Abstract
The similarities of two major peanut allergens, Ara h 2 and Ara h 6, in molecular size, amino acid sequence, and structure have made it difficult to obtain natural Ara h 6 free of Ara h 2. The objectives of this study were to purify natural Ara h 6 that is essentially free of Ara h 2 and to compare its IgE reactivity and potency in histamine release assays to Ara h 2. SDS-PAGE of the highly purified allergen (<0.01% Ara h 2) revealed a single 14.5kD band and the identity of Ara h 6 was confirmed by LC-MS/MS. Ara h 6 showed a higher seroprevalence in chimeric-IgE ELISA (n=54), but a weaker biological activity in basophil histamine release assays than Ara h 2. Purified Ara h 6 will be useful for diagnostic IgE antibody assays, as well as molecular and cellular studies to investigate the immunological mechanisms of peanut allergy.
Keywords: Peanut, allergen, IgE reactivity, histamine release
Introduction
Peanut allergy is an important food allergy in the United States that affects 1.4 % of children and 0.6% of adults.(1, 2) In the United Kingdom, approximately 1.8% of children have an allergy to peanut.(3, 4) While most children outgrow food allergies to milk, egg, or wheat; allergy to peanut is more persistent and often continues into adulthood.(5) Minute amounts of peanut, as little as 0.4 g, are enough to elicit milder allergic symptoms that include rashes, angioedema, and gastrointestinal symptoms.(5, 6) However, peanut is also one of the main triggers of severe anaphylactic reactions that can be fatal.(7)
Currently, 12 peanut allergens have been documented by the WHO/International Union of Immunological Societies (IUIS) Allergen Nomenclature committee.(8) Ara h 1 and Ara h 2 have been well-studied and are recognized as major allergens. Ara h 2 has a higher predictive value for diagnosis of clinical peanut allergy than Ara h 1, Ara h 3, Ara h 8 and Ara h 9.(9) Ara h 2 is also more potent than Ara h 1 or Ara h 3 in histamine release assays and skin prick tests.(10–12)
Another peanut allergen, Ara h 6, has recently emerged as an important allergen which, together with Ara h 2, has been associated with clinical peanut allergy.(13) Ara h 6 has approximately the same seroprevalence as Ara h 2 and thus is considered a major peanut allergen.(13, 14) Ara h 6 and Ara h 2 are of similar molecular size – Ara h 2 is 17–19 kDa and Ara h 6 is 14.5 kDa. Both allergens are 2S albumins that are heat-stable, immunogenic and resistant to digestion in the gut.(15–17) The nucleotide (or amino acid) sequences of Ara h 2.0101 and Ara h 6.0101 are 50% identical and 58% similar (EMBOSS Needle alignment). The structure of the protease-resistant core of Ara h 6 has previously been determined by NMR and the folds of this allergenic core were found to be virtually identical to that of Ara h 2.(15) The 3-dimensional folds determine the IgE epitopes which are lost upon unfolding.(18) More recently, the structure of Ara h 2 was determined by X-ray crystallography and molecular modeling studies predict that half of the residues on the surfaces of both proteins are conserved.(19) These factors contribute to the difficulty in obtaining purified natural Ara h 6 (nAra h 6) that is free of Ara h 2. Ara h 7, the third peanut allergen of the 2S albumin family also shows sequence homology to Ara h 2 and Ara h 6.(20) However, attempts to identify natural Ara h 7 protein in peanut extracts have failed. Recently, Schmidt et al found low abundance of natural Ara h 7.0201 and Ara h 7.0202 in peanut extracts after enrichment of the low molecular mass peanut proteins.(21)
Highly purified allergens are important for allergen standardization.(22, 23) There is extensive variability in allergen composition and potency between different commercially available peanut extracts, including those used for skin prick testing that could lead to misdiagnosis.(24, 25) ‘Component-resolved’ or molecular diagnostics is based on measuring IgE antibodies to multiple individual allergens rather than to heterogeneous extracts. The use of molecular diagnostics tends to lower false-positive IgE antibody results caused by interaction with profilins and cross-reactive carbohydrate determinants that are present in diverse plant-based food.(26–29) Some birch pollen-allergic patients were found to be cross-sensitized to peanut through cross-reactivity between Bet v 1 and Ara h 8, which are homologous proteins that belong to the PR-10 family.(30) Moreover, pea-allergic patients who later developed peanut allergy recognized only Ara h 1, but not Ara h 2 or Ara h 3.(31) Vicilin homologues in pea and peanut (Ara h 1) are the molecular basis for this cross-reactivity. Most peanut-allergic patients that react to Ara h 2 also have IgE against Ara h 6; however, a recent case study reported that Ara h 6 caused severe reactions to peanut in the absence of sensitization to Ara h 2.(32) Thus, it is important to have pure, single-component peanut allergens for accurate diagnosis of peanut allergy.
Our objective in this study was to obtain purified nAra h 6 that is essentially free of Ara h 2 and to compare its IgE reactivity and potency in histamine release assays to Ara h 2.
Materials and Methods
Purified Natural Ara h 6
Peanut flour was extracted into Phosphate buffered saline, pH 7.4 (PBS) containing 1 M sodium chloride for 2 hours at 60 °C. The mixture was centrifuged at 13,500 x g for 25 minutes and the supernatant was sterile filtered. Ammonium sulfate precipitation was used to remove contaminating Ara h 1. The 70–100% pellet containing Ara h 6 and Ara h 2 was redissolved in PBS and passed over a 1C4 monoclonal antibody column to remove the contaminating Ara h 2. Size exclusion chromatography (HiPrep 16/60 Sephacryl S-100 HR, GE Life Sciences, Uppsala, Sweden) was used as a final polishing step. The identity of purified nAra h 6 was confirmed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and its purity was analyzed by SDS-PAGE and silver staining. The concentration of the purified protein was determined by the Advanced Protein Assay (Cytoskeleton, Denver, CO.) and the Ara h 2 content was measured using a two-site antibody ELISA with monoclonal antibody 1C4 as the capture antibody and rabbit-polyclonal antibody anti-Ara h 2 for detection.(33) Ara h 6 was measured by ELISA using capture monoclonal antibody 3B8 and detection antibody 3E12. Another nAra h 6 preparation available from TNO Quality of Life, Zeist, The Netherlands (T-Ara h 6) was tested for comparison.
