Abstract
Severe congenital neutropenia (SCN) is a rare hematopoietic disorder, with estimated incidence of 1 in 200,000 individuals of European descent, many cases of which are inherited in an autosomal dominant pattern. Despite the fact that several causal genes have been identified, the genetic basis for >30% of cases remains unknown. We report a five generation family segregating a novel single nucleotide variant (SNV) in TCIRG1. There is perfect co-segregation of the SNV with congenital neutropenia in this family; all 11 affected, but none of the unaffected, individuals carry this novel SNV. Western blot analysis show reduced levels of TCIRG1 protein in affected individuals, compared to healthy controls. Two unrelated patients with SCN, identified by independent investigators, are heterozygous for different, rare, highly conserved, coding variants in TCIRG1.
Keywords: TCIRG1, Congenital neutropenia, SCN, V-ATPase
Severe congenital neutropenia (SCN) is a hematological condition characterized by blood neutrophil counts (ANC) < 0.5 ×109/L and recurrent bacterial infections usually beginning very early in childhood. In 1956, Kostmann described an autosomal recessive form of SCN. [Kostmann R, 1956] This recessive form of SCN is now attributable to mutations in HAX1, a Bcl-2 family-related-gene [Carlsson and Fasth, 2001; Klein et al., 2007]. Much more frequently, SCN is an autosomal dominant disorder caused by mutations in ELA2, a gene encoding the protein neutrophil elastase, an enzyme of the neutrophil’s primary granules [Horwitz et al., 1999; Dale et al., 2000]. About 60% of cases of SCN in the North American population are attributable to ELA2 mutations [Xia et al., 2009]. Mutations in other genes, e.g., genes affecting glucose homeostasis (SLC37A4, G6PC3), lysosomal function (LYST, RAB27A, ROBLD3/p14, AP3B1, VPS13B), ribosomal proteins (SBDS, RMRP), mitochondrial proteins (HAX1, AK2, TAZ), immune functions (STK4, GFI1, CXCR4) and X-linked (WAS), also cause neutropenia [Boztug and Klein, 2009]. However, many families with autosomal dominant SCN have no identifiable mutation, suggesting that other SCN genes exist. We used high density SNP chips to detect identity by descent (IBD) regions among affecteds in a large SCN family, followed by exome sequencing to identify coding single nucleotide variants (SNVs) in the IBD regions. This strategy implicates TCIRG1 (MIM# 604592) as a novel SCN gene.
Primary analysis was performed on 18 members of a single five generation family of European-American descent ascertained for neutropenia (ANC < 1.5 × 109/L) and severe neutropenia (ANC < 0.5 × 109/L) utilizing DNA extracted from peripheral blood mononuclear cells and saliva (Supp. Figure S1). In 13 of the affected individuals, the median ANC was 0.524 × 109/L, with range 0.074 to 1.1 × 109/L and 6 of the 13 had severe neutropenia based on all available clinical data. For 8 unaffected members, the median was 3.819 × 109/L, with range 2.343 to 6.5 × 109/L. There were no family members with congenital anomalies of the heart, lungs, neurological, gastrointestinal or urogenital systems and no history of bone disease, frequent fractures or hearing loss. Two patients had prominent hemangiomas that became more prominent during treatment with granulocyte colony stimulating factor (G-CSF). Genes associated with SCN, i.e., ELA2, HAX1, G6PC3, WAS, and GFI1, had been previously sequenced in the index case for this family and no likely causal variants were identified [Xia et al., 2009].
IBD analysis, carried out using fast IBD from the software package BEAGLE 3.3.2 [Browning and Browning, 2011] on SNPs from the ~200K SNP HumanCytoSNP-12 BeadChip reduced the possible regions underlying the shared phenotype to chromosomes (chrs.) 3 and 11. The five affected cousins in the lowest generations (IDs 410, 411, 504, 505, and 510) shared regions IBD on chrs. 1, 3 and 11. The unaffected cousin (ID 412) also shared the region on chr. 1, and parts of the regions on chr. 3q and 11q. This narrowed the regions of interest to chr. 3 (between 193801183bp and the q terminus, map build GRCh37/hg18) and 11 (66842469 to 76462540 bp).
