Abstract
Ascorbic acid has been shown to kill various cancer cell lines at pharmacologic concentrations. We found that Epstein-Barr virus (EBV)-positive Burkitt lymphoma (BL) cells were more susceptible to ascorbic acid-induced cell killing than EBV-negative BL cells or EBV-transformed lymphoblastoid cells (LCLs). Ascorbic acid did not induce apoptosis in any of the tested cells but did induce the production of reactive oxygen species and cell death. Previously, we showed that bortezomib, a proteasome inhibitor, induces cell death in LCLs and EBV-positive BL cells. We found that ascorbic acid is strongly antagonistic for ascorbic acid-induced cell death in LCLs and EBV-positive BL cells. Finally, ascorbic acid did not prolong survival of severe combined immunodefiency mice inoculated with LCLs either intraperitoneally or subcutaneously. Thus, while ascorbic acid was highly effective at killing EBV-positive BL cells and LCLs in vitro, it antagonized cell killing by bortezomib and was ineffective in an animal model.
Keywords: Ascorbic acid, Epstein Barr virus, Burkitt, lymphoma, reactive oxygen species, bortezomib
INTRODUCTION
Epstein-Barr virus (EBV) is one of the most successful human viruses, infecting over 90% of adults and persisting for the lifetime of the individual [1]. The virus infects both B cells and epithelial cells. EBV is associated with a number of malignancies. These include most cases of post-transplant lymphoproliferative disease, anaplastic nasopharyngeal carcinoma, and about 50% of cases of Hodgkin's disease in the United States and B cell lymphomas in AIDS patients [2,3]. In nearly all of these diseases EBV latent membrane protein 1 (LMP1) is expressed. This protein is an oncogene and activates the NF-κB pathway to inhibit apoptosis in the cell and drive B cell proliferation. LMP1 binds the tumor necrosis factor receptor associated factors (TRAFs) and to tumor necrosis factor receptor-associated death domain protein (TRADD) which results in activation of NF-κB to mediate growth transformation [3].
Three different patterns of latency have been associated with EBV infection [2]. In latency pattern 1, Epstein-Barr virus nuclear antigen-1 (EBNA-1) is the only EBV protein expressed. This is the pattern of gene expression seen in tissues from patients with Burkitt lymphoma [3] and gastric carcinoma [4,5]. In latency pattern 2, EBNA-1, LMP1, and EBV latent membrane protein 2 (LMP2) are expressed. This pattern is seen in tissues from patients with EBV-positive Hodgkin lymphoma [6], nasopharyngeal carcinoma [7], NK/T cell lymphoma [8], and peripheral T cell lymphoma [9]. All of the EBV-associated latency proteins are expressed in latency pattern 3. This pattern of latency is seen in EBV-associated post-transplant lymphoproliferative disease [10], immunoblastic lymphomas of the CNS in AIDS patients [11], some non-Hodgkin lymphomas in patients with AIDS [10,12,13], and in EBV-transformed lymphoblastoid cell lines in vitro.
Several agents (BAY 11-7082, high dose simvastatin, and bortezomib) have been shown to induce apoptosis of EBV-transformed B cells as well as inhibit development EBV-induced lymphomas in severe combined immunodeficiency (SCID) mice [1,14-16]. However, since these compounds have multiple effects on the cell and are often associated with toxicity, it is important to examine other compounds that may be used as potential treatments for EBV-associated malignancies. Ascorbic acid (vitamin C) is an essential vitamin and a potent water-soluble antioxidant which functions as an electron donor. At pharmacologic concentrations ascorbic acid can also exert pro-oxidant effects through the reduction of transition metal ions, such as iron and copper. Ascorbic acid functions as an electron donor for hydroxylating enzymes. Infection of B cells by EBV induces oxidative stress [17]. Furthermore, hydrogen peroxide, a potent oxidizer, inhibits induction of EBV immediate-early gene expression and therefore is critical for maintaining EBV latency [18]. Previous studies have shown that addition of pharmacologic concentrations of ascorbic acid to various cancer cell lines, including EBV-negative Burkitt lymphoma cell lines, causes cell death at concentrations less than 5 mM [19-21]. Plasma levels of ascorbic acid of less than 100 μM can be achieved after oral dosing, whereas, levels of 20 mM are obtained with intravenous dosing [22-24]. Therefore, we examined whether ascorbic acid would kill EBV-positive Burkitt lymphoma cells as well as EBV-transformed B cell lines, and if ascorbic acid could be a potential treatment for EBV-associated B cell lymphomas.
We found that pharmacologic concentrations of ascorbic acid induced cell death in all cell lines tested through the production of reactive oxygen species (ROS). However, when used in combination with bortezomib, ascorbic acid was highly antagonistic to bortezomib. Surprisingly, ascorbic acid had no effect on the development of EBV-lymphomas in SCID mice. These finding suggest that while ascorbic acid is potent in vitro, it may not be effective for treatment for EBV-associated B cell lymphomas.
MATERIALS AND METHODS
Cell Lines
Two EBV-transformed lymphoblastoid cell lines (LCLa and LCLb), three EBV positive Burkitt lymphoma cell lines (Akata, Kem I, and Mutu I), and two EBV negative Burkitt lymphoma cells lines (BJAB and BL30) were grown in RPMI 1640 medium supplemented with 10 % heat-inactivated fetal bovine serum (FBS), penicillin, and streptomycin.
Reagents
Ascorbic acid and bovine liver catalase were obtained from Sigma-Aldrich (St. Louis, MO) and alamar blue was from Invitrogen Biosource (Carlsbad, CA). The general caspase inhibitor, Q-VD-OPH, was obtained from R&D Systems (Minneapolis, MN). Bortezomib was obtained from Millennium Pharmaceuticals Inc. (Cambridge, MA).
Cell Viability Assay
Cells (4 × 104 cells) were plated and cultured in RPMI 1640 media with 10% FBS in the presence of ascorbic acid at 37°C for 3 days. On the third day, 10μL of a 1:1 mixture of alamar blue (Invitrogen Biosource, Carlsbad, CA) and RPMI 1640 media with 10% FBS were added to the cells and incubated at 37°C for 2 hr. Proliferating cells reduce alamar blue to a highly fluorescent compound which can be quantified using a multiwell plate reader with excitation of 535 nm and emission of 595 nm.