Sera
Sera for the immunoblotting, chimeric-IgE ELISA and cross-inhibition ELISA were obtained from Bioreclamation, Inc. (East Meadow, NY) (n=24), which operates in full compliance with the Food and Drug Administration guidelines, the Department of Allergy and Clinical Immunology at Johns Hopkins University (n=25) and kindly provided by Dr. Peter Heymann, University of Virginia (n=8). Sera for the Basophil Histamine Release Assay were provided by the Department of Allergy, Asthma and Clinical Immunology at the University of Colorado (n=20). All donors signed informed consent and, for minors, assent. Sera were collected with approval from the respective institutions’ Human Investigation Committees.
Immunoblotting
IgE reactivity of purified nAra h 6 was compared to natural Ara h 2 (nAra h 2), peanut extract and T-Ara h 6 by immunoblot using pooled sera from six peanut-allergic patients (≥ 100 kU/L by chimeric-IgE ELISA, PWH6, BR13, BR21, BR22, BR23, BR24). Proteins loaded at 0.5 mg/ml and separated by SDS-PAGE under non-reducing conditions (Homogenous 20%, GE Lifesciences, Uppsala, Sweden) were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories, Hercules, CA) by electroblotting. The membrane was blocked with 5% non-fat dry milk in 25 mM Tris, 150 mM NaCl, pH 7.4, 0.05% Tween-20 for 16 hours at 4 °C and then incubated with the pooled sera at a 1:2 dilution in blocking buffer for 2 hours at room temperature. Bound IgE was detected with peroxidase-labeled goat anti-human IgE (Kirkegaard & Perry Laboratories, Inc, Gaithersburg, MD) followed by ECL™ Western Blotting Analysis System (GE Lifesciences, Uppsala, Sweden), which produced a detectable chemiluminescent signal.
Chimeric-IgE ELISA
IgE antibody binding to nAra h 6 was compared to IgE binding to T-Ara h 6 and nAra h 2 by a modified chimeric-IgE ELISA as described previously.(34–36) Briefly, microtiter plates coated to saturation with one allergen at 0.5 Vg/well were incubated with sera from peanut-allergic patients (ImmunoCAP values ranged from 0.45 to > 100 kUA/L; average 15.2 ± 21.4 kUA/L, n = 54). Bound IgE antibody was detected using biotinylated goat anti-human IgE (Kierkegaard and Perry, Gaithersburg, MD) followed by streptavidin-peroxidase. The intensity of the color developed by ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) was measured at 405 nm. A chimeric anti-Der p 2 antibody, 2B12-IgE, was used to establish a standard curve from 0.4 – 115 U/ml IgE to quantify the bound specific IgE as previously reported.(36) Ara h 6 and Ara h 2-specific IgE for each serum sample were extrapolated from the linear portion of the 2B12-IgE curve. Levels of specific IgE antibody reactive with nAra h 6 and nAra h 2 or T-Ara h 6 were plotted and modeled using linear regression. Pearson correlations were used to analyze the relationship between specific IgE to nAra h 6 and nAra h 2 or T-Ara h 6 in peanut allergic patients sera.
Cross-inhibition ELISA
The chimeric-IgE ELISA was modified for cross-inhibition assays. Microtiter plates were saturated with nAra h 6 or nAra h 2 at 0.5 Vg/well for 16 hours at 4 °C.(34) A serum pool of the same sera used in the immunoblot was incubated with increasing concentrations of the inhibitor (either nAra h 6 or nAra h 2) for one hour prior to performing the assays. Protein concentration of the inhibiting allergen was increased in 10-fold increments from 10 to 10,000 ng/ml. The pooled sera with or without the inhibitor were added to their respective wells and incubated at room temperature for one hour. Bound IgE was detected as described for the Chimeric-IgE ELISA. The percent inhibition was calculated based on the optical density at 405 nm. 0% inhibition was assumed for the wells that had no inhibitor.
Basophil Histamine Release Assay
The stripped basophil histamine release assay was performed as previously described.(37) Briefly, basophil cells were stripped of membrane-bound IgE and sensitized with each serum from peanut-allergic patients (Ara h 6 specific IgE values ranged from 5 to 480 kUA/L; average 87.8 ± 125 kUA/L, n=20). The cells were then stimulated with increasing concentrations of allergen and histamine release was measured by fluorometric analysis. Histamine release was expressed as a percentage of the total amount of histamine in the cells determined after lysis of the cells with perchloric acid (100% release). The EC50 was the allergen concentration required to stimulate 50% of the maximum release. Histamine release dose response curves for nAra h 6 and nAra h 2 were generated for concentrations between 0.001 and 1000 ng/ml.
Results
Protein Characterization
Purified nAra h 6 (Lane 4) and T-Ara h 6 (Lane 5) migrated as single bands on silver-stained SDS-PAGE at the predicted molecular weight of 14.5 kDa (Figure 1A). Ara h 6 content was 1.05 mg/ml while the concentration of Ara h 2 was less than 0.1 Vg/ml (0.01 %) as measured by two-site antibody ELISA. The results of LC-MS/MS confirmed the identity of the purified allergen as Ara h 6.0101 with 74.5% sequence coverage (Figure 2).
Figure 1.
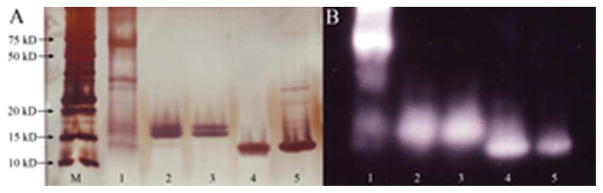
Silver-stain and immunoblot of nAra h 2, nAra h 6, and peanut extract using pooled sera from six peanut-allergic patients. A) Silver-stained gel; B) Proteins transferred onto a PVDF membrane were probed with sera, and bound IgE was detected with a peroxidase-labeled goat anti-human IgE. Lane M: Marker, Lane 1: Peanut extract, Lane 2 and 3: nAra h 2, Lane 4: nAra h 6 and Lane 5: T-Ara h 6.
