Abstract
Objectives
Non–small cell lung cancer (NSCLC) is a heterogenous group of disorders that can be subclassified based upon molecular characterization. Anaplastic lymphoma kinase translocation and MET aberrations occur in a subset of NSCLC. Anaplastic lymphoma kinase/ MET have been shown to be inhibited by the small molecule tyrosine kinase inhibitor crizotinib. Recently, crizotinib was shown to decrease testosterone in males. Herein, we describe the effects of crizotinib on multiple hormonal axes.
Materials and Methods
Seven consecutive patients with NSCLC who were receiving crizotinib as part of their standard care were evaluated for hormonal disruptions.
Results
Primary hypogonadism was detected in 4/5 of males, whereas mildly elevated prolactin was observed in 4/7 patients. Hypocalcemia was observed in 3/7 patients. Interestingly, 5/7 patients had elevated levels of insulin-like growth factor-1 (IGF-1) levels, and the remaining 2 individuals had levels that were near the upper limits of the normal range.
Conclusions
Because of cellular cross-talk between MET and IGF-1 signaling, elevated IGF-1 levels induced by crizotinib treatment may have implications for long-term drug efficacy. Furthermore, this finding suggests a potential avenue of therapeutic synergy, namely coordinate inhibition of the MET and IGF-1 signaling pathways. Finally, as crizotinib has been recently approved, it is prudent to check hormone and calcium biomarkers and correct noted deficiencies for improved outcomes and quality of life.
Keywords: anaplastic lymphoma kinase, crizotinib, hypocalcemia, hypogonadism, insulin-like growth factor-1, MET, non–small cell lung carcinoma
Advances in knowledge regarding the molecular basis of cancer have led to the development of a wide range of novel pharmacologic agents for use in oncology. Crizotinib (PF-02341066; Xalkori; Pfizer, La Jolla, CA) is a small molecule receptor tyrosine kinase inhibitor developed to antagonize MET and anaplastic lymphoma kinase (ALK).1 Activating mutations of ALK are thought to result in approximately 4% of non–small cell lung cancer (NSCLC) cases,2–4 and recent evidence has shown crizotinib to be superior to intravenous chemotherapy in NSCLC patients with ALK rearrangements.5 In addition to activity against ALK, crizotinib also has selective activity against MET6 as well as action on ROS,7 suggesting that this agent may find broader use in the treatment of various malignancies. It is important to recognize that inhibition of these signaling pathways to address cancers may corrupt other cellular processes critical for normal cellular function. Likewise, the inhibition of one signal transduction cascade may impact many others through intracellular crosstalk between various signaling networks.
Evidence of potential endocrine disruption by the ALK/ MET inhibitor crizotinib was shown in male patients with NSCLC who developed hypogonadism.8 The mechanism of hypogonadism was unclear, with gonadotropin (follicle stimulating hormone [FSH] and luteinizing hormone [LH]) levels at the upper end of the normal range. In this study, the crizotinib-induced androgen deficiency appeared to be both rapid and reversible. The observed hypogonadism resulting from crizotinib therapy may have resulted from inhibition of its two main targets, ALK and MET, which are both expressed in the testes.9,10 Interestingly, both of these tyrosine kinase receptors are also expressed in the brain, including in the hypothalamus and pituitary.11–14 As such, inhibition of these receptors may result in the disruption of other hormonal axes. To interrogate this supposition, 7 consecutive patients on crizotinib therapy underwent evaluation of pituitary function and calcium homeostasis. The results presented concur with the previous report of testosterone deficiency in male patients; however, the present analysis also suggests the disruption of other hormonal systems, including calcium homeostasis and the growth hormone (GH)/insulin-like growth factor-1 (IGF-1) axis.
MATERIALS AND METHODS
Seven consecutive patients with NSCLC seen in the Oncology Clinic at the University of Chicago who were taking crizotinib underwent hormonal evaluation for pituitary and calcium disruption. The Institutional Review Board had previously approved the research study. On the basis of patient preference, laboratory studies were performed in the Clinical Chemistry Laboratory at the University of Chicago or at a commercial facility near the patient’s home.
