Abstract
Matrix metalloproteinase-9 (MMP-9) plays key roles in the brain pathophysiology, especially in blood-brain barrier (BBB) breakdown. Therefore, inhibiting MMP-9 activity may be a promising therapy for protecting brains in cerebrovascular diseases. Here we show that in a mouse prolonged cerebral hypoperfusion model, a clinically proven radical scavenger edaravone suppressed MMP-9 and reduced BBB damage in cerebral white matter. Prolonged cerebral hypoperfusion was induced by bilateral common carotid artery stenosis in male adult C57BL/6J mice (10 weeks old). After 7 days of cerebral hypoperfusion, white matter region (e.g. corpus callosum) exhibited significant BBB leakage, assessed by IgG staining. Correspondingly, immunostaining and western blotting showed that MMP-9 was upregulated in the white matter. Edaravone treatment (3 mg/kg, i.p. at day 0 and 3) inhibited both BBB leakage and MMP-9 increase. Under the early phase of cerebral hypoperfusion conditions, oligodendrocyte precursor cells (OPCs) mainly contribute to the MMP-9 increase, but our immunostaining data showed that very little OPCs expressed MMP-9 in the edaravone-treated animals at day 7. Therefore, in vitro studies with primary rat OPCs were conducted to examine whether edaravone would directly suppressed MMP-9 expressions in OPCs. OPC cultures were exposed to sub-lethal CoCl2 for 7 days to induce prolonged chemical hypoxic stress. Prolonged chemical hypoxic stress increased MMP-9 expression in OPCs, and radical scavenging with edaravone (10 μM for 7 days) ameliorated the increase. Taken together, our proof-of-concept study demonstrates that radical scavengers may provide a potential therapeutic approach for white matter injury by suppressing BBB damage.
Keywords: white matter injury, prolonged cerebral hypoperfusion, matrix metalloproteinase-9, blood-brain barrier, edaravone
Introduction
Matrix metalloproteinases (MMPs) are zinc endopeptidases that degrade most extracellular matrix proteins, and uncontrolled expression of MMPs results in tissue injury and inflammation [8, 10, 35, 45]. Among the large family of MMPs, MMP-9 is well known as one of the major contributors for the pathogenesis of acute brain injury. Under normal conditions, MMP-9 expression and activation in the brain are tightly regulated in low levels. But after brain injury, MMP-9 is upregulated to degrade neurovascular substrates, resulting in blood-brain barrier (BBB) breakdown [23, 30]. BBB plays an important role to maintain the homeostasis of the central nervous system, and BBB damage is a major hallmark for most brain diseases [49]. Past studies using animal models demonstrate the close correlation between MMP-9 upregulation and BBB breakdown; stroke [6, 41], intracerebral hemorrhage [17, 39], and brain trauma [41].
Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) is a radical scavenging drug that is clinically used in Japan for acute ischemic stroke [1, 20]. In vivo studies with rodent stroke models suggest that the neuroprotective effects of edaravone may take place in penumbral-like regions where oxygen radicals are typically generated [3, 18]. The efficacy of edaravone in protecting brain cells is also confirmed in cell culture studies [7, 19, 21, 46, 47]. Recently, we reported that edaravone has shown to have a protective effect in a mouse model of prolonged cerebral hypoperfusion, wherein cerebral white matters are selectively degenerated [27]. In that study, we showed that edaravone treatment ameliorated oligodendrocyte death and myelin degradation, which would ease the cognitive dysfunction. The stress caused by prolonged cerebral hypoperfusion is also known to induce BBB damage due to MMP-9 upregulations in the mouse white matter [37], but efficacy of edaravone on white matter BBB damage is still mostly unknown. Therefore, in this study, we test effects of edaravone on MMP-9 upregulation and BBB breakdown in the mouse model of prolonged cerebral hypoperfusion.