Figure 2.

Sequence coverage of purified nAra h 6 with Ara h 6.0101. Underlined red letters indicate identical amino acids between the purified allergen and the published sequence.
IgE Binding Analysis
Immunoblotting demonstrated strong IgE reactivity to nAra h 2 (Lanes 2 and 3), nAra h 6 (Lanes 4 and 5) and peanut extract (Lane 1, Figure 1B). The major allergens, Ara h 1 (63 kDa), Ara h 2 (18–20 kDa), and Ara h 6 can be seen as major bands in the peanut extract. Ara h 2 was not detected in the purified nAra h 6 preparation.
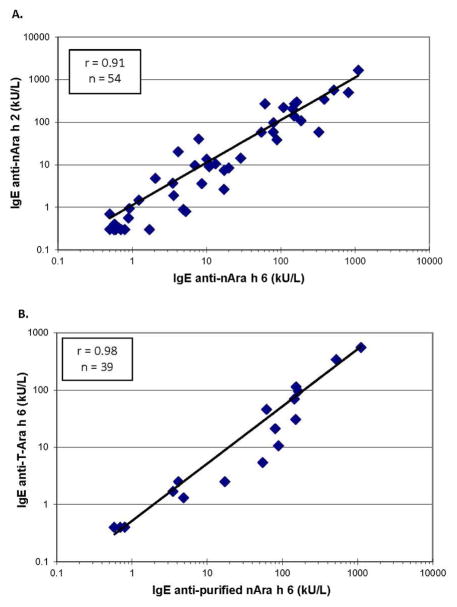
More sera showed IgE binding to nAra h 6 than to nAra h 2 (45 vs. 39) using a chimeric IgE-ELISA; however, the levels of Ara h 2-specific IgE were on average 13 % higher than the levels of Ara h 6-specific IgE. Twenty-five of these patients had higher levels of specific-IgE to one allergen but not to the other (Table 1). For example, patient BR1 had four-fold higher Ara h 2- than Ara h 6-specific IgE while patient BH25 had five-fold higher Ara h 6- than Ara h 2-specific IgE (Table 1). There was a strong quantitative correlation between the levels of specific IgE to nAra h 6 and specific IgE to nAra h 2 (r = 0.91, n = 54, p < 0.001; Figure 3A). The levels of Ara h 6-specific IgE binding to purified nAra h 6 correlated well with the same Ara h 6-specific IgE binding to T-Ara h 6 (r = 0.98, n = 39, p < 0.001; Figure 3B). On average, the IgE binding to the purified nAra h 6 material was 46% higher than that observed interacting with T-Ara h 6.
Table 1.
Ara h 2 and Ara h 6 specific IgE concentrations of sera of peanut allergic patients that were positive to at least one of the two allergens as measured by Chimeric ELISA. Sera with a ratio of Ara h 2-specific IgE: Ara h 6-specific IgE > 2 or <0.5 are highlighted in bold.
| INDOOR ID# | Ara h 2 (kUA/L) | nAra h 6 (kUA/L) |
|---|---|---|
| BH1 | 8.96 | 10.9 |
| BH2 | 223 | 109 |
| BH3 | 9.59 | 7.02 |
| BH4 | 3.61 | 8.7 |
| BH5 | 8.43 | 20 |
| BH6 | 345 | 383 |
| BH7 | 499 | 814 |
| BH8 | 1.03 | 10.8 |
| BH9 | 40.1 | 7.9 |
| BH10 | 0.56 | 0.89 |
| BH11 | <0.4 | 0.7 |
| BH12 | 58.3 | 79.5 |
| BH13 | 108 | 187 |
| BH14 | 1.89 | 3.62 |
| BH15 | 0.93 | 0.92 |
| BH16 | <0.4 | 1.71 |
| BH17 | 13.6 | 10.1 |
| BH18 | 1.05 | 13.3 |
| BH19 | 7.32 | 17.4 |
| BH21 | 0.35 | 0.6 |
| BH22 | 1.47 | 1.23 |
| BH23 | 14.2 | 28.9 |
| BH24 | 4.83 | 2.06 |
| BH25 | 58.3 | 326 |
| BR1 | 270 | 61.7 |
| BR2 | 38.4 | 89.2 |
| BR4 | <0.4 | 0.7 |
| BR5 | 20.2 | 4.17 |
| BR6 | 3.67 | 3.51 |
| BR8 | 0.89 | 4.89 |
| BR11 | <0.4 | 0.6 |
| BR12 | 0.4 | <0.4 |
| BR13 | 568 | 520 |
| BR14 | 0 | 0.8 |
| BR16 | 2.63 | 3.07 |
| BR17 | 58.4 | 6.71 |
| BR18 | 96.8 | 36.1 |
| BR21 | 208 | 145 |
| BR22 | 140 | 151 |
| BR23 | 299 | 164 |
| BR24 | 269 | 93.1 |
| PWH1 | <0.4 | 0.57 |
| PWH3 | 0.8 | 5.31 |
| PWH4 | 0.33 | 0.66 |
| PWH5 | <0.4 | 0.71 |
| PWH6 | 1657 | 1113 |
Figure 3.
Correlation between IgE-binding to purified nAra h 6 and purified nAra h 2 (A) and between purified nAra h 6 and T-Ara h 6 (B). Allergen coated onto microtiter plates was incubated with sera from peanut-allergic patients and bound IgE was detected by biotin-labeled goat anti-human IgE followed by streptavidin-peroxidase. The intensity of the color development of ABTS was measured at 405 nm.