Immunoblots
Cells were lysed in RIPA buffer containing 10 mM Tris-HCl pH 8, 100 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% deoxycholate, 0.5% SDS, and protease inhibitors (Roche, Indianapolis, IN). Equal amounts of protein were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and incubated with antibodies. Caspase-3, cleaved caspase 3 (Cell Signaling Technology, Danvers, MA), and BZLF-1 (Santa Cruz Biotechnology) antibodies were used in immunoblots.
Annexin V Apoptosis Assay
Apoptosis was measured using annexin V/7-AAD staining according to the manufacturer's instructions (BD Pharmingen Biosciences, San Diego, CA). Cells (2 × 105) were incubated with various concentrations of ascorbic acid for 24 or 48 hr. The cells were washed, resuspended, and incubated with binding buffer containing annexin V –PE for 15 min at room temperature. Flow cytometry was used to analyze the labeled cells.
Combination Studies
Combination studies were performed and analyzed as previously described [25]. Briefly, cells were treated with ascorbic acid, bortezomib, or a combination of ascorbic acid and bortezomib for 72 hr and analyzed for viability. The Chou-Talalay equation was used to determine if the drug combinations were synergistic, additive, or antagonistic [26]. Additional combination studies were performed in which cells were pretreated with bortezomib for 4 or 8 hr before the addition of 1 mM ascorbic acid to the cells and analyzed for viability after 72 hr.
Animal Experiments
Female SCID mice, aged 4-6 weeks, were inoculated intraperitoneally (i.p.) with 1 × 106 LCLs and treatment with ascorbic acid was initiated 7-10 days later. Animals were treated with either 4 g/kg of ascorbic acid in H20 (pH 7.0) or 0.5M NaCl (isotonic with the dose of ascorbic acid) 5 days a week for 6 weeks (for a total of 30 days of treatment). Mice were observed daily for ascites and solid tumors. Alternatively, female SCID mice, aged 4-6 weeks, were inoculated subcutaneously (s.c.) with 5 × 106 LCLs and 10 to 17 days post-inoculation, mice were divided into treatment groups dependent on the size of the masses. Treatment was begun as described in the i.p. studies; alternatively, treatment continued for the entire course of the experiment. The s.c. masses were measured three times per week and animals were euthanized when the masses exceeded 2 centimeters in diameter or when the masses ulcerated. Upon euthanization, the masses were excised and measured for weight and volume.
Measurement of Ascorbic Acid Levels in Plasma
Peripheral blood was collected in heparinized tubes from animals injected either i.p. or s.c. with LCLs. Plasma was separated and mixed 1:1 with a solution of 10% EDTA/90% methanol, incubated on ice for 5 min, and the resulting supernatant was analyzed by HPLC as previously described [27].
RESULTS
Burkitt Lymphoma Cell Lines are Generally More Susceptible to Killing by Ascorbic Acid than EBV-transformed Lymphoblastoid Cell Lines
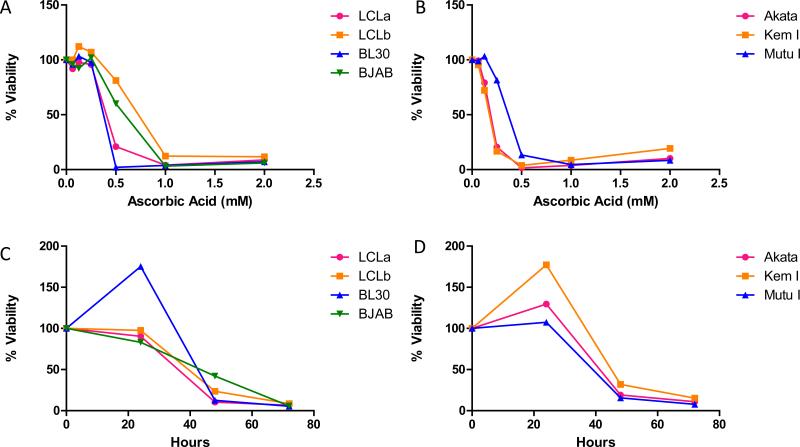
Several EBV-positive and EBV-negative cells were treated with various concentrations of ascorbic acid for 72 hr. All cell lines tested showed > 60% loss of viability with 2 mM ascorbic acid (Figures 1A and B). However, EBV-positive Burkitt lymphoma cells with a type I latency pattern (Akata, Kem I, and Mutu I) were generally more sensitive to ascorbic acid than EBV-transformed LCLs (LCLa, LCLb) or EBV-negative Burkitt lymphoma cells (BJAB, BL30) (Table I). The average effective concentration at which 50% of the cells were killed (EC50) was < 0.5 mM for two EBV-positive Burkitt lymphoma cell lines (Akata and Kem I cells), while the EBV-transformed LCLs had an EC50 that ranged from 0.66 mM to 0.78 mM. Treatment of cell lines with ascorbic acid over a three day time period showed that cell death increased with length of treatment (Figures 1C and D).
Figure 1. Ascorbic acid kills EBV-positive and EBV-negative cell lines.
A and B. Cells were treated with various concentrations of ascorbic acid for 72 hr and cell viability was analyzed using alamar blue. Ascorbic acid killed EBV transformed (LCLa and LCLb), EBV-negative Burkitt lymphoma (BL30 and BJAB), and EBV-positive Burkitt lymphoma (Akata, Kem I, and Mutu I) cell lines in a dose-dependent manner. A representative experiment is shown in which each point represents the mean of triplicate replicates. C and D. Cells were treated with treated with 2 mM ascorbic acid for 24, 48, or 72 hr and cell viability was analyzed using alamar blue. For all cell lines tested, ascorbic acid induced cell death increased with length of treatment. A representative experiment is shown in which each point represents the mean of triplicate replicates.
Table I. EC50s for EBV-positive and negative B cell lines treated with ascorbic acid.
