SUMMARY
Background
The ability to predict the development of venous thromboembolism is highly desirable.
Objective
We aim to determine the association between hyperglycemia and venous thromboembolism in non-diabetic critically ill children.
Patients/Methods
We conducted a retrospective cohort study that included children in the pediatric intensive care unit on vasopressor or on mechanical ventilator and without history of diabetes mellitus or prior diagnosis of thrombosis. Based on maximum blood glucose >150 mg/dl while admitted to the unit, children were categorized as hyperglycemic or non-hyperglycemic. Primary outcome was development of venous thromboembolism while admitted to the unit. We determined the association between hyperglycemia and venous thromboembolism using logistic regression models adjusting for selected subject characteristics.
Results
Of the 789 subjects analyzed, 34 subjects developed venous thromboembolism (incidence: 4.3%; 95% confidence interval: 3.0%–6.0%). Venous thromboembolism was more likely to develop in hyperglycemic subjects compared with non-hyperglycemic subjects. A total of 31 subjects (6.2%; 95% confidence interval: 4.2%–8.7%) developed venous thromboembolism after becoming hyperglycemic compared with 3 non-hyperglycemic subjects with venous thromboembolism (1.0%, 95% confidence interval: 0.2%–3.0%). When adjusted for age, diagnosis, presence of central venous catheter, prophylactic antithrombotic use and severity of illness, the odds ratio of venous thromboembolism with hyperglycemia was 4.1 (95% confidence interval: 1.2–14.1). For every 10 mg/dl increase in maximum blood glucose, adjusted odds ratio of venous thromboembolism was 1.04 (95% confidence interval: 1.01–1.06).
Conclusion
Hyperglycemia is associated with venous thromboembolism in critically ill non-diabetic children. Maximum blood glucose is a potential predictor of venous thromboembolism in this population.
Keywords: blood glucose, deep venous thrombosis, hyperglycemia, pulmonary embolism, risk factors
INTRODUCTION
Venous thromboembolism (VTE) can occur in as many as 16% of children in the intensive care unit (ICU) [1]. Critically ill children with VTE are supported on mechanical ventilation and stay in the ICU longer than similar children with no VTE [2]. Some pediatric ICUs have developed guidelines to reduce the incidence of VTE [3–5]. These guidelines recommend the use of pharmacologic thromboprophylaxis. Because the efficacy and safety of pharmacologic thromboprophylaxis in children is unclear, its use may be associated with increased bleeding. In order to improve the risk-benefit ratio of thromboprophylaxis, it is highly desirable to identify children at high risk of VTE [1].
Clinical risk factors are poor predictors of VTE. Only the presence of inherited thrombophilia and use of central venous catheter (CVC) predicted the development of VTE in critically ill children [6]. The use of biomarkers, such as blood glucose, may improve our predictive ability. Children with diabetic ketoacidosis (DKA) are at increased risk of CVC-related VTE [7, 8]. Hyperglycemia, not necessarily from diabetes mellitus [9], is associated with VTE in adults [10, 11]. It is unclear whether hyperglycemia is also associated with VTE in non-diabetic critically ill children. In this study, we aim to determine the association between hyperglycemia and VTE in non-diabetic critically ill children.
METHODS
Study design
We performed a retrospective cohort study of children admitted to the pediatric ICU at Yale-New Haven Children’s Hospital (YNHCH) from January 1, 2007 to December 31, 2010. Our pediatric ICU is a 19-bed mixed medical-surgical and cardiac ICU located within a tertiary not-for-profit medical center in Connecticut. The Human Investigation Committee at Yale School of Medicine approved the study including waiver of consent.
Subjects
We included children <18 years old admitted to the ICU who were on invasive mechanical ventilation or on vasopressor support with at least one blood glucose measurement during their ICU stay. These children represented the sickest patients in the ICU [12] and are more likely to benefit from thromboprophylaxis. Vasopressor support was defined as dopamine or dobutamine ≥5 mcg/kg/min, or any dose of epinephrine, norepinephrine, phenylephrine, milrinone or vasopressin if used for hypotension. We excluded children with diabetes mellitus or pre-existing thromboembolism. We identified potential subjects using the hospital’s cost-accounting database then reviewed their medical records to confirm eligibility.