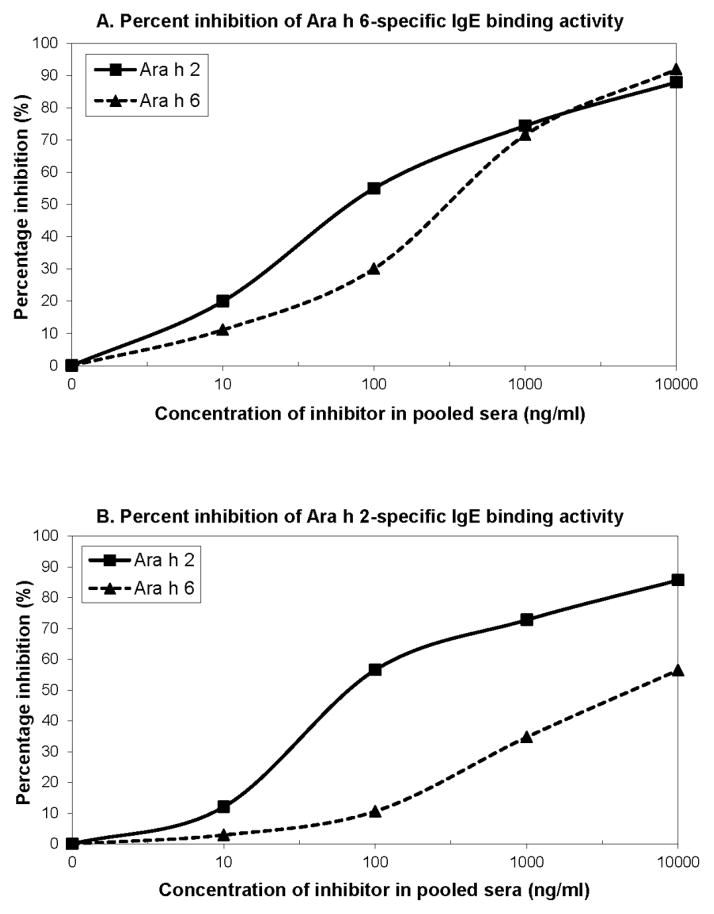
Results obtained in the cross-inhibition assay indicate that nAra h 2 inhibited IgE-allergen binding more effectively than nAra h 6. At 70 ng/ml, Ara h 2 inhibited 50% of IgE-Ara h 2 and IgE-Ara h 6 interactions (Figure 4). However, 600 ng/ml and 5000 ng/ml of Ara h 6 were required to inhibit 50% of IgE binding to Ara h 6 and Ara h 2, respectively.
Figure 4.
Specific IgE cross-inhibition assay with increasing inhibitor allergen concentrations of Ara h 2 and 6. A) Percent inhibition of Ara h 6-specific IgE binding activity. B) Percent inhibition of Ara h 2-specific IgE binding activity.
Biological Activity
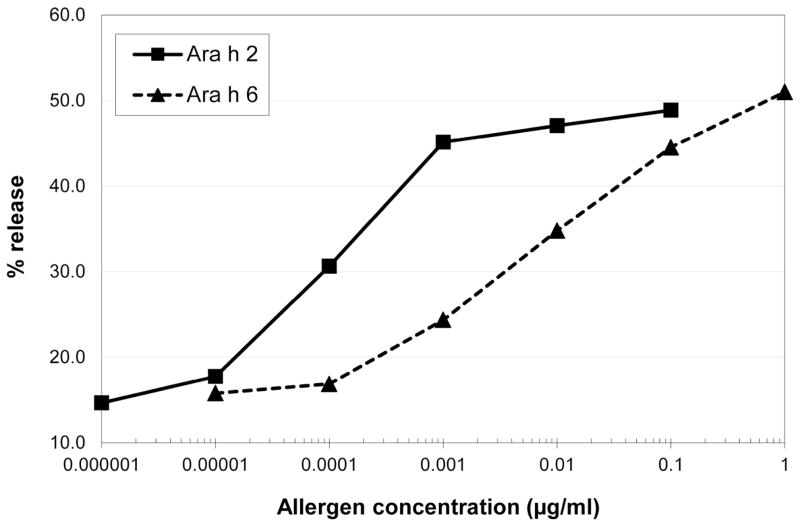
Natural Ara h 6 showed less biological activity than nAra h 2 in the basophil histamine release assay (Fig.5). At a concentration of 0.1 Vg/ml, Ara h 2 stimulated 50% of the maximum histamine release, while 1 Vg/ml of Ara h 6 was required to achieve 50% of maximum histamine release. In general, histamine release was 10% lower for nAra h 6 than nAra h 2 at the same allergen concentration. The dose-response curve of histamine release by nAra h 6 was shifted to the right of the dose response curve for nAra h 2 (Figure 5). On average, histamine release began to plateau at 0.001 μg/ml of Ara h 2 and continued an upward trend even to a concentration of 1 Vg/ml of Ara h 6.
Figure 5.
Average basophil histamine release by nAra h 2 and nAra h 6 (n = 20) using increasing concentrations of allergen.
