| Intrapartum nevirapine, n (%) | 473 (67) | 29 (91) | 444 (66) | 0.003 |
| Median ALTd, U/mL (IQR) | 14.6 (11.4–18.1) | 17.5 (14.3–21.5) | 14.4 (11.3–18.0) | 0.02 |
| Median ASTd, U/mL (IQR) | 23.9 (20.2–28.8) | 24.9 (20.2–32.0) | 23.8 (20.1–28.8) | 0.58 |
| Median bilirubind, mg/dL (IQR) | 0.55 (0.43–0.76) | 0.63 (0.44–0.78) | 0.55 (0.43–0.76) | 0.24 |
| Median Hb, g/dL (IQR) | 10.6 (9.4–11.9) | 10.9 (9.0–12.4) | 10.8 (9.4–11.9) | 0.86 |
| Maternal mortality at one year postpartum, n (%) | 10 (1) | 1 (3) | 9 (1) | 0.38 |
| Infant Characteristics | ||||
| Low birth weighte, n (%) | 433 (63) | 23 (71) | 410 (62) | 0.28 |
| Small for gestational age, n (%) | 73 (11) | 3 (9) | 70 (11) | >0.95 |
| Preterm birthe, n (%) | 96 (16) | 5 (16) | 91 (16) | 0.95 |
| Median Apgar Score at one minute, score (IQR) | 6 (5, 7) | 7 (5, 8) | 6 (5, 7) | 0.40 |
| Median Apgar Acore at five minutes, score (IQR) | 8 (7, 9) | 8 (8, 9) | 8 (5, 9) | 0.80 |
| Congenital malformation, n (%) | 7 (1) | 0 (0) | 7 (1) | >0.95 |
| Median breastfeeding duration, months (IQR) | 3.4 (3.2, 6.0) | 3.3 (3.2, 6.0) | 3.4 (3.2, 6.0) | 0.86 |
| HIV infection at 12 months, n (%) | 90 (13) | 3 (9) | 87 (13) | 0.79 |
| All-cause infant mortality at 12 months, n (%) | 27 (4) | 3 (9) | 24 (4) | 0.13 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; AZT, zidovudine; HAART, highly active antiretroviral therapy; Hb, hemoglobin; HBV, hepatitis B virus; HIV, human immunodeficiency virus.
Data are presented for study participants with hepatitis B surface antigen screening results (N=689).
Baseline values assessed at the time of delivery.
Received at any time during the study period.
Baseline values assessed at enrollment.
Low birth weight was defined as < 2500 g, and preterm birth was defined as < 37 weeks gestation
Maternal HBV DNA
None of the HIV-HBV coinfected women received lamivudine-containing HAART during the study period. Serum samples (+/− 6 months of delivery) from 28 (88%) of the 32 HIV-HBV co-infected women were available for retrospective HBV DNA assessment. Of these, 18 (64%) had detectable HBV DNA (defined as > 51 copies/mL) with a median HBV viral load of 155 copies/mL (IQR <51–6741). Four women had high HBV viral loads (>100000 copies/mL) (Table 2).
Table 2.