Material and methods
Cerebral prolonged hypoperfusion model
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. For inducing cerebral prolonged hypoperfusion stress, a microcoil (0.18 mm diameter, Sawane Spring Co.) was applied to bilateral common carotid arteries (CCAs) according to previous reports [15, 38]. Briefly, male C57BL/6J mice (10 weeks old, Charles River Institute) were anesthetized with 4.0% isoflurane and then maintained on 1.5% isoflurane in 70% N2O and 30% O2, maintaining the rectal temperature between 36.5°C and 37.5°C. Through a midline cervical incision, the microcoil was applied to the right CCA by rotating it around the artery. Then, another microcoil was applied to the left CCA. The cerebral blood flow was measured before/after the micro-coil placement as described previously [27]. The radical scavenger edaravone (3 mg/kg ip) or vehicle was treated twice at day 0 and 3, and the animals were sacrificed on day 7. All in vivo experiments and measurements were performed in a blinded and randomized manner. Animal numbers for each experiment are described in the figure legends.
Immunohistochemistry
Mouse brain was removed at day 7, and postfixed for 24 h in 4% paraformaldehyde (4% PFA) in phosphate-buffered saline (PBS) at 4°C before cryoprotection by bathing in 30% sucrose. Coronal sections with 16 μm thickness (3 sections for each animal) were incubated overnight with primary antibodies. After washing with PBS, they were incubated with secondary antibodies (1:200; Jackson Immunoresearch Laboratories) for 1 hour at room temperature, and the slides were covered with VECTASHIELD with DAPI (Vector Laboratories). Immunostaining was analyzed with a fluorescence microscope (Nikon). Information for primary antibodies is as follows - anti-PDGFRα (1:100; SantaCruz, or anti-CD140a, 1:100, BD phamamigen; OPC maker), and anti-MMP-9 (1:100, Calbiochem).
IgG staining
Mouse brains were taken out after perfusion with 0.9% saline and quickly frozen using powdered dry ice. Coronal sections with 20 μm thickness were cut on cryostat at -20°C and collected on glass slides. Three sections for each animal were used for IgG staining. Sections were fixed by 4% PFA and rinsed 3 times in PBS (pH 7.4). Subsequently, the sections were incubated in 3% H2O2, followed by blocking with 10% Brockace (AbD serotec) in PBS. Then the sections were incubated overnight at 4°C with antibody against donkey anti-mouse IgG (1:300; Jackson Immunoresearch Laboratories). Immunoreactivity was visualized using fluorescence-conjugated streptavidin. Staining was analyzed with a fluorescence microscope (Nikon) interfaced with a digital charge-coupled device camera and an image analysis system. Area densities of structures stained with IgG were calculated as the proportion of pixels having a fluorescence intensity value equal to or greater than the threshold: this ratio was expressed as %area.
Cell counting for brain sections
An investigator blinded to the experimental groups counted the number of stained cells in lateral side of corpus callosum (0.25 mm2) of PDGFRα-stained sections.
Cell culture
Primary cortical OPCs were prepared and maintained according to our previous work [4]. To differentiate OPCs to myelin basic protein-positive oligodendrocytes, the culture medium was switched to include sub-lethal CoCl2 (1 μM for 7 days) for treatment to induce a prolonged chemical hypoxic conditions as described before [28, 37]. Dulbecco’s Modified Eagle’s Medium (DMEM) containing 1% penicillin/streptomycin, 10 ng/ml ciliary neurotrophic factor (CNTF), 15 nM triiodo-L-thyronine (T3), and 2% B27 supplement (DMEM medium) was used. Hypoxic conditions were confirmed by increase of HIF-1α expression. Edaravone (Mitsubishi Tanabe Pharma) were dissolved in dimethysulphoxide. The final concentration of dimethysulphoxide in the culture medium was less than 0.1%, which had no effects on OPC survival and function.
Western blotting
Tissue samples of corpus callosum and cell culture were dissected in Pro-PREPTM Protein Extraction Solution (Boca scientific). Samples were heated with equal volumes of SDS sample buffer (Novex) and 10 mM dithiothreitol (DTT) at 95 °C for 5 min, then each sample (20 μg per lane) was loaded onto 4–20% Tris–glycine gels. After electrophoresis and transferring to polyvinylidene difluoride membranes (Novex), the membranes were blocked in Brockace (AbD serotec), then incubated overnight at 4°C with primary antibodies against HIF1α (1:3000, abcam; a marker for hypoxic conditions), MMP-9 (1:1000, Calbiochem), or β-actin (1:10000, Sigma Aldrich) followed by incubation with peroxidase-conjugated secondary antibodies and visualization by enhanced chemiluminescence (Amersham).