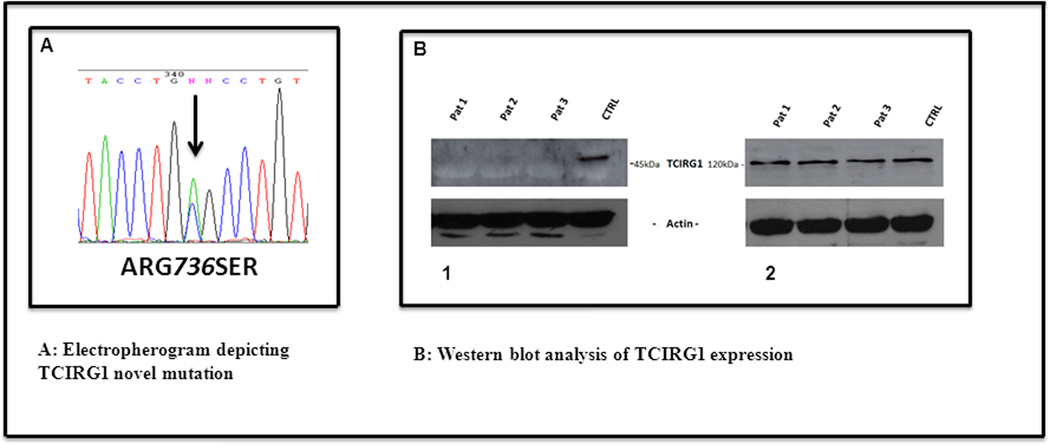
A search of the exome sequences of IDs 410 and 504 in these regions on chrs. 3 and 11 led to two genes of interest. Two novel (no rsID and does not exist in the Exome Variant Server (EVS) at SeattleSNPs [http://evs.gs.washington.edu/EVS/] as of April 2013) missense SNVs were shared by these two exome sequenced affecteds in the genes TCIRG1 and MAP6 on chr. 11 (Table 1). Both SNVs are conserved across mammals (Genome Evolutionary Rate Profiling (GERP) score > 2 [Adzubei et al., 2010]. However, only TCIRG1 is expressed in blood tissue (monocytes) [Pontius et al., 2003] and is directly involved in T-cell activation [Utku et al., 1998], making it the likely etiological candidate. The TCIRG1 SNV NM_006019.3:c.2206C>A (ClinVar SCV000120004), which causes the amino acid change – p.Arg736Ser (Figure 1A), has a GERP score of 3.78. Sanger sequencing of 20 members (14 affected and 6 unaffected) of this family reveals a perfect segregation of the novel TCIRG1 SNV with affection status (Figure 1A). Known congenital neutropenia genes (ELA2, HAX1, G6PC3, WAS, and GFI1) were checked for sequence variation that might have been missed by the earlier Sanger sequencing, and no known causal or novel variation was shown to be shared among these two affected individuals.
Table 1.
Novel missense variants detected in regions of interest with GERP > 2
| Chr | Position | HGVS annotation | Ref | Alt | Gene | AA change | GERP |
|---|---|---|---|---|---|---|---|
| 11 | 67075198 | NM_017857.3:c.781G>A; | G | A | SSH3 | p.Glu261Lys | 3.49 |
| 11 | 67817691 | NM_006019.3:c.2206C>A; | C | A | TCIRG1 | p.Arg736Ser | 3.78 |
| 11 | 71712875 | NM_173042.2:c.553T>C; | T | C | IL18BP | p.Ser185Pro, | 2.97 |
| 11 | 75317003 | NM_033063.1:c.1166C>G; | G | C | MAP6 | p.Ala389Gly | 2.48 |
RS identifiers do not exist for these SNVs at the time of publication. Sites in bold are those for which both affected individuals carry the minor (Alt) allele.
Figure 1.
