Abstract
Background
The threadworm, Strongyloides stercoralis, endemic in tropical and temperate climates, is a neglected tropical disease. Its diagnosis requires specific methods, and accurate information on its geographic distribution and global burden are lacking. We predicted prevalence, using Bayesian geostatistical modeling, and determined risk factors in northern Cambodia.
Methods
From February to June 2010, we performed a cross-sectional study among 2,396 participants from 60 villages in Preah Vihear Province, northern Cambodia. Two stool specimens per participant were examined using Koga agar plate culture and the Baermann method for detecting S. stercoralis infection. Environmental data was linked to parasitological and questionnaire data by location. Bayesian mixed logistic models were used to explore the spatial correlation of S. stercoralis infection risk. Bayesian Kriging was employed to predict risk at non-surveyed locations.
Principal Findings
Of the 2,396 participants, 44.7% were infected with S. stercoralis. Of 1,071 strongyloidiasis cases, 339 (31.6%) were among schoolchildren and 425 (39.7%) were found in individuals under 16 years. The incidence of S. stercoralis infection statistically increased with age. Infection among male participants was significantly higher than among females (OR: 1.7; 95% CI: 1.4–2.0; P<0.001). Participants who defecated in latrines were infected significantly less than those who did not (OR: 0.6; 95% CI: 0.4–0.8; P = 0.001). Strongyloidiasis cases would be reduced by 39% if all participants defecated in latrines. Incidence of S. stercoralis infections did not show a strong tendency toward spatial clustering in this province. The risk of infection significantly decreased with increasing rainfall and soil organic carbon content, and increased in areas with rice fields.
Conclusions/Significance
Prevalence of S. stercoralis in rural Cambodia is very high and school-aged children and adults over 45 years were the most at risk for infection. Lack of access to adequate treatment for chronic uncomplicated strongyloidiasis is an urgent issue in Cambodia. We would expect to see similar prevalence rates elsewhere in Southeast Asia and other tropical resource poor countries.
Author Summary
Data on the prevalence and distribution of Strongyloides stercoralis (threadworm) is scarce in many resource-poor countries. We carried out a cross-sectional study during the dry season among 2,396 rural Cambodians of all ages. We used a rigorous diagnostic approach, involving two stool samples per person and two examination techniques, namely, Koga agar plate culture and the Baermann method. We predicted the spatial distribution of S. stercoralis using Bayesian Kriging analysis. Almost half of the participants (44.7%) were infected with S. stercoralis. Of the S. stercoralis cases, 39.7% involved participants under 16 years old. S. stercoralis infection prevalence was significantly higher in males than in females. Participants younger than 10 years old had a lower risk of infection than did older participants. Furthermore, our study showed that toilet use could prevent threadworm infections by 39%. Infection prevalence in the province was negatively associated with rainfall and soil organic content and positively associated with land covered by rice fields. We conclude that access to adequate treatment for S. stercoralis must be addressed in Cambodia. Infection prevalence is likely to be similar in other countries of the region and the developing world.
Introduction
Strongyloides stercoralis, a soil-transmitted nematode, is a neglected tropical helminthiasis [1], [2] and endemic in tropical, subtropical and temperate settings where sanitary and hygiene conditions are poor [3], [4]. However, the worldwide prevalence of S. stercoralis is heterogeneously distributed [2] and the current estimation of infection remains underestimated due to the use of inadequate diagnostic method [5].The available information about S. stercoralis infection in developing countries mostly comes from studies in Brazil and Thailand [2].
The gastrointestinal symptoms of the disease include diarrhea and abdominal pain, while dermatological symptoms include itching, rash (urticaria) and migrating larvae in the skin (larva currens) [6]–[9]. However, more than 50% of all infections remain asymptomatic [4]. Due to its particular ability for autoinfection, S. stercoralis is the only soil-transmitted helminth (STH) that can lead to systemic infection with high parasite densities and severe to potentially fatal complications, especially in immunosuppressed hosts [3], [7], [10]. Ivermectin is recommended as the most effective treatment [11].
The presence of S. stercoralis larvae in stool specimens is proof of infection [12]. Koga-agar plate (KAP) culture [13] and the Baermann method [14] are specific diagnostic methods for strongyloidiasis. However, their sensitivity is not satisfactory when testing a single stool sample in cases of chronic, uncomplicated strongyloidiasis [15]–[18].
In Cambodia, data from several cross-sectional studies in community and hospital settings revealed S. stercoralis prevalences between 2.6% and 31.5%. However, in all but three studies, a diagnostic approach with low sensitivity was used on a single stool sample [19]–[22]. Three recent studies used a combined diagnostic approach (KAP culture and Baermann technique) on two [9], [23] and three stool samples [16].
We aimed to determine the prevalence, risk factors and spatial distribution of S. stercoralis infection in Preah Vihear province. We conducted a cross-sectional study of S. stercoralis infection, using KAP culture and the Baermann method on two stool samples from each participant in 60 villages of Preah Vihear province, northern Cambodia.
Materials and Methods
Ethical considerations
The research was approved by the Ethics Committee of the Cantons of Basel-Stadt and Baselland (EKBB, #16/10, dated 1 February 2010), Switzerland, and by the National Ethics Committee for Health Research, Ministry of Health, Cambodia (NECHR, #004, dated 5 February 2010). Written informed consent was obtained from each participant prior to the start of the study. For participants between the ages of 1 and 18 years, written informed consent was obtained from the parents, legal guardian or appropriate literate substitute. All participants were informed of the study's purpose and procedures prior to enrolment.
All participants infected with S. stercoralis were treated with a single oral dose of ivermectin (200 µg/kg BW) [24]. All other parasitic infections were treated according to the guidelines of the National Helminth Control Program of Cambodia [25].