During the study period, the frequency, source of blood (i.e., venous, capillary or arterial) and method for testing blood glucose (i.e. bedside glucose meter vs. laboratory) in our unit were not standardized. However, in children with persistent hyperglycemia, blood glucose was controlled to a range of 90–119 mg/dl using intravenous insulin [13]. The decision to provide and the choice of thromboprophylaxis were not standardized and left to the attending physician’s discretion.
Data Management
We collected all blood glucose values measured once the eligibility criteria were fulfilled until the diagnosis of VTE, or until discharge from the ICU in those with no VTE. Subjects with maximum blood glucose >150 mg/dl were categorized as having hyperglycemia. The risk of mortality and duration of ICU stay significantly increase above this threshold [14].
Each subject’s medical record was reviewed for suspicion of VTE. These included signs and symptoms of inflammation such as pain, tenderness, erythema, swelling of the ipsilateral limb or signs of venous obstruction [15]. All images were reviewed by one of the co-authors (CTS) to confirm the diagnosis.
We collected demographics, interventions, glucose measurements and outcomes (Table 1). We categorized the subjects into 3 age groups (i.e. <1 year old, 1–13 years old, and >13 years old) to reflect the bimodal distribution of VTE in children [2]. We used the Pediatric Index of Mortality 2 (PIM2) score to measure severity of illness [16]. The score predicts the risk of mortality using physical examination findings, diagnoses and biochemical markers collected within the first hour of the patient’s contact with the critical care team. Prophylactic antithrombotic included unfractionated heparin, low molecular weight heparin, warfarin or aspirin. The typical work up for thrombophilia in our center included testing for functional levels of antithrombin, protein C and S, factor VIII, lipoprotein (a) and homocysteine; presence of activated protein C resistance and lupus anticoagulants; and prothrombin G20210A, factor V Leiden and methylenetetrahydrofolate reductase mutations.
Table 1.
Comparison of selected characteristics of hyperglycemic and non-hyperglycemic subjects.
| Hyperglycemic N=498 |
Non-Hyperglycemic N=291 |
p value | ||
|---|---|---|---|---|
| Demographics | ||||
| Age group | 0.39 | |||
| <1 year old | 194 (39.0%) | 121 (41.6%) | ||
| 1–13 years old | 228 (45.8%) | 119 (40.9%) | ||
| >13 years old | 76 (15.3%) | 51 (17.5%) | ||
| Weight (kg) | 12.8 (5.6–32.0) | 11.0 (5.0–29.2) | 0.34 | |
| Gender (male) | 215 (43.2%) | 117 (40.2%) | 0.42 | |
| Race/ethnicity | 0.29 | |||
| White | 220 (44.2%) | 147 (50.5%) | ||
| Black | 108 (21.7%) | 50 (17.2%) | ||
| Hispanic | 123 (24.7%) | 66 (22.7%) | ||
| Others | 47 (9.4%) | 28 (9.6%) | ||
| Pediatric index of mortality 2 | 0.044 (0.019–0.089) | 0.036 (0.015–0.057) | <0.001 | |
| Admitting diagnosis | <0.001 | |||
| Trauma | 39 (7.8%) | 19 (6.5%) | ||
| Infection | 114 (22.9%) | 76 (26.1%) | ||
| Congenital heart disease | 179 (35.9%) | 58 (19.9%) | ||
| Cancer | 22 (4.4%) | 5 (1.7%) | ||
| Others | 144 (28.9%) | 133 (45.7%) | ||
| Recent surgery | 260 (52.2%) | 118 (40.6%) | 0.002 | |
| Interventions | ||||
| Mechanical ventilation | 464 (93.2%) | 266 (91.4%) | 0.36 | |
| Vasopressor | 248 (49.8%) | 65 (22.3%) | <0.001 | |
| Central venous catheter | 318 (63.8%) | 87 (29.9%) | <0.001 | |
| Antithrombotic agent | 59 (11.9%) | 12 (4.1%) | <0.001 | |
| Insulin | 86 (17.3%) | 0 (0%) | <0.001 | |
| Total parenteral nutrition | 146 (29.3%) | 32 (11.0%) | <0.001 | |
| Glucose measurements | ||||
| Number (during entire ICU stay) | 10 (4–26) | 3 (2–6) | <0.001 | |
| Maximum blood glucose (mg/dl) | 209 (175–262) | 120 (101–134) | <0.001 | |
| Mean blood glucose (mg/dl) | 140 (124–163) | 108 (96–119) | <0.001 | |
| Outcomes | ||||
| Venous thromboembolism | 31 (6.2%) | 3 (1.0%) | 0.001 | |
| Duration of mechanical ventilation (days) | 2 (1–7) | 1 (1–3) | <0.001 | |
| Duration of ICU stay (days) | 5 (3–12) | 3 (2–6) | <0.001 | |
| Duration of hospital stay (days) | 10 (5–23) | 6 (3–10) | <0.001 | |
| Mortality | 62 (12.5%) | 6 (2.1%) | <0.001 | |
ICU – intensive care unit. Data presented as N (%) or median (interquartile range).