RESULTS
Crizotinib Results in Primary Hypogonadism
A previous report described the development of hypogonadism in male patients taking the drug crizotinib.8 Because the targets of crizotinib are expressed in the hypothalamus and pituitary, patients underwent comprehensive pituitary evaluation to evaluate for hypogonadism as well as other hormonal disturbances (Table 1). Of the 5 male patients in the study, 4 had frankly low testosterone (patients 1 and 4 to 6), whereas the fifth patient (patient 2) had a level near the lower end of the normal range. Of these patients, 4 had elevated FSH levels (patients 1, 2, 4, and 5); one had an elevated LH level (patient 2), and one had LH levels near the upper end of normal (patient 1). One patient with high-normal LH (patient 5) was on high-dose dexamethasone, which may have limited his LH response to testosterone deficiency.15,16 One female patient (patient 3) in the cohort was evaluated during the luteal phase of her menstrual cycle (based on menstrual history) and found to have a normal estradiol and FSH level with a high-normal LH level. The menstrual cycle phase of a second female patient was unknown (patient 7); however, she had low estradiol level and LH levels with an FSH that was low or inappropriately normal. Patient 7 was subsequently found to have metastatic involvement of her pituitary gland that may have resulted in central hypogonadism. These results suggest that crizotinib induces testicular dysfunction in male patients. In contrast, the effects in women are less clear.
TABLE 1.
Effects of Crizotinib on Pituitary Function
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | |
|---|---|---|---|---|---|---|---|
| Age | 46 | 70 | 31 | 54 | 39 | 61 | 30 |
| Race/ethnicity | White | African-American | White | White | White | White | White |
| Sex | Male | Male | Female | Male | Male | Male | Female |
| Menstrual status | NA | NA | Premenopausal (luteal phase) | NA | NA | NA | Amenorrheic |
| Mutation | EML4-ALK | EML4-ALK | ROS translocation | EML4-ALK | EML4-ALK | EML4-ALK | EML4-ALK |
| Disease status at evaluation | Metastatic disease with stability on crizotinib | Metastatic disease with stability on crizotinib | Metastatic disease with stability on crizotinib | Metastatic disease with stability on crizotinib | Metastatic disease with progression on crizotinib | Metastatic disease with stability on crizotinib | Metastatic disease (including pituitary involvement) with stability on crizotinib |
| History of chemotherapy | Cisplatin-pemetrexed (2009); bevacizumab (2009–2010); carboplatin-paclitaxel (2010); gemcitabine (2011) | Carboplatin-paclitaxel (2005); cisplatin-pemetrexed (2007); tarceva (2010) | None | Carboplatin-paclitaxel-bevacizumab (2008); pemetrexed-bevacizumab (2008); pemetrexed (2009); erlotinib-bevacizumab (2010); erlotinib (2011) | Cisplatin-pemetrexed (2011) | None | Carboplatin-pemetrexed (2012) |
| Duration of crizotinib | 9 mo | 18 mo | 7 wk | 14 mo | 10 mo | 8 mo | 2 wk |
| Time of testing | 07:55 am | 08:02 am | 08:30 am | 08:24 am | 09:07 am | 02:32 pm | 4:28 am |
| LH (mIU/mL) | 2.0–6.8 | 2.0–6.8 | 1.0–11 | 2.0–6.8 | 1.7–8.6 | 2.0–6.8 | * |
| 6.7 | 7.8 | 10.7 | 4.9 | 7.2 | 2.3 | 0.4 | |
| FSH (mIU/mL) | 1.2–8.0 | 1.2–8.0 | 1.7–7.7 | 1.2–8.0 | 0.8–13.9 | 1.2–8.0 | * |
| 18.3 | 14.3 | 4.5 | 12.6 | 40.8 | 3.3 | 3.5 | |