Study setting and population
The study was conducted in 60 rural villages of Preah Vihear province, Northern Cambodia (Figure 1). The villages were randomly selected from a list of all villages in six of the seven districts in Preah Vihear province (total number of villages: 184). The district of Chhaeb was not included as most villages in this district are difficult to access by car, which was necessary to ensure the rapid transfer (three hours by car) of stool samples to one of the two temporary laboratories established in the health centers of Kulen and Rovieng districts.
Figure 1. Map of the study villages in Preah Vihear province, Cambodia, 2010.
A cross-sectional study was carried out from February to June 2010 among all the population living in 60 villages. Fifteen households were randomly selected from the list of all households in the selected villages. All household members one year of age and older were eligible for inclusion in the study and all household members present on the day of the survey were enrolled.
Field and laboratory procedures
After obtaining written informed consent from participants, an individual questionnaire was administered to obtain demographic information (age, gender, educational level and profession), personal risk-perception (knowledge about worm infections), and behavioral data (personal hygiene practices, wearing shoes, and latrine use). The head of household was interviewed, based on a household questionnaire, about socioeconomic indicators such as house type, household assets, latrine and livestock. All questionnaires were pre-tested. After the interview, each participant was given a pre-labeled plastic container (ID code, name, sex, age and date) for stool sample collection. The next morning, the filled stool container was collected and a second empty, pre-labeled one was handed out for a second stool sample of the following day.
Stool samples were transported at ambient temperature and arrived at the laboratory within three hours of collection. Laboratory technicians from the National Center for Parasitology, Entomology and Malaria Control (CNM), Phnom Penh, processed the stool specimens in one of two laboratories established in Kulen and Rovieng health centers, respectively. First, a single Kato-Katz thick smear [26] was prepared using the WHO standard template and examined under a light microscope to detect helminth eggs. Eggs were counted and recorded for each helminth species separately. Second, KAP culture [13] was used to detect S. stercoralis larvae. A hazelnut-sized stool sample was placed in the middle of the agar plate and the closed Petri dish was incubated in a humid chamber for 48 hours at 28°C. Afterwards, the plates were visually examined for the presence of larval tracks. The plates were then rinsed with sodium acetate-acetic acid-formalin (SAF) solution. The eluent was centrifuged and the sediment was examined under a microscope for the presence of larvae. Based on morphology, larvae were identified (i.e., size of buccal cavity, presence of genital primordium (L1), presence of forked tail-end (L3)) as either S. stercoralis or hookworm larvae. Finally, the Baermann technique [14] was performed to detect S. stercoralis larvae. A walnut-sized stool sample was placed on gauze inserted into a glass funnel and covered with water. The apparatus was exposed for two hours to artificial light directed from below. After centrifuging the collected liquid, the sediment was examined under a microscope for the presence of S. stercoralis larvae.
Quality control
For quality control, the technicians were specifically trained on the morphological criteria for distinguishing hookworm and S. stercoralis larvae. Throughout the study period, technicians were rigorously supervised by a qualified microscopist from the Swiss Tropical and Public Health Institute (Swiss TPH), Basel, Switzerland. Any unclear diagnosis was immediately discussed with both the qualified microscopist and the study supervisor.
Environmental data collection
Day and night land surface temperature (LST), enhanced vegetation index (EVI) and land use/land cover (LULC) were extracted at 1×1 km resolution from Moderate Resolution Imaging Spectroradiometer (MODIS) Land Processes Distributed Active Archive Center (LP DAAC), U.S. Geological Survey (USGS) Earth Resources Observation and Science (EROS) Center (http://lpdaac.usgs.gov). Rainfall estimates (RFE) at 0.1 degree (about 10×11 km) resolution were obtained from the National Oceanic and Atmospheric Administration's (NOAA) Climate Prediction Center (CPC) Famine Early Warning System (FEWS) Rainfall Estimates South Asia, version 2.0 (http://www.cpc.ncep.noaa.gov/products/fews/SASIA/rfe.shtml). Digital elevation data at a resolution of 90×90 m were retrieved from the NASA Shuttle Radar Topographic Mission's (SRTM) Consortium for Spatial Information of the Consultative Group for International Agricultural Research (CGIAR-CSI) database. Soil type data at a spatial resolution of 9×9 km, including bulk density, soil organic carbon content and pH, was extracted from the International Soil Reference and Information Center's (ISRIC) World Inventory Soil Emission Potentials (WISE), version 1.0 (http://www.isric.org). The 18 land cover type 1 classes (IGBP) were merged into five categories according to similarity and respective frequencies. Yearly means, as well as minima and maxima of EVI, monthly LST and RFE were calculated for May 2009 to April 2010.
Statistical analyses
Questionnaire and laboratory data
Questionnaire and laboratory data collected from each participant were entered twice and validated in EpiData version 3.1 (EpiData Association; Odense, Denmark). Statistical analyses were performed with STATA version 12.1 (StataCorp.; College Station, TX, USA). Only participants with complete records (two stool samples examined with all diagnostic methods and completed questionnaires) were included in the final analyses. Smoothed age prevalence was used to present the infection prevalence distribution by participant's age. P-values less than 0.05 were considered to indicate a significant association.
Data on household assets and livestock were used to build the socioeconomic status (SES), employing principle component analysis (PCA). SES status was defined according to one of three wealth tiers, from poor to least poor [27].
Generalized Estimating Equations (GEE) were used to assess the association between infection status and demographic variables, hygienic status, knowledge, recent medical history of participants and environmental factors. Variables with odds ratios below 0.80 and above 1.25 in the bivariate models were selected for inclusion in the multivariate GEE model. Population attributable fraction was calculated for significantly associated risks.