Each value represents the average of four experiments that were performed in triplicate as well as the SD for the data. The final column shows the EBV latency state of each cell line.
| Cell Line | EC50 (mM) | SD | EBV Latency State |
|---|---|---|---|
| LCLa | 0.66 | 0.42 | 3 |
| LCLb | 0.78 | 0.21 | 3 |
| BL30 | 0.41 | 0.03 | EBV-negative |
| BJAB | 0.66 | 0.13 | EBV-negative |
| Akata | 0.25 | 0.07 | 1 |
| Kem I | 0.31 | 0.10 | 1 |
| Mutu I | 0.63 | 0.46 | 1 |
Ascorbic Acid Does Not Induce Apoptosis or EBV Lytic Replication in B Cell Lines
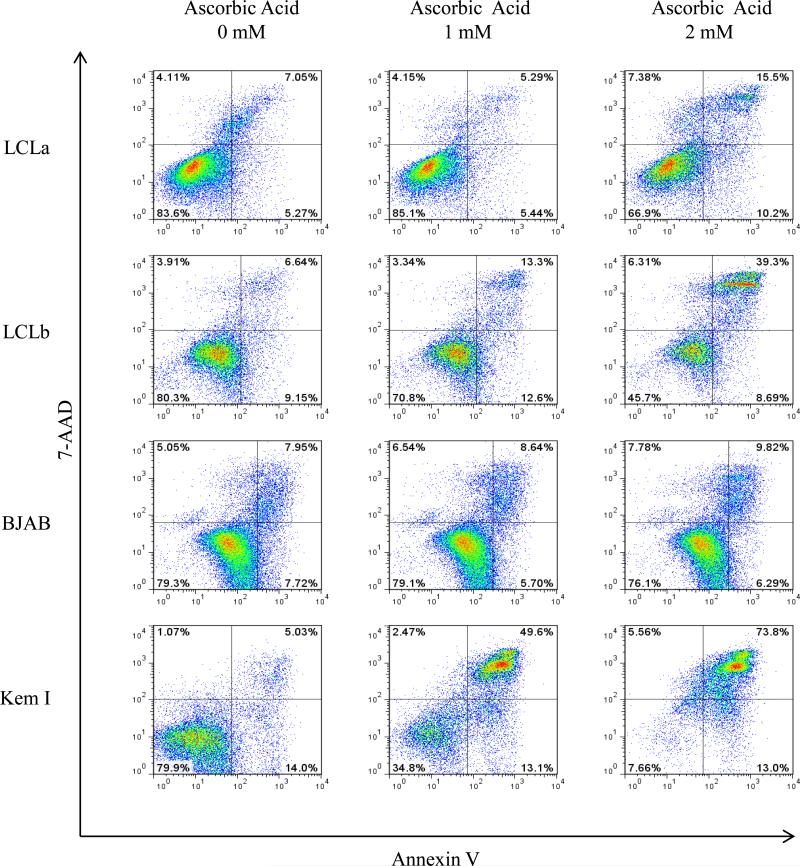
To determine if ascorbic acid kills B cells tested by inducing apoptosis, cells were treated with various concentrations of ascorbic acid for either 24 or 48 hr, stained with annexin V and 7-AAD, and analyzed by flow cytometry. Cells that were annexin V/7-AAD negative were considered viable, cells that were annexin V positive and 7-AAD positive were considered necrotic, and cells that were annexin V positive and 7-AAD negative were considered apoptotic,. LCLa, LCLb, BJAB, and Kem I showed little or no increase in the percentage of apoptotic cells (Figure 2 and data not shown).
Figure 2. Ascorbic acid does not induce apoptosis in EBV-positive and negative B cell lines.
LCLa, LCLb, BJAB, and Kem I cells were treated with various concentrations of ascorbic acid and apoptosis was evaluated by annexin V/7-AAD staining and flow cytometry after 24 hr. Similar results were obtained 48 hr after treatment with ascorbic acid.
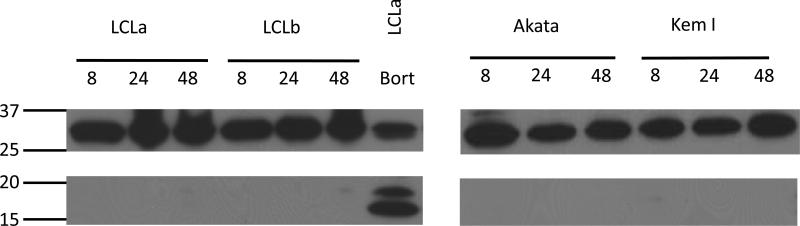
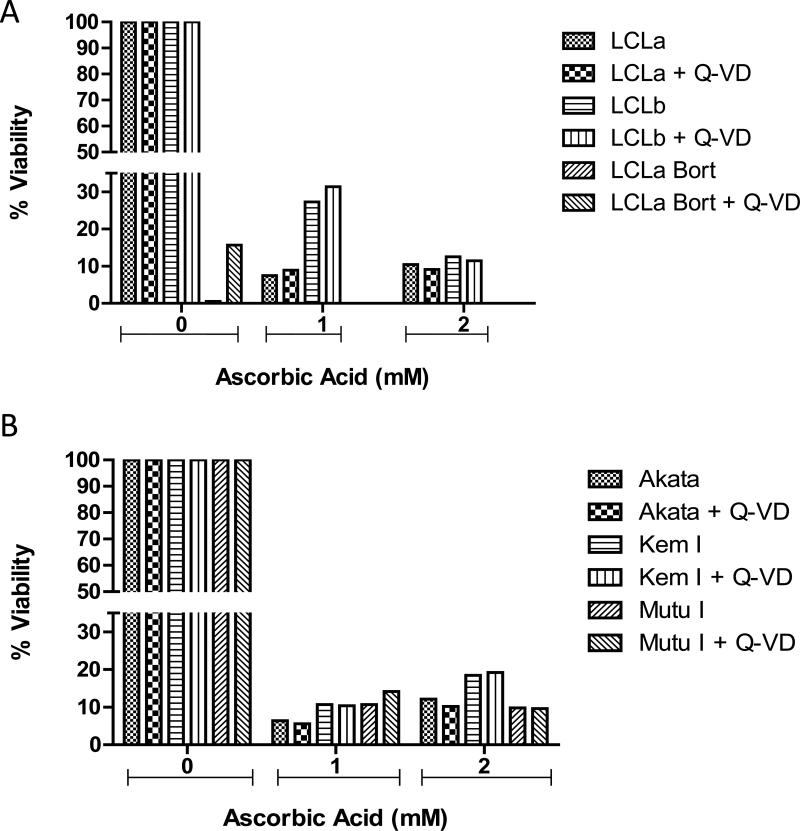
In order to confirm that ascorbic acid does not induce apoptosis in the cells studied, caspase 3 cleavage was examined. Treatment of LCLs and Burkitt lymphoma cell lines with ascorbic acid for various periods of time did not induce cleavage of caspase 3 (Figure 3). Bortezomib, a known inducer of apoptosis of LCLs [14], served as a positive control. For further confirmation that apoptosis is not induced in the cells treated with ascorbic acid, cells were pretreated with the general caspase inhibitor, Q-VD-OPH and then treated with ascorbic acid for 72 hr. The addition of the caspase inhibitor did not protect any of the cells tested from ascorbic acid induced cell death (Figure 4A and B).