| Total testosterone (ng/dL) | 240–950 | 180–800 | 240–950 | 280–800 | 180–800 | ||
| 141 | 209 | Not drawn | 151 | 45 | 145 | Not drawn | |
| Free testosterone (pg/mL) | 90–300 | No range for adult males | 90–300 | No range for adult males | |||
| 87 | 107 | Not drawn | 81 | Not drawn | 73 | Not drawn | |
| Estradiol (pg/mL) | 30–400 | 30–400 | |||||
| Not drawn | Not drawn | 92 | Not drawn | Not drawn | Not drawn | 14 | |
| Prolactin (ng/mL) | 4.0–15.2 | 4.0–15.2 | 4.8–23.3 | 4.0–15.2 | 4.0–15.2 | 4.0–15.2 | 4.8–23.3 |
| 16.51 | 6.27 | 29.30 | 11.46 | 16.3 | 6.61 | 15.79/33.02† | |
| ACTH (pg/mL) | <52 | <52 | <52 | <52 | 0–46 | <52 | <52 |
| 18.4 | 65.3 | 33.4 | 10.6 | <5‡ | 9.4 | 29.5 | |
| Cortisol (mcg/dL) | 6.8–26 | 6.8–26 | 6.8–26 | 6.8–26 | 6.0–25.0 | 3.8–14§ | 6.8–26 |
| 12.8 | 16.1 | 23.9 | 9.6 | 0.3‡ | 6.5 | 21.1 | |
| Growth hormone (ng/mL) | 0–4.2 | 0–4.2 | 0–4.2 | 0–4.2 | 0–0.80 | 0–4.2 | 0–4.2 |
| 0.3 | 0.8 | 0.1 | 0.2 | 0.96 | 0.1 | 0.9/4.2 | |
| IGF-1 (ng/mL) | 94–252 | 69–200 | 115–307 | 87–238 | 109–284 | 75–212 | 117–329 |
| 358 | 190 | 414 | 228 | 371 | 254 | 155/437† | |
| TSH (mcU/mL) | 0.3–4.0 | 0.3–4.0 | 0.3–4.0 | 0.3–4.0 | 0.27–4.20 | 0.3–4.0 | 0.3–4.0 |
| 2.57 | 2.64 | 1.81 | 2.25 | 0.72 | 3.29 | 2.92 | |
| Free T4 (ng/dL) | 0.9–1.7 | 0.9–1.7 | 0.9–1.7 | 0.9–1.7 | >0.80–1.80 | 0.9–1.7 | 0.9–1.7 |
| 1.03 | 1.19 | 1.33 | 1.03 | 1.11 | 1.12 | 2.92 |
Normal range dependent on menstrual status (FSH: 3.9–8.3 mIU/mL [follicular phase]; 1.7–7.7 mIU/mL [luteal phase]; LH: 2.0–6.2 mIU/mL [follicular phase]; 1.0–11mIU/mL [luteal phase]).
Laboratories drawn twice: 8 days and 26 days after initiation of therapy.
Patient on supraphysiological doses of dexamethasone.
Laboratories drawn in the afternoon.
Values in bold are outside of the reference range.
ACTH indicates adrenocorticotropic hormone; FSH, follicle stimulating hormone; GH, growth hormone; IGF-1, insulin-like growth factor-1; LH, luteinizing hormone; T4, tetraiodothyronine; TSH, thyroid stimulating hormone.
Crizotinib Therapy is Associated With Elevated IGF-1 Levels
Because ALK and MET are expressed in the hypothalamus and pituitary, a comprehensive analysis of pituitary function was undertaken to ascertain whether other hormonal axes were disrupted by drug treatment. Six of the 7 patients had normal cortisol levels. The seventh patient (#5) had low adrenocorticotropic hormone and cortisol as a result of continued treatment with dexamethasone for brain metastases. Of the 6 patients with normal cortisol levels, one had an elevated adrenocorticotropic hormone level (patient 2). All patients appeared to have normal thyroid function. Four of the 7 patients had mildly elevated prolactin levels (patients 1, 3, 5, and 7), one of whom had a normal prolactin of 11.30 ng/mL on repeat testing (patient 3). Interestingly, 5 of the 7 patients (patients 1, 3, 5, 6, and 7) had elevated IGF-1 levels, whereas the other two had IGF-1 levels near the upper limit of the normal range (patients 2 and 4). In only one of those patients, however, was the GH level mildly elevated (patient 5), whereas a second patient had a GH level at the upper limit of normal (patient 7). Three patients who underwent glucose tolerance tests (patients 1, 3, and 5) showed normal suppression of GH in response to the glucose load (data not shown).