Environmental data
ArcGIS version 10.0 (ESRI; Redlands, CA, USA) was used for environmental data processing, geo-referencing and map making. Environmental data was linked to parasitological and questionnaire data according to location. Data management and bivariate regressions were performed in STATA version 12.1. Bayesian multivariate models were fitted using WinBUGS version 1.4.3 (Imperial College & Medical Research Council; London, UK). Spatial analysis was performed using mixed logistic regression models. The association between infection risk and environmental covariates was assessed at a 15% significance level, as determined by the likelihood ratio test (LRT), with mixed bivariate logistic regressions accounting for village-level correlation with an exchangeable random effect. To explore the village-level correlation of S. stercoralis infection risk, Bayesian mixed logistic models were run in absence of covariates. Bayesian models with or without environmental covariates were run alternately with a spatial and a non-spatial exchangeable random effect. Spatial models assumed a stationary isotropic process, with village-specific random effects following a normal distribution with mean zero and a variance-covariance matrix being an exponential function of the distance between pairs of locations. Non-informative prior distributions were chosen for all other parameters. Markov chain Monte Carlo (MCMC) simulation was used to estimate model parameters [28]. Convergence was assessed by examining the ergodic averages of selected parameters. For all models, a burn-in of 5,000 was followed by 50,000 iterations, after which convergence was reached. Results were withdrawn for the last 10,000 iterations of each chain, with a thinning of 10. Model fit was appraised with the Deviance Information Criterion (DIC). A lower DIC indicates a better model [29]. DIC was retrieved after 10,000 additional iterations.
S. stercoralis infection risk was predicted at non-surveyed locations using Bayesian Kriging [30]. For model validation, 48 randomly selected villages were used for fitting and the 12 remaining were used as test locations. Models were run with either a spatial random effect, using the WinBUGS “spatial.unipred” function, or with an exchangeable random effect [31]. Predictive ability was assessed using the probability coverage of the shortest Bayesian credible interval, the Mean Squared Error and a χ2 test analogue [32], [33]. Based on environmental factors only, predictions were made at 16,532 pixels of a 1×1 km resolution, using an exchangeable random effect.
Results
Compliance and study population
Overall, 3,560 individuals from 616 households (average household size: 5; range: 1–12) were enrolled, of which 2,748 (77.2%) participants submitted two stool samples. The final analysis included 2,396 (67.3%) participants with complete data records, i.e., two stool specimens examined with all diagnostic tests and all questionnaires completed.
The median age of the participants was 20 years, with a range from 1 to 85 years. One thousand three hundred and fifty-five (56.5%) participants were females. Half of the participants (48.5%) were farmers and 33.0% were pupils. The majority of participants (58.3%) had attended primary school; one third (32.2%) had not received primary education.
Parasitological findings
Seven intestinal parasite species were found in the stool samples. Hookworm and S. stercoralis were most common, with a prevalence of 46.7% and 44.7%, respectively. Taenia sp. was found in 0.4% of participants, while Hymenolepis nana and Enterobius vermicularis were observed in 0.2% and 0.1% of participants, respectively. Both Ascaris lumbricoides and Trichuris trichiura were observed in 0.3% of participants. Of the 1,071 S. stercoralis cases, 642 (59.9%) were co-infected with hookworm.
Performance of the diagnostic methods
Table 1 summarizes the results of KAP culture and Baermann tests for the 1,071 S. stercoralis cases (44.7%) detected. KAP culture and the Baermann technique detected 877 and 823 cases, respectively. The total of all positive cases diagnosed by any of the two methods was considered the “diagnostic gold standard”. The sensitivity of the KAP culture was 81.9%, and that of the Baermann technique, 76.8%. The negative predictive values were 87.2% and 84.2%, while the positive predictive values were 81.8% and 76.8% for KAP culture and Baermann technique, respectively.
Table 1. Koga agar plate (KAP) culture and Baermann method for the detection of S. stercoralis in 2,396 participants, Preah Vihear province, Cambodia, 2010.
| Combined Methods (KAP culture and Baermann) | Total | |||
| Positive | Negative | |||
| Positive | 877 | 0 | 877 | |
| KAP culture | Negative | 194 | 1,325 | 1,519 |
| Total | 1,071 | 1,325 | 2,396 | |
| Positive | 823 | 0 | 823 | |
| Baermann Technique | Negative | 248 | 1,325 | 1,573 |
| Total | 1,071 | 1,325 | 2,396 | |
Characteristics of Strongyloides stercoralis cases
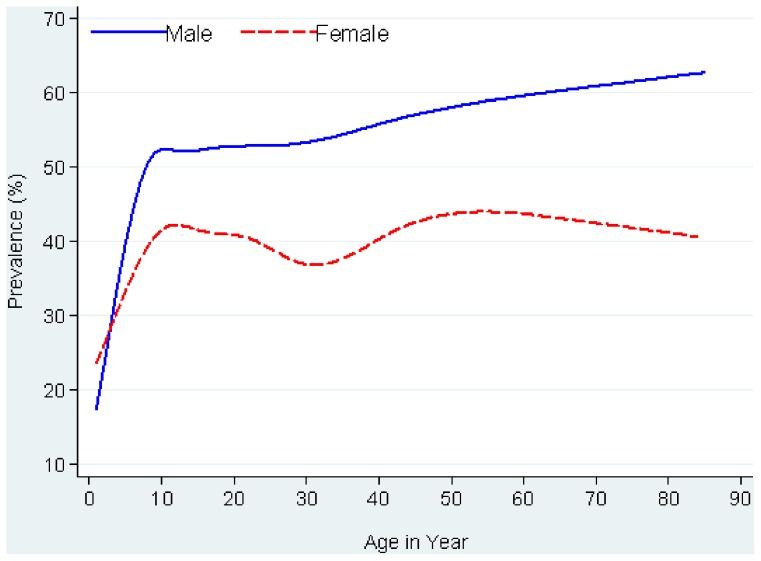
Of 1,071 S. stercoralis cases, half were females (50.1%), half were farmers (51.1%), and 425 (39.7%) cases were diagnosed in individuals under 16 years. The majority (57.0%) attended primary school, while one third (33.6%) reported no schooling. Figure 2 shows the smoothed age prevalence stratified by gender. The prevalence of S. stercoralis increased rapidly with age, particularly in the first eight years of life, where after it leveled off in females but continued to rise slowly in males. Prevalence rose from 31.4% in children, aged five, to 51.2% in participants older than 50. In all age groups, prevalence was higher in males than in females.