Hyperglycemic subjects defined as maximum blood glucose >150 mg/dl.
Statistical analysis
Characteristics and outcomes for hyperglycemic and non-hyperglycemic subjects were presented as medians (interquartile ranges [IQR]) for continuous variables and counts (percentages) for categorical variables. The incidence of VTE was presented as proportion of children with VTE (95% confidence interval [CI]). Wilcoxon rank sum and chi-squared tests were used as appropriate.
In our main analysis, we included subjects with VTE that were confirmed radiologically with ultrasound, computed tomography scan, magnetic resonance imaging or echocardiography, or visualized grossly during surgery. We used logistic regression to model the likelihood of developing VTE with hyperglycemia [17]. We included all potential predictors in the model to avoid selection bias [17]. We included as predictors, known risk factors for VTE (i.e, age, cancer, congenital heart disease, infection, trauma, and CVC) [2], prophylactic antithrombotic use for its potential effect on the development of VTE, and PIM2 score to control for severity of illness. Inclusion of these predictors allowed us to predict VTE as accurately as possible [18]. We did not include thrombophilia because it was differentially tested only in those with VTE. Although length of hospitalization may be associated with VTE, we did not include it in the model because of difficulty in precisely predicting it [19]. We also analyzed the association with blood glucose treated as a continuous variable. Using similar models, we conducted sensitivity analyses to account for excluded subjects with suspected, but unconfirmed, VTE and for subjects with single blood glucose measurement. Associations were reported as odds ratios (OR; 95% CI). Statistical tests were performed using Stata 13 (College Station, TX). Statistical significance was assumed when p <0.05.
RESULTS AND DISCUSSION
In this retrospective cohort study, we report that hyperglycemia is associated with VTE in non-diabetic critically ill children. Maximum blood glucose is dose-dependently associated with increased incidence of VTE. This is the first study to document the association between hyperglycemia and VTE in non-diabetic children.
A total of 34 of the 789 subjects included in the main analysis had VTE for an incidence of 4.3% (95% CI: 3.0%–6.0%) (Table 1). There were 5 additional eligible subjects who were excluded because of missing charts. The incidence is significantly higher than the 0.7% reported by Higgerson et al [15]. In contrast to our study, Higgerson et al included all children admitted to the ICU regardless of organ support. Majority of subjects with VTE were <1 year old (n=21, 61.8%), had CVC (n=28, 82.4%) or recent surgery (n=22, 64.7%), and were on vasopressor support (n=25; 73.5%) or on total parenteral nutrition (n=20, 58.8%). Only one subject had thrombophilia (protein C and S deficiencies). Most of the subjects with VTE presented with swelling (n=24, 70.6%) of the ipsilateral limb. The sites of the VTE were lower extremity (n=17, 50.0%), upper extremity (n=7, 20.6%), inferior vena cava (n=6, 17.6%), atrium (n=4, 11.8%), and pulmonary artery (n=1, 2.9%). One subject had VTE in both upper and lower extremities.