Crizotinib Treatment May Contribute to Hypocalcemia
Five of the 7 patients on crizotinib (patients 1, 2, 4, 6, and 7) had low calcium levels with three of these patients having low calcium levels after correcting for serum albumin (patients 1, 2, and 4) (Table 2). Patients 1 and 2 had elevated parathyroid hormone (PTH) levels, whereas patient 6 had a PTH level that was near the upper limit of normal. The low calcium level in patient 2 may have been partly the result of low calcium intake; a fact supported by his low 24-hour urinary calcium excretion. Calcium supplementation for 2 weeks raised patient 4’s calcium level into the normal range. The low calcium in patient 7 likely reflected a low serum albumin (2.6 g/dL; normal: 3.5 to 5.0 g/dL); however, she also had a low 1,25-di-OH vitamin D level and an elevated alkaline phosphatase (129 U/L; normal: 30 to 120 U/L). The etiology in patients 1 and 6 was not apparent. Although no pattern of vitamin D disturbances could be discerned from the 7 patients, 1 (patient 5) had a low 25-OH vitamin D level, whereas another (patient 3) had a high 1,25-dihydroxy vitamin D. Given the commonality of low 25-OH vitamin D levels in the general population,17 this may not reflect drug effect. The etiology of the elevated 1,25-dihydroxy vitamin D level in patient 3 was not clear.
TABLE 2.
Effects of Crizotinib on Calcium Homeostasis
| Calcium ( mg/dL) | 8.4–10.2 | 8.4–10.2 | 8.4–10.2 | 8.4–10.2 | 8.2–10.4 | 8.4–10.2 | 8.4–10.2 |
| 7.6 | 8.0 | 10.0 | 7.9/8.8* | 8.9 | 8.3 | 8.3 | |
| Albumin (g/dL) | 3.5–5.0 | 3.5–5.0 | 3.5–5.0 | 3.5–5.0 | 3.5–5.2 | 3.5–5.2 | 3.5–5.2 |
| 3.5 | 3.8 | 4.8 | 3.6/3.8 | 3.9 | 3.7 | 2.6 | |
| Corrected Ca (mg/dL)† | 8.4–10.2 | 8.4–10.2 | 8.4–10.2 | 8.4–10.2 | 8.2–10.4 | 8.4–10.2 | 8.4–10.2 |
| 8.0 | 8.2 | 9.4 | 8.2/9.0 | 9.0 | 8.5 | 9.4 | |
| Phosphate (mg/dL) | Not performed | 2.5–4.4 | 2.5–4.4 | 2.5–4.4 | Not performed | Not performed | Not performed |
| 3.1 | 3.2 | 2.4/2.9 | |||||
| PTH (pg/mL) | 15–75 | 15–75 | 15–75 | 15–75 | 15–72 | 15–75 | 15–75 |
| 125 | 102 | 34 | 58 | 37 | 69 | 51 | |
| 25-OH vitamin D (ng/mL) | 10–52 | 10–52 | 10–52 | 10–52 | 30–80 | 10–52 | 10–52 |
| 23 | 27 | 29 | 25 | 24 | 42 | 24 | |
| 1,25-di-OH vitamin D (pg/mL) | 18–64 | 18–64 | 18–64 | 18–64 | 38–88 | 18–78 | |
| 32 | 40 | 98 | 33 | 82 | 14 | ||
| 24 h urine calcium excretion | 100–300 mg/24 h | ||||||
| Not performed | 32 | Not performed | Not performed | Not performed | Not performed | Not performed |
Patient initially hypocalcemic with subsequent improvement on calcium supplementation when endocrine studies drawn.
Calcium levels were corrected to an albumin level of 4.0 g/dL.
Values in bold are outside of the reference range.
PTH indicates parathyroid hormone.