Discussion
Peanut allergy is on the rise as evidenced by a 1% increase in just over an 11 year period (1997–2008) in the US.(2) Traces of peanut can be found in many processed foods and this poses a danger to peanut allergic patients as miniscule amounts can trigger an allergic reaction.(6) Previous studies have demonstrated significant variability in the protein content and allergenicity of commercially available peanut extracts.(38) There is a critical need for ultra-pure allergens to clarify research results and improve the accuracy of diagnostic tests in an attempt to better understand the mechanisms associated with the induction of allergic diseases. Analysis of single components allows more effective dissection of cross-reactivity among structurally similar allergens and aids in identifying the cross-reactive allergen from the allergen that initially elicited the sensitization. For food allergy studies in particular, molecular diagnostics more effectively reveal patterns of sensitization and cross-reactivity among allergenic homologues across food groups.(39)
Ara h 2 has been extensively studied and established as a major allergen, while Ara h 6 has only recently gained interest in peanut allergy research. Ara h 2 and Ara h 6 belong to the 2S-albumin family that are characterized by having a stable core formed by four disulfide bridges, which makes these proteins resistant to high-temperature processing and proteolytic digestion.(16, 40) The stability of these proteins and their resistance to digestive enzymes in the gut has been identified as major factors contributing to their allergenicity.(15, 41) The sequence and structural similarity between Ara h 6 and Ara h 2 has made it difficult to produce nAra h 6 that is free of nAra h 2.(19) Recombinant Ara h 6 has been produced but conformational differences between the natural and recombinant forms have yielded recombinant Ara h 6 with low IgE reactivity.(20) More recently, a recombinant Ara h 6 produced in Pichia pastoris has shown improved biological potency similar to that of nAra h 6.(42)
Highly purified nAra h 6, has analytically been shown to be free of Ara h 2. Similar to natural Ara h 2, natural Ara h 7 also shares sequence similarity with Ara h 6 and thus may have the potential to co-purify with the Ara h 6. However, natural Ara h 7 has a very low abundance in peanut extracts, which greatly minimizes the risk of contamination.(21)
Purified natural Ara h 6 displayed its own unique immunological activity patterns. While a good correlation was shown between the relative levels of Ara h 2-specific IgE and Ara h 6-specific IgE, sera from twenty-five individuals reacted more strongly to one allergen than the other, as has been reported in previous studies.(16, 32, 43) Interestingly, only eight out of the twenty-five tested sera had higher Ara h 2-specific IgE even though Ara h 2 is the predominantly-recognized allergen by peanut allergic patients.(13)
In our cross-inhibition analyses, Ara h 2 inhibited Ara h 6-specific-IgE binding more effectively than Ara h 6 inhibited Ara h 2-specific IgE binding, and has been reported previously.(16) The inhibitory effect of Ara h 2 may be due to its unique IgE-binding epitopes that are not present in Ara h 6 while Ara h 6 contains IgE-binding epitopes that are also present on Ara h 2.(44) However, we did not achieve complete inhibition of Ara h 6-IgE binding by Ara h 2 even at 10 Vg/ml, which suggests that Ara h 6 could have unique IgE-binding epitope(s). This is supportive of the report by Lehmann et al. who performed an enzyme allergosorbent test and found that maximal inhibition by recombinant Ara h 2 and recombinant Ara h 6 was only 70% and 60%, respectively.(15, 45)
Ara h 2 consistently induced higher levels of histamine release than Ara h 6 in basophil studies. In general, a 10-fold lower concentration of Ara h 2 than Ara h 6 was required to induce the same amount of histamine release. Similarly, Ara h 2 was recently found to be more potent than Ara h 6 using the RBL SX-38 cells assay although the magnitude of the difference was less than we report here with the stripped basophil assay.(46) Ara h 2 also elicited a higher magnitude of mediator release at lower concentrations than Ara h 6.(15) However, as the allergen concentration increased, Ara h 6 induced the release of higher percentages of β-Hexosaminidase than Ara h 2.(47)
While most published data suggest that Ara h 2 is more potent than Ara h 6, there are exceptions. Ara h 6 had a higher seroprevalence (83.3%) than Ara h 2 (72.2%) in our study that involved the testing of 54 sera from peanut-allergic patients. In another study, Ara h 6 was shown to produce a stronger Th2 response than Ara h 2 in peripheral blood mononuclear cells of peanut-allergic children.(48) The depletion of Ara h 2 or Ara h 6 alone from crude peanut extract did not cause a significant reduction in the maximal net level of mediator release from SBX-38 cells, but the removal of both allergens decreased effector activity by approximately 20 %.(49) Previously, Ara h 2 and 6 together were shown to be responsible for over 60% of the effector activity.(50) Furthermore, in vivo studies using murine models showed that desensitization with Ara h 2/6 mixture and crude peanut extract produced comparable results.(51, 52)
Peanut allergy associated with sensitization to storage proteins (Ara h1, Ara h 2, Ara h 3, Ara h 6 and Ara h 7) presents the most serious form of peanut allergy.(53) While Ara h 1 and Ara h 3 are the most abundant storage proteins in peanut (11–31% and 38–76% of protein content in peanut extracts respectively), patients with peanut allergy recognize predominantly Ara h 2 and Ara h 6.(13, 14, 54) Molecular diagnostics have shown that the combined results of IgE reactivity to the two storage proteins Ara h 2 and Ara h 6 yielded the highest diagnostic sensitivity and specificity for detecting clinically evident peanut allergy.(13) By themselves, Ara h 2 and Ara h 6 had high diagnostic sensitivity and specificity compared to Ara h 1 and Ara h 3, but together, the two 2S albumin proteins were able to predict peanut allergy with 98% sensitivity and 85% specificity at a predictive threshold of 0.1 kU/L. The addition of specific IgE tests for other storage proteins such as Ara h 6 or Ara h1 and Ara h 3, will increase specificity and sensitivity of molecular diagnosis, as not all patients with clinical peanut allergy show sensitization to Ara h 2 (32, 53, 55). Thus, purified nAra h 6 should be considered an important diagnostic reagent for both in vitro and in vivo assays, that, together with nAra h 2 will improve diagnostic, immunologic, and biologic assays used in the investigation of peanut allergy, T-cell studies and mouse models of asthma.
Acknowledgments
We are grateful to Dr. Peter Heymann for the peanut allergic patients’ sera. We would also like to thank Dr. Govardus A.H. de Jong for providing the T-Ara h 6. SCD received funding from HAL Allergy for part of the research carried out in this work. This work was also partially supported by a grant, RO1-AI052164, to SCD from the National Institute of Allergy and Infectious Diseases (National Institutes of Health) 89130-898.