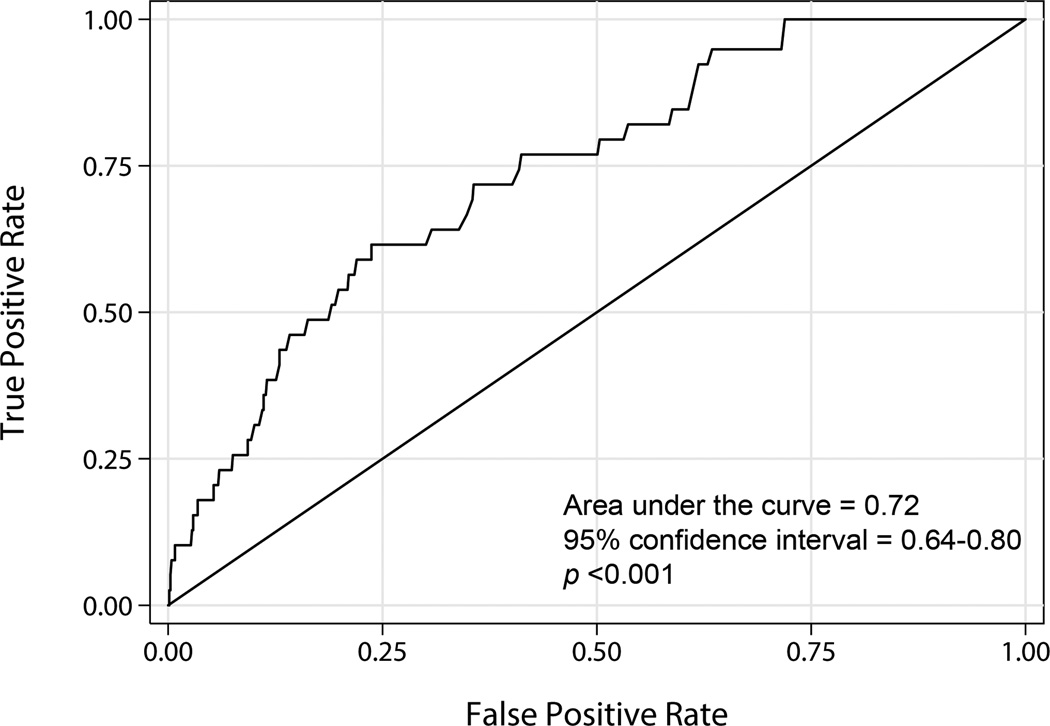
Hyperglycemia is associated with VTE. A total of 31 of 498 hyperglycemic subjects developed VTE (6.2%, 95% CI: 4.2%–8.7%) while 3 of 291 non-hyperglycemic subjects developed VTE (1.0%, 95% CI: 0.2%–3.0%). The adjusted OR of VTE with hyperglycemia was 4.1 (95% CI: 1.2–14.1; p=0.02) (Table 2). The area under the receiver operating characteristic curve was 0.72 (95% CI: 0.64–0.80) (Figure 1), which is above the minimum acceptable clinical threshold of 0.70 [20]. The sensitivity and specificity of hyperglycemia for predicting VTE was 91.2% (95% CI: 76.3%–98.1%) and 38.1% (95% CI: 34.7%–41.7%), respectively. Hyperglycemia alone is more sensitive, though less specific, when compared to a prediction tool that includes clinical variables alone [19]. Inclusion of subjects with suspected, but unconfirmed, VTE (n=5), with single blood glucose >150 mg/dl (n=24), or with single blood glucose (n=54) did not significantly affect the association. The adjusted OR of VTE with hyperglycemia in these scenarios remained statistically significant and ranged from 3.8 to 4.8.
Table 2.