A. Electropherogram depicting TCIRG1 novel mutation (the N above the G nucleotide at pos 342 in this electropherogram is computer generated error and should be labeled as G)
- down regulated levels of 45kDa TCIRG1 product in PB mononuclear cells of 3 patients (26%, 49%, 35% respectively) compared to healthy individual.
- Identical expression level of TCIRG1-isoa. (n=3)
Western blot analysis confirmed a possible deregulatory involvement of TCIRG1 protein in the pathogenesis of neutropenia in this family. Previous studies have shown that Western blot analysis from patient derived cells harboring homozygous mutations in this gene have undetectable concentrations of TCIRG1 protein products [Frattini et al., 2000]. TCIRG1, through alternate splicing, gives rise to two separate proteins: Isoforms (-iso) a and b. previously known as OC-116 and TIRC7, respectively [Heinemann et al., 1999; Frattini et al., 2000; Susani et al., 2004]. TCIRG1-isob lacks the first 5 exons of the longer variant, TCIRG1-isoa. We performed western blot analysis using lysed peripheral blood mononuclear cells from 3 affected individuals of this family and healthy volunteers. Two different commercially available antibodies against TCIRG1 protein were used. A polyclonal antibody developed against the N-terminal cytoplasmic domain of human TCIRG1-isoa protein (Santa Cruz Biotechnology), detects reduced amounts of ~45kDa TCIRG1 product fragment in all 3 individuals harboring the ARG736SER mutation, compared to healthy controls (Figure 1, B1). This protein fragment is most likely the shorter TCIRG1-isob. A monoclonal antibody raised against the partial recombinant TCIRG1 isoa 121aa-220aa (Abnova) detects a distinct protein band at ~120 kDa. Presumably, this is the full-length TCIRG1-isoa protein, with similar expression levels in both affected individuals and healthy controls (Figure 1, B2). In addition, we have performed TCIRG1 transcripts analysis (data not shown) by RT-PCR using the total RNA from the same source as the WB analysis and found no alterations in mRNA expression compared to the controls.
It is unclear how the p.Arg736Ser mutation causes a reduction of the shorter isoform, but not the longer isoform a. It is possible that the reduced protein in our patients is one of the not yet fully characterized novel isoforms of TCIRG1 as previously reported by Smirnova et al. [Smirnova et al., 2005].. Apparently, this missense SNV within exon 18 does not cause any detectable alterations in the TCIRG1 transcripts. However, the change does affect the observed level of protein. The arginine is changed to serine, a major shift based on amino acids molecular size and properties, and this change is located at an evolutionary highly conserved site. It is possible that the reduced levels of protein is due to post-translational folding defects of the mutant protein and subsequent endoplasmic reticulum associated degradation and elimination [Meusser B et al., 2005; Ding WX and Yin XM, 2008; Waters PJ, 2001]. The p.Arg736Ser mutation in this family has never been reported, either as a variant causal for autosomal recessive malignant osteopetrosis (arOP) or associated with neutropenia.
As a follow-up, TCIRG1 mutational analysis in 20 unrelated SNC individuals known to be negative for mutations in the known SCN genes was conducted by standard PCR amplification using HotStarTaq DNA Polymerase (Qiagen, Inc) and custom designed primer sets. Products of PCR amplification were purified with QIAquick PCR Purification Kit (Qiagen, Inc.). The amplicons were sequenced in the forward and reverse directions using BigDye Terminator V3.1 Cycle Sequencing Kits (Applied Biosystems, Foster City, CA) and a capillary DNA analyzer (Applied Biosystems). Primer set sequences will be provided upon request. Two SNVs in two individuals were implicated in SCN based on minor allele frequency (MAF) and GERP score. The first mutation, rs139617644:G>A, [described in Sobacchi et al, 2001] is located in the acceptor splice site of intron 14, and predicted to alter the splicing (data not shown). This SNV occurs in only 3 of 12988 chromosomes in EVS (July 2013), has GERP=4.12 and is highly conserved among vertebrates [Siepel A et al., 2005]. The second mutation, rs186758849:G>A, causes p.GLY160GLU and is located in exon 5. This SNV has MAF=0.006, is predicted to be possibly damaging [Adzhubei IA et al., 2010] and is highly evolutionarily conserved (GERP=4.42). No other SNV’s predicted to alter protein structure were found.
