Abstract
Excitatory amino acid carrier 1 (EAAC1, also called EAAT3) is a Na+-dependent glutamate transporter expressed by both glutamatergic and GABAergic neurons. It provides precursors for the syntheses of glutathione and GABA and contributes to the clearance of synaptically released glutamate. Mice deleted of EAAC1 are more susceptible to neurodegeneration in models of ischemia, Parkinson’s disease, and aging. Antisense knock-down of EAAC1 causes an absence seizure-like phenotype. Additionally, EAAC1 expression increases after chemonvulsant-induced seizures in rodent models and in tissue specimens from patients with refractory epilepsy. The goal of the present study was to determine if the absence of EAAC1 affects the sensitivity of mice to seizure-induced cell death. A chemoconvulsant dose of pilocarpine was administered to EAAC1−/− mice and to wild-type controls. Although EAAC1−/− mice experienced increased latency to seizure onset, no significant differences in behavioral seizure severity or mortality were observed. We examined EAAC1 immunofluorescence 24 hours after pilocarpine administration and confirmed that pilocarpine causes an increase in EAAC1 protein. Forty-eight hours after induction of seizures, cell death was measured in hippocampus and in cortex using Fluoro-Jade C. Surprisingly, there was ~2-fold more cell death in area CA1 of wild-type mice than in the corresponding regions of the EAAC1−/− mice. Together, these studies indicate that absence of EAAC1 results in either a decrease in pilocarpine-induced seizures that is not detectable by behavioral criteria (surprising, since EAAC1 provides glutamate for GABA synthesis), or that the absence of EAAC1 results in less pilocarpine/seizure-induced cell death, possible explanations as discussed.
Keywords: glutamate transport, EAAC1, EAAT3, seizure, cell death, pilocarpine
1. Introduction
A family of five Na+-dependent transporters (EAAT 1-5) clears extracellular glutamate, and it is generally thought that the astroglial transporters GLT-1 and GLAST (also known as EAAT2 and EAAT1, respectively) mediate most of this activity in brain (for reviews see Danbolt, 1994; Robinson and Dowd, 1997). EAAC1 (also called EAAT3) is found throughout the mammalian forebrain but is localized to neurons (Rothstein et al., 1994; Holmseth et al., 2012) and oligodendroglia (Kugler and Schmitt, 1999) and probably does not contribute significantly to clearance of glutamate. Synaptosomes from GLT-1 null mice display a 95% reduction of the Na+-dependent transport activity observed in wild-type animals (Tanaka et al., 1997). Finally, mice deleted of EAAC1 display a relatively normal phenotype until 11 months of age (Peghini et al., 1997; Aoyama et al., 2006). Together these observations suggest the role of EAAC1 is more important for neuronal cellular function than global CNS glutamate uptake.
Three main physiological functions have been attributed to EAAC1: limitation of spillover at excitatory synapses (Rothstein et al., 1994; Diamond, 2001; Scimemi et al., 2009), provision of glutamate for GABA synthesis (Sepkuty et al., 2002; Mathews and Diamond, 2003), and contribution to neuronal uptake of cysteine for synthesis of glutathione (Chen and Swanson, 2003; Himi et al., 2003; Aoyama et al., 2008; Escartin et al., 2011; Aoyama and Nakaki, 2012; Aoyama et al., 2012a; for reviews see Aoyama et al., 2012b; Aoyama and Nakaki, 2013). Most studies suggest that EAAC1 may be neuroprotective. For example, aging mice deleted of EAAC1 display progressive neurodegeneration that is attenuated with N-acetylcysteine (Aoyama et al., 2006; Berman et al., 2011). EAAC1−/− mice are also more sensitive to ischemia-induced cell death than wild-type mice (Won et al., 2010). EAAC1 null mice display increased cell death, and overexpression of EAAC1 decreases cell death, after axotomy (Kiryu-Seo et al., 2006). However, there is also evidence that EAAC1 may exacerbate the effects of acute insults to the nervous system as mice deleted of EAAC1 display a reduced anoxic depolarization shift, suggesting the EAAC1 can contribute to the release of glutamate by reverse transport when energy stores are depleted (Gebhardt et al., 2002).
Several groups have determined that seizures are associated with changes in transporter expression using a variety of animal models and in examination of surgically resected tissue from humans with temporal lobe epilepsy (for review, see Sheldon and Robinson, 2007). One of the more consistent findings is an increase in EAAC1 mRNA and protein within hours to days after an acute seizure in rodent models (Miller et al., 1997; Ghijsen et al., 1999; Ueda et al., 2001; Zhang et al., 2004; Voutsinos-Porche et al., 2006; Ross et al., 2011). There is also evidence that EAAC1 mRNA and protein are elevated in humans with temporal lobe epilepsy (Crino et al., 2002). Although pan-inhibitors of Na+-dependent glutamate transporters increase depolarization and/or cause seizures in rodents (Demarque et al., 2004; Shimamoto et al., 2004; Campbell and Hablitz, 2005; Montiel et al., 2005; Cattani et al., 2007; Campbell and Hablitz, 2008), it is not known if mice deleted of EAAC1 display differential sensitivity to seizure-induced cell death. Pilocarpine is a muscarinic receptor agonist commonly used to induce an acute seizure (Turski et al., 1983; Curia et al., 2008) and results in an increase in EAAC1 mRNA and/or protein (Crino et al., 2002; Zhang et al., 2004; Voutsinos-Porche et al., 2006; Ross et al., 2011). Within the first few days after an acute pilocarpine-induced seizure, neuronal damage has been documented in hippocampus; this cell death is attenuated by excitatory amino acid receptor antagonists, consistent with an excitotoxic mechanism (Milhaud et al., 2003; Mikati et al., 2008; Schauwecker, 2012). The goal of the present study was to determine if mice deleted of EAAC1 display differential sensitivity to pilocarpine/seizure-induced cell death.