Abbreviations Used
- nAra h 6
natural Ara h 6
- nAra h 2
natural Ara h 2
- T-Ara h 6
TNO-produced Ara h 6 used as a reference
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- LC-MS/MS
Liquid chromatography-tandem mass spectrometry
- ELISA
Enzyme-linked immunosorbent assay
- WHO
World Health Organization
- PR-10
Pathogenesis-related class 10 protein
- PVDF
Polyvinylidene difluoride
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
Reference List
- 1.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, Holl JL. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128(1):e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125(6):1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 3.Hourihane JO, Aiken R, Briggs R, Gudgeon LA, Grimshaw KE, DunnGalvin A, Roberts SR. The impact of government advice to pregnant mothers regarding peanut avoidance on the prevalence of peanut allergy in United Kingdom children at school entry. J Allergy Clin Immunol. 2007;119(5):1197–1202. doi: 10.1016/j.jaci.2006.12.670. [DOI] [PubMed] [Google Scholar]
- 4.Du TG, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, Fox AT, Turcanu V, Amir T, Zadik-Mnuhin G, Cohen A, Livne I, Lack G. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122(5):984–991. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 5.Finkelman FD. Peanut allergy and anaphylaxis. Curr Opin Immunol. 2010;22(6):783–788. doi: 10.1016/j.coi.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perry TT, Matsui EC, Conover-Walker MK, Wood RA. Risk of oral food challenges. J Allergy Clin Immunol. 2004;114(5):1164–1168. doi: 10.1016/j.jaci.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 7.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119(4):1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 8.Chapman MD, Pomes A, Breiteneder H, Ferreira F. Nomenclature and structural biology of allergens. J Allergy Clin Immunol. 2007;119(2):414–420. doi: 10.1016/j.jaci.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaou N, Murray C, Belgrave D, Poorafshar M, Simpson A, Custovic A. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol. 2011;127(3):684–685. doi: 10.1016/j.jaci.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Astier C, Morisset M, Roitel O, Codreanu F, Jacquenet S, Franck P, Ogier V, Petit N, Proust B, Moneret-Vautrin DA, Burks AW, Bihain B, Sampson HA, Kanny G. Predictive value of skin prick tests using recombinant allergens for diagnosis of peanut allergy. J Allergy Clin Immunol. 2006;118(1):250–256. doi: 10.1016/j.jaci.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 11.Koppelman SJ, Wensing M, Ertmann M, Knulst AC, Knol EF. Relevance of Ara h 1, Ara h 2 and Ara h 3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h 2 is the most important peanut allergen. Clin Exp Allergy. 2004;34(4):583–590. doi: 10.1111/j.1365-2222.2004.1923.x. [DOI] [PubMed] [Google Scholar]
- 12.Palmer GW, Dibbern DA, Jr, Burks AW, Bannon GA, Bock SA, Porterfield HS, McDermott RA, Dreskin SC. Comparative potency of Ara h 1 and Ara h 2 in immunochemical and functional assays of allergenicity. Clin Immunol. 2005;115(3):302–312. doi: 10.1016/j.clim.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Codreanu F, Collignon O, Roitel O, Thouvenot B, Sauvage C, Vilain AC, Cousin MO, Decoster A, Renaudin JM, Astier C, Monnez JM, Vallois P, Morisset M, Moneret-Vautrin DA, Brulliard M, Ogier V, Castelain MC, Kanny G, Bihain BE, Jacquenet S. A novel immunoassay using recombinant allergens simplifies peanut allergy diagnosis. Int Arch Allergy Immunol. 2011;154(3):216–226. doi: 10.1159/000321108. [DOI] [PubMed] [Google Scholar]
- 14.Flinterman AE, van Hoffen E, den Hartog Jager CF, Koppelman S, Pasmans SG, Hoekstra MO, Bruijnzeel-Koomen CA, Knulst AC, Knol EF. Children with peanut allergy recognize predominantly Ara h2 and Ara h6, which remains stable over time. Clin Exp Allergy. 2007;37(8):1221–1228. doi: 10.1111/j.1365-2222.2007.02764.x. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann K, Schweimer K, Reese G, Randow S, Suhr M, Becker WM, Vieths S, Rosch P. Structure and stability of 2S albumin-type peanut allergens: implications for the severity of peanut allergic reactions. Biochem J. 2006;395(3):463–472. doi: 10.1042/BJ20051728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suhr M, Wicklein D, Lepp U, Becker WM. Isolation and characterization of natural Ara h 6: evidence for a further peanut allergen with putative clinical relevance based on resistance to pepsin digestion and heat. Mol Nutr Food Res. 2004;48(5):390–399. doi: 10.1002/mnfr.200400028. [DOI] [PubMed] [Google Scholar]
- 17.Starkl P, Krishnamurthy D, Szalai K, Felix F, Lukschal A, Oberthuer D, Sampson HA, Swoboda I, Betzel C, Untersmayr E, Jensen-Jarolim E. Heating Affects Structure, Enterocyte Adsorption and Signalling, As Well as Immunogenicity of the Peanut Allergen Ara h 2. Open Allergy J. 2011;4:24–34. doi: 10.2174/1874838401104010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starkl P, Felix F, Krishnamurthy D, Stremnitzer C, Roth-Walter F, Prickett SR, Voskamp AL, Willensdorfer A, Szalai K, Weichselbaumer M, O’Hehir RE, Jensen-Jarolim E. An unfolded variant of the major peanut allergen Ara h 2 with decreased anaphylactic potential. Clin Exp Allergy. 2012;42(12):1801–1812. doi: 10.1111/cea.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller GA, Gosavi RA, Pomes A, Wunschmann S, Moon AF, London RE, Pedersen LC. Ara h 2: crystal structure and IgE binding distinguish two subpopulations of peanut allergic patients by epitope diversity. Allergy. 2011;66(7):878–885. doi: 10.1111/j.1398-9995.2010.02532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleber-Janke T, Crameri R, Appenzeller U, Schlaak M, Becker WM. Selective cloning of peanut allergens, including profilin and 2S albumins, by phage display technology. Int Arch Allergy Immunol. 1999;119(4):265–274. doi: 10.1159/000024203. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt H, Krause S, Gelhaus C, Petersen A, Janssen O, Becker WM. Detection and structural characterization of natural Ara h 7, the third peanut allergen of the 2S albumin family. J Proteome Res. 2010;9(7):3701–3709. doi: 10.1021/pr1002406. [DOI] [PubMed] [Google Scholar]