Figure 3. Caspase 3 cleavage is not induced by ascorbic acid.
Cells were treated with 2 mM ascorbic acid for 8, 24, or 48 hr, lysates were prepared and analyzed by immunoblotting with anti-caspase 3 antibodies. Numbers at left indicate molecular masses in kilodaltons. Top panel shows uncleaved caspase 3 and lower panel shows caspase 3 cleavage products. Caspase 3 is 35 kD and cleaved caspase 3 is 15 and 17 kD.
Figure 4. Q-VD, a general caspase inhibitor, does not protect from ascorbic acid-induced cell death.
A. LCLa and LCLb (type III latency) and B. Akata and Kem I (type I latency). Cells were pretreated with 50 μM Q-VD for one hr prior to treatment with ascorbic acid for 72 hr and then cell viability was analyzed using alamar blue. LCLa treated with bortezomib +/−pretreatment with Q-VD was used as a positive control for apoptosis-induced cell death. Samples were analyzed in triplicate and a representative experiment is shown.
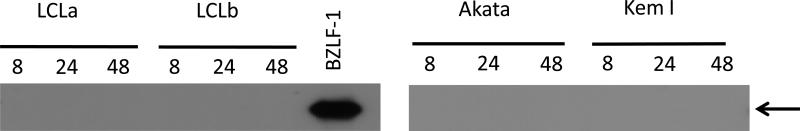
Since lytic replication of EBV can induce cell death, we determined if ascorbic acid might induce virus replication in the EBV-infected cells lines. LCLs and EBV-positive Burkitt lymphoma cell lines were treated with ascorbic acid and immunoblotted to look at expression of BZLF1 protein, a marker of virus lytic replication. No evidence for lytic replication was detected (Figure 5).
Figure 5. Ascorbic acid does not induce EBV lytic reactivation.
Cells were treated with 2 mM ascorbic acid for 8, 24, or 48 hr, and lysates were analyzed by immunoblotting with an anti-BZLF-1 antibody. Arrow indicates BZLF-1 protein. Cell lysate from 293T cells transfected with a BZLF-1 plasmid was used as a positive control.
Ascorbic Acid Induces Production of ROS
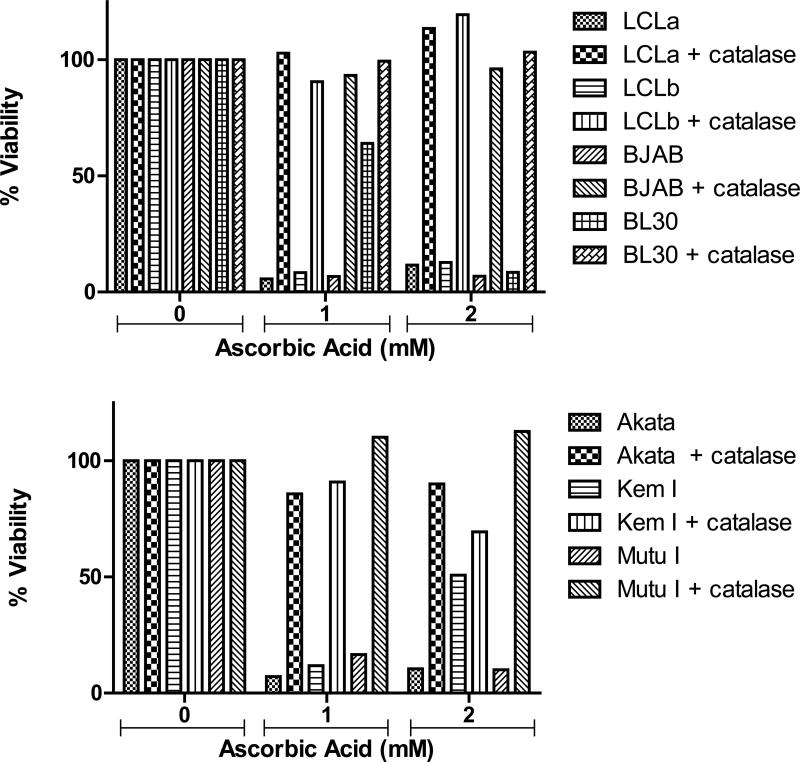
Catalase is a powerful antioxidant and scavenger of hydrogen peroxide which previously was shown to be protective against ascorbic acid-induced cell death caused by the production of ROS [19]. To determine that ascorbic acid-induced cell death was due to the production of ROS, cells were treated ascorbic acid in the presence or absence of 0.1 mg/mL of catalase for 72 hr and viability was analyzed by alamar blue staining. The addition of catalase protected both EBV-positive and EBV-negative B cells from ascorbic acid-induced cell death confirming that generation of ROS is the cause of cell death in these cells (Figure 6).
Figure 6. Catalase protects EBV-positive and EBV-negative B cells from ascorbic acid-induced cell death.
Cells were treated with 0.1 mg/mL catalase and ascorbic acid for 72 hr then cell viability was analyzed using alamar blue. Samples were analyzed in triplicate and a representative experiment is shown. Catalase protection was more evident for BL30 cells treated with 1mM ascorbic acid and Kem I cells treated with 2mM ascorbic acid in other experiments (data not shown).
Ascorbic Acid is Strongly Antagonistic Towards Bortezomib
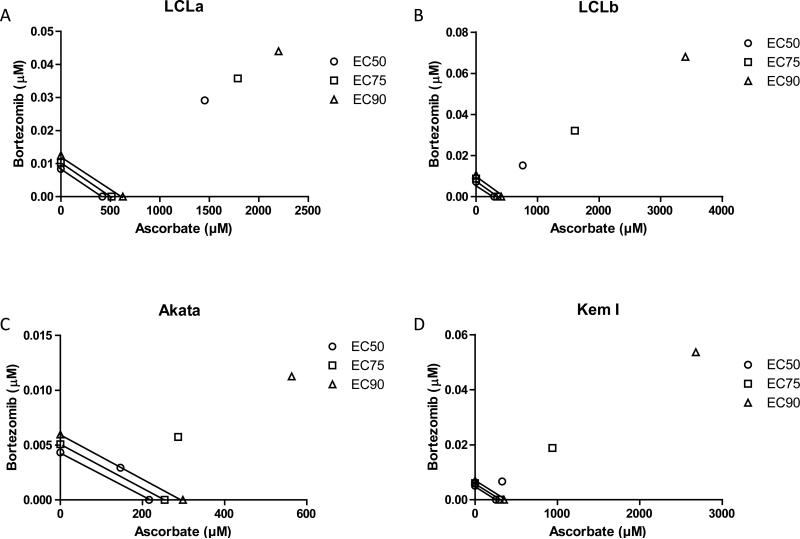
Previously, we showed that bortezomib, an inhibitor of the proteasome, induced cell death in LCLs with a greater efficiency than in EBV-positive or EBV-negative Burkitt lymphoma cells [14]. Therefore, we determined whether a combination of ascorbic acid and bortezomib would enhance cell death in EBV-positive and EBV-negative cells when compared to either ascorbic acid or bortezomib alone. A synergistic, additive, or antagonistic effect of bortezomib on ascorbic acid was determined by calculation of the combination index as well as by isobologram analysis. A combination index of less than 1 is indicative of synergy, a combination index equal to 1 is indicative of an additive effect, and a combination index greater than 1 is indicative of antagonism. For all cell lines tested, ascorbic acid was antagonistic to bortezomib at each effective concentration tested (EC50, 75, 90) (Table II). An isobologram analysis confirmed that ascorbic acid was strongly antagonistic towards bortezomib at concentrations of ascorbic acid greater than approximately 100 μM in all cells tested (Figure 7.