2. Materials and Methods
2.1. Pilocarpine-induced seizure model
This work was reviewed and approved by the Institutional Animal Care and Use Committee of the Children’s Hospital of Philadelphia. EAAC1 null mice on a CD-1 background were used for most of the studies presented (Peghini et al., 1997). In addition, we back-crossed this mutation onto a C57Bl/6 background (Jackson Laboratories) for 11 generations, and some of these animals were also used for experiments (noted in the results). Heterozygote male and female mice were used to produce homozygous knock-out (EAAC1−/−) and wild-type age-matched controls. Adult male mice (mean age 100 days) were first injected with intraperitoneal (i.p.) scopolamine methyl nitrate (1 mg/kg in sterile water) to reduce peripheral cholinergic effects. After 30 minutes, mice received either 210 mg/kg pilocarpine hydrochloride i.p. to induce seizures or a 1/10 dose of pilocarpine (sham control). Under these conditions, 49 out of 85 animals developed spontaneous seizures within 20 min, and of these 29 animals died during the observation time. Animals that died or that failed to develop status epilepticus (n = 36) were excluded. Status epilepticus (SE) was defined as an uninterrupted convulsive seizure state. Mice were continuously monitored for 3 hours for seizure activity that was scored using the Racine scale (Racine, 1972). Three hours after pilocarpine injection, mice were injected with diazepam (0.25 mg/50 μl) i.p. to stop seizure activity and given a single dose of 0.9% saline (1 mL subcutaneous) to reduce dehydration. Mice were singly housed until the end of the experiment. Mice were allowed ad libitum access to moistened mouse chow placed in the bottom of the cage.
2.2. Tissue preparation and cell death staining
Animals were sacrificed 48 hours after SE for Fluoro-Jade staining and 24 hours after initiation of SE for EAAC1 staining. Mice were anesthetized with isoflorane through a nose cone and transcardially perfused with 15 mL phosphate buffered saline (PBS), pH 7.4 followed by 15 mL of freshly prepared 4% paraformaldehyde in PBS. Brains were post-fixed in 4% paraformaldehyde overnight after dissection, allowed to equilibrate in 30% sucrose, then flash-frozen in dry ice-chilled 2-methylbutane and stored at −80°C until sectioning. Coronal sections (30 μm) were prepared using a freezing sliding microtome.
Fluoro-Jade C staining was performed as previously described with minor modifications (Schmued et al., 2005). In brief, sections (bregma −1.25 to −2.80) were baked onto slides at 50°C for 30 minutes prior to staining. Slides were then sequentially immersed in 100% ethanol for 3 minutes, 70% ethanol for one minute, followed by distilled water for one minute. Slides were transferred to 0.06% potassium permanganate for 15 minutes at room temperature, washed in distilled water, then stained with Fluoro-Jade C for 30 minutes in the dark at 4°C. Slides were then washed in distilled water, dried, and dehydrated with Safe-Clear xylene substitute (Protocol) for 6 minutes prior to mounting with DPX mounting medium (Sigma-Aldrich).
2.3. Data collection and analysis
Using a Zeiss Axioplan epifluorescent microscope (Oberkochen, Germany), Fluoro-Jade C positive cells were counted in 3-7 sections per animal in four hippocampal regions (CA1, CA3, dentate gyrus and hilus) and in cortex. The average number of dead cells per square micrometer was determined in each animal. Overall group comparisons were done by two-way ANOVA with a Bonferroni post-hoc test (Graphpad Prism).
2.4. Immunofluorescent staining and microscopy
Matched hippocampal sections (bregma −1.5 to −2.5 mm) from wild-type and EAAC1−/− animals (seizure and sham) were used for these analyses. Sections were washed three times in phosphate buffered saline (PBS) and blocked for 1 hour in 10% normal goat serum and 0.4% Triton X-100. Sections were then incubated overnight at 4°C with one of four anti-EAAC1 antibodies: rabbit-anti-EAAC1 (Santa Cruz, cat # sc-25658, Dallas, TX, 1:100), rabbit-anti-EAAC1 (Alpha Diagnostics, cat # EAAC11-A, San Antonio, TX, 1:200), mouse-anti-EAAT3 (Abcam, cat # ab78395, Cambridge, MA, 1:500) and rabbit-anti-EAAC1 (Rothstein et al., 1994), 1:200). Sections were washed and incubated with Alexa-Fluor 546-conjugated goat anti-rabbit or 488-conjugated goat anti-mouse (Molecular Probes) 1:400 for 2 hours at room temperature. Photomicrographs of all stained sections were acquired using a Leica DM 6000 fluorescent microscope (Solms, Germany).