Cell death/survival assay
Cell proliferation/survival was assessed by water-soluble-tetrazolium (WST) assay (Dojindo) according to the manufacturer’s instruction. Cytotoxicity was quantified by lactate dehydrogenase (LDH) assay.
Statistical analysis
Statistical significance was evaluated using the unpaired t-test to compare differences between the two groups and a one-way ANOVA followed by Tukey’s honestly significant difference test for multiple comparisons. Data are expressed as mean ± S.D. A p-value of <0.05 was considered statistically significant.
Results
We started this study with in vivo experiments using a mouse model of prolonged hypoperfusion, which is now relatively well accepted as a clinically-relevant white matter injury model [15]. Prolonged cerebral hypoperfusion was induced by bilateral common carotid artery stenosis (BCAS) using micro-coils. Firstly, BBB integrity in the white matter (corpus callosum) was assessed with IgG staining. Under normal conditions, BBB is intact and IgG is rarely found in the brain parenchyma. But under pathological conditions, BBB is permeable and IgG goes in the brain parenchyma from the circulation. Our IgG staining confirmed that no IgG signals were detected in the sham groups, but the cerebral hypoperfusion group exhibited significant IgG signals after 7 days of cerebral hypoperfusion (Figure 1). Importantly, edaravone treatment (3 mg/Kg, i.p. at days 0 and 3) reduced the BBB leakage (Figure 1). As our previous report showed that this edaravone treatment schedule was effective in radical scavenging for this mouse model [27], and these data suggest that edaravone is BBB-protective in white matter.
Figure 1. Edaravone reduced BBB leakage in a mouse model of prolonged cerebral hypoperfusion model A.
Representative IgG staining images of the white matter region (corpus callosum) at day 7 after the bilateral common artery stenosis (BCAS). Edaravone (3 mg/Kg, i.p.) was treated at days 0 and 3. Scale bar = 50 μm. B. Quantitative data for the IgG staining. Values are mean ± SD. N=6. *P<0.05 vs vehicle-treated BCAS group. Ve: vehicle-treated animals, Eda: edaravone-treated animals.
Next, we assessed MMP-9 upregulation using immunostaining and western blotting. As reported previously [37], significant upregulation in MMP-9 was observed in the corpus callosum at day 7 (Figure 2A-C). And again, edaravone inhibited the MMP-9 upregulation (Figure 2A-C). In this mouse model of cerebral hypoperfusion, early MMP-9 expression is mostly derived from OPCs, and the OPC-derived MMP-9 contributes to the early BBB damage and secondary MMP-9 upregulation in cerebral endothelium [37]. Therefore, we then checked if edaravone suppressed MMP-9 upregulation in OPCs. Double staining of MMP-9 with PDGF-R-α (a marker for OPCs) showed that brain sections of edaravone-treated mice exhibited smaller area of PDGF-R-α/MMP-9-double positive signal compared to the ones from vehicle-treated group (Figure 2D).
Figure 2. Edaravone decreased MMP-9 upregulation in a mouse model of prolonged cerebral hypoperfusion model A.
Representative MMP-9 staining images of the white matter region (corpus callosum) at day 7 after the bilateral common artery stenosis (BCAS). Edaravone (3 mg/Kg, i.p.) was treated at days 0 and 3. Scale bar = 50 μm. B. Quantitative data for the MMP-9 staining. Values are mean ± SD. N=6. *P<0.05 vs vehicle-treated BCAS group. C. Representative images and quantitative data for MMP-9 western blotting using in vivo samples from the white matter region. Values are mean ± SD. N=5. *P<0.05 vs vehicle-treated BCAS group. D. Representative images and quantitative data for the double staining of MMP-9 with a OPC marker PDGF-R-α. Arrows indicate PDGF-R-α-positive cells. Scale bar = 20 μm. Values are mean ± SD. N=5. *P<0.05. Ve: vehicle-treated animals, Eda: edaravone-treated animals.