Table II. Combination Index values for bortezomib and ascorbic acid regimens in lymphoma cells.
Combination Index (CI) is a quantitative measure of the degree of bortezomib:ascorbic acid interaction in terms of synergism (CI < 1), additive effect (CI = 1), or antagonism (CI > 1) for a given measurement of cytotoxicity (EC50, 75, 90). Drugs were tested as either a single agent or 1:50,000 bortezomib:ascorbic acid ratio. Cytotoxicity was determined in at least 3 individual experiments per cell type by triplicate alamar blue viability assay determinations at 72 post-exposure. r2: linear regression.
| Cell line | CI EC50 | CI EC75 | CI EC90 | r2 |
|---|---|---|---|---|
| LCLa | 6.9 | 7.0 | 7.0 | 0.889 |
| LCLb | 4.7 | 8.3 | 14.7 | 0.973 |
| Akata | 1.4 | 2.3 | 3.8 | 0.885 |
| Kem I | 2.6 | 6.3 | 15.3 | 0.944 |
Figure 7. Ascorbic acid is antagonistic to bortezomib based on isobologram plots of lymphoma cell lines.
A. LCLa. B. LCLb. C. Akata. D. Kem I. Lines connect the dose required to achieve EC50, 75, 90 levels of cytotoxicity for exposure to either bortezomib (y axis) or ascorbic acid (x axis) alone. Single points not on the x or y axes indicate the dose required to achieve EC50, 75, 90 levels of cytotoxicity with bortezomib and ascorbic acid combined at molar ratio of 1:50,000. Distance above the lines indicates the degree of antagonism. Cytotoxicity was determined in at least 3 individual experiments per cell type by triplicate alamar blue viability assay determinations at 72 hr post-exposure.
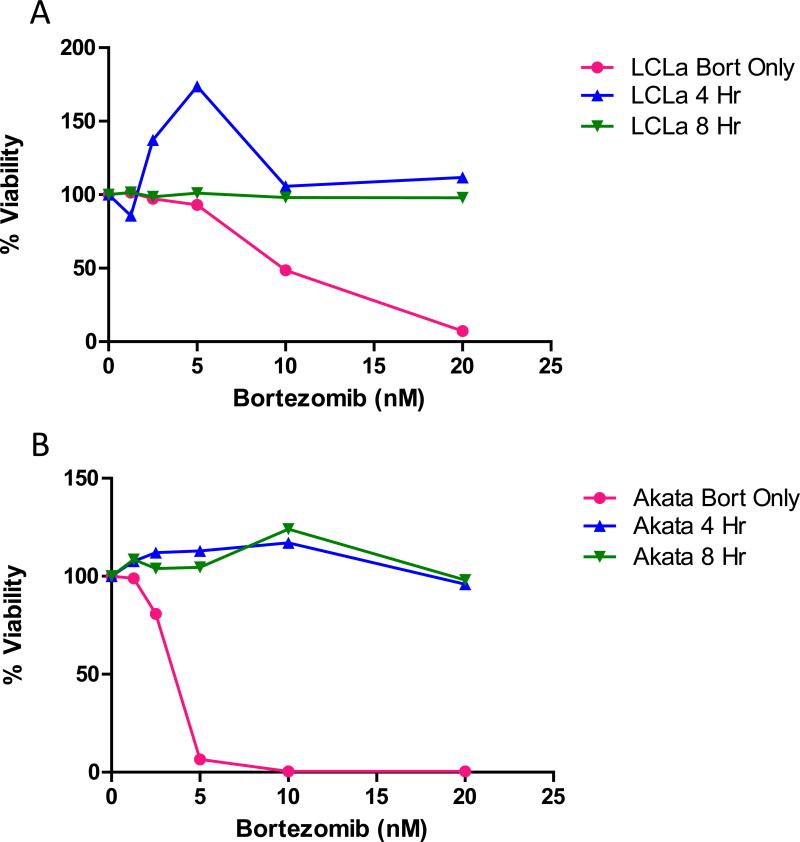
A previous study showed that delaying ascorbic acid treatment of multiple myeloma cells by 4 to 8 hr reduced the antagonist effect of ascorbic acid on bortezomib-induced cell death in vitro [28]. Therefore, we determined if delaying ascorbic acid treatment would also reduce the antagonistic effect of ascorbic acid on bortezomib-induced death of EBV-positive B cell lines. For all cell lines tested, addition ascorbic acid 4 or 8 hr after bortezomib treatment still inhibited bortezomib-induced cell death (Figure 8 and data not shown).
Figure 8. Ascorbic acid inhibits the activity of bortezomib even when treatment with ascorbic acid is delayed.
A. LCLa, B. Akata. Cells were treated with various concentrations of bortezomib for 4 or 8 hr before the addition of 1mM ascorbic acid (4 hr or 8 hr), and after 72 hr viability was analyzed using alamar blue. Samples were analyzed in triplicate and a representative experiment is shown.