3. Results
Several groups have observed increases in EAAC1 protein staining within hours to days after a chemoconvulsant-induced seizure (Miller et al., 1997; Ghijsen et al., 1999; Ueda et al., 2001; Zhang et al., 2004; Voutsinos-Porche et al., 2006; Ross et al., 2011). Recent studies have demonstrated that many of the commercially available anti-EAAC1 antibodies cross-react with non-specific proteins (Holmseth et al., 2005; Holmseth et al., 2012), raising the possibility that at least some of these prior observations may be related to increased expression of a protein other than EAAC1. Therefore, we first examined the specificity of several different anti-EAAC1 antibodies. Four antibodies were evaluated. Three of the antibodies (Alpha Diagnostics, Santa Cruz, and Abcam) yielded similar or identical immunofluorescent staining in knock-out and control animals. This staining was dependent upon primary antibody and therefore is likely due to cross-reactivity of these anti-EAAC1 antibodies (data not shown, n = 3 independent observations). The final antibody (Rothstein et al., 1994) yielded clear staining in wild-type animals with no staining observed in the EAAC1−/− animals (Fig. 1A & B). Using this antibody, the level of EAAC1 immunofluorescence was examined 24 hours after pilocarpine-induced status epilepticus. To minimize potential variability along the rostral-caudal axis, sections were limited to the portion of the hippocampus between bregma −1.5 and −2.5 mm. We confirmed that EAAC1 immunofluorescence was higher after seizures than in the sham control in the CA1 region and in cortex (Fig. 1 C-F). We also note that occasional cells in stratum oriens expressed high levels of EAAC1 protein. These cells were only observed in animals that received convulsive doses of pilocarpine.
Figure 1. Photomicrograph of representative EAAC1 staining from wild-type and EAAC1 knock-out animals.
EAAC1 staining is observed in tissue from wild-type animals (A) under conditions that result in no staining in tissue from EAAC1−/− mice (B). Some increase can be observed in staining intensity at 24 hours after SE (D) compared to wild-type control in CA1 (C) (sham n = 3, SE n = 2). This effect is greater in cortex (F, E).
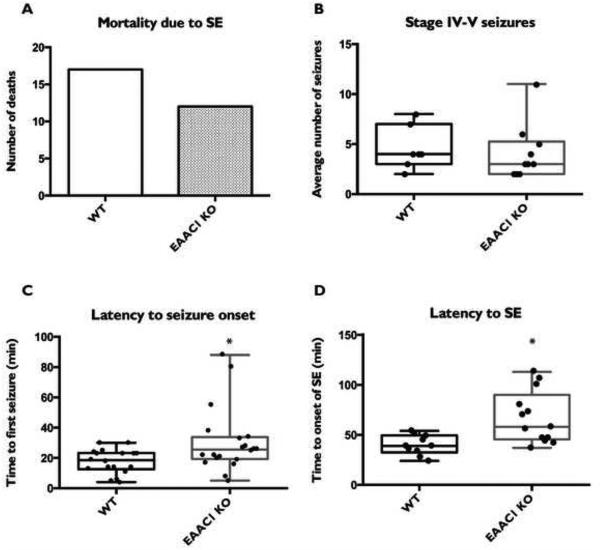
We sought to determine if the absence of EAAC1 influences the cell death observed after a pilocarpine-induced seizure. Wild-type or EAAC1−/− mice on a CD-1 background were injected with either a convulsive or sub-convulsive dose of pilocarpine and monitored for seizure activity for 3 hours. All animals received diazepam to eliminate any signs of behavioral seizures 3 hours after pilocarpine administration. As has been observed by others (Mazzuferi et al., 2012), ~60% of animals experience seizures (Racine scale stage IV-V) within 40 min after the administration of pilocarpine, and ~50% of these animals die shortly thereafter. It should be noted that these rates vary significantly with the strain of mouse (Schauwecker, 2012). There was no effect of genotype on the percentage of animals that failed to have seizures (44.2% wild-type; 40.5% EAAC1−/−). There was no significant difference in mortality due to seizures between wild-type animals and EAAC1−/− and no difference in the total number of seizures (Fig. 2A & B). We observed a mild, though significant, increase in latency to onset of the first stage IV-V seizure (p < 0.05), as well as latency to develop status epilepticus (p < 0.01) (Fig. 2C & D), defined as a constant stage IV seizure state. These differences suggest that it takes longer for the EAAC1 null mice to develop SE. Once they start having convulsive seizures the number of stage IV-V seizures and mortality was similar between wild-type animals and EAAC1−/−, indicating delayed seizure onset in the EAAC1−/− mice but equivalent behavioral seizure activity.
Figure 2. Comparison of observable seizure activity and mortality in mice injected with pilocarpine.
A total of 85 mice (CD-1 background) received pilocarpine injections (43 wild-type, 42 EAAC1 knock-out). No significant differences were seen between wild-type and EAAC1−/− mice in either the number of deaths due to seizures, as measured by a binomial test (A) or in observable stage IV-V seizures, as measured by a Student’s t-test (B). There was a mild though significant increase in latency to seizure onset (C) as well as induction of SE (D) in EAAC1−/− compared to control as measure by a Student’s t-test.