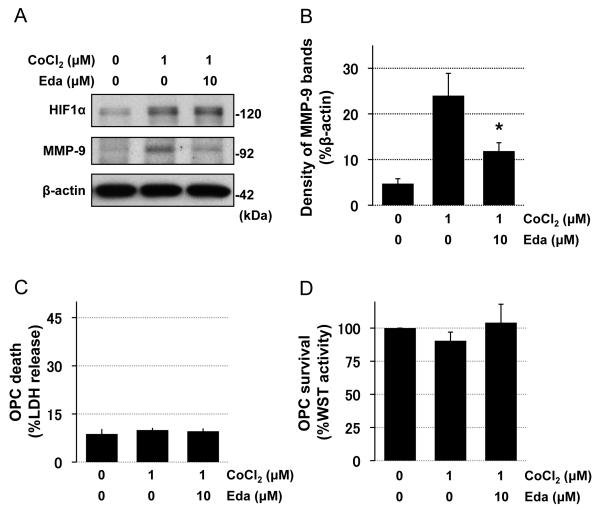
Finally, to evaluate whether edaravone directly works on OPCs and inhibits hypoxia-induced MMP-9 upregulation, we conducted in vitro cell culture experiments using cultured rat primary OPCs. OPC cultures were prepared from neonatal rat cortex, and our previous reports confirmed that our OPC cultures were pure and functional [4, 31]. When OPCs were subjected to sub-lethal chemical hypoxia (1 μM CoCl2 for 7 days) to induce prolonged hypoxic conditions [27, 37], MMP-9 expression was significantly increased (Figure 3A-B). And once again, edaravone treatment inhibited the hypoxia-induced MMP-9 upregulation (Figure 3A-B). LDH and WST assays confirmed that neither the chemical hypoxic stress nor edaravone treatment induced overt cell death in our OPC cultures (Figure 3C-D).
Figure 3. Edaravone decreased hypoxia-induced MMP-9 expression in cultured rat OPCs A-B.
Representative images and quantitative data for MMP-9 western blotting using in vitro OPC samples. CoCl2 was used to induce chemical hypoxic stress. HIF-1α western blotting was used as an indicator for hypoxic conditions. β-actin was used as an internal control. Prolonged sub-lethal CoCl2 treatment (1 μM for 7 days) increased MMP-9 expression in OPC cultures, and edaravone (10 μM for 7 days) ameliorated the change. Values are mean ± SD. N=5. *P<0.05 vs CoCl2 group. C-D. LDH and WST assays confirmed that the CoCl2 exposure (1 μM for 7 days) did not induce overt cell death in OPC cultures. Values are mean ± SD. N=5. Eda: edaravone.
Discussion
We have shown that edaravone, a free radical scavenger, protected white matter BBB against prolonged cerebral hypoperfusion stress in mice. This BBB-protective effect partly resulted from the decrease of MMP-9. Our cell culture experiments also confirmed that edaravone inhibited hypoxia-induced MMP-9 upregulation in OPCs, which are major cell type for regulating white matter homeostasis. These findings support the previous reports that edaravone inhibits MMP-9 upregulation and reduce BBB damage in a rat focal stroke model [43] and an in vitro endothelial cell culture model [12]. Patients with vascular dementia exhibit cerebral hypoperfusion [44], and the mouse model of prolonged cerebral hypoperfusion with micro-coils show similar white matter dysfunction and cognitive decline as observed in clinic [15, 38]. White matters are vulnerable to oxidative stress due to the low levels of intrinsic antioxidant properties [33, 36]. Therefore, our current proof-of-concept study supports the well-accepted idea that oxidative stress is still a viable target for vascular dementia or other white matter-related diseases [16, 30, 40].