- 22.van Ree R, Chapman MD, Ferreira F, Vieths S, Bryan D, Cromwell O, Villalba M, Durham SR, Becker WM, Aalbers M, Andre C, Barber D, Cistero BA, Custovic A, Didierlaurent A, Dolman C, Dorpema JW, Di FG, Eberhardt F, Fernandez CE, Fernandez RM, Fiebig H, Focke M, Fotisch K, Gadermaier G, Das RG, Gonzalez ME, Himly M, Kinaciyan T, Knulst AC, Kroon AM, Lepp U, Marco FM, Mari A, Moingeon P, Monsalve R, Neubauer A, Notten S, Ooievaar-de Heer P, Pauli G, Pini C, Purohit A, Quiralte J, Rak S, Raulf-Heimsoth M, San Miguel Moncin MM, Simpson B, Tsay A, Vailes L, Wallner M, Weber B. The CREATE project: development of certified reference materials for allergenic products and validation of methods for their quantification. Allergy. 2008;63(3):310–326. doi: 10.1111/j.1398-9995.2007.01612.x. [DOI] [PubMed] [Google Scholar]
- 23.Chapman MD, Ferreira F, Villalba M, Cromwell O, Bryan D, Becker WM, Fernandez-Rivas M, Durham S, Vieths S, van Ree R. The European Union CREATE project: a model for international standardization of allergy diagnostics and vaccines. J Allergy Clin Immunol. 2008;122(5):882–889. doi: 10.1016/j.jaci.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 24.Hefle SL, Helm RM, Burks AW, Bush RK. Comparison of commercial peanut skin test extracts. J Allergy Clin Immunol. 1995;95(4):837–842. doi: 10.1016/s0091-6749(95)70127-3. [DOI] [PubMed] [Google Scholar]
- 25.Akkerdaas JH, Wensing M, Knulst AC, Krebitz M, Breiteneder H, de VS, Penninks AH, Aalberse RC, Hefle SL, van Ree R. How accurate and safe is the diagnosis of hazelnut allergy by means of commercial skin prick test reagents? Int Arch Allergy Immunol. 2003;132(2):132–140. doi: 10.1159/000073714. [DOI] [PubMed] [Google Scholar]
- 26.Mari A, Ooievaar-de Heer P, Scala E, Giani M, Pirrotta L, Zuidmeer L, Bethell D, van Ree R. Evaluation by double-blind placebo-controlled oral challenge of the clinical relevance of IgE antibodies against plant glycans. Allergy. 2008;63(7):891–896. doi: 10.1111/j.1398-9995.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 27.Valenta R, Duchene M, Ebner C, Valent P, Sillaber C, Deviller P, Ferreira F, Tejkl M, Edelmann H, Kraft D. Profilins constitute a novel family of functional plant pan-allergens. J Exp Med. 1992;175(2):377–385. doi: 10.1084/jem.175.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Ree R. Carbohydrate epitopes and their relevance for the diagnosis and treatment of allergic diseases. Int Arch Allergy Immunol. 2002;129(3):189–197. doi: 10.1159/000066770. [DOI] [PubMed] [Google Scholar]
- 29.Santos A, van Ree R. Profilins: mimickers of allergy or relevant allergens? Int Arch Allergy Immunol. 2011;155(3):191–204. doi: 10.1159/000321178. [DOI] [PubMed] [Google Scholar]
- 30.Mittag D, Akkerdaas J, Ballmer-Weber BK, Vogel L, Wensing M, Becker WM, Koppelman SJ, Knulst AC, Helbling A, Hefle SL, van Ree R, Vieths S. Ara h 8, a Bet v 1-homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J Allergy Clin Immunol. 2004;114(6):1410–1417. doi: 10.1016/j.jaci.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Wensing M, Knulst AC, Piersma S, O’Kane F, Knol EF, Koppelman SJ. Patients with anaphylaxis to pea can have peanut allergy caused by cross-reactive IgE to vicilin (Ara h 1) J Allergy Clin Immunol. 2003;111(2):420–424. doi: 10.1067/mai.2003.61. [DOI] [PubMed] [Google Scholar]
- 32.Asarnoj A, Glaumann S, Elfstrom L, Lilja G, Lidholm J, Nilsson C, Wickman M. Anaphylaxis to Peanut in a Patient Predominantly Sensitized to Ara h 6. Int Arch Allergy Immunol. 2012;159(2):209–212. doi: 10.1159/000336027. [DOI] [PubMed] [Google Scholar]
- 33.Chapman MD, Tsay A, Vailes LD. Home allergen monitoring and control--improving clinical practice and patient benefits. Allergy. 2001;56(7):604–610. doi: 10.1034/j.1398-9995.2001.00049.x. [DOI] [PubMed] [Google Scholar]
- 34.Trombone AP, Tobias KR, Ferriani VP, Schuurman J, Aalberse RC, Smith AM, Chapman MD, Arruda LK. Use of a chimeric ELISA to investigate immunoglobulin E antibody responses to Der p 1 and Der p 2 in mite-allergic patients with asthma, wheezing and/or rhinitis. Clin Exp Allergy. 2002;32(9):1323–1328. doi: 10.1046/j.1365-2745.2002.01455.x. [DOI] [PubMed] [Google Scholar]
- 35.Schuurman J, Perdok GJ, Aalberse RC. Mouse/human chimeric antibodies directed to two nonoverlapping epitopes of the house dust mite allergen, Der p 2. Int Arch Allergy Immunol. 1997;113(1–3):262–263. doi: 10.1159/000237566. [DOI] [PubMed] [Google Scholar]
- 36.Schuurman J, Perdok GJ, Lourens TE, Parren PW, Chapman MD, Aalberse RC. Production of a mouse/human chimeric IgE monoclonal antibody to the house dust mite allergen Der p 2 and its use for the absolute quantification of allergen-specific IgE. J Allergy Clin Immunol. 1997;99(4):545–550. doi: 10.1016/s0091-6749(97)70083-6. [DOI] [PubMed] [Google Scholar]