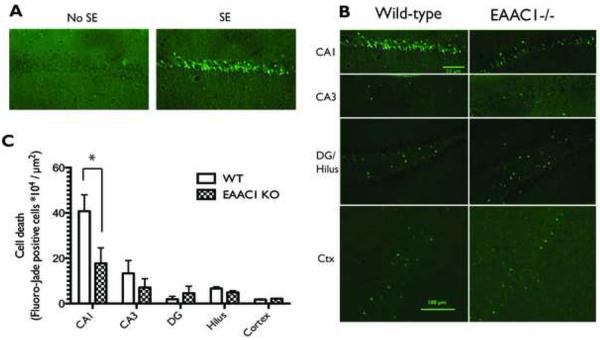
Previous studies have demonstrated that cell death, detected using Fluoro-Jade C staining, is maximal between 1 and 7 days after pilocarpine-induced seizures (Poirier et al., 2000). Therefore, cell death was compared in wild-type and knock-out animals 48 hours after pilocarpine-induced seizures. Fluoro-Jade C positive cells were observed in hippocampal sections from mice that experienced status epilepticus, but were not observed in sections from CD-1 wild-type or EAAC1−/− mice that received pilocarpine treatment but did not experience SE (Fig. 3A). As has been previously observed (Mohajeri et al., 2004), the level of cell death was greater in area CA1 than in area CA3, dentate gyrus, or in the hilar regions of the hippocampus with between 2- to 20-fold more Fluoro-Jade stained cells in area CA1 than in other subregions (Fig. 3B & C). A comparison across all regions of the hippocampus revealed an overall group difference between wild-type and knock-out animals with significantly fewer Fluoro-Jade stained cells observed in EAAC1−/− animals compared to wild-type controls (P < 0.05). A post-hoc comparison of the number of Fluoro-Jade positive cells revealed significantly less cell death in area CA1 in EAAC1−/− animals. This is not due to variability in the size of the area measured, as area did not vary between groups (data not shown). We also counted the number of Fluoro-Jade stained cells in cortex (layers 1-6), and there was no significant difference between the two groups of animals. Together, these studies demonstrate that the absence of EAAC1 does not exacerbate cell death observed after a pilocarpine-induced seizure. In fact, our data demonstrate that deletion of EAAC1 reduces the amount of cell death observed in area CA1. As the EAAC1−/− mice displayed a prolonged latency to the first seizure, we asked if cell death in area CA1 was correlated with latency to the first seizure; there was no correlation (r2= 0.056, P > 0.3). Similarly, cell death in area CA1 was not correlated with latency to onset of status epilepticus (r2 = 0.0033, P > 0.8).
Figure 3. Photomicrograph of representative Fluoro-Jade C staining from wild-type and EAAC1−/− animals 48 hours after pilocarpine-induced seizures and comparison of cell death.
(A) Fluoro-Jade staining is evident in the hippocampi of mice that have experienced SE, whereas it is absent in the hippocampi of mice that received pilocarpine but did not experience SE. CA1 pyramidal cells in wild-type animals were more sensitive to pilocarpine-induced cell death than those in CA3, the dentate gyrus, the hilus, or the cortex. (B) In EAAC1−/− mice, less cell death was consistently observed in CA1 than was observed in the wild-type mice. (C) A two-way ANOVA shows a significant effect of genotype on the amount of cell death following pilocarpine-induced SE, which a post hoc test shows to be due to a significant difference in CA1. EAAC1−/− mice had significantly less cell death in the CA1 region of the hippocampus than wild-type animals (WT = 40.77 ± 7.27 * 104 cells/μm2, KO = 17.76 ± 6.80 * 104 cells/μm2, p = 0.03). Data are presented as mean ± SEM, WT n=7, KO n = 10 animals.
As different strains of mice are known to display varied responses to chemoconvulsant-induced seizures and cell death (Schauwecker, 2002, 2012), we back-crossed the EAAC1−/− deletion onto a C57Bl/6 background (Jackson Laboratories). We first examined the effects of i.p. injections of kainate in C57Bl/6 mice. At 30 mg/kg, kainate caused 100% mortality (WT n = 4, KO n = 4 mice), and only slightly lower doses (20 or 25 mg/kg) caused seizures but no cell death in either genotype (WT n = 4, KO n = 4 mice). The latter was also the case with i.p. injections of kainate (30 mg/kg) in the CD-1 background (WT n = 17, KO n = 16). Because we observed high mortality with kainate and other groups have observed very high mortality in C57Bl/6 mice from Jackson Laboratories with pilocarpine (Borges et al., 2003; Schauwecker, 2012), we did no further experiments with these mice in an effort to minimize animal suffering. We also tested the effects of direct i.c.v. injections of kainate in mice on the CD-1 background, but found too much variability in the numbers of dead cells to confidently quantify cell death (WT n = 9, KO n = 10 mice).
4. Discussion
While many groups have examined associations between the levels of EAAC1 mRNA or protein and seizure activity (Miller et al., 1997; Ghijsen et al., 1999; Ueda et al., 2001; Zhang et al., 2004; Voutsinos-Porche et al., 2006; Ross et al., 2011) and one previous study reported an absence seizure phenotype in antisense knock-down of EAAC1 (Rothstein et al., 1996), the present study is the first to investigate the effects of genetic deletion of EAAC1 on seizure outcomes and chemoconvulsant-induced neurodegeneration. While no difference was observed in cortical neuronal death, significantly fewer dead cells were observed in the CA1 region of the hippocampus. We therefore conclude that genetic deletion of EAAC1 decreases the levels of cell death seen after prolonged seizure activity. We also find an increase in EAAC1 immunoreactivity in pyramidal neurons of the cortex and the CA1 region of the hippocampus following SE.