Brain endothelium/vessels show specialized function and structure to form the BBB, which is essential for maintenance of brain homeostasis and proper neuronal activities [34, 49]. Endothelial tight junctions play important roles in BBB integrity. Also, BBB function is known to be supported by neighboring cells such as astrocytes and pericytes [2, 5, 9, 32, 42]. Besides cells, extracellular matrix is a critical for keeping BBB tightness [13], and therefore, uncontrolled MMP-9 leads to BBB damage under pathological conditions. Recently, we have shown that OPCs are also one of the important cell types to modulate the BBB integrity [29]. After white matter damage, OPC-derived MMP-9 may cause BBB dysfunction [37]. Hence, our current study would support and expand our previous findings; OPC-derived MMP-9 is a therapeutic target for white matter injury, and radical scavenging can be a promising tool for reducing MMP-9 upregulation.
Another important finding of this study is that edarvone can directly inhibit MMP-9 expression in OPCs. Edaravone is a clinically used drug, and past studies extensively examined the effects of edaravone on several types of brain cells. However, efficacies of edaravone on OPCs are still mostly unknown. OPCs work as precursor cells even in the adulthood [25, 48]. They monitor neighboring environments and proliferate/differentiate as needed for maintaining and repairing white matter function [14]. But OPCs are susceptible to stress as neurons are [11, 26]. Although OPCs potentially mediate a compensatory response that underlies white matter repair after damage and disease, OPCs tend to die easily under pathological conditions. We previously showed that edaravone protected OPCs and promoted the OPC-to-oliogdendrocyte differentiation under prolonged cerebral hypoperfusion in mice [27]. Therefore our current study may demonstrate another important facet of edaravone on OPC function under white matter injury.
Nevertheless, there are important caveats in this study. Firstly, we tested edaravone only at one time point (e.g. day 7). Nonetheless, the BBB leakage can be observed at least from day 3 to day 28 in the prolonged cerebral hypoperfusion model (data not shown). Therefore, assessing BBB permeability on other time points should be carefully analyzed in future studies. Second, our in vitro experiments used only OPC cultures. But at the later time points of day 7, endothelial cells would also contribute to MMP-9 upregulation [37]. Hence, using cerebral endothelial cultures is warranted for future studies. And finally, MMP-9 upregulation and BBB leakage at the acute phase may not always be deleterious for brains. These responses may be important processes leading to brain repair/remodeling at the chronic phase [22, 24]. Therefore, long-term effects of anti-oxidant drugs on white matter remodeling after injury should be carefully examined to determine the balance between beneficial versus potentially detrimental effects.
In summary, this proof-of-concept study demonstrates that during prolonged cerebral hypoperfusion, oxidative stress upregulates MMP-9 and disrupts BBB function in the white matter. Hypoperfusion may lead to white matter damage in stroke and vascular dementia, and therefore, anti-oxidant radical scavengers would provide a broad therapeutic approach for cerebrovascular disorders.
Conclusion
A radical scavenger edaravone inhibited MMP-9 upregulation and reduced BBB damage in a mouse model of prolonged cerebral hypoperfusion. These results may support the idea that anti-oxidants are promising therapeutic tools for white matter-related diseases such as vascular dementia or stroke.
Highlights.
Prolonged cerebral hypoperfusion induced MMP-9 increase and BBB damage in mice.
A radical scavenger edaravone ameliorated these changes.
Prolonged chemical hypoxic stress increased MMP-9 expression in OPC cultures.
Edaravone inhibited the MMP-9 upregulation in OPCs in vitro.