TCIRG1 is located at 11q13, consists of 20 exons, and through alternate splicing gives rise to two main isoforms: TCIRG1-isoa and TCIRG1-isob. TCIRG1-iso-a is a full length isoform and encodes a3 subunit of vacuolar H+-ATPase. TCIRG1-iso-b is a shorter isoform, lacking the first 5 exons of the longer isoform. Vacuolar H+-ATPases are well described large protein complexes composed of two main functional domains: peripheral V1 and membrane associated V0. Both domains consist of multiple subunits. V-ATPases are involved in regulation of the pH of intracellular compartments and organelles of eukaryotic cells, including the pH of neutrophil phagocytic vacuoles (Supp. Figure S2) [Nanda et al., 1996; Yao et al., 2007; Hinton et al., 2009].
TCIRG1 isoa is highly expressed in osteoclasts and is essential for bone resorption. Homozygous or compound heterozygous mutations in this gene cause arOP characterized by bone resorption defect due to osteoclast malfunction through impairing acidification at the ruffle border interface between osteoclasts and bone [Frattini et al., 2000]. More than 50% of all cases of arOP are attributable to mutations in TCIRG1, and more than 90 different mutations have been described.
Although congenital neutropenia due to mutations in TCIRG1 have not been previously described, there is biological evidence for a role in SCN. Experimental data reveals that Arginine 735 in yeast TCIRG1 protein product (homologous to human ARG736) is essential for proton translocation [Kawasaki-Nishi et al., 2001]. Studies in mice have shown that although a homozygous mutation at amino acid 740 (homologous to human amino acid 736) is lethal in mice, a heterozygous mutation of the V-ATPase a3 subunit R740S causes dominant negative osteopetrosis [Ochotny et al., 2011]. As mentioned above, through an alternative splicing and usage of an alternative initiation codon in exon 7, TCIRG1 codes another protein, TIRC7-isob, which is a T cell-specific membrane protein demonstrated to play an essential role for T-lymphocyte activation and immune response. RNA interference to silence TCIRG1-isob, Ap6i +/− mice reduced inflammatory responses in a mouse model of periodontal disease and decreased osteoclasts and monocytes [Jiang et al., 2013]. However, these reports do not comment on blood neutrophil counts or granulocytopoiesis in the mice. Although the secondary hematological consequences of osteopetrosis are well known, there are no comments in the literature on blood neutrophil counts or granulocytopoiesis in individuals harboring heterozygous variants in this gene. Interestingly, there are multiple novel splice variants of TCIRG1 with unknown functional effects and the proteins are expressed in numerous human tissues (heart, liver, kidney, lung, and pancreas) [Susani et al., 2004; Smirnova et al., 2005].
It is possible that TCIRG1 is relevant to SCN through other pathways. Besides regulating bone resorption, osteoclasts regulate osteoblasts, a cell population implicated in hematopoietic stem cell maintenance [Zhang et al., 2002; Calvi et al., 2003]. Indeed, osteoclast activation has been implicated in the mobilization of hematopoietic progenitor cells from the bone marrow to blood [Kollet et al., 2006]. Moreover, prior published studies of RNA profiling of murine osteoblasts indicates that TCIRG1 is also expressed in this cell population [Eash et al., 2010]. Thus, it is possible that TCIRG1 p.Arg736Ser may indirectly disrupt granulopoiesis by altering the bone marrow microenvironment. Alternatively, TCIRG1 products are broadly expressed in hematopoietic cells. RNA expression profiling of granulocytic precursors from healthy donors suggests that TCIRG1 isoa is highly expressed in promyelocytes. Thus, TCIRG1 p.Arg736Ser may also directly act on granulocytic precursors to impair their differentiation or maturation. Further studies will be directed to defining the mechanisms for neutropenia and the importance of the vATPases in maintaining the integrity of the pathway for neutrophil production and deployment. Understanding the pathogenesis of TCIRG1-associated neutropenia will require several methodological approaches and more studies involving both individual patients and families.