The effect of the absence of EAAC1 is the opposite of that predicted based on the effects observed with general inhibitors of Na+-dependent transporters or with specific manipulation of the astroglial transporters. Several studies show that pan-inhibition of the Na+-dependent glutamate transporters results in seizure-like discharges in vitro or seizures in vivo (Demarque et al., 2004; Shimamoto et al., 2004; Campbell and Hablitz, 2005; Montiel et al., 2005; Cattani et al., 2007; Campbell and Hablitz, 2008). Genetic deletion of GLT-1 results in spontaneous seizures and premature death in mice (Tanaka et al., 1997). Conversely, over-expression of GLT-1 in transgenic mice using the GFAP promoter to drive astrocytic expression results in decreased mortality and decreased cell death due to pilocarpine-induced status epilepticus (Kong et al., 2012). Up-regulation of GLT-1 induced by the beta-lactam antibiotic, ceftriaxone, increases latency to seizure onset and decreases mortality associated with administration of pentylenetetrazole (Jelenkovic et al., 2008). Genetic deletion of GLAST results in a more severe pentylenetetrazole-induced seizure phenotype compared to controls (Watanabe et al., 1999) and more intraictal spikes as a result of kindling, though more stimulations were required to obtain kindling and a shorter after-discharges were observed (Tsuru et al., 2002).
We report that mice deleted of the neuronal glutamate transporter, EAAC1, experience less cell death than control animals after pilocarpine-induced seizures. Although we detected no differences in mortality and stage IV-V seizures in wild-type and EAAC1−/− mice, it is possible that pilocarpine causes less intense electrographic seizures in the knock-out animals. This would be an unexpected result as EAAC1 provides glutamate to inhibitory neurons for the synthesis of GABA (Sepkuty et al., 2002; Mathews and Diamond, 2003) and limits spillover of glutamate to neighboring synapses (Rothstein et al., 1994; Diamond, 2001; Scimemi et al., 2009); both of these actions would tend to decrease excitability. We favor the possibility that EAAC1 contributes to cell death through reverse transport of glutamate. During a seizure, ATP levels fall and the Na+ gradients collapse (Streck et al., 2006; Kovac et al., 2012). Under these conditions these transporters will not only fail to clear extracellular glutamate, they can move glutamate from the cytosol into the extracellular space (Szatkowski et al., 1990; Taylor et al., 1992; Gemba et al., 1994; Katsumori et al., 1999). There is strong evidence that reversed operation of the Na+-dependent glutamate transporters contributes to the rise of extracellular glutamate observed after an ischemic insult (Longuemare and Swanson, 1995; Yamaguchi et al., 1998; Koch et al., 1999). Neuronal transporters appear to be more important as a source for this increase in extracellular glutamate, in part because cytoplasmic pools of glutamate are thought to be higher in neurons than in astrocytes (Rossi et al., 2000). There is a 3-fold delay in the depolarization shift observed in response to anoxia from EAAC1−/− mice compared to wild-type mice (Gebhardt et al., 2002), providing evidence that EAAC1 can contribute to the rise in extracellular glutamate observed during energy depletion.
The result of the present study is also surprising in light of the effects of EAAC1 deletion in other neurodegenerative models. One of the most striking examples of this is that EAAC1−/− mice experience more cell death as a result of ischemia than do controls (Won et al., 2010). There are a few possible explanations for the opposite effect in a seizure model. EAAC1−/− mice display markedly decreased levels of the antioxidant glutathione, synthesized from glutamate and cysteine, both of which are transported into neurons by EAAC1 (Kanai and Hediger, 1992; Rothstein et al., 1994; Rothstein et al., 1996; Diamond, 2001; Sepkuty et al., 2002; Chen and Swanson, 2003; Himi et al., 2003; Mathews and Diamond, 2003; Aoyama et al., 2008; Scimemi et al., 2009; Escartin et al., 2011; Aoyama and Nakaki, 2012; Aoyama et al., 2012a). It is possible that maintenance of neuronal glutathione is more important for attenuating ischemia-mediated cell death than for attenuation of seizure-induced cell death. Another difference between ischemia and seizures are the effects on total EAAC1 protein levels. In contrast to the increases in EAAC1 protein that are observed shortly after a seizure (and prior to cell death), transient focal ischemia causes a decrease in EAAC1 protein that precedes cell death (Martin et al., 1997; Rao et al., 2001). Finally, EAAC1 is unique compared to the other glutamate transporters in that it rapidly and dramatically traffics on and off the plasma membrane (for reviews see Nieoullon et al., 2006; Gonzalez et al., 2007). In fact, recent studies suggest that under baseline conditions most EAAC1 protein is found within cells in vivo (Holmseth et al., 2012). As only plasma membrane pools of EAAC1 could contribute to a rise in extracellular glutamate, it is possible that EAAC1 trafficking is differentially affected in seizures and after ischemia. This was not examined.
In summary, we report evidence that mice deleted of EAAC1 are less susceptible to pilocarpine-induced cell death in area CA1. Based on the analyses performed, we suggest that the presence of EAAC1 either causes an increase in seizure burden that was not detectable behaviorally or the presence of EAAC1 increases the amount of cell death that is observed as a consequence of SE. While we favor the latter explanation, either interpretation suggests that EAAC1 contributes to pathology observed with seizures.
Highlights.
We examined the difference in cell death between wild-type and EAAC1−/− mice after status epilepticus.
Neuronal death was less in EAAC1−/− mice after status epilepticus than in wild-type controls.
EAAC1 protein was observed to increase in CA1 and cortex after SE in wild-type animals.