Figure 2. Smoothed age prevalence of S. stercoralis infection by sex among 2,396 participants in Preah Vihear province, Cambodia, 2010.
The multivariate GEE found that gender was significantly associated with S. stercoralis infection (mOR: 1.7; 95% CI: 1.4–2.0; P<0.001). Compared to children under six years old, all age groups had a higher risk for infection. Participants who reported having been treated for worms were less frequently infected with S. stercoralis than those who did not report taking anthelminthic drugs (mOR: 0.7; 95% CI: 0.6–0.8; P<0.001). In addition, participants who usually defecated in latrines were significantly less infected with S. stercoralis than those who did not use latrines (mOR: 0.6; 95% CI: 0.4–0.8; P = 0.001). No additional predictor of S. stercoralis infection relating to personal disease perception and hygiene was found in the multiple regression analysis. Looking at environmental factors, risk significantly decreased with increasing rainfall (mOR: 0.8; 95% CI: 0.7–0.9; P = 0.004) and soil organic carbon content (mOR: 0.6; 95% CI: 0.5–0.9; P = 0.003). The land cover class corresponding to croplands was associated with an increased risk for infection (mOR: 1.7, 95%CI: 1.2–2.4; P = 0.004) (Table 2).
Table 2. Risk factors for S. stercoralis infection in the multivariate GEE among 2,396 participants, Preah Vihear province, Cambodia, 2010.
| S. stercoralis Negative | S. stercoralis Positive | mOR (95% CI) | p-Value | |
| (N = 1325) | (N = 1071) | |||
| n (%) | n (%) | |||
| Gender (men compared to women) | 507 (38.37) | 534 (49.9) | 1.7 (1.4–2.0) | <0.001 |
| Age group | ||||
| 1–5 years | 129 (9.7) | 59 (5.5) | Reference | |
| 6–15 years | 453 (34.2) | 366 (34.2) | 2.3 (1.4–3.6) | <0.001 |
| 16–30 years | 352 (26.6) | 287 (26.8) | 1.8 (1.2–3.0) | 0.01 |
| 31–45 years | 220 (16.6) | 174 (16.2) | 1.7 (1.0–2.8) | 0.04 |
| >45 years | 171 (12.9) | 185 (17.3) | 2.2 (1.4–3.7) | 0.001 |
| Profession | ||||
| Farmer/Rice-Grower | 615 (46.4) | 547 (51.1) | Reference | |
| Schoolchildren | 451 (34.0) | 339 (31.6) | 0.8 (0.6–1.2) | 0.275 |
| Others | 259 (19.6) | 185 (17.3) | 1.0 (0.7–1.3) | 0.859 |
| Has been treated for worms (yes) | 450 (33.9) | 279 (26.0) | 0.7 (0.6–0.8) | <0.001 |
| Knows about worms/infection with worms (yes) | 240 (18.1) | 234 (21.8) | 1.3 (1.1–1.7) | 0.017 |
| Usually defecates in toilet (yes) | 195 (14.7) | 81 (7.5) | 0.6 (0.4–0.8) | 0.001 |
| Has shoes (yes) | 1203 (90.8) | 999 (93.3) | 1.1 (0.8–1.6) | 0.657 |
| Night temperature (year mean) | - | - | 0.9 (0.8–1.1) | 0.359 |
| Rainfall (year mean) | - | - | 0.8 (0.7–0.9) | 0.004 |
| Soil organic carbon content (10–20 g/kg) | 773 (58.3) | 485 (45.3) | 0.6 (0.5–0.9) | 0.003 |
| Land cover | ||||
| Savanna and shrubland | 441 (33.3) | 283 (26.4) | Reference | |
| Forest | 155 (11.7) | 130 (12.1) | 1.3 (0.8–2.1) | 0.233 |
| Grassland | 114 (8.6) | 70 (6.6) | 1.4 (0.8–2.4) | 0.315 |
| Cropland and crop-natural vegetation mosaic | 615 (46.4) | 588 (54.9) | 1.7 (1.2–2.4) | 0.004 |
mOR: Multiple Odds Ratio; 95% CI: 95% Confidence Interval.
During the two weeks preceding examinations for S. stercoralis, 50.5% of participants reported an episode of diarrhea, 12.7% had experienced nausea and 59.1% complained about abdominal pain. However, none of these clinical symptoms was significantly associated with S. stercoralis infection.
Population attributable risk analysis found that the number of strongyloidiasis cases would be reduced by 39% if all participants used a latrine for defecation.
Spatial analysis of Strongyloides stercoralis infection risk
The spatial model run without covariates indicated very little spatial correlation of infection risk, as indicated by the 1 km range. The small residual (unexplained) within village variance (σ) also indicated a weak clustering tendency of S. stercoralis infection risk. Parameters of these models are presented in Table 3. After introducing LST night, rainfall, soil carbon content and land cover, the model with an exchangeable random effect fitted the data slightly better, as indicated by the lower DIC. Environmental covariates explained 45% of the village-level variability and the range dropped under a kilometer after covariates were introduced in the model.