DISCUSSION
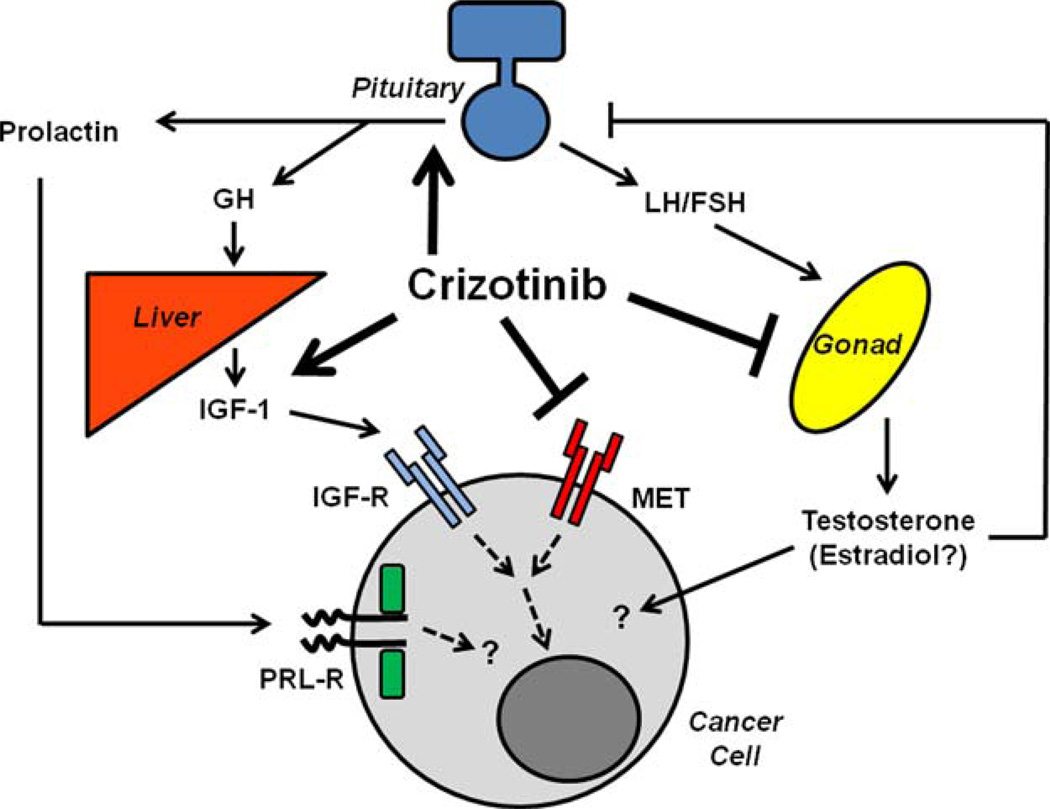
Crizotinib is a small molecule inhibitor of tyrosine kinase receptors that was developed to target MET but has activity against ALK and ROS,1,6,7 and now appears to induce disruptions in multiple hormonal cascades (Fig. 1). Consistent with previous reports,8 the majority of male patients on crizotinib exhibited hypogonadism. The elevated or high-normal levels of gonadotropins in the present study suggest that this finding principally results from testicular dysfunction; it is less likely to be related to the coordinate hyperprolactinemia seen in several patients because prolactin is believed to induce hypogonadism through suppression of gonadatropins. Although ALK is expressed in the testes,10 its function in mediating testosterone production is not known. In contrast, MET is expressed in the Leydig cells, and stimulation of this receptor with hepatocyte growth factor (HGF), the endogenous MET ligand, increases testosterone secretion and inhibits Leydig cell apoptosis.18 Furthermore, HGF signaling regulates Leydig cell expression of extracellular matrix proteins19 as well as Leydig-Sertoli cell interactions.20 Collectively, these findings suggest that crizotinib likely induces hypogonadism through testicular MET disruption. Further, the dual effects on Leydig and Sertoli cells would be predicted to also impair spermatogenesis; however, this was not directly tested in the present cohort. Although the hypogonadism in one of the female patients was likely a consequence of metastatic disease to the pituitary, HGF signaling is also important for ovarian function21; however, whether crizotinib-mediated inhibition of MET affects female reproduction requires further study.
FIGURE 1.
Schematic depiction of crizotinib-mediated endocrine disruption. FSH indicates follicle stimulating hormone; GH, growth hormone; IGF-1, insulin-like growth factor-1; IGF-R, insulin-like growth factor receptor; LH, luteinizing hormone; PRL-R, prolactin receptor.