Acknowledgements
Supported in part by National Institutes of Health, Research Abroad from the Uehara Memorial Foundation. We thank Dr. Eng H. Lo for many helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: none
References
- [1].Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222–229. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- [2].Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- [3].Amemiya S, Kamiya T, Nito C, Inaba T, Kato K, Ueda M, Shimazaki K, Katayama Y. Anti-apoptotic and neuroprotective effects of edaravone following transient focal ischemia in rats. Eur J Pharmacol. 2005;516:125–130. doi: 10.1016/j.ejphar.2005.04.036. [DOI] [PubMed] [Google Scholar]
- [4].Arai K, Lo EH. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J Neurosci. 2009;29:4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- [6].Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen H, Wang S, Ding JH, Hu G. Edaravone protects against MPP+ -induced cytotoxicity in rat primary cultured astrocytes via inhibition of mitochondrial apoptotic pathway. J Neurochem. 2008;106:2345–2352. doi: 10.1111/j.1471-4159.2008.05573.x. [DOI] [PubMed] [Google Scholar]
- [8].Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- [9].Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].del Zoppo GJ. The neurovascular unit, matrix proteases, and innate inflammation. Ann N Y Acad Sci. 2010;1207:46–49. doi: 10.1111/j.1749-6632.2010.05760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].French HM, Reid M, Mamontov P, Simmons RA, Grinspan JB. Oxidative stress disrupts oligodendrocyte maturation. J Neurosci Res. 2009;87:3076–3087. doi: 10.1002/jnr.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harada K, Suzuki Y, Yamakawa K, Kawakami J, Umemura K. Combination of reactive oxygen species and tissue-type plasminogen activator enhances the induction of gelatinase B in brain endothelial cells. Int J Neurosci. 2012;122:53–59. doi: 10.3109/00207454.2011.623808. [DOI] [PubMed] [Google Scholar]
- [13].Hermann DM, ElAli A. The abluminal endothelial membrane in neurovascular remodeling in health and disease. Sci Signal. 2012;5:re4. doi: 10.1126/scisignal.2002886. [DOI] [PubMed] [Google Scholar]
- [14].Hughes EG, Kang SH, Fukaya M, Bergles DE. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat Neurosci. 2013;16:668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ihara M, Taguchi A, Maki T, Washida K, Tomimoto H. A mouse model of chronic cerebral hypoperfusion characterizing features of vascular cognitive impairment. Methods Mol Biol. 2014;1135:95–102. doi: 10.1007/978-1-4939-0320-7_8. [DOI] [PubMed] [Google Scholar]
- [16].Irving EA, Yatsushiro K, McCulloch J, Dewar D. Rapid alteration of tau in oligodendrocytes after focal ischemic injury in the rat: involvement of free radicals. J Cereb Blood Flow Metab. 1997;17:612–622. doi: 10.1097/00004647-199706000-00003. [DOI] [PubMed] [Google Scholar]
- [17].Katsu M, Niizuma K, Yoshioka H, Okami N, Sakata H, Chan PH. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood-brain barrier dysfunction in vivo. J Cereb Blood Flow Metab. 2010;30:1939–1950. doi: 10.1038/jcbfm.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kawai H, Nakai H, Suga M, Yuki S, Watanabe T, Saito KI. Effects of a novel free radical scavenger, MCl-186, on ischemic brain damage in the rat distal middle cerebral artery occlusion model. J Pharmacol Exp Ther. 1997;281:921–927. [PubMed] [Google Scholar]
- [19].Kawasaki T, Kitao T, Nakagawa K, Fujisaki H, Takegawa Y, Koda K, Ago Y, Baba A, Matsuda T. Nitric oxide-induced apoptosis in cultured rat astrocytes: protection by edaravone, a radical scavenger. Glia. 2007;55:1325–1333. doi: 10.1002/glia.20541. [DOI] [PubMed] [Google Scholar]