Table 3. Model parameters in absence or presence of covariates for the spatial models and their non-spatial counterparts.
| No covariates | Environmental covariates | |||||||
| Model parameters | non spatial | spatial | non spatial | spatial | ||||
| Median | 95% CI | Median | 95% CI | Median | 95% CI | Median | 95% CI | |
| DIC | 3136.4 | 3135.6 | 3130.6 | 3130.8 | ||||
| Σ | 0.4 | (0.2–0.7) | 0.4 | (0.2–0.7) | 0.2 | (0.1–0.4) | 0.2 | (0.1–0.4) |
| Ρ | n.a. | n.a. | 317.8 | (23.6–1558.0) | n.a. | n.a. | 812.5 | (98.8–1594.0) |
| Range (km) | n.a. | n.a. | 1.1 | (0.2–14.0) | n.a. | n.a. | 0.4 | (0.2–3.2) |
CI, credible interval; DIC, deviance information criterion (a measure of model fit; a lower DIC indicates a better fit).
σ is the location-specific unexplained variance.
ρ is the decay parameter. The range (range = 3/ρ) is the distance at which the spatial correlation becomes less than 5%.
Prediction of Strongyloides stercoralis infection risk and model validation
Mixed bivariate logistic regressions revealed no association at 15% significance level between S. stercoralis infection risk and any yearly summary measure of altitude, LST day, EVI, soil pH or bulk density. LST night (P = 0.072), yearly means of rainfall estimates (P<0.0001), soil organic carbon content (P = 0.002) and land cover (P = 0.107) were associated with infection risk and were used to predict S. stercoralis infection risk throughout Preah Vihear province. Apart from LST night, all covariates remained significant in the multivariate model and ORs were similar to those obtained in the multivariate GEE for the risk factor analysis (data not shown). Maps of the covariates used predict infection in Preah Vihear province are presented in Figure S1.
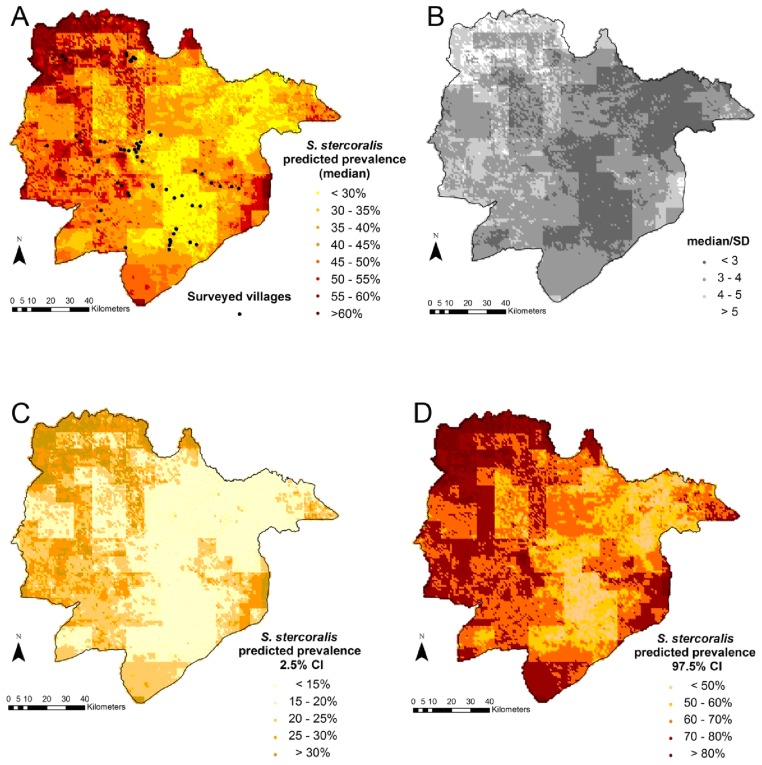
Model validation revealed that both models were able to correctly predict prevalence for 100% of the test locations, within a 95% credible interval. However the non-spatial model, i.e. with an exchangeable random effect, had slightly better predictive ability (MSE: 0.0226 and 0.0229, χ2: 13.22 and 13.59 for the non-spatial and spatial models, respectively). Therefore, the non-spatial model was used to predict S. stercoralis infection risk in Preah Vihear province, Cambodia. Figure 3 displays the S. stercoralis predicted median prevalence in Preah Vihear province (Figure 3A), together with the uncertainty of the estimates (Figure 3B) as expressed by the error coefficient (the ratio between the predicted median and its standard deviation). The lower (2.5%) and upper (97.5%) credible intervals of the predicted S. stercoralis prevalence are presented in Figure (3C) and (3D), respectively. Results were consistent with observed prevalence at surveyed locations.
Figure 3. Predicted S. stercoralis median prevalence (a), error coefficient (b); lower (c), and higher (d) estimates of S. stercoralis predicted prevalence in Preah Vihear province, Cambodia.
Legend: The error coefficient is the ratio of the predicted median over its standard deviation; a higher value indicates a higher precision. The lower and upper estimates correspond to the 2.5% and 97.5% borders of the Bayesian credible interval, respectively.
Discussion
Many epidemiological aspects of S. stercoralis infection are poorly understood [34]. The available information on the prevalence of S. stercoralis comes from studies on other STHs, where diagnostic methods with low-sensitivity for S. stercoralis and only a single stool sample were mostly used [1], [2], [16]. To reach an acceptable estimate of the “true” prevalence of S. stercoralis, Siddiqui and Beck [12], and Khieu et al. [16] proposed analyzing multiple stool samples with multiple diagnostic techniques simultaneously.
In our study of S. stercoralis among a rural population living in 60 villages in northern Cambodia, we examined two stool samples using two diagnostic techniques (KAP culture and Baermann method) specifically targeting S. stercoralis and found that 44.7% of the participants were infected. Children under the age of six accounted for 5.5% of the infections, while prevalence increased with age.