Ascorbic Acid Does Not Consistently Protect From EBV Lymphoma in an Animal Model
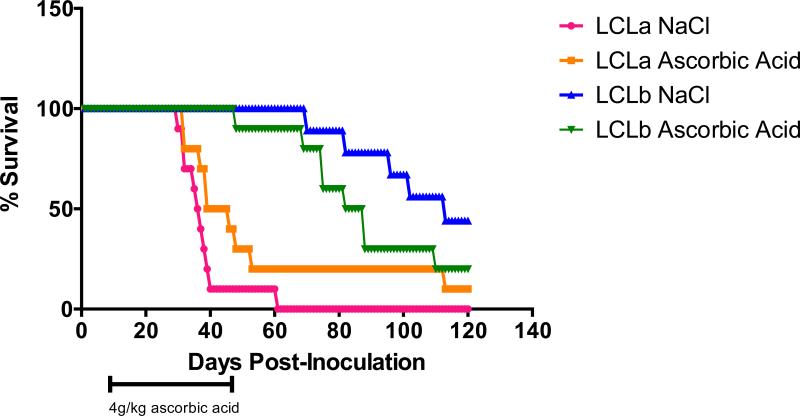
SCID mice injected i.p. with EBV-transformed B cells (LCLs), develop EBV-positive lymphomas that express viral proteins similar to those found in immunocompromised patients with EBV lymphoproliferative disease [29]. Groups of 10 animals each were inoculated with 1 × 106 LCLs i.p. and 7-10 days later treatment with ascorbic acid (4g/kg) or NaCl (0.5M) was initiated. The animals were treated 5 days a week for 6 weeks. There was no improvement in survival of mice that received ascorbic acid as compared to the controls (Figure 9).
Figure 9. Ascorbic acid does not increase survival of SCID mice inoculated intraperitoneally (i.p.) with LCLs.
Groups of 10 animals each were inoculated with 1 × 106 LCLs i.p. and 7-10 days later treatment with ascorbic acid or NaCl was begun. The animals were treated with 4 g/kg of ascorbic acid or 0.5 M NaCl 5 days a week for 6 weeks and survival of the animals was followed for 120 days.
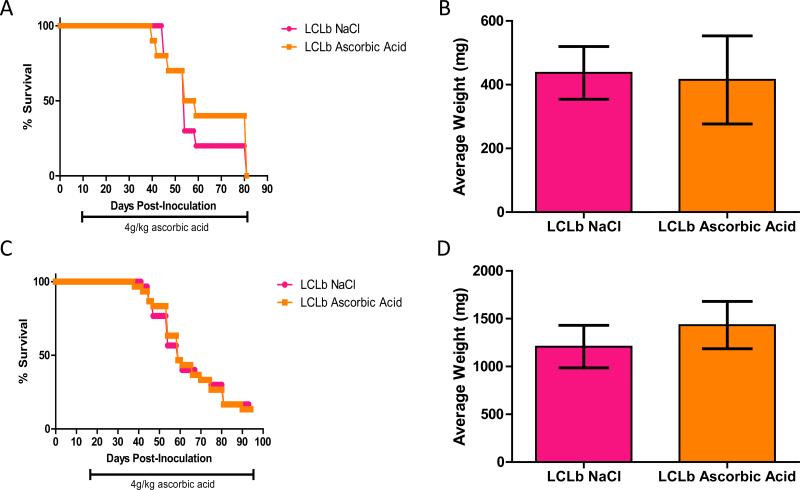
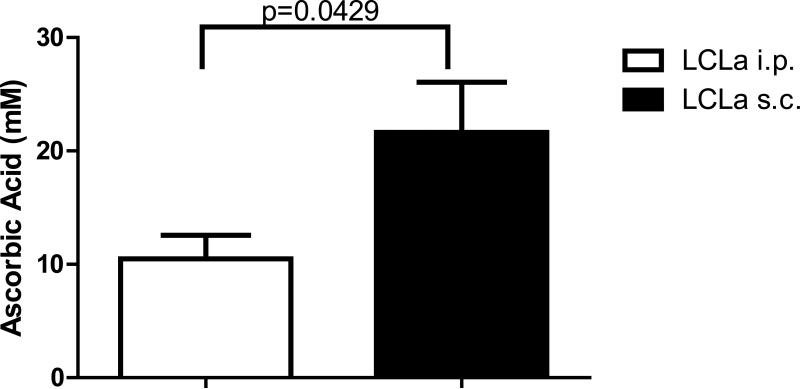
SCID mice injected s.c. with EBV-transformed B cells also develop EBV-positive tumors at the location of the injection [30]. Therefore, animals were inoculated s.c. with 5 × 106 LCLb s.c. and 10 or 17 days after inoculation, the animals were divided into 2 treatment groups of at least 10 animals per group based on the size of palpable masses. Animals were then treated with either ascorbic acid (4 g/kg) or NaCl (0.5M) for 5 days a week for 70 or more days and then sacrificed. Neither of the ascorbic acid treatment groups showed a difference in survival or a difference in average tumor mass compared to the NaCl treated groups (Figure 10). Similar results were seen in two additional experiments (data not shown). Since we did not see an effect of ascorbic acid after i.p. injection of EBV transformed B cells, we determined if the location of the B cells might have an effect on plasma levels of ascorbic acid in the treated animals. Animals were inoculated with 1 × 106 cells i.p. or 5 × 106 cells s.c. and 7 days later they received ascorbic acid (4g/kg) i.p. 5 days a week. On the 10th day of treatment, the animals were bled 2 hr after injection with ascorbic acid and plasma levels of ascorbic acid were measured. Animals that received EBV transformed B cells i.p. had significantly lower plasma levels of ascorbic acid compared to those injected with cells s.c. (Figure 11). This suggests that accelerated ascorbic acid degradation in the peritoneal cavity is induced when EBV transformed B cells are inoculated i.p. thereby decreasing formation of hydrogen peroxide which is necessary for ROS formation.
Figure 10. Ascorbic acid does not prolong survival or affect the size of masses in SCID mice inoculated subcutaneously (s.c.) with LCLs.
Animals were inoculated with 5 × 106 LCLb s.c and 10 or 17 days after inoculation, animals were divided into treatment groups of 10 animals (10 days post-inoculation, Panels A, B) or of 30 animals (17 days post-inoculation, Panels C, D). Animals were treated with either 4 g/kg of ascorbic acid or 0.5M NaCl until the end of the experiment. At the time of euthanasia, the masses were excised and weighed. Panels A and C show survival curves and Panels B and D show average weight of excised masses. Error bars in panels B and D represent standard errors.
Figure 11. Mice inoculated intraperitoneally (i.p.) with LCLs have significantly lower levels of ascorbic acid in plasma compared to mice inoculated subcutaneously (s.c.) with LCLs.