While the androgen receptor has been shown to promote growth in prostate cancer, its role in other malignancies is still under significant investigation. A recent report suggests that mice lacking the androgen receptor developed more undifferentiated and larger tumors in a model of hepatocellular carcinoma. 22 As such, testosterone deficiency in patients treated with crizotinib or other MET/ALK inhibitors could modulate tumor response, and further research examining whether androgens are antiproliferative or proproliferative in NSCLC are warranted to determine the appropriateness of hormone replacement therapy in hypogonadal patients
Disruption of calcium homeostasis with crizotinib has not been described. Although the present cohort is small, 3 of the 7 patients had episodes of low serum calcium after correction for serum albumin, and 2 had appropriate compensatory increases in PTH. In a single report, HGF treatment resulted in the downregulation of the calcium sensing receptor in rat cardiomyocytes. 23 While it remains unclear whether similar effects occur in the parathyroid gland, antagonism of HGF signaling through MET may increase expression of the calcium sensing receptor, an effect predicted to lower calcium through a decrease in PTH secretion. The fact that 2 of the 3 hypocalcemic patients had elevated PTH levels, however, speaks against such a mechanism. Whether extracellular calcium levels modulate signaling through MET in vivo is not known. In hepatocellular carcinoma cells, disruptions in intracellular calcium levels resulted in the synthesis of an alternative c-Met that was found to be active independent of HGF.24 Thus, alterations in calcium homeostasis could modulate cancer growth through disruption of this pathway. Interestingly, one normocalcemic patient in this cohort had elevated levels of 1,25-dihydroxy vitamin D. In rat interstitial fibroblasts, 1,25-dihdyroxy vitamin D was found to upregulate expression of HGF,25 suggesting that enhanced vitamin D signaling may promote MET signaling and antagonize the effects of crizotinib. Further study is warranted to ascertain whether crizotinib plays a pathogenic role in hypocalcemia and if low calcium levels modulate disease progression.
Perhaps of greatest interest in the present study is the finding that all patients studied had high or high-normal levels of IGF-1. IGF-1 is an important growth factor that has received a significant amount of attention from the pharmaceutical industry as a potential molecular target in cancer; however, results in clinical trials have been disappointing.26–29 Interestingly, levels of IGF-1 appear to correlate with cancer risk in human populations,30 and high levels of IGF-1 are associated with increased mortality in the general population.31 Although IGF-1 can directly promote cell growth, recent evidence suggests that it engages in important cross-talk with the HGF/ MET signaling cascade. In a pancreatic carcinoma cell line, IGF-1 and HGF, signaling through MET, resulted in more than additive increases in cell migration and invasion,32 an effect mediated through expression of urokinase-type plasminogen activator and its receptor.33 Using a colon cancer cell line (CRC cells), this same group showed similar cooperativity between IGF-1-stimulated and HGF-stimulated migration and invasion, while suggesting that c-MET was required for IGF-1-mediated migration and invasion.34 In an interesting study of a rat hepatocellular carcinoma model, IGF-1 was found to be a comitogen for HGF.35 In this model, IGF-1 increased mitogen-activated protein kinase activity resulting in increased c-fos and c-jun expression; however, this did not increase mitogenesis. In contrast, HGF stimulation in this model led to a decrease in mitogenesis; however, when HGF treatment followed pretreatment with IGF-1, mitogenesis was stimulated. These data suggest that the higher than normal levels of IGF-1 may be important in the pathogenesis of these patients, raising several interesting questions. First, is the elevated IGF-1 a primary contributor to cancer development in these patients or is it a compensatory response to MET inhibition? Second, if this is compensation, does the rise in IGF-1 antagonize the effectiveness of MET inhibitors? Third, if elevated IGF-1 antagonizes the effectiveness of MET inhibition, would dual therapy against both these molecular targets result in synergistic therapeutic responses? The posttherapy analysis of these patients limits inferences from being drawn regarding whether IGF-1 increases result as a response to therapy; however, patient 7 did exhibit a rise in IGF-1 from 8 to 26 days posttherapy initiation.