Almost every second individual in our study population was infected with S. stercoralis. To our knowledge, this is one of the highest prevalence ever reported, compared to other studies in highly endemic areas like Cambodia [2], [16], [35], Laos [36], Thailand [37], Brazil [34] and China [38], or in other countries. The main reason for such high prevalence is likely to be due to the more rigorous diagnostic approach employed in our study (number of stool specimen, multiple diagnostic methods), compared to the other studies, where a single method to examine a single fecal sample was used. Yet, the prevalence we observed is also substantially higher than that of other studies using the similar diagnostic approaches. Two recent studies in Kandal and Takeo provinces in Cambodia reported that about a quarter (24.4%) of schoolchildren and 21.0% of the general population were infected, respectively [16], [35]; while Steinmann et al., and Knopp et al. found a prevalence rate of 11.7% in a village in Yunnan, China and of 10.8% among schoolchildren in Zanzibar, respectively [39], [40]. Hence, other factors such socioeconomic and sanitary conditions are likely to contribute to the differences observed.
In the absence of a gold standard for diagnosing S. stercoralis, KAP culture [13] and the Baermann method [14] are widely used for detecting the parasite microscopically. Our study found that KAP culture was more sensitive than the Baermann method, which is consistent with reports from Cambodia [16], [35], rural Côte d′Ivoire [41], Brazil [42] and Honduras [43]. However, the opposite was observed in studies in south-central Côte d′Ivoire [44], Zanzibar [40], China [39] and Uganda [45]. This seems to indicate that neither method is superior. As either technique will fail to identify a certain number of infections, the combined use of both methods is recommended for optimal sensitivity.
We found that about one third of children under six (59 of 188 children) were already infected with S. stercoralis. This hints at a high contamination of the environment, such that children easily become infected when playing on the ground around the house or barefoot in the village. The fact that prevalence steadily increases with age can be explained by the fact that once infected at a young age, an infection can persist in an untreated individual for their entire life [46], [47]. Personal hygiene (not using a toilet for defecating) as a significant predictor of S. stercoralis infection was also observed in a study in south-central Cambodia [16]. This connection is obvious: with proper disposal of the feces, contamination of the surrounding area with infective larvae decreases. We calculated that 39.0% of S. stercoralis cases in the study area could be prevented if everyone were to defecate in a toilet. The cycle of S. stercoralis transmission could thus be interrupted by improving personal hygiene and sanitation. Strongyloidiasis is almost non-existent in countries where sanitation and human waste disposal have improved [48].
S. stercoralis infections were ubiquitous the study setting and exhibited a weak tendency to spatial clustering in the Preah Vihear province, as indicated by the low location-specific variance parameter. A low clustering tendency was also observed for hookworm, in the Region of Man, Côte d′Ivoire and Ghana [49], [50]. However, the lack of spatial correlation in this analysis is likely due to the study's small scale. This does not preclude S. stercoralis infection risk from spatially clustering at country or regional level, since environmental factors delimit suitable ecological zones for parasites at larger scales. [51].
Still, even at this provincial scale, we found significant associations with rainfall, soil organic carbon content and croplands both in the predictive model and after adjusting for demographic and behavioral factors. Our risk predictions yielded two broad risk zones: a lower risk zone in the East of the province and a higher risk zone in the West, characterized by lower rainfall and soil organic carbon content and a higher proportion of zones occupied by cropland. Since there was no indication of spatial correlation, risk prediction was carried out using an exchangeable random effect and relied on the predictors only. While a negative association between rainfall and infection risk was also identified in Thailand, a laboratory study found that S. stercoralis development was impaired by submersion of stools in water [52]. Hypothetically, the decreased risk of S. stercoralis infection in the East of the Province where rainfall was higher, might relate to more extensive or long lasting flooding that could negatively affect S. stercoralis transmission. Another possibility might be that higher rainfall in the East reduces parasite survival rates, as parasites are washed away by run-off water down steeper slopes. We found that lower soil carbon content was associated with increased risk of infection (in the West). A full profile of soil type information was unavailable for this setting and soil organic carbon content depends on a complex interplay of environmental and soil features, so interpretation is limited. But, in general, soil organic content tends to decrease with increased forest destruction, burning of savannas and land use for agriculture [53]. Hence, the association of increased risk of infection with lower soil carbon contents in our setting might relate to human activities such as slash-and-burn practices that destroy forests to create agricultural lands. Moreover, risk of infection was found to increase in croplands, a MODIS land cover category that specifically corresponds to soils that are alternately bare and cultivated. In our setting, these are rice fields [54]. Half (51.7%) of the study villages are surrounded by rice fields and 54.9% of participants infected with S. stercoralis live in such environments. Risk might be increased further by regular soil contamination by defecation around the fields and exposure during agricultural activities. Indeed, open defecation was the usual habit for 88.5% of participants. Finally, the small cluster size (1 km) of infection risk suggests that S. stercoralis transmission occurs within villages rather than between them and may relate to the location of defecation sites within and close to the villages.
We conclude that S. stercoralis infection is highly prevalent in rural communities of Cambodia. School-aged children and adults over 45 years were the most at risk for infection. Almost 40% of infections could be avoided by proper personal hygiene. Access to adequate treatment for chronic uncomplicated strongyloidiasis is low. Given its potential to produce potentially fatal disseminated infections, further epidemiological data on this parasite in other endemic areas are urgently needed
Supporting Information
Distribution of environmental factors used to predict S. stercoralis prevalence in Preah Vihear province, Cambodia.
(TIF)
Results of bivariate risk analysis for S. stercoralis infection.
(DOCX)
Acknowledgments
We are grateful to the participants and local authorities of Preah Vihear province. We deeply thank the laboratory technicians from the Helminth Control Program of the National Centre for Parasitology, Entomology and Malaria Control for their valuable laboratory work, and the staff from Preah Vihear Provincial Health Department for their great field work under difficult field conditions. We acknowledge the help of Ms. Nadja Cereghetti in the laboratory. The authors thank Mrs. Amena Briet for her efficient language editing.