Mice were inoculated with LCLs and commencing ten days post-inoculation, animals were treated for 10 days with 4g/kg of ascorbic acid. Peripheral blood was collected and plasma isolated and tested for levels of ascorbic acid by HPLC.
DISCUSSION
We have shown that ascorbic acid induces cell death in EBV-positive and EBV-negative cell lines and that Burkitt lymphoma cell lines are more susceptible than EBV-transformed B cell lines. The EC50s for EBV-positive B cells were lower than that reported for other human tumor cell lines that have been reported previously [19-21]. In these prior studies pharmacological doses of ascorbic acid decreased the growth rate of tumors in mice injected with human tumor cell lines [20,21]. Therefore, we tested EBV-positive cell lines that were more sensitive to ascorbic acid-mediated cell death than other cell lines to determine if the drug would reduce the growth of tumors in SCID mice. Surprisingly, we did not observe a consistent prolongation of survival in animals treated with ascorbic acid. Such disparity between in vitro sensitivity and in vivo efficacy for experimental cancer cell lines and pharmacologic levels of ascorbic acid has not been previously reported. This may have been due to lower plasma levels of ascorbic acid after i.p. inoculation of animals with EBV-transformed B cells in the peritoneal cavity. Protein levels in peritoneal fluid are markedly increased in the presence of tumor cells (i.e. exudative ascites) and this could result in increased protein binding to ascorbic acid which could reduce its activity, or increase the level of a host factor that consumes ascorbic acid, thereby lowering the concentration for generation of ROS and reducing death of EBV-transformed cells.
Ascorbic acid-induced cell death was due to the production of ROS. It is known that ROS are essential for biological function and that the levels of ROS in cells are tightly regulated. However, significant increases in cellular ROS levels are able to cause cell damage and cell death (reviewed in [31]). Ascorbic acid-induced production of ROS, leads to the production of hydrogen peroxide which in turn can be converted to highly reactive hydroxyl radicals. Several studies have shown that when various cancer cell lines are treated with ascorbic acid at pharmacological concentrations, cell death is through ROS and hydrogen peroxide production [20,21,32] and that pretreating cells with catalase, a powerful antioxidant which destroys hydrogen peroxide, can protect cells from ascorbic acid-induced cell death [19]. Similarly, we found that EBV-positive Burkitt lymphoma cells as well as EBV-positive transformed B cells were protected from ascorbic acid-induced cell death when treated with catalase. In contrast, we did not detect any evidence that cell death was induced through apoptosis.
Although recent results are promising [24], there is currently much controversy about the role of ascorbic acid in the treatment of various cancers, including the route of delivery, the levels in the blood, and as a component of combination therapy. As reviewed elsewhere, plasma concentrations after oral dosing of ascorbic acid are tightly controlled and do not exceed 100 μM; however, when administered intravenously, plasma concentrations of ascorbic acid can reach 20 mM [22,23]. We found that EBV-positive Burkitt lymphoma cells and EBV-transformed cell lines were killed in vitro with EC50s ranging from 0.25-0.80 mM, well within the range of serum levels after intravenous (but not oral) dosing in humans. We previously showed that bortezomib, a proteasome inhibitor used to treat certain B cell tumors (multiple myeloma and mantle cell lymphoma), kills EBV-transformed B cells and EBV-positive Burkitt lymphomas cells. Previous studies examining combination of bortezomib and ascorbic acid in multiple myeloma cells in vitro have yielded conflicting results. One study showed ascorbic acid at physiological concentrations is not antagonistic to bortezomib in vivo for prostate cancer cells [33], while another study showed that ascorbic acid at physiological concentrations is antagonistic to bortezomib in vivo for multiple myeloma cells [34]. Our results show that at pharmacological concentrations, ascorbic acid treatment is highly antagonistic towards bortezomib in vitro for EBV-positive B cells. Interestingly, ascorbic acid was not antagonistic to bortezomib in multiple myeloma cells in vitro when ascorbic acid treatment was delayed 4-8 hr after treatment with bortezomib [28]. Ascorbic acid binds to bortezomib with a dissociation constant of 645 μmol/L that likely inactivates bortezomib [35].
We have shown that although pharmacologic concentrations of ascorbic acid induced cell death of EBV-positive Burkitt lymphoma cells and EBV-transformed cell lines in vitro, ascorbic acid did not consistently delay development of EBV tumors in SCID mice. These studies emphasize the importance of testing promising in vitro treatment in animal models to learn whether the same effects are observed in vivo as in vitro.
ACKNOWLEDGEMENTS
This work was supported by the intramural research programs of the National Institute of Allergy and Infectious Diseases and the National Institute of Digestive Diseases and Kidney Diseases.