Acknowledgements
This research was supported by an NIH grant R01 HD060132. This work was also supported by the Neuroscience Core of the Institutional Intellectual and Developmental Disabilities Research Center (P30 HD26979).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoyama K, Matsumura N, Watabe M, Nakaki T. Oxidative stress on EAAC1 is involved in MPTP-induced glutathione depletion and motor dysfunction. The European journal of neuroscience. 2008;27:20–30. doi: 10.1111/j.1460-9568.2007.05979.x. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Nakaki T. Inhibition of GTRAP3-18 May Increase Neuroprotective Glutathione (GSH) Synthesis. Int J Mol Sci. 2012;13:12017–12035. doi: 10.3390/ijms130912017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K, Nakaki T. Neuroprotective properties of the excitatory amino acid carrier 1 (EAAC1) Amino Acids. 2013;45:133–142. doi: 10.1007/s00726-013-1481-5. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nature Neuroscience. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Wang F, Matsumura N, Kiyonari H, Shioi G, Tanaka K, Kinoshita C, Kikuchi-Utsumi K, Watabe M, Nakaki T. Increased neuronal glutathione and neuroprotection in GTRAP3-18-deficient mice. Neurobiology of disease. 2012a;45:973–982. doi: 10.1016/j.nbd.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Aoyama K, Watabe M, Nakaki T. Modulation of neuronal glutathione synthesis by EAAC1 and its interacting protein GTRAP3-18. Amino Acids. 2012b;42:163–169. doi: 10.1007/s00726-011-0861-y. [DOI] [PubMed] [Google Scholar]
- Berman AE, Chan WY, Brennan AM, Reyes RC, Adler BL, Suh SW, Kauppinen TM, Edling Y, Swanson RA. N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1−/−mouse. Annals of Neurology. 2011;69:509–520. doi: 10.1002/ana.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Experimental neurology. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Campbell S, Hablitz JJ. Modification of epileptiform discharges in neocortical neurons following glutamate uptake inhibition. Epilepsia. 2005;46(Suppl 5):129–133. doi: 10.1111/j.1528-1167.2005.01020.x. [DOI] [PubMed] [Google Scholar]
- Campbell SL, Hablitz JJ. Decreased glutamate transport enhances excitability in a rat model of cortical dysplasia. Neurobiology of disease. 2008;32:254–261. doi: 10.1016/j.nbd.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattani AA, Bonfardin VD, Represa A, Ben-Ari Y, Aniksztejn L. Generation of slow network oscillations in the developing rat hippocampus after blockade of glutamate uptake. J Neurophysiol. 2007;98:2324–2336. doi: 10.1152/jn.00378.2007. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. Journal of Neurochemistry. 2003;84:1332–1339. doi: 10.1046/j.1471-4159.2003.01630.x. [DOI] [PubMed] [Google Scholar]
- Crino PB, Jin H, Shumate MD, Robinson MB, Coulter DA, Brooks-Kayal AR. Increased expression of the neuronal glutamate transporter (EAAT3/EAAC1) in hippocampal and neocortical epilepsy. Epilepsia. 2002;43:211–218. doi: 10.1046/j.1528-1157.2002.35001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. Journal of neuroscience methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. The high affinity uptake system for excitatory amino acids in the brain. Prog Neurobiol. 1994;44:377–396. doi: 10.1016/0301-0082(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Demarque M, Villeneuve N, Manent JB, Becq H, Represa A, Ben-Ari Y, Aniksztejn L. Glutamate transporters prevent the generation of seizures in the developing rat neocortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:3289–3294. doi: 10.1523/JNEUROSCI.5338-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:8328–8338. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Won SJ, Malgorn C, Auregan G, Berman AE, Chen PC, Deglon N, Johnson JA, Suh SW, Swanson RA. Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7392–7401. doi: 10.1523/JNEUROSCI.6577-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C, Korner R, Heinemann U. Delayed anoxic depolarizations in hippocampal neurons of mice lacking the excitatory amino acid carrier 1. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22:569–575. doi: 10.1097/00004647-200205000-00008. [DOI] [PubMed] [Google Scholar]
- Gemba T, Oshima T, Ninomiya M. Glutamate efflux via the reversal of the sodium-dependent glutamate transporter caused by glycolytic inhibition in rat cultured astrocytes. Neuroscience. 1994;63:789–795. doi: 10.1016/0306-4522(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Ghijsen WE, da Silva Aresta Belo AI, Zuiderwijk M, Lopez da Silva FH. Compensatory change in EAAC1 glutamate transporter in rat hippocampus CA1 region during kindling epileptogenesis. Neuroscience Letters. 1999;276:157–160. doi: 10.1016/s0304-3940(99)00824-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, Susarla BT, Fournier KM, Sheldon AL, Robinson MB. Constitutive endocytosis and recycling of the neuronal glutamate transporter, excitatory amino acid carrier 1. Journal of Neurochemistry. 2007;103:1917–1931. doi: 10.1111/j.1471-4159.2007.04881.x. [DOI] [PubMed] [Google Scholar]
- Himi T, Ikeda M, Yasuhara T, Nishida M, Morita I. Role of neuronal glutamate transporter in the cysteine uptake and intracellular glutathione levels in cultured cortical neurons. J Neural Transm. 2003;110:1337–1348. doi: 10.1007/s00702-003-0049-z. [DOI] [PubMed] [Google Scholar]