Funding Statement
The study was funded by UBS Optimus Foundation, Zürich, Switzerland. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, et al. (2009) Strongyloidiasis—the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg 103: 967–972. [DOI] [PubMed] [Google Scholar]
- 2. Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, et al. (2013) Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl Trop Dis 7: e2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marcos LA, Terashima A, Dupont HL, Gotuzzo E (2008) Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans R Soc Trop Med Hyg 102: 314–318. [DOI] [PubMed] [Google Scholar]
- 4. Foreman EB, Abraham PJ, Garland JL (2006) Not your typical Strongyloides infection: a literature review and case study. Southern Medical Journal 99: 847–852. [DOI] [PubMed] [Google Scholar]
- 5. Bisoffi Z, Buonfrate D, Montresor A, Requena-Mendez A, Munoz J, et al. (2013) Strongyloides stercoralis: A Plea for Action. PLoS Negl Trop Dis 7: e2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ly MN, Bethel SL, Usmani AS, Lambert DR (2003) Cutaneous Strongyloides stercoralis infection: an unusual presentation. J Am Acad Dermatol 49: S157–160. [DOI] [PubMed] [Google Scholar]
- 7. Vadlamudi RS, Chi DS, Krishnaswamy G (2006) Intestinal strongyloidiasis and hyperinfection syndrome. Clin Mol Allergy 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koczka CP, Hindy P, Goodman A, Gress F (2012) Strongyloidiasis: a diagnosis more common than we think. Eur J Gastroenterol Hepatol 24: 860–862. [DOI] [PubMed] [Google Scholar]
- 9. Khieu V, Srey S, Schär F, Muth S, Marti H, et al. (2013) Strongyloides stercoralis is a cause of abdominal pain, diarrhea and urticaria in rural Cambodia. BMC Res Notes 6: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Basile A, Simzar S, Bentow J, Antelo F, Shitabata P, et al. (2010) Disseminated Strongyloides stercoralis: hyperinfection during medical immunosuppression. J Am Acad Dermatol 63: 896–902. [DOI] [PubMed] [Google Scholar]
- 11. WHO (2002) Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser 912: i-vi, 1–57. [PubMed] [Google Scholar]
- 12. Siddiqui AA, Berk SL (2001) Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis 33: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 13. Koga K, Kasuya S, Khamboonruang C, Sukhavat K, Ieda M, et al. (1991) A modified agar plate method for detection of Strongyloides stercoralis . Am J Trop Med Hyg 45: 518–521. [DOI] [PubMed] [Google Scholar]
- 14.Garcia L, Bruckner D (2001) Diagnostic medical parasitology. eds Washington DC: Am Soc Microbiol: 1–179.
- 15. Marchi Blatt J, Cantos GA (2003) Evaluation of techniques for the diagnosis of Strongyloides stercoralis in human immunodeficiency virus (HIV) positive and HIV negative individuals in the city of Itajai, Brazil. Braz J Infect Dis 7: 402–408. [DOI] [PubMed] [Google Scholar]
- 16. Khieu V, Schär F, Marti H, Sayasone S, Duong S, et al. (2013) Diagnosis, Treatment and Risk Factors of Strongyloides stercoralis in Schoolchildren in Cambodia. PLoS Negl Trop Dis 7: e2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Requena-Mendez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, et al. (2013) The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis 7: e2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schär F, Hattendorf J, Khieu V, Muth S, Char MC, et al. (2014) Strongyloides stercoralis larvae excretion patterns before and after treatment. Parasitology 141: 892–897. [DOI] [PubMed] [Google Scholar]
- 19. Koga-Kita K (2004) Intestinal parasitic infections and socioeconomic status in Prek Russey Commune, Cambodia. Nihon Koshu Eisei Zasshi 51: 986–992. [PubMed] [Google Scholar]
- 20. Longfils P, Heang UK, Soeng H, Sinuon M (2005) Weekly iron and folic acid supplementation as a tool to reduce anemia among primary school children in Cambodia. Nutr Rev 63: S139–145. [DOI] [PubMed] [Google Scholar]
- 21. Chhakda T, Muth S, Socheat D, Odermatt P (2006) Intestinal parasites in school-aged children in villages bordering Tonle Sap Lake, Cambodia. Southeast Asian J Trop Med Public Health 37: 859–864. [PubMed] [Google Scholar]
- 22. Moore CE, Hor PC, Soeng S, Sun S, Lee SJ, et al. (2012) Changing Patterns of Gastrointestinal Parasite Infections in Cambodian Children: 2006-2011. J Trop Pediatr 58(6): 509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schär F, Inpankaew T, Traub RJ, Khieu V, Dalsgaard A, et al. (2014) The prevalence and diversity of intestinal parasitic infections in humans and domestic animals in a rural Cambodian village. Parasitol Int 63: 597–603. [DOI] [PubMed] [Google Scholar]
- 24. Marti H, Haji HJ, Savioli L, Chwaya HM, Mgeni AF, et al. (1996) A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. Am J Trop Med Hyg 55: 477–481. [DOI] [PubMed] [Google Scholar]
- 25.CNM (2004) National Policy and Guideline for Helminth Control in Cambodia. Phnom Penh, Cambodia: National Center for Parasitology, Entomology and Malaria Control, Ministry of Health. 44 p. [Google Scholar]
- 26. Katz N, Chaves A, Pellegrino J (1972) A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni . Rev Inst Med Trop Sao Paulo 14: 397–400. [PubMed] [Google Scholar]
- 27. Vyas S, Kumaranayake L (2006) Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan 21: 459–468. [DOI] [PubMed] [Google Scholar]
- 28. Gelfand AE, Smith AFM (1990) Sampling-Based Approaches to Calculating Marginal Densities. Am Stat Ass 85: 398–409. [Google Scholar]