Multivariable logistic regression model demonstrating the association between hyperglycemia and the development of venous thromboembolism.
| Unadjusted Odds Ratio (95% Confidence Interval) |
p value | Adjusted Odds Ratio (95% Confidence Interval) |
p value | ||
|---|---|---|---|---|---|
| Presence of hyperglycemia | 6.4 (1.9–21.0) | 0.002 | 4.1 (1.2–14.2) | 0.02 | |
| Age group | |||||
| <1 year old | 2.7 (1.2–5.9) | 0.02 | 2.6 (1.1–6.1) | 0.03 | |
| 1–13 years old | Referent | Referent | |||
| >13 years old | 1.2 (0.4–4.0) | 0.74 | 1.3 (0.4–4.3) | 0.70 | |
| Pediatric index of mortality 2 | 4.4 (1.0–19.1) | 0.05 | 1.8 (0.3–9.5) | 0.50 | |
| Presence of infection | 0.4 (0.1–1.2) | 0.10 | 0.6 (0.2–1.8) | 0.34 | |
| Presence of congenital heart disease | 2.1 (1.1–4.3) | 0.03 | 1.0 (0.4–2.2) | 0.95 | |
| Use of central venous catheter | 4.7 (1.9–11.4) | 0.001 | 3.2 (1.3–8.1) | 0.01 | |
| Use of antithrombotic agent | 4.0 (1.8–9.0) | 0.001 | 2.5 (1.1–6.0) | 0.04 | |
Trauma and cancer as admitting diagnoses were dropped from the model because no subjects with these diagnoses developed venous thromboembolism.
Hyperglycemia is defined as maximum blood glucose >150 mg/dl.
Figure 1.
The receiver operating characteristic curve for maximum blood glucose demonstrating its ability to predict venous thromboembolism developing during the subject’s stay in the intensive care unit.
The association between VTE in critically ill children with CVC and DKA is well recognized. Gutierrez et al. and Worly et al reported that children with DKA were more likely to develop CVC-related VTE, defined as VTE in the same extremity as the CVC, compared with similar age-matched control children with CVC and circulatory shock [7, 8]. In our study, only 22 of the 28 subjects with VTE and CVC had CVC-related VTE. A total of 20 (64.5%) of 31 cases of VTE in hyperglycemic subjects and 2 (66.7%) of 3 cases of VTE in non-hyperglycemic subjects were CVC-related. In subjects without CVC, the OR of VTE for every 10 mg/dl increase in maximum blood glucose was 1.07 (95% CI: 1.01–1.14, p=0.03). We, therefore, postulate that hyperglycemia may be associated with VTE even in children without CVC.
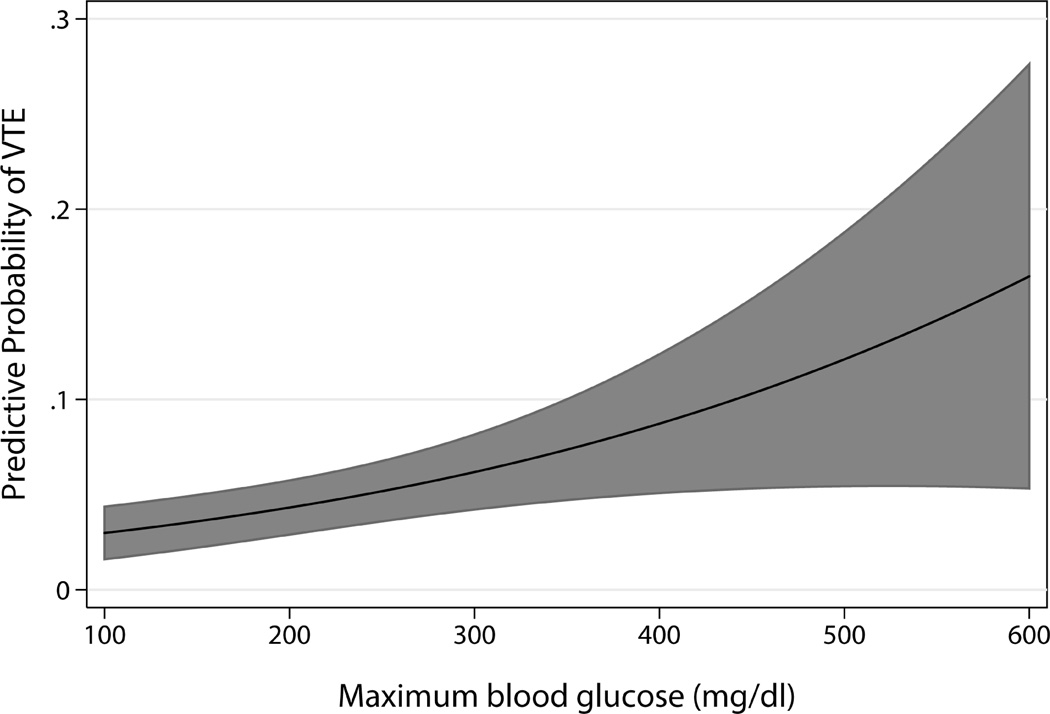
Some evidence suggests that the association between hyperglycemia and VTE may be causal. First, the association is biologically plausible. In healthy non-diabetic volunteers, experimentally increasing blood glucose levels activated factor VII activity, and increased thrombin-antithrombin complexes and soluble tissue factor [21, 22]. Second, our results are consistent with data from non-critically ill adults. Hyperglycemia was associated with VTE in adult outpatients [23], prior to major orthopedic surgery [11], and after total hip replacement [10]. Third, our study suggests a dose-response curve. For every 10 mg/dl increase in maximum blood glucose, adjusted OR of VTE was 1.04 (95% CI: 1.01–1.06; p=0.003) (Figure 2). In addition, for every 10 mg/dl increase in mean blood glucose, adjusted OR of VTE was 1.09 (95% CI: 1.00–1.18; p=0.06). Fourth, a temporal relationship seems to exist. VTE was diagnosed at a median of 6 days (IQR: 1–14 days) after the maximum blood glucose.