- Holmseth S, Dehnes Y, Bjornsen LP, Boulland JL, Furness DN, Bergles D, Danbolt NC. Specificity of antibodies: unexpected cross-reactivity of antibodies directed against the excitatory amino acid transporter 3 (EAAT3) Neuroscience. 2005;136:649–660. doi: 10.1016/j.neuroscience.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Holmseth S, Dehnes Y, Huang YH, Follin-Arbelet VV, Grutle NJ, Mylonakou MN, Plachez C, Zhou Y, Furness DN, Bergles DE, Lehre KP, Danbolt NC. The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6000–6013. doi: 10.1523/JNEUROSCI.5347-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenkovic AV, Jovanovic MD, Stanimirovic DD, Bokonjic DD, Ocic GG, Boskovic BS. Beneficial effects of ceftriaxone against pentylenetetrazole-evoked convulsions. Exp Biol Med (Maywood) 2008;233:1389–1394. doi: 10.3181/0803-RM-83. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Katsumori H, Baldwin RA, Wasterlain CG. Reverse transport of glutamate during depolarization in immature hippocampal slices. Brain Research. 1999;819:160–164. doi: 10.1016/s0006-8993(98)01352-3. [DOI] [PubMed] [Google Scholar]
- Kiryu-Seo S, Gamo K, Tachibana T, Tanaka K, Kiyama H. Unique anti-apoptotic activity of EAAC1 in injured motor neurons. EMBO J. 2006;25:3411–3421. doi: 10.1038/sj.emboj.7601225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HP, Chamberlin AR, Bridges RJ. Nontransportable inhibitors attenuate reversal of glutamate uptake in synaptosomes following a metabolic insult. Mol Pharmacol. 1999;55:1044–1048. doi: 10.1124/mol.55.6.1044. [DOI] [PubMed] [Google Scholar]
- Kong Q, Takahashi K, Schulte D, Stouffer N, Lin Y, Lin CL. Increased glial glutamate transporter EAAT2 expression reduces epileptogenic processes following pilocarpine-induced status epilepticus. Neurobiology of disease. 2012;47:145–154. doi: 10.1016/j.nbd.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovac S, Domijan AM, Walker MC, Abramov AY. Prolonged seizure activity impairs mitochondrial bioenergetics and induces cell death. J Cell Sci. 2012;125:1796–1806. doi: 10.1242/jcs.099176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler P, Schmitt A. Glutamate transporter EAAC1 is expressed in neurons and glial cells in the rat nervous system. Glia. 1999;27:129–142. doi: 10.1002/(sici)1098-1136(199908)27:2<129::aid-glia3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Longuemare MC, Swanson RA. Excitatory amino acid release from astrocytes during energy failure by reversal of sodium-dependent uptake. J Neurosci Res. 1995;40:379–386. doi: 10.1002/jnr.490400312. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink AM, Lehmann C, Portera-Cailliau C, Koehler R, Rothstein J, Traystman RJ. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Annals of Neurology. 1997;42:335–348. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- Mathews GC, Diamond JS. Neuronal glutamate uptake Contributes to GABA synthesis and inhibitory synaptic strength. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:2040–2048. doi: 10.1523/JNEUROSCI.23-06-02040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuferi M, Kumar G, Rospo C, Kaminski RM. Rapid epileptogenesis in the mouse pilocarpine model: video-EEG, pharmacokinetic and histopathological characterization. Experimental neurology. 2012;238:156–167. doi: 10.1016/j.expneurol.2012.08.022. [DOI] [PubMed] [Google Scholar]
- Mikati MA, Rizk E, El Dada S, Zeinieh M, Kurdi R, El Hokayem J, Rahmeh A, Koubeissi M, Azzam D, Usta J, El Sabban M, Dbaibo G. Programmed cell death in the lithium pilocarpine model: evidence for NMDA receptor and ceramide-mediated mechanisms. Brain Dev. 2008;30:513–519. doi: 10.1016/j.braindev.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Milhaud D, Rondouin G, Lerner-Natoli M, Bockaert J, Lafon-Cazal M. Neuroprotective activity of antazoline against neuronal damage induced by limbic status epilepticus. Neuroscience. 2003;120:475–484. doi: 10.1016/s0306-4522(03)00268-9. [DOI] [PubMed] [Google Scholar]
- Miller HP, Levey AI, Rothstein JD, Tzingounis AV, Conn PJ. Alterations in glutamate transporter protein levels in kindling-induced epilepsy. Journal of Neurochemistry. 1997;68:1564–1570. doi: 10.1046/j.1471-4159.1997.68041564.x. [DOI] [PubMed] [Google Scholar]
- Mohajeri MH, Madani R, Saini K, Lipp HP, Nitsch RM, Wolfer DP. The impact of genetic background on neurodegeneration and behavior in seizured mice. Genes, brain, and behavior. 2004;3:228–239. doi: 10.1111/j.1601-1848.2004.00073.x. [DOI] [PubMed] [Google Scholar]
- Montiel T, Camacho A, Estrada-Sanchez AM, Massieu L. Differential effects of the substrate inhibitor l-trans-pyrrolidine-2,4-dicarboxylate (PDC) and the non-substrate inhibitor DL-threo-beta-benzyloxyaspartate (DL-TBOA) of glutamate transporters on neuronal damage and extracellular amino acid levels in rat brain in vivo. Neuroscience. 2005;133:667–678. doi: 10.1016/j.neuroscience.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Canolle B, Masmejean F, Guillet B, Pisano P, Lortet S. The neuronal excitatory amino acid transporter EAAC1/EAAT3: does it represent a major actor at the brain excitatory synapse? Journal of Neurochemistry. 2006;98:1007–1018. doi: 10.1111/j.1471-4159.2006.03978.x. [DOI] [PubMed] [Google Scholar]
- Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier JL, Capek R, De Koninck Y. Differential progression of Dark Neuron and Fluoro-Jade labelling in the rat hippocampus following pilocarpine-induced status epilepticus. Neuroscience. 2000;97:59–68. doi: 10.1016/s0306-4522(00)00026-9. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalography and clinical neurophysiology. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rao VL, Bowen KK, Dempsey RJ. Transient focal cerebral ischemia down-regulates glutamate transporters GLT-1 and EAAC1 expression in rat brain. Neurochem Res. 2001;26:497–502. doi: 10.1023/a:1010956711295. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Dowd LA. Heterogeneity and functional properties of subtypes of sodium-dependent glutamate transporters in the mammalian central nervous system. Adv Pharmacol. 1997;37:69–115. doi: 10.1016/s1054-3589(08)60948-5. [DOI] [PubMed] [Google Scholar]
- Ross JR, Porter BE, Buckley PT, Eberwine JH, Robinson MB. mRNA for the EAAC1 subtype of glutamate transporter is present in neuronal dendrites in vitro and dramatically increases in vivo after a seizure. Neurochemistry International. 2011;58:366–375. doi: 10.1016/j.neuint.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Schauwecker PE. Complications associated with genetic background effects in models of experimental epilepsy. Progress in brain research. 2002;135:139–148. doi: 10.1016/s0079-6123(02)35014-3. [DOI] [PubMed] [Google Scholar]
- Schauwecker PE. Strain differences in seizure-induced cell death following pilocarpine-induced status epilepticus. Neurobiology of disease. 2012;45:297–304. doi: 10.1016/j.nbd.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Research. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Tian H, Diamond JS. Neuronal transporters regulate glutamate clearance, NMDA receptor activation, and synaptic plasticity in the hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:14581–14595. doi: 10.1523/JNEUROSCI.4845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, Coulter DA, Rothstein JD. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:6372–6379. doi: 10.1523/JNEUROSCI.22-15-06372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochemistry International. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto K, Sakai R, Takaoka K, Yumoto N, Nakajima T, Amara SG, Shigeri Y. Characterization of novel L-threo-beta-benzyloxyaspartate derivatives, potent blockers of the glutamate transporters. Mol Pharmacol. 2004;65:1008–1015. doi: 10.1124/mol.65.4.1008. [DOI] [PubMed] [Google Scholar]
- Streck EL, Feier G, Burigo M, Franzon R, Dal-Pizzol F, Quevedo J, Wyse AT. Effects of electroconvulsive seizures on Na(+),K(+)-ATPase activity in the rat hippocampus. Neuroscience Letters. 2006;404:254–257. doi: 10.1016/j.neulet.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Geer JJ, Burke SP. Endogenous extracellular glutamate accumulation in rat neocortical cultures by reversal of the transmembrane sodium gradient. Neuroscience Letters. 1992;145:197–200. doi: 10.1016/0304-3940(92)90021-x. [DOI] [PubMed] [Google Scholar]
- Tsuru N, Ueda Y, Doi T. Amygdaloid kindling in glutamate transporter (GLAST) knockout mice. Epilepsia. 2002;43:805–811. doi: 10.1046/j.1528-1157.2002.36601.x. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behavioural Brain Research. 1983;9:315–335. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Doi T, Tokumaru J, Yokoyama H, Nakajima A, Mitsuyama Y, Ohya-Nishiguchi H, Kamada H, Willmore LJ. Collapse of extracellular glutamate regulation during epileptogenesis: down-regulation and functional failure of glutamate transporter function in rats with chronic seizures induced by kainic acid. Journal of Neurochemistry. 2001;76:892–900. doi: 10.1046/j.1471-4159.2001.00087.x. [DOI] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Koning E, Clement Y, Kaplan H, Ferrandon A, Motte J, Nehlig A. EAAC1 glutamate transporter expression in the rat lithium-pilocarpine model of temporal lobe epilepsy. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:1419–1430. doi: 10.1038/sj.jcbfm.9600295. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Morimoto K, Hirao T, Suwaki H, Watase K, Tanaka K. Amygdala-kindled and pentylenetetrazole-induced seizures in glutamate transporter GLAST-deficient mice. Brain Research. 1999;845:92–96. doi: 10.1016/s0006-8993(99)01945-9. [DOI] [PubMed] [Google Scholar]
- Won SJ, Yoo BH, Brennan AM, Shin BS, Kauppinen TM, Berman AE, Swanson RA, Suh SW. EAAC1 gene deletion alters zinc homeostasis and exacerbates neuronal injury after transient cerebral ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:15409–15418. doi: 10.1523/JNEUROSCI.2084-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Endo K, Kitajima T, Ogata H, Hori Y. Involvement of the glutamate transporter and the sodium-calcium exchanger in the hypoxia-induced increase in intracellular Ca2+ in rat hippocampal slices. Brain Research. 1998;813:351–358. doi: 10.1016/s0006-8993(98)01037-3. [DOI] [PubMed] [Google Scholar]
- Zhang G, Raol YS, Hsu FC, Brooks-Kayal AR. Long-term alterations in glutamate receptor and transporter expression following early-life seizures are associated with increased seizure susceptibility. Journal of Neurochemistry. 2004;88:91–101. doi: 10.1046/j.1471-4159.2003.02124.x. [DOI] [PubMed] [Google Scholar]