Maternal and Infant HBV and HIV Viremia Characteristics (n=32)
| Patient | Maternal HBV Viral Load(copies/ml) | Documented receipt of HBV vaccination and IVIG at birth | Infant HBV Viral Load(Copies/ml) | Maternal HIV Viral Load (Copies/ ml) | Infant HIV status in 12 months |
|---|---|---|---|---|---|
| 1 | 1,684,972,136 | Y | 934,458,295 | 17700 | Negative |
| 2 | 576,498,407 | N | 358 | 8326 | Negative |
| 3 | 41,046,040 | Y | <51 Copies/mL | 1040 | Negative |
| 4 | 211,060 | Y | <51 Copies/mL | < 400 | Negative |
| 5 | 89,999 | N | NA | 13529 | Negative |
| 6 | 8,947 | Y | <51 Copies/mL | < 400 | Negative |
| 7 | 7,028 | N | NA | 24427 | Negative |
| 8 | 6,454 | N | <51 Copies/mL | 27600 | Negative |
| 9 | 4,125 | N | 227 | 90766 | Negative |
| 10 | 3,981 | Y | <51 Copies/mL | < 400 | Negative |
| 11 | 883 | N | 99 | 24213 | Negative |
| 12 | 440 | N | NA | 94100 | Negative |
| 13 | 195 | N | <51 Copies/mL | < 400 | Negative |
| 14 | 183 | N | NA | 1689 | Negative |
| 15 | 128 | Y | <51 Copies/mL | 3130 | Negative |
| 16 | 105 | N | NA | < 400 | Negative |
| 17 | 96 | Y | <51 Copies/mL | < 400 | Negative |
| 18 | 58 | N | <51 Copies/mL | 1610 | Negative |
| 19 | <51 Copies/mL | N | 139 | < 400 | Negative |
| 20 | <51 Copies/mL | N | NA | 1720 | Negative |
| 21 | <51 Copies/mL | Y | 220 | 3060 | Negative |
| 22 | <51 Copies/mL | N | <51 Copies/mL | 25900 | Negative |
| 23 | <51 Copies/mL | Y | NA | 686 | Negative |
| 24 | <51 Copies/mL | Y | NA | 1300 | Positive |
| 25 | <51 Copies/mL | N | <51 Copies/mL | 15639 | Negative |
| 26 | <51 Copies/mL | N | NA | 53863 | Negative |
| 27 | <51 Copies/mL | Y | <51 Copies/mL | < 399 | Negative |
| 28 | <51 Copies/mL | N | 59 | < 400 | Negative |
| 29 | NA | N | NA | 291600 | Negative |
| 30 | NA | Y | NA | < 400 | Negative |
| 31 | NA | Y | NA | 30900 | Positive |
| 32 | NA | N | NA | 325281 | Positive |
NA=sample not available, ND= virus not detected
Infant HBV viremia
Thirteen of 32 live-born infants of mothers with known antepartum HBV co-infection had documented receipt of hepatitis B immunoglobulin and HBV vaccination at birth. We had archived plasma samples from 19 (59%) of these 32 infants who underwent viral load testing; 7 (37%) of which had detectable HBV viremia defined as >51 copies/ml (Table 2). Of these 19 infants, 9 infants had documented receipt of HBV vaccination with hepatitis B immunoglobulin and 2 of 9 had detectable viremia. Only one infant had significant HBV viremia of 9.3× 108 copies/ml. Six of 19 had viremia raging between 51 copies/ml and 400 copies/ml.
HIV transmission and infant mortality
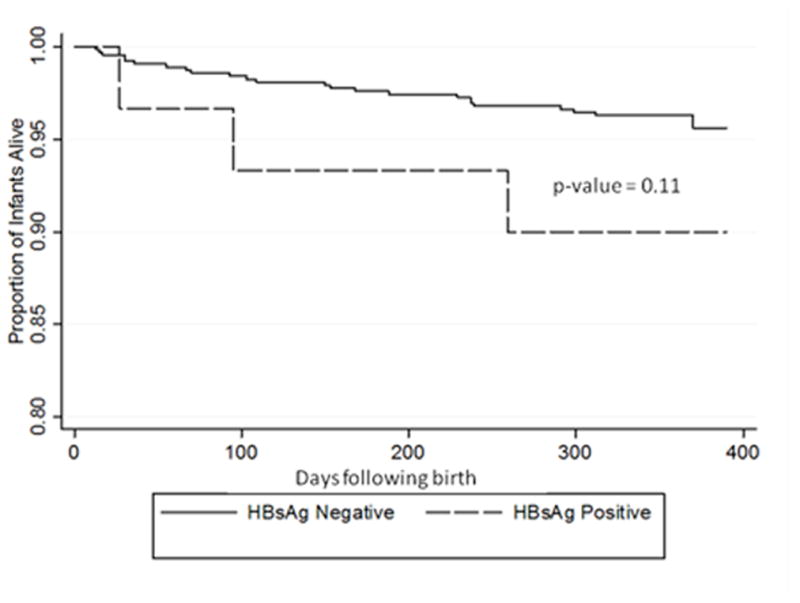