Figure 2.
Relationship between maximum blood glucose and the probability of developing venous thromboembolism (VTE). Solid line indicates the predicted probability of developing VTE at different levels of maximum blood glucose while the shaded area indicates the 95% confidence interval of the predicted probability.
Alternatively, hyperglycemia and VTE may be separate markers of severity of illness. This seems less likely based on the biological relationship between hyperglycemia and the coagulation system [24]. Hyperglycemia and VTE may also be related through another factor. For example, both may have occurred iatrogenically and not directly related.
The adjusted OR of VTE with the use of prophylactic antithrombotic agents was 2.5 (95% CI: 1.1–6.0; p=0.04) (Table 2). Although the efficacy of thromboprophylaxis in children is unclear [25], the association is likely confounded by indication. In the absence of a systematic approach to thromboprophylaxis in our unit, children at higher risk of VTE were more likely to receive antithrombotic agents.
The results of our study should be evaluated in view of certain limitations. The frequency, blood source and method of blood glucose measurement may have affected our ability to detect the maximum blood glucose and may have resulted in subject misclassification [14]. This is unlikely because the incidence of hyperglycemia in our study is consistent with other studies where blood glucose was routinely measured with consistent blood source and method [26–28]. Also, our sensitivity analyses provided results consistent with our main analysis. Systematic radiologic screening for VTE that would have detected the more common asymptomatic cases [1] was not performed as part of clinical care. Because clinical suspicion has low sensitivity for detecting VTE [1], some subjects without VTE might have been misclassified. This bias was likely non-differential and would have resulted in an underestimation of the reported associations. We were not able to adjust the OR of VTE for the presence of obesity because height was not consistently documented in the medical records. This is unlikely to affect our results because obesity has not been associated with VTE in hospitalized children [19]. Lastly, despite the large cohort of children in our study, only 34 children had VTE. This increased the likelihood of type II error.
CONCLUSION
In this cohort of non-diabetic critically ill children, blood glucose is associated with increased risk of VTE. Maximum blood glucose is a potential marker for VTE and may be used to identify children more likely to develop VTE. Future prospective studies to confirm our findings should include a systematic approach in measuring blood glucose and the use of antithrombotic agents.
Acknowledgments
FUNDING SOURCE: No external funding was secured for this study.
EVSF received funding NIH (CTSA Grant Number UL1 RR024139 and KL2 RR024138).
Footnotes
FINANCIAL DISCLOSURES: The other authors have no financial relation relevant to this article.
CONFLICT OF INTEREST: The authors of this study have no conflicts of interest to disclose.