- 29. Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A (2002) Bayesian measures of model complexity and fit. Roy Stat Soc: Series B (Statistical Methodology) 64: 583–639. [Google Scholar]
- 30. Diggle PJ, Tawn JA, Moyeed RA (1998) Model-based geostatistics. J Roy Stat Soc: Series C (Applied Statistics) 47: 299–350. [Google Scholar]
- 31. Lunn D, Thomas A, Best N, Spiegelhalter D (2000) WinBUGS - A Bayesian modelling framework: Concepts, structure, and extensibility. Statistics and Computing 10: 325–337. [Google Scholar]
- 32. Gosoniu L, Vounatsou P, Sogoba N, Smith T (2006) Bayesian modelling of geostatistical malaria risk data. Geospat Health 1: 127–139. [DOI] [PubMed] [Google Scholar]
- 33. Riedel N, Vounatsou P, Miller JM, Gosoniu L, Chizema-Kawesha E, et al. (2010) Geographical patterns and predictors of malaria risk in Zambia: Bayesian geostatistical modelling of the 2006 Zambia national malaria indicator survey (ZMIS). Malar J 9: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paula FM, Costa-Cruz JM (2011) Epidemiological aspects of strongyloidiasis in Brazil. Parasitology 138: 1331–1340. [DOI] [PubMed] [Google Scholar]
- 35. Khieu V, Schär F, Marti H, Bless PJ, Char MC, et al. (2014) Prevalence and risk ractors of Strongyloides stercoralis in Takeo province, Cambodia. Parasit Vectors (In Press).. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sayasone S, Vonghajack Y, Vanmany M, Rasphone O, Tesana S, et al. (2009) Diversity of human intestinal helminthiasis in Lao PDR. Trans R Soc Trop Med Hyg 103: 247–254. [DOI] [PubMed] [Google Scholar]
- 37. Nontasut P, Muennoo C, Sa-nguankiat S, Fongsri S, Vichit A (2005) Prevalence of strongyloides in Northern Thailand and treatment with ivermectin vs albendazole. Southeast Asian J Trop Med Public Health 36: 442–444. [PubMed] [Google Scholar]
- 38. Wang C, Xu J, Zhou X, Li J, Yan G, et al. (2013) Strongyloidiasis: an emerging infectious disease in China. Am J Trop Med Hyg 88: 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steinmann P, Zhou XN, Du ZW, Jiang JY, Wang LB, et al. (2007) Occurrence of Strongyloides stercoralis in Yunnan Province, China, and comparison of diagnostic methods. PLoS Negl Trop Dis 1: e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, et al. (2008) Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis 2: e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glinz D, N′Guessan NA, Utzinger J, N′Goran EK (2010) High prevalence of Strongyloides stercoralis among school children in rural Cote d′Ivoire. J Parasitol 96: 431–433. [DOI] [PubMed] [Google Scholar]
- 42. Ines Ede J, Souza JN, Santos RC, Souza ES, Santos FL, et al. (2011) Efficacy of parasitological methods for the diagnosis of Strongyloides stercoralis and hookworm in faecal specimens. Acta Trop 120: 206–210. [DOI] [PubMed] [Google Scholar]
- 43. de Kaminsky RG (1993) Evaluation of three methods for laboratory diagnosis of Strongyloides stercoralis infection. J Parasitol 79: 277–280. [PubMed] [Google Scholar]
- 44. Becker SL, Sieto B, Silue KD, Adjossan L, Kone S, et al. (2011) Diagnosis, clinical features, and self-reported morbidity of Strongyloides stercoralis and hookworm infection in a Co-endemic setting. PLoS Negl Trop Dis 5: e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stothard JR, Pleasant J, Oguttu D, Adriko M, Galimaka R, et al. (2008) Strongyloides stercoralis: a field-based survey of mothers and their preschool children using ELISA, Baermann and Koga plate methods reveals low endemicity in western Uganda. J Helminthol 82: 263–269. [DOI] [PubMed] [Google Scholar]
- 46. Concha R, Harrington W Jr, Rogers AI (2005) Intestinal strongyloidiasis: recognition, management, and determinants of outcome. J Clin Gastroenterol 39: 203–211. [DOI] [PubMed] [Google Scholar]
- 47. Prendki V, Fenaux P, Durand R, Thellier M, Bouchaud O (2011) Strongyloidiasis in man 75 years after initial exposure. Emerg Infect Dis 17: 931–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.WHO (2013) Neglected tropical diseases. Strongyloidiasis.: www.who.int/neglected_diseases/diseases/strongyloidiasis/en/.
- 49. Soares Magalhaes RJ, Biritwum NK, Gyapong JO, Brooker S, Zhang Y, et al. (2011) Mapping helminth co-infection and co-intensity: geostatistical prediction in ghana. PLoS Negl Trop Dis 5: e1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raso G, Vounatsou P, Gosoniu L, Tanner M, N′Goran EK, et al. (2006) Risk factors and spatial patterns of hookworm infection among schoolchildren in a rural area of western Cote d′Ivoire. Int J Parasitol 36: 201–210. [DOI] [PubMed] [Google Scholar]
- 51. Brooker S (2007) Spatial epidemiology of human schistosomiasis in Africa: risk models, transmission dynamics and control. Trans R Soc Trop Med Hyg 101: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Anamnart W, Pattanawongsa A, Intapan PM, Morakote N, Janwan P, et al. (2013) Detrimental Effect of Water Submersion of Stools on Development of Strongyloides stercoralis . PLoS One 8: e82339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buringh P (1984) Organic carbon in soils of the world. The Role of Terrestrial Vegetation in the Global Carbon Cycle Measurement by Remote Sensing, Vol SCOPE 23.
- 54. Friedl MA, McIver DK, Hodges JCF, Zhang XY, Muchoney D, et al. (2002) Global land cover mapping from MODIS: algorithms and early results. Remote Sens of Environ 83: 287–302. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of environmental factors used to predict S. stercoralis prevalence in Preah Vihear province, Cambodia.
(TIF)
Results of bivariate risk analysis for S. stercoralis infection.
(DOCX)