HIV transmission at 12 months was similar in the HIV-HBV coinfected and HIV mono-infected study groups (9% vs. 13%, p=0.79). Multivariate analysis adjusted for maternal CD4 cell count, maternal HIV-1 viral load, maternal ART, and maternal hemoglobin concentration. Compared to HIV mono-infection, maternal HIV-HBV coinfection was not associated with increased odds of HIV transmission (aOR 1.06; 95% CI 0.30, 3.66; p=0.93) (Table 3). All-cause infant mortality at 12 months was higher in the HIV-HBV co-infected group than the HIV mono-infected group, but the difference was not statistically significant (9% vs. 4%, p=0.13). In multivariate analysis that adjusted for maternal CD4 cell count, maternal HIV-1 viral load, maternal ART, maternal hemoglobin concentration, maternal mortality at one year, and infant HIV infection, a trend towards increased odds of infant mortality at 12 months was again noted but was not statistically significant (aOR 3.12; 95% CI 0.67, 14.57; p=0.15) (Table 3). Although there was no significant difference in overall infant mortality, a Kaplan-Meier plot shows that the HIV-HBV coinfection group had consistently higher infant mortality than the maternal HIV mono-infection group (p=0.11) (Figure 1).
Table 3.
Risk Factors Associated with HIV Transmission and Infant Mortalitya
| HIV Transmission OR (95% CI) p-value | HIV Transmission aORb (95% CI) p-value | Infant Mortality OR (95% CI) p-value | Infant Mortality aORc (95% CI) p-value | |
|---|---|---|---|---|
| Risk Factor | ||||
| Maternal age | 1.05 (0.98, 1.12) 0.16 | 1.07 (0.96, 1.19) 0.22 | ||
| Maternal Education | ||||
| < Primary | Ref | Ref | Ref | Ref |
| > Primary | 0.67 (0.44, 1.07) 0.10 | 0.84 (0.52, 1.37) 0.49 | 0.51 (0.24, 1.12) 0.09 | 0.56 (0.23, 1.35) 0.20 |
| Maternal CD4 cell countd | ||||
| >350 cells/μL | Ref | Ref | Ref | Ref |
| 200–350 cells/μL | 1.45 (0.85, 2.25) 0.17 | 1.28 (0.73, 2.32) 0.22 | 1.77 (0.71, 4.40) 0.22 | 1.71 (0.64, 4.61) 0.28 |
| <200 cells/μL | 4.28 (2.14, 8.56) 0.001 | 4.85 (2.45, 9.59) 0.001 | 5.81 (2.18, 15.5) 0.001 | 3.45 (1.01, 11.8) 0.047 |
| Maternal HIV-1 viral loadd | ||||
| <100,000 copies/mL | Ref | Ref | Ref | Ref |
| >100,000 copies/mL | 4.82 (2.75, 8.47) <0.001 | 3.27 (1.74, 6.14) <0.001 | 4.38 (1.84, 10.5) 0.001 | 1.71 (0.60, 4.85) 0.31 |
| Antepartum zidovudine | 0.61 (0.37, 1.01) 0.05 | 0.87 (0.49, 1.54) 0.63 | 0.50 (0.20, 1.25) 0.14 | |
| Intrapartum nevirapine | 0.67 (0.42, 1.05) 0.08 | 0.88 (0.51, 1.53) 0.65 | 0.57 (0.26, 1.25) 0.16 | |
| Maternal Hbd concentration | 0.86 (0.77, 0.96) 0.008 | 0.93 (0.83, 1.05) 0.24 | 0.72 (0.60, 0.87) 0.001 | |
| Maternal HIV-HBV coinfection | 0.68 (0.20, 2.27) 0.53 | 1.06 (0.30, 3.66) 0.93 | 2.73 (0.78, 9.59) 0.12 | 3.12 (0.67, 14.57) 0.15 |
| Maternal mortality at one year | 2.92 (0.74, 11.49) 0.13 | 11.7 (2.85, 48.03) 0.001 | 2.41 (0.40, 14.5) 0.34 | |
| Breastfeeding duration | 1.14 (1.07, 1.21) <0.001 | 3.57 (1.06, 1.21) <0.001 | 0.69 (0.55, 0.86) 0.001 | 0.67 (0.53, 0.83) <0.001 |
| Infant HIV infection at one year | 7.05 (3.20, 15.57) <0.001 | 8.14 (3.26, 20.30) <0.001 | ||
aOR, adjusted odds ratio; CI, confidence interval; Hb, hemoglobin; HBV, hepatitis B virus; HIV, human immunodeficiency virus; OR, odds ratio.