ADDENDUM
J. A. Tala and E. V. S. Faustino contributed substantially to the concept and design, analysis and interpretation of the data, and critical writing of the manuscript. C. T. Silva, S. Pemira and E. Vidal collected and interpreted the data. All authors revised the manuscript for intellectual content and approved the final manuscript submitted for publication.
REFERENCES
- 1.Faustino EV, Spinella PC, Li S, Pinto MG, Stoltz P, Tala J, Card ME, Northrup V, Baker KE, Goodman TR, Chen L, Silva CT. Incidence and acute complications of asymptomatic central venous catheter-related deep venous thrombosis in critically ill children. J Pediatr. 2013:387–391. doi: 10.1016/j.jpeds.2012.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faustino EV, Lawson KA, Northrup V, Higgerson RA. Mortality-adjusted duration of mechanical ventilation in critically ill children with symptomatic central venous line-related deep venous thrombosis. Crit Care Med. 2011:1151–1156. doi: 10.1097/CCM.0b013e31820eb8a1. 2011/02/22 edn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raffini L, Trimarchi T, Beliveau J, Davis D. Thromboprophylaxis in a pediatric hospital: A patient-safety and quality-improvement initiative. Pediatrics. 2011;127:e1326–e1332. doi: 10.1542/peds.2010-3282. [DOI] [PubMed] [Google Scholar]
- 4.Hanson SJ, Punzalan RC, Arca MJ, Simpson P, Christensen MA, Hanson SK, Yan K, Braun K, Havens PL. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72:1292–1297. doi: 10.1097/TA.0b013e31824964d1. [DOI] [PubMed] [Google Scholar]
- 5.Faustino EV, Patel S, Thiagarajan RR, Cook DJ, Northrup V, Randolph AG. Survey of pharmacologic thromboprophylaxis in critically ill children. Crit Care Med. 2011:1773–1778. doi: 10.1097/CCM.0b013e3182186ec0. 2011/03/23 edn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiter PD, Wathen B, Valuck RJ, Dobyns EL. Thrombosis risk factor assessment and implications for prevention in critically ill children. Pediatr Crit Care Med. 2012;13:381–386. doi: 10.1097/PCC.0b013e31823893f5. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez JA, Bagatell R, Samson MP, Theodorou AA, Berg RA. Femoral central venous catheter-associated deep venous thrombosis in children with diabetic ketoacidosis. Crit Care Med. 2003;31:80–83. doi: 10.1097/00003246-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Worly JM, Fortenberry JD, Hansen I, Chambliss CR, Stockwell J. Deep venous thrombosis in children with diabetic ketoacidosis and femoral central venous catheters. Pediatrics. 2004;113:e57–e60. doi: 10.1542/peds.113.1.e57. [DOI] [PubMed] [Google Scholar]
- 9.Heit J, Leibson C, Ashrani A, Petterson T, Bailey K, Melton Is diabetes mellitus an independent risk factor for venous thromboembolism?: A population-based case-control study. Arterioscler Thromb Vasc Biol. 2009;29:1399–1405. doi: 10.1161/ATVBAHA.109.189290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn DM, Hermanides J, DeVries JH, Kamphuisen PW, Kuhls S, Homering M, Hoekstra JB, Lensing AW, Buller HR. Stress-induced hyperglycaemia and venous thromboembolism following total hip or total knee arthroplasty: Analysis from the RECORD trials. Thromb Haemost. 2012;107:225–231. doi: 10.1160/TH11-07-0447. [DOI] [PubMed] [Google Scholar]
- 11.Mraovic B, Hipszer B, Epstein R, Pequignot E, Parvizi J, Joseph J. Preadmission hyperglycemia is an independent risk factor for in-hospital symptomatic pulmonary embolism after major orthopedic surgery. J Arthroplasty. 2010;25:64–70. doi: 10.1016/j.arth.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5:329–336. doi: 10.1097/01.pcc.0000128607.68261.7c. [DOI] [PubMed] [Google Scholar]