- [20].Lapchak PA. A critical assessment of edaravone acute ischemic stroke efficacy trials: is edaravone an effective neuroprotective therapy? Expert Opin Pharmacother. 2010;11:1753–1763. doi: 10.1517/14656566.2010.493558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee BJ, Egi Y, van Leyen K, Lo EH, Arai K. Edaravone, a free radical scavenger, protects components of the neurovascular unit against oxidative stress in vitro. Brain research. 1307:22–27. doi: 10.1016/j.brainres.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- [23].Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- [24].Maki T, Hayakawa K, Pham LD, Xing C, Lo EH, Arai K. Biphasic mechanisms of neurovascular unit injury and protection in CNS diseases. CNS Neurol Disord Drug Targets. 2013;12:302–315. doi: 10.2174/1871527311312030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maki T, Liang AC, Miyamoto N, Lo EH, Arai K. Mechanisms of oligodendrocyte regeneration from ventricular-subventricular zone-derived progenitor cells in white matter diseases. Front Cell Neurosci. 2013;7:275. doi: 10.3389/fncel.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- [27].Miyamoto N, Maki T, Pham LD, Hayakawa K, Seo JH, Mandeville ET, Mandeville JB, Kim KW, Lo EH, Arai K. Oxidative stress interferes with white matter renewal after prolonged cerebral hypoperfusion in mice. Stroke. 2013;44:3516–3521. doi: 10.1161/STROKEAHA.113.002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Miyamoto N, Pham LD, Hayakawa K, Matsuzaki T, Seo JH, Magnain C, Ayata C, Kim KW, Boas D, Lo EH, Arai K. Age-Related Decline in Oligodendrogenesis Retards White Matter Repair in Mice. Stroke. 2013 doi: 10.1161/STROKEAHA.113.001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Miyamoto N, Pham LD, Seo JH, Kim KW, Lo EH, Arai K. Crosstalk between cerebral endothelium and oligodendrocyte. Cell Mol Life Sci. 2014;71:1055–1066. doi: 10.1007/s00018-013-1488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pham LD, Hayakawa K, Seo JH, Nguyen MN, Som AT, Lee BJ, Guo S, Kim KW, Lo EH, Arai K. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012;60:875–881. doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Quaegebeur A, Lange C, Carmeliet P. The neurovascular link in health and disease: molecular mechanisms and therapeutic implications. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- [33].Ravindranath V, Shivakumar BR, Anandatheerthavarada HK. Low glutathione levels in brain regions of aged rats. Neurosci Lett. 1989;101:187–190. doi: 10.1016/0304-3940(89)90528-4. [DOI] [PubMed] [Google Scholar]
- [34].Ronaldson PT, Davis TP. Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Curr Pharm Des. 2012;18:3624–3644. doi: 10.2174/138161212802002625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- [36].Sasaki T, Senda M. Evaluation of glutathione localization in brain using 99mTc meso-HMPAO. J Nucl Med. 1999;40:1056–1060. [PubMed] [Google Scholar]
- [37].Seo JH, Miyamoto N, Hayakawa K, Pham LD, Maki T, Ayata C, Kim KW, Lo EH, Arai K. Oligodendrocyte precursors induce early blood-brain barrier opening after white matter injury. J Clin Invest. 2013;123:782–786. doi: 10.1172/JCI65863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- [39].Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- [40].Ueno Y, Zhang N, Miyamoto N, Tanaka R, Hattori N, Urabe T. Edaravone attenuates white matter lesions through endothelial protection in a rat chronic hypoperfusion model. Neuroscience. 2009;162:317–327. doi: 10.1016/j.neuroscience.2009.04.065. [DOI] [PubMed] [Google Scholar]
- [41].Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yagi K, Kitazato KT, Uno M, Tada Y, Kinouchi T, Shimada K, Nagahiro S. Edaravone, a free radical scavenger, inhibits MMP-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke. 2009;40:626–631. doi: 10.1161/STROKEAHA.108.520262. [DOI] [PubMed] [Google Scholar]
- [44].Yao H, Sadoshima S, Kuwabara Y, Ichiya Y, Fujishima M. Cerebral blood flow and oxygen metabolism in patients with vascular dementia of the Binswanger type. Stroke. 1990;21:1694–1699. doi: 10.1161/01.str.21.12.1694. [DOI] [PubMed] [Google Scholar]
- [45].Yong VW, Agrawal SM, Stirling DP. Targeting MMPs in acute and chronic neurological conditions. Neurotherapeutics. 2007;4:580–589. doi: 10.1016/j.nurt.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N, Tada N. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS drug reviews. 2006;12:9–20. doi: 10.1111/j.1527-3458.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yuan WJ, Yasuhara T, Shingo T, Muraoka K, Agari T, Kameda M, Uozumi T, Tajiri N, Morimoto T, Jing M, Baba T, Wang F, Leung H, Matsui T, Miyoshi Y, Date I. Neuroprotective effects of edaravone-administration on 6-OHDA-treated dopaminergic neurons. BMC Neurosci. 2008;9:75. doi: 10.1186/1471-2202-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zhang R, Chopp M, Zhang ZG. Oligodendrogenesis after cerebral ischemia. Front Cell Neurosci. 2013;7:201. doi: 10.3389/fncel.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]