Logistic regression analysis was used to calculate unadjusted and adjusted odds ratios. Analyses were restricted to study participants with hepatitis B surface antigen screening results (N=689).
Adjusted for maternal HIV-1 viral load, maternal CD4 cell count, breastfeeding duration, maternal antiretroviral therapy, maternal Hb.
Adjusted for maternal HIV-1 viral load, maternal CD4 cell count, breastfeeding duration, maternal antiretroviral therapy, maternal Hb, maternal mortality at one year, and infant HIV infection.
Baseline value assessed at the time of delivery.
Figure 1.
Discussion
To our knowledge, this is the first study to evaluate the impact of maternal HIV-HBV coinfection on MTCT of HIV and infant mortality. Maternal HBV coinfection was found in 4.6% of HIV-infected pregnant women in India. Notably, active maternal HIV-HBV co-infection did not independently increase the risk of MTCT of HIV, but may contribute to increased all-cause infant mortality.
In different parts of India, the overall prevalence of HBV mono-infection (defined as HBsAg positive) ranges from 1–9%. 19 Our observed 4.6% HIV-HBV coinfection prevalence among pregnant women in Pune, Maharashtra, is almost 5 times higher than the 1% HBV mono-infection prevalence previously observed at our center during routine antenatal surveillance.23 Increased HBV prevalence among HIV-infected pregnant women has been previously reported, and our finding is consistent with a statistic representing the mean of various studies that have included high-risk populations. 24,25 HIV-HBV co-infection in pregnancy ranges between 5% and 20% in other parts of Asia2 and ranges between 4% and 27% in Africa.26–29
Among the HIV-HBV coinfected women who were tested for HBV DNA in our study, nearly two-thirds had detectable HBV DNA. A similar prevalence of detectable HBV DNA was described in a population of HIV-HBV coinfected adults in South Africa.30 Interestingly, the median HBV viral load in our study is low at 155 copies/mL, which is surprising as HIV-HBV coinfection tends to be associated with higher HBV DNA levels, and none of our HIV-HBV coinfected women received lamivudine-containing HAART. In fact, only four women had high HBV DNA levels (> 100000 copies/mL). A recent multinational study that included HBV-endemic regions also found low HBV DNA levels prior to HAART initiation in a large proportion of the HIV-HBV coinfected study population. 30 That study found that the majority of HBeAg negative patients had low HBV DNA levels while HBeAg positivity was independently associated with high HBV DNA levels. 30 Our HBV DNA findings may also be a function of the HBV serotype, which was not assessed.
Seven (37%) infants had detectable HBV viremia at 6–12 months after birth as timely vaccination against HBV was not provided, however only one infant had very high levels of viremia providing evidence that only one potential transmission likely occurred. This infant had documented receipt of HBV vaccine and hepatitis B immunoglobulin. Not surprisingly, the mother of this infant also had the highest measured HBV viral load. These findings support that high maternal viremia can cause MTCT of HBV even if vaccination was provided in a timely manner. There is some debate whether antepartum antiviral suppression of HBV is warranted to prevent HBV transmission to infants in addition to HBV vaccine and anti-HBV immunoglobulin specifically for mothers with high HBV viremia. 31 Studies are now ongoing to evaluate the role of HAART with dual antiviral activity among HIV-HBV co-infected pregnant women with high HBV viremia to prevent vertical transmission of HIV and HBV.