REFERENCES
- 1.Keller SA, Hernandez-Hopkins D, Vider J, et al. NF-kappaB is essential for the progression of KSHV- and EBV-infected lymphomas in vivo. Blood. 2006;107(8):3295–3302. doi: 10.1182/blood-2005-07-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen JI. Epstein-Barr Virus Infection. N Engl J Med. 2000;343(7):481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 3.Cohen JI, Bollard CM, Khanna R, Pittaluga S. Current understanding of the role of Epstein-Barr virus in lymphomagenesis and therapeutic approaches to EBV-associated lymphomas. Leukemia and Lymphoma. 2008;49(1 supp 1):27–34. doi: 10.1080/10428190802311417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai S, Koizumi S, Sugiura M, et al. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci U S A. 1994;91(19):9131–9135. doi: 10.1073/pnas.91.19.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugiura M, Imai S, Tokunaga M, et al. Transcriptional analysis of Epstein-Barr virus gene expression in EBV-positive gastric carcinoma: unique viral latency in the tumour cells. Br J Cancer. 1996;74(4):625–631. doi: 10.1038/bjc.1996.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pallesen G, Hamilton-Dutoit SJ, Rowe M, Young LS. Expression of Epstein-Barr virus latent gene products in tumour cells of Hodgkin's disease. Lancet. 1991;337(8737):320–322. doi: 10.1016/0140-6736(91)90943-j. [DOI] [PubMed] [Google Scholar]
- 7.Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333(11):693–698. doi: 10.1056/NEJM199509143331103. [DOI] [PubMed] [Google Scholar]
- 8.Chiang AK, Tao Q, Srivastava G, Ho FC. Nasal NK- and T-cell lymphomas share the same type of Epstein-Barr virus latency as nasopharyngeal carcinoma and Hodgkin's disease. Int J Cancer. 1996;68(3):285–290. doi: 10.1002/(SICI)1097-0215(19961104)68:3<285::AID-IJC3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 9.Chen CL, Sadler RH, Walling DM, et al. Epstein-Barr virus (EBV) gene expression in EBV-positive peripheral T-cell lymphomas. J Virol. 1993;67(10):6303–6308. doi: 10.1128/jvi.67.10.6303-6308.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JI. Epstein-Barr virus lymphoproliferative disease associated with acquired immunodeficiency. Medicine (Baltimore) 1991;70(2):137–160. doi: 10.1097/00005792-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 11.MacMahon EM, Glass JD, Hayward SD, et al. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet. 1991;338(8773):969–973. doi: 10.1016/0140-6736(91)91837-k. [DOI] [PubMed] [Google Scholar]
- 12.Rea D, Delecluse HJ, Hamilton-Dutoit SJ, et al. Epstein-Barr virus latent and replicative gene expression in post-transplant lymphoproliferative disorders and AIDS-related non-Hodgkin's lymphomas. French Study Group of Pathology for HIV-associated Tumors. Ann Oncol. 1994;5(Suppl 1):113–116. doi: 10.1093/annonc/5.suppl_1.s113. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton-Dutoit SJ, Rea D, Raphael M, et al. Epstein-Barr virus-latent gene expression and tumor cell phenotype in acquired immunodeficiency syndrome-related non-Hodgkin's lymphoma. Correlation of lymphoma phenotype with three distinct patterns of viral latency. Am J Pathol. 1993;143(4):1072–1085. [PMC free article] [PubMed] [Google Scholar]
- 14.Zou P, Kawada J, Pesnicak L, Cohen JI. Bortezomib Induces Apoptosis of Epstein-Barr Virus (EBV)-Transformed B Cells and Prolongs Survival of Mice Inoculated with EBV-Transformed B Cells. J Virol. 2007;81(18):10029–10036. doi: 10.1128/JVI.02241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahir-McFarland ED, Carter K, Rosenwald A, et al. Role of NF-kappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J Virol. 2004;78(8):4108–4119. doi: 10.1128/JVI.78.8.4108-4119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katano H, Pesnicak L, Cohen JI. Simvastatin induces apoptosis of Epstein-Barr virus (EBV)-transformed lymphoblastoid cell lines and delays development of EBV lymphomas. Proc Natl Acad Sci U S A. 2004;101(14):4960–4965. doi: 10.1073/pnas.0305149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassoued S, Ben Ameur R, Ayadi W, et al. Epstein-Barr virus induces an oxidative stress during the early stages of infection in B lymphocytes, epithelial, and lymphoblastoid cell lines. Mol Cell Biochem. 2008;313(1-2):179–186. doi: 10.1007/s11010-008-9755-z. [DOI] [PubMed] [Google Scholar]
- 18.Osipova-Goldberg HI, Turchanowa LV, Adler B, Pfeilschifter JM. H2O2 inhibits BCR-dependent immediate early induction of EBV genes in Burkitt's lymphoma cells. Free Radic Biol Med. 2009;47(8):1120–1129. doi: 10.1016/j.freeradbiomed.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Espey MG, Krishna MC, et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(38):13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Q, Espey MG, Sun AY, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proceedings of the National Academy of Sciences. 2008;105(32):11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verrax J, Calderon PB. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic Biol Med. 2009;47(1):32–40. doi: 10.1016/j.freeradbiomed.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Ohno S, Ohno Y, Suzuki N, Soma G, Inoue M. High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res. 2009;29(3):809–815. [PubMed] [Google Scholar]
- 23.Levine M, Padayatty SJ, Espey MG. Vitamin C: a concentration-function approach yields pharmacology and therapeutic discoveries. Adv Nutr. 2011;2(2):78–88. doi: 10.3945/an.110.000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monti DA, Mitchell E, Bazzan AJ, et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PLoS One. 2012;7(1):e29794. doi: 10.1371/journal.pone.0029794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawada J, Zou P, Mazitschek R, Bradner JE, Cohen JI. Tubacin kills epstein-barr virus (EBV)-Burkitt lymphoma cells by inducing reactive oxygen species and EBV lymphoblastoid cells by inducing apoptosis. J Biol Chem. 2009;284(25):17102–17109. doi: 10.1074/jbc.M809090200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 27.Washko PW, Welch RW, Dhariwal KR, Wang Y, Levine M. Ascorbic acid and dehydroascorbic acid analyses in biological samples. Analytical Biochemistry. 1992;204(1):1–14. doi: 10.1016/0003-2697(92)90131-p. [DOI] [PubMed] [Google Scholar]
- 28.Nakano A, Abe M, Oda A, et al. Delayed treatment with vitamin C and N-acetyl-L-cysteine protects Schwann cells without compromising the anti-myeloma activity of bortezomib. Int J Hematol. 2011;93(6):727–735. doi: 10.1007/s12185-011-0850-7. [DOI] [PubMed] [Google Scholar]
- 29.Rowe M, Young LS, Crocker J, et al. Epstein-Barr virus (EBV)-associated lymphoproliferative disease in the SCID mouse model: implications for the pathogenesis of EBV-positive lymphomas in man. J Exp Med. 1991;173(1):147–158. doi: 10.1084/jem.173.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng WH, Kenney SC. Valproic acid enhances the efficacy of chemotherapy in EBV-positive tumors by increasing lytic viral gene expression. Cancer Res. 2006;66(17):8762–8769. doi: 10.1158/0008-5472.CAN-06-1006. [DOI] [PubMed] [Google Scholar]
- 31.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8(7):579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 32.Chen Q, Espey MG, Sun AY, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proceedings of the National Academy of Sciences. 2007;104(21):8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bannerman B, Xu L, Jones M, et al. Preclinical evaluation of the antitumor activity of bortezomib in combination with vitamin C or with epigallocatechin gallate, a component of green tea. Cancer Chemother Pharmacol. 2011;68(5):1145–1154. doi: 10.1007/s00280-011-1591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrone G, Hideshima T, Ikeda H, et al. Ascorbic acid inhibits antitumor activity of bortezomib in vivo. Leukemia. 2009;23(9):1679–1686. doi: 10.1038/leu.2009.83. [DOI] [PubMed] [Google Scholar]
- 35.Zou W, Yue P, Lin N, et al. Vitamin C inactivates the proteasome inhibitor PS-341 in human cancer cells. Clin Cancer Res. 2006;12(1):273–280. doi: 10.1158/1078-0432.CCR-05-0503. [DOI] [PubMed] [Google Scholar]