Supplementary Material
Acknowledgments
Grant support received for this study from NIH/NIAID, grant # 5R 24AI049393-09. Sequencing was provided by the University of Washington Center for Mendelian Genomics (UW CMG), funded by NIH grant 1U54HG006493 to Drs. Debbie Nickerson, Jay Shendure and Michael Bamshad.
Footnotes
Conflict of Interest Disclosure:
All authors of this manuscript declare no competing financial interests.
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boztug K, Klein C. Novel genetic etiologies of severe congenital neutropenia. Curr Opin Immunol. 2009;21:472–480. doi: 10.1016/j.coi.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Browning BL, Browning SR. A fast, powerful method for detecting identity by descent. Am J Hum Genet. 2011;88:173–182. doi: 10.1016/j.ajhg.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Carlsson G, Fasth A. Infantile genetic agranulocytosis, morbus Kostmann: presentation of six cases rom the original "Kostmann family" and a review. Acta Paediatr. 2001;90:757–764. [PubMed] [Google Scholar]
- Dale DC, Person RE, Bolyard AA, Aprikyan AG, Bos C, Bonilla MA, Boxer LA, Kannourakis G, Zeidler C, Welte K, Benson KF, Horwitz M. Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood. 2000;96:2317–2322. [PubMed] [Google Scholar]
- Ding WX, Yin XM. Sorting, recognition and activation of the misfolded protein degradation pathways through macroautophagy and the proteasome. Autophagy. 2008;4:141–150. doi: 10.4161/auto.5190. [DOI] [PubMed] [Google Scholar]
- Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J Clin Invest. 2010;120:2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK, Wallbrandt P, Zecca L, Notarangelo LD, Vezzoni P, et al. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet. 2000;25:343–346. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- Heinemann T, Bulwin GC, Randall J, Schneiders B, Sandoff K, Volk HD, Milford E, Gullans SR, Utku N. Genomic organization of the gene coding for TIRC7, a novel membrane protein essential for T-Cell activation. Genomics. 1999;57:398–406. doi: 10.1006/geno.1999.5751. [DOI] [PubMed] [Google Scholar]
- Hinton A, Bond S, Forgac M. ATPase functions in normal and disease processes. Pflugers Arch. 2009;457:589–598. doi: 10.1007/s00424-007-0382-4. [DOI] [PubMed] [Google Scholar]
- Horwitz M, Benson KF, Person RE, Aprikyan AG, Dale DC. Mutations in ELA2, encoding neutrophil elastase, define a 21-day biological clock in cyclic haematopoiesis. Nat Genetics. 1999;23:433–436. doi: 10.1038/70544. [DOI] [PubMed] [Google Scholar]
- Jiang H, Chen W, Zhu G, Zhang L, Tucker B, Hao L, Feng S, Ci H, Ma J, Wang L, Stashenko P, Li YP. RNAi-mediated silencing of Atp6i and Atp6i haploinsufficiency prevents both bone loss and inflammation in a mouse model of periodontal disease. PLoS One. 2013;8:e58599. doi: 10.1371/journal.pone.0058599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki-Nishi S, Nishi T, Forgac M. Arg-735 of the 100-kDa subunit a of the yeast V-ATPase is essential for proton translocation. Proc Natl Acad Sci U S A. 2001;98:12397–12402. doi: 10.1073/pnas.221291798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Grudzien M, Appaswamy G, Germeshausen M, Sandrock I, Schäffer AA, Rathinam C, Boztug K, Schwinzer B, Rezaei N, Bohn G, Melin M, et al. HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease) Nat Genet. 2007;39:86–92. doi: 10.1038/ng1940. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- Kostmann R. Infantile genetic agranulocytosis; agranulocytosis infantilis hereditaria. Acta Paediatr Suppl. 1956;45(Suppl 105):1–78. [PubMed] [Google Scholar]
- Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- Nanda A, Brumell JH, Nordström T, Kjeldsen L, Sengelov H, Borregaard N, Rotstein OD, Grinstein S. Activation of proton pumping in human neutrophils occurs by exocytosis of vesicles bearing vacuolar-type H+-ATPases. J Biol Chem. 1996;271:15963–15970. doi: 10.1074/jbc.271.27.15963. [DOI] [PubMed] [Google Scholar]
- Ochotny N, Flenniken AM, Owen C, Voronov I, Zirngibl RA, Osborne LR, Henderson JE, Adamson SL, Rossant J, Manolson MF, Aubin JE. The V-ATPase a3 subunit mutation R740S is dominant negative and results in osteopetrosis in mice. J Bone Miner Res. 2011;26:1484–1493. doi: 10.1002/jbmr.355. [DOI] [PubMed] [Google Scholar]
- Pontius JU, Wagner L, Schuler GD. UniGene: a unified view of the transcriptome. In: McEntyre J, Ostell J, editors. The NCBI Handbook. Bethesda: National Center for Biotechnology Information; 2003. pp. 1–11. [Google Scholar]
- Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, Rosenbloom K, Clawson H, Spieth J, Hillier LW, Richards S, Weinstock GM, Wilson RK, et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005;15:1034–1050. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova AS, Morgun A, Shulzhenko N, Silva I, Gerbase-DeLima G. Identification of new alternative spice events in the TCIRG1 gene in different human tissues. Biochem Biophys Res Commun. 2005;330:943–949. doi: 10.1016/j.bbrc.2005.03.065. [DOI] [PubMed] [Google Scholar]
- Sobacchi C, Frattini A, Orchard P, Porras O, Tezcan I, Andolina M, Babul-Hirji R, Baric I, Canham N, Chitayat D, Dupuis-Girod S, Ellis I, et al. The mutational spectrum of human malignant autosomal recessive osteopetrosis. Hum Mol Genet. 2001;10:1767–1773. doi: 10.1093/hmg/10.17.1767. [DOI] [PubMed] [Google Scholar]
- Susani L, Pangrazio A, Sobacchi C, Taranta A, Mortier G, Savarirayan R, Villa A, Orchard P, Vezzoni P, Albertini A, Frattini A, Pagani F. TCIRG1-dependent recessive osteopetrosis: mutation analysis, functional identification of the splicing defects, and in vitro rescue by snRNA. Hum Mutat. 2004;24:225–235. doi: 10.1002/humu.20076. [DOI] [PubMed] [Google Scholar]
- Utku N, Heinemann T, Tulius SG, Bulwin GC, Beinke S, Blumberg RS, Beato F, Randall J, Kojima R, Busconi L, Robertson ES, Schülein R, et al. Prevention of acute allograft rejection by antibody targeting of TIRC7, a novel T cell membrane protein. Immunity. 1998;9:509–518. doi: 10.1016/s1074-7613(00)80634-2. [DOI] [PubMed] [Google Scholar]
- Waters PJ. Degradation of mutant proteins, underlying "loss of function" phenotypes, plays a major role in genetic disease. Curr Issues Mol Biol. 2001:57–65. [PubMed] [Google Scholar]
- Xia J, Bolyard AA, Rodger E, Stein S, Aprikyan AA, Dale DC, Link DC. Prevalence of mutations in ELANE, GFI1, HAX1, SBDS, WAS and G6PC3 in patients with severe congenital neutropenia. Br J Haematol. 2009;147:535–542. doi: 10.1111/j.1365-2141.2009.07888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao G, Feng H, Cai Y, Qi W, Kong K. Characterization of vacuolar-ATPase and selective inhibition of vacuolar-H(+)-ATPase in osteoclasts. Biochem Biophys Res Commun. 2007;357:821–827. doi: 10.1016/j.bbrc.2007.04.082. [DOI] [PubMed] [Google Scholar]
- Zhang M, Xuan S, Bouxsein ML, von Stechow D, Akeno N, Faugere MC, Malluche H, Zhao G, Rosen CJ, Efstratiadis A, Clemens TL. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.