Whether the elevated IGF-1 levels reflect direct effects of crizotinib on hepatic synthesis or indirect effects mediated through GH secretion remain enigmatic. One individual had an elevated GH level, whereas another had a level at the upper end of the normal range. Because GH secretion is episodic, static measurements may not reflect elevated GH levels integrated over time. Interestingly, 4 of the 7 patients with elevated IGF-1 levels also had elevated prolactin levels (patients 1, 3, 5, and 7). As a result of their common embryological origins, a subset of pituitary cells can cosecrete GH and prolactin,36,37 suggesting that crizotinib may be acting centrally to alter endocrine axes. Whether the observed elevations in prolactin could play a role in the physiology of the patient’s cancer remains an open question as prolactin signals through the JAK/ STAT pathway38,39 and mutations in this cascade have been identified in NSCLC.40 Future studies will need to prospectively determine how ALK/MET inhibition alters secretion of growth-promoting hormones as well as the extent to which receptors for these pathways are expressed in patients with NSCLC.
The current report is limited by the small sample size and the fact that all endocrine measurements were taken while the patients were on crizotinib, which prevents definitive assessment of cause-and-effect relationships. In addition, IGF-1 binding proteins were not measured to provide an index of free hormone levels. Furthermore, paraneoplastic evaluation of these patients was not performed, and 5/7 patients had previously received other chemotherapeutic agents, raising the possibility that other factors may have contributed to the endocrine disturbances. As such, prospective studies are required to understand the potential role of crizotinib and other MET inhibitors in promoting IGF-1 secretion and whether augmentation of IGF signaling promotes disease progression. Likewise, additional examination of crizotinib effects on calcium homeostasis is warranted. From a clinical perspective, oncologists should be aware of potential endocrine disrupting effects of crizotinib, and remain vigilant to symptoms of hormonal disturbances. Furthermore, current evidence supports specific monitoring for the development of hypogonadism in male patients and potentially periodic calcium assessment as hypocalcemia may exacerbate the QT prolongation observed with crizotinib therapy.
Increasing understanding of the signal transduction pathways that regulate cell growth, survival, and migration has permitted the development of a vast array of new, targeted anticancer agents. Because of the significant cross-talk between pathways regulating cancer growth and normal physiology, these drugs may provide important new insights into the molecular mechanisms regulating endogenous hormonal signaling. Furthermore, hormonal disruptions induced by anticancer agents may partially counteract the therapeutic efficacy of some of those agents. With recent evidence suggesting that agents such as crizotinib may be superior to standard chemotherapy,5 use of targeted therapies will continue to increase. Therefore, it is incumbent upon prescribing physicians to remain alert to the potential endocrine disrupting effects of their pharmacological armamentarium, especially as these observations may provide insights into potential avenues for developing complimentary therapies.
Acknowledgments
Supported in part by the National Institutes of Health K08-ES019176 to RMS; Guy Geleerd Memorial Foundation to R.S.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 3.Martelli MP, Sozzi G, Hernandez L, et al. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol. 2009;174:661–670. doi: 10.2353/ajpath.2009.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–1733. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 5.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 6.Ou SH. Crizotinib: a novel and first-in-class multitargeted tyrosine kinase inhibitor for the treatment of anaplastic lymphoma kinase rearranged non-small cell lung cancer and beyond. Drug Des Devel Ther. 2011;5:471–485. doi: 10.2147/DDDT.S19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsang Shaw A, Camidge DR, Engelman JA, et al. Clinical activity of crizotinib in advanced non-small cell lung cancer (NSCLC) harboring ROS1 gene rearrangement. J Clin Oncol. 2012;30 (suppl, abstr 7508). [Google Scholar]