Our study is among the first to evaluate and identify that maternal HIV-HBV co-infection is not a significant risk factor for MTCT of HIV. To date, reports regarding the impact of HBV mono-infection on pregnancy and infant outcomes are conflicting, and data specific to maternal HIV-HBV coinfection are lacking. Higher rates of congenital malformations, low birth weight and mortality among infants born to HBV mono-infected mothers have been observed in what are mostly retrospective case-control studies. Wong et al showed no difference in the incidence of preterm birth, but a small increased risk for small for gestational age, low birth weight, premature rupture of membranes, prenatal asphyxia and perinatal mortality in HBV mono-infected mothers.8 Interestingly, these associations are hypothesized to be consequences of increased levels of pro-inflammatory cytokines and an exaggerated systemic inflammatory response associated with HBV infection.7–17 Other studies have established that coinfection with chronic illnesses such as malaria, hepatitis C viral infection, and tuberculosis increases the risk of HIV transmission.3–6 However, maternal HIV infection likely plays a more significant role than HBV infection regarding HIV transmission, adverse pregnancy and infant morbidity outcomes.20, 32–40
In conclusion, our study identified a 4.6% prevalence of active HBV coinfection among HIV-infected pregnant women in India. Although limited by small sample size, our study suggests that HIV-HBV coinfection has minimal to no significant effect on MTCT of HIV but may contribute to increased all-cause infant mortality. Since HBV DNA was detected among a large percentage of pregnant women, routine antenatal HBV screening, the use of ART with dual antiviral activity among HIV-HBV co-infected pregnant women, and the timely vaccination of HBV-exposed infants are warranted as a public health policy to prevent MTCT of both HIV and HBV in endemic countries, including India.
Acknowledgments
SOURCES OF FUNDING
This work was supported by the US National Institutes of Health, the US National Institute of Allergy and Infectious Diseases [R01AI45462 to RCB]; the National Institutes of Health-Fogarty International Center Program of International Training Grants in Epidemiology Related to AIDS [D43-TW0000 to Chris Beyrer]; the National Institutes of Health BJMC HIV Clinical Trials Unit [U01 AI069497 to AG]; and the National Institutes of Health-NIAID [K23 AI066983A and R01 AI100748-01 to DB]. This work is also supported in part by the Gilead Foundation and the Ujala Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
The authors thank the SWEN study participants and staff for their immense contributions and acknowledge the contributions of the following investigators: VM, AG, RCB, and RB conceived and designed the study. DB, AK, NG, RB, US, VK, NS, PD, and JS contributed to data collection and analysis. VM, NG, DB, KM, RCB, AG and RB prepared the manuscript.
Footnotes
AUTHOR DISCLOSURE
All authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and were fully responsible for all aspects of manuscript development. The authors declare no conflicts of interest.
References
- 1.Alter M. Epidemiology of viral hepatitis and HIV coinfection. J Hepatol. 2006;44:S6–S9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J, Dore G, Zhang F, et al. Hepatitis B and C virus coinfection in the TREAT Asia HIV observational database. J Gastroenterol Hepatol. 2007;22:1510–1518. doi: 10.1111/j.1440-1746.2007.05062.x. [DOI] [PubMed] [Google Scholar]
- 3.Reddick K, Javeri R, Gandhi M, et al. Pregnancy outcomes associated with viral hepatitis. J Viral Hepat. 2011;18:e394–e398. doi: 10.1111/j.1365-2893.2011.01436.x. [DOI] [PubMed] [Google Scholar]
- 4.Gindes L, Teperberg-Oikawa M, Sherman D, et al. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG. 2008;115:830–835. doi: 10.1111/j.1471-0528.2007.01651.x. [DOI] [PubMed] [Google Scholar]