- 37.Kleine BI, de Heer PG, van der Zee JS, Aalberse RC. The stripped basophil histamine release bioassay as a tool for the detection of allergen-specific IgE in serum. Int Arch Allergy Immunol. 2001;126(4):277–285. doi: 10.1159/000049524. [DOI] [PubMed] [Google Scholar]
- 38.Hourihane JO. Peanut allergy. Pediatr Clin North Am. 2011;58(2):445–58. xi. doi: 10.1016/j.pcl.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Steckelbroeck S, Ballmer-Weber BK, Vieths S. Potential, pitfalls, and prospects of food allergy diagnostics with recombinant allergens or synthetic sequential epitopes. J Allergy Clin Immunol. 2008;121(6):1323–1330. doi: 10.1016/j.jaci.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Koppelman SJ, Hefle SL, Taylor SL, de Jong GA. Digestion of peanut allergens Ara h 1, Ara h 2, Ara h 3, and Ara h 6: a comparative in vitro study and partial characterization of digestion-resistant peptides. Mol Nutr Food Res. 2010;54(12):1711–1721. doi: 10.1002/mnfr.201000011. [DOI] [PubMed] [Google Scholar]
- 41.Moreno FJ, Clemente A. 2S Albumin Storage Proteins: What Makes them Food Allergens? Open Biochem J. 2008;2:16–28. doi: 10.2174/1874091X00802010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhuang Y, Durrani S, Hodges BD, Dreskin SC, Chen X. Expression of recombinant Ara h 6 in Pichia pastoris but not in Escherichia coli preserves allergic effector function and allows assessment of specific mutations. Mol Nutr Food Res. 2012;56(6):986–995. doi: 10.1002/mnfr.201100827. [DOI] [PubMed] [Google Scholar]
- 43.Bernard H, Mondoulet L, Drumare MF, Paty E, Scheinmann P, Thai R, Wal JM. Identification of a new natural Ara h 6 isoform and of its proteolytic product as major allergens in peanut. J Agric Food Chem. 2007;55(23):9663–9669. doi: 10.1021/jf071424g. [DOI] [PubMed] [Google Scholar]
- 44.Koppelman SJ, de Jong GA, Laaper-Ertmann M, Peeters KA, Knulst AC, Hefle SL, Knol EF. Purification and immunoglobulin E-binding properties of peanut allergen Ara h 6: evidence for cross-reactivity with Ara h 2. Clin Exp Allergy. 2005;35(4):490–497. doi: 10.1111/j.1365-2222.2005.02204.x. [DOI] [PubMed] [Google Scholar]
- 45.Bernard H, Drumare MF, Guillon B, Paty E, Scheinmann P, Wal JM. Immunochemical characterisation of structure and allergenicity of peanut 2S albumins using different formats of immunoassays. Anal Bioanal Chem. 2009;395(1):139–146. doi: 10.1007/s00216-009-2842-5. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Wang Q, El-Mezayen R, Zhuang Y, Dreskin SC. Ara h 2 and Ara h 6 Have Similar Allergenic Activity and Are Substantially Redundant. Int Arch Allergy Immunol. 2012;160(3):251–258. doi: 10.1159/000341642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blanc F, Adel-Patient K, Drumare MF, Paty E, Wal JM, Bernard H. Capacity of purified peanut allergens to induce degranulation in a functional in vitro assay: Ara h 2 and Ara h 6 are the most efficient elicitors. Clin Exp Allergy. 2009;39(8):1277–1285. doi: 10.1111/j.1365-2222.2009.03294.x. [DOI] [PubMed] [Google Scholar]
- 48.Flinterman AE, Pasmans SG, den Hartog Jager CF, Hoekstra MO, Bruijnzeel-Koomen CA, Knol EF, van Hoffen E. T cell responses to major peanut allergens in children with and without peanut allergy. Clin Exp Allergy. 2010;40(4):590–597. doi: 10.1111/j.1365-2222.2009.03431.x. [DOI] [PubMed] [Google Scholar]
- 49.Chen X, Zhuang Y, Wang Q, Moutsoglou D, Ruiz G, Yen SE, Dreskin SC. Analysis of the effector activity of Ara h 2 and Ara h 6 by selective depletion from a crude peanut extract. J Immunol Methods. 2011;372(1–2):65–70. doi: 10.1016/j.jim.2011.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Porterfield HS, Murray KS, Schlichting DG, Chen X, Hansen KC, Duncan MW, Dreskin SC. Effector activity of peanut allergens: a critical role for Ara h 2, Ara h 6, and their variants. Clin Exp Allergy. 2009;39(7):1099–1108. doi: 10.1111/j.1365-2222.2009.03273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulis M, Chen X, Lew J, Wang Q, Patel OP, Zhuang Y, Murray KS, Duncan MW, Porterfield HS, Burks W, Dreskin SC. The 2S albumin allergens of Arachis hypogaea, Ara h 2 and Ara h 6, are the major elicitors of anaphylaxis and can effectively desensitize peanut-allergic mice. Clin Exp Allergy. 2012;42(2):326–336. doi: 10.1111/j.1365-2222.2011.03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhuang Y, Dreskin SC. Redefining the major peanut allergens. Immunol Res. 2012 doi: 10.1007/s12026-012-8355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vereda A, van HM, Ahlstedt S, Ibanez MD, Cuesta-Herranz J, van OJ, Wickman M, Sampson HA. Peanut allergy: Clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol. 2011;127(3):603–607. doi: 10.1016/j.jaci.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Koppelman S, Apostolovic D, Warmenhoven H, Verbart D, Taylor S, Isleib T, Maleki S. The content of allergens Ara h1, Ara h2, Ara h3, and Ara h6 in different peanut cultivars commonly consumed in Europe and the USA. Allergy. 2012;67 (Suppl 96):548. [Google Scholar]
- 55.Pedrosa M, Boyano-Martinez T, Garcia-Ara MC, Caballero SQ. Peanut seed storage proteins are responsible for clinical reactivity in Spanish peanut-allergic children. Pediatr Allergy Immunol. 2012:654–659. doi: 10.1111/j.1399-3038.2012.01337.x. [DOI] [PubMed] [Google Scholar]