- 13.Faraon-Pogaceanu C, Banasiak KJ, Hirshberg EL, Faustino EV. Comparison of the effectiveness and safety of two insulin infusion protocols in the management of hyperglycemia in critically ill children. Pediatr Crit Care Med. 2010;11:741–749. doi: 10.1097/PCC.0b013e3181e88cfb. [DOI] [PubMed] [Google Scholar]
- 14.Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. 2005;146:30–34. doi: 10.1016/j.jpeds.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 15.Higgerson RA, Lawson KA, Christie LM, Brown AM, McArthur JA, Totapally BR, Hanson SJ for the National Association of Children's Hospitals and Related Institution's Pediatric Intensive Care Unit FOCUS Group. Incidence and risk factors associated with venous thrombotic events in pediatric intensive care unit patients. Pediatr Crit Care Med. 2011;12:628–634. doi: 10.1097/PCC.0b013e318207124a. [DOI] [PubMed] [Google Scholar]
- 16.Slater A, Shann F, Pearson G. PIM2: A revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29:278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 17.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr., Habbema JD. Prognostic modelling with logistic regression analysis: A comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059–1079. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: What, why, and how? BMJ. 2009;338:b375. doi: 10.1136/bmj.b375. [DOI] [PubMed] [Google Scholar]
- 19.Branchford BR, Mourani P, Bajaj L, Manco-Johnson M, Wang M, Goldenberg NA. Risk factors for in-hospital venous thromboembolism in children: a case-control study employing diagnostic validation. Haematologica. 2012;97:509–515. doi: 10.3324/haematol.2011.054775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd Edition. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 21.Ceriello A, Giugliano D, Quatraro A, Dello Russo P, Torella R. Blood glucose may condition factor VII levels in diabetic and normal subjects. Diabetologia. 1988;31:889–891. doi: 10.1007/BF00265372. [DOI] [PubMed] [Google Scholar]
- 22.Stegenga ME, van der Crabben SN, Blumer RM, Levi M, Meijers JC, Serlie MJ, Tanck MW, Sauerwein HP, van der Poll T. Hyperglycemia enhances coagulation and reduces neutrophil degranulation, whereas hyperinsulinemia inhibits fibrinolysis during human endotoxemia. Blood. 2008;112:82–89. doi: 10.1182/blood-2007-11-121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hermanides J, Cohn DM, Devries JH, Kamphuisen PW, Huijgen R, Meijers JC, Hoekstra JB, Buller HR. Venous thrombosis is associated with hyperglycemia at diagnosis: A case-control study. J Thromb Haemost. 2009;7:945–949. doi: 10.1111/j.1538-7836.2009.03442.x. [DOI] [PubMed] [Google Scholar]
- 24.Langouche L, Meersseman W, Vander Perre S, Milants I, Wouters PJ, Hermans G, Gjedsted J, Hansen TK, Arnout J, Wilmer A, Schetz M, Van den Berghe G. Effect of insulin therapy on coagulation and fibrinolysis in medical intensive care patients. Crit Care Med. 2008;36:1475–1480. doi: 10.1097/CCM.0b013e31816f7bae. [DOI] [PubMed] [Google Scholar]
- 25.Monagle P, Chan AK, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Gottl U, Vesely SK. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e737S–e801S. doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preissig CM, Hansen I, Roerig PL, Rigby MR. A protocolized approach to identify and manage hyperglycemia in a pediatric critical care unit. Pediatr Crit Care Med. 2008;9:581–588. doi: 10.1097/PCC.0b013e31818d36cb. [DOI] [PubMed] [Google Scholar]
- 27.Branco RG, Garcia PC, Piva JP, Casartelli CH, Seibel V, Tasker RC. Glucose level and risk of mortality in pediatric septic shock. Pediatr Crit Care Med. 2005;6:470–472. doi: 10.1097/01.PCC.0000161284.96739.3A. [DOI] [PubMed] [Google Scholar]
- 28.Branco RG, Tasker RC. Glycemic level in mechanically ventilated children with bronchiolitis. Pediatr Crit Care Med. 2007;8:546–550. doi: 10.1097/01.PCC.0000288712.67749.45. [DOI] [PubMed] [Google Scholar]