- 5.McGready R, Lee S, Wiladphaingern J, et al. Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: a population based study. Lancet Infect Dis. 2012;12(5):388–396. doi: 10.1016/S1473-3099(11)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin H, Chen H. Increased risk of low birth weight and small for gestational age infants among women with tuberculosis. BJOG. 2010;117:585–590. doi: 10.1111/j.1471-0528.2010.02504.x. [DOI] [PubMed] [Google Scholar]
- 7.Tse K, Ho L, Lao T. The impact of maternal HBsAg carrier status on pregnancy outcomes: a case-control study. J Hepatol. 2005;43(Suppl 5):771–775. doi: 10.1016/j.jhep.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 8.Wong S, Chan L, Yu V, Ho L. Hepatitis B carrier and perinatal outcome in singleton pregnancy. Am J Perinatol. 1999;16(Suppl 9):485–488. doi: 10.1055/s-1999-6802. [DOI] [PubMed] [Google Scholar]
- 9.Safir A, Levy A, Sikuler E, Sheiner E. Maternal hepatitis B virus or hepatitis C virus carrier status as an independent risk factor for adverse perinatal outcome. Liver international. 2010;30(5):765–770. doi: 10.1111/j.1478-3231.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 10.Wong V, Ip H, Reesink H, et al. Prevention of HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and immunoglobulin: a double-blind randomized placebo-controlled study. Lancet. 1984;28:921–926. doi: 10.1016/s0140-6736(84)92388-2. [DOI] [PubMed] [Google Scholar]
- 11.Pastorek J, Miller J, Summers P. The effect of hepatitis B antigenemia on pregnancy outcome. AJOG. 1998;158:486–489. doi: 10.1016/0002-9378(88)90010-5. [DOI] [PubMed] [Google Scholar]
- 12.To W, Cheung W, Mok K. Hepatitis B surface antigen carrier status and its correlation to gestational hypertension. Aust N Z J Obstet Gynaecol. 2003;43:110–122. doi: 10.1046/j.0004-8666.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 13.Hieber J, Dalton D, Shorey J, Combes B. Hepatitis and pregnancy. J Pediatr. 1977;91:545–549. doi: 10.1016/s0022-3476(77)80499-x. [DOI] [PubMed] [Google Scholar]
- 14.Medhat A, el-Sharkawy M, Shaaban M, et al. Acute viral hepatitis in pregnancy. Int J Gynaecol Obstet. 1993;40:25–31. doi: 10.1016/0020-7292(93)90768-r. [DOI] [PubMed] [Google Scholar]
- 15.Pavel A, Tirsia E, Maior E, Cristae A. Detrimental effects of hepatitis virus infection on the development of the product of conception. Virologie. 1983;34:35–40. [PubMed] [Google Scholar]
- 16.Wands J. Viral hepatitis and its effect on pregnancy. Clin Obstet Gynecol. 1979;22:310–311. doi: 10.1097/00003081-197906000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Hak S, Do Y, Kyun R. The influence of hepatitis B virus on the fetus in pregnancy. Acta Paediatr Jpn. 1987;29:449–454. doi: 10.1111/j.1442-200x.1987.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Bhosale R, Kinikar A, et al. Maternal tuberculosis: a risk factor for mother-to-child transmission of human immunodeficiency virus. J Infect Dis. 2011;203(3):358–63. doi: 10.1093/jinfdis/jiq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paintsil E, Andiman W. Update on successes and challenges regarding mother-to-child transmission of HIV. Curr Opin Pediatr. 2009;21(1):94–101. doi: 10.1097/MOP.0b013e32831ec353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bedri A, Gudetta B, Isehak A, et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–313. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann C, Desmond Martin D, et al. Hepatitis B Virus Infection and Response to Antiretroviral Therapy (ART) in a South African ART Program. Clin Infect Dis. 2008;47(11):1479–148. doi: 10.1086/593104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spradling P, Richardson J, Buchacz K, et al. Prevalence of chronic hepatitis B virus infection among patients in the HIV outpatient study, 1996–2007. J Viral Hepat. 2010;17(12):879–886. doi: 10.1111/j.1365-2893.2009.01249.x. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee S, Ravishankari K, Narang A, Kinikar A. Hepatitis B prevalence during pregnancy. Indian Pediatr. 2009;46(11):1005–1008. [PubMed] [Google Scholar]
- 24.Lodha R, Jain Y, Anand K, et al. Hepatitis B in India. A review of disease epidemiology. Indian Pediatr. 2001;38:349–371. [PubMed] [Google Scholar]
- 25.Indian Association for Study of the Liver (INSAL) Hepatitis B in India; therapeutic options and prevention strategies-Consensus statement. Indian J Gastroenterol. 2000;19:C4–C66. [Google Scholar]
- 26.Pirollo M, Bassani L, Germinario E, et al. Seroprevalence of hepatitis B and C viruses among HIB-infected pregnant women in Uganda and Rwanda. J Med virol. 2007;79(12):1797–1801. doi: 10.1002/jmv.21007. [DOI] [PubMed] [Google Scholar]
- 27.SImpore J, Savadogo A, Ilboudo D, et al. Toxoplasma gondii, HCV, and HBV seroprevalence and co-infection among HIV-positive and -negative pregnant women in Burkina Faso. J Med Virol. 2006;78(6):730–733. doi: 10.1002/jmv.20615. [DOI] [PubMed] [Google Scholar]
- 28.Rouet F, Chaix M, Inwoley A, et al. HBV and HCV prevalence and viraemia in HIV-positive and HIV-negative pregnant women in Abidjan, Côte d’Ivoire: the ANRS 1236 study. J Med Virol. 2004;74 (1):34–40. doi: 10.1002/jmv.20143. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann C, Thio C. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7:402–409. doi: 10.1016/S1473-3099(07)70135-4. [DOI] [PubMed] [Google Scholar]
- 30.Thio C, Smeaton L, Saulynas M, et al. Characterization of HIV-HBV coinfection in a multinational HIV- infected cohort. AIDS. 2013;27:191–201. doi: 10.1097/QAD.0b013e32835a9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu WM, Cui YT, Wang L, et al. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebocontrolled study. J Viral Hepat. 2009;16:94–103. doi: 10.1111/j.1365-2893.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- 32.Braddick M, Kreiss J, Embree J, et al. Impact of maternal HIV infection on obstetrical and early neonatal outcome. AIDS. 1990;4:1001–1005. doi: 10.1097/00002030-199010000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Temmerman M, Chomba E, Ndinya-Achola J, et al. Maternal HIV infection and pregnancy outcome. Obstet Gynecol. 1994;83:495–501. doi: 10.1097/00006250-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Leroy V, Ladner J, Nyiraziraje M, et al. Effect of HIV-1 infection on pregnancy outcome in Kigali, Rawanda, 1992–1994. AIDS. 1998;12:643–650. doi: 10.1097/00002030-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 35.Dreyfus M, Msamanga G, Spiegelman D, et al. Determinants of low birth weight among HIV infected pregnant women in Tanzania. Am J Clin Nutr. 2001;74:814–826. doi: 10.1093/ajcn/74.6.814. [DOI] [PubMed] [Google Scholar]
- 36.Mitgitti R, Seanchaisuriya P, Schelp F, et al. Low birth weight infants born to HIV-seropositive mothers and HIV-seronegative mothers in Chiang Rai, Thailand. Southeast Asian J Trop Med Public Health. 2008;39(2):273–278. [PubMed] [Google Scholar]
- 37.Gomutbutra V. Characteristics of pregnancy with human immuno-deficiency virus (HIV) and perinatal transmission in Nakornping Hospital. J Med Assoc Thai. 2008;91(2):142–145. [PubMed] [Google Scholar]
- 38.Rollins N, Coovadia H, Bland R, et al. Pregnancy outcomes in HIV-infected and uninfected women in rural and urban South Africa. J Acquir Immune Defic Syndr. 2007;44(3):321–328. doi: 10.1097/QAI.0b013e31802ea4b0. [DOI] [PubMed] [Google Scholar]
- 39.Taha T, Dallabetta G, Canner J, et al. The effect of HIV infection on birth rate and infant and child mortality in urban Malawi. Int J Epidemiol. 1995;24(5):1022–1029. doi: 10.1093/ije/24.5.1022. [DOI] [PubMed] [Google Scholar]
- 40.Stratton P, Tuomala R, Abboud R, et al. Obstetric and newborn outcomes in cohort of HIV infected pregnant women: a report of the women and infants transmission study. J Acquir Immune Defic Syndr. 1999;20:179–186. doi: 10.1097/00042560-199902010-00011. [DOI] [PubMed] [Google Scholar]