- 8.Weickhardt AJ, Rothman MS, Salian-Mehta S, et al. Rapid-onset hypogonadism secondary to crizotinib use in men with metastatic nonsmall cell lung cancer. Cancer. 2012;118:5302–5309. doi: 10.1002/cncr.27450. [DOI] [PubMed] [Google Scholar]
- 9.Depuydt CE, Zalata A, de Potter CR, et al. The receptor encoded by the human C-MET oncogene is expressed in testicular tissue and on human spermatozoa. Mol Hum Reprod. 1996;2:2–8. doi: 10.1093/molehr/2.1.2. [DOI] [PubMed] [Google Scholar]
- 10.Vernersson E, Khoo NK, Henriksson ML, et al. Characterization of the expression of the ALK receptor tyrosine kinase in mice. Gene Expr Patterns. 2006;6:448–461. doi: 10.1016/j.modgep.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Jung W, Castren E, Odenthal M, et al. Expression and functional interaction of hepatocyte growth factor-scatter factor and its receptor c-met in mammalian brain. J Cell Biol. 1994;126:485–494. doi: 10.1083/jcb.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwahara T, Fujimoto J, Wen D, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 13.Giacobini P, Giampietro C, Fioretto M, et al. Hepatocyte growth factor/scatter factor facilitates migration of GN-11 immortalized LHRH neurons. Endocrinology. 2002;143:3306–3315. doi: 10.1210/en.2002-220146. [DOI] [PubMed] [Google Scholar]
- 14.Hou XZ, Liu W, Fan HT, et al. Expression of hepatocyte growth factor and its receptor c-Met in human pituitary adenomas. Neuro Oncol. 2010;12:799–803. doi: 10.1093/neuonc/noq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breen KM, Thackray VG, Hsu T, et al. Stress levels of glucocorticoids inhibit LHbeta-subunit gene expression in gonadotrope cells. Mol Endocrinol. 2012;26:1716–1731. doi: 10.1210/me.2011-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breen KM, Karsch FJ. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology. 2004;145:692–698. doi: 10.1210/en.2003-1114. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell DM, Henao MP, Finkelstein JS, et al. Prevalence and predictors of vitamin d deficiency in healthy adults. Endocr Pract. 2012;18:914–923. doi: 10.4158/EP12072.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Bravo J, Catizone A, Ricci G, et al. Hepatocyte growth factor-modulated rat Leydig cell functions. J Androl. 2007;28:866–874. doi: 10.2164/jandrol.107.002865. [DOI] [PubMed] [Google Scholar]
- 19.Catizone A, Ricci G, Tufano MA, et al. Hepatocyte growth factor (HGF) modulates Leydig cell extracellular matrix components. J Androl. 2010;31:306–313. doi: 10.2164/jandrol.109.007658. [DOI] [PubMed] [Google Scholar]
- 20.Catizone A, Ricci G, Galdieri M. Hepatocyte growth factor modulates Sertoli-Sertoli tight junction dynamics. J Cell Physiol. 2008;216:253–260. doi: 10.1002/jcp.21400. [DOI] [PubMed] [Google Scholar]
- 21.Zachow R, Uzumcu M. The hepatocyte growth factor system as a regulator of female and male gonadal function. J Endocrinol. 2007;195:359–371. doi: 10.1677/JOE-07-0466. [DOI] [PubMed] [Google Scholar]
- 22.Ma WL, Hsu CL, Yeh CC, et al. Hepatic androgen receptor suppresses hepatocellular carcinoma metastasis through modulation of cell migration and anoikis. Hepatology. 2012;56:176–185. doi: 10.1002/hep.25644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan L, Zhu TB, Wang LS, et al. Inhibitory effect of hepatocyte growth factor on cardiomyocytes apoptosis is partly related to reduced calcium sensing receptor expression during a model of simulated ischemia/reperfusion. Mol Biol Rep. 2011;38:2695–2701. doi: 10.1007/s11033-010-0412-8. [DOI] [PubMed] [Google Scholar]
- 24.Dai R, Li J, Fu J, et al. Disturbance of Ca2 + homeostasis converts pro-Met into non-canonical tyrosine kinase p190MetNC in response to endoplasmic reticulum stress in MHCC97 cells. J Biol Chem. 2012;287:14586–14597. doi: 10.1074/jbc.M111.333435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Spataro BC, Yang J, et al. 1,25-dihydroxyvitamin D inhibits renal interstitial myofibroblast activation by inducing hepatocyte growth factor expression. Kidney Int. 2005;68:1500–1510. doi: 10.1111/j.1523-1755.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 26.Dziadziuszko R, Camidge DR, Hirsch FR. The insulin-like growth factor pathway in lung cancer. J Thorac Oncol. 2008;3:815–818. doi: 10.1097/JTO.0b013e31818180f5. [DOI] [PubMed] [Google Scholar]
- 27.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12:159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 28.Fidler MJ, Shersher DD, Borgia JA, et al. Targeting the insulin-like growth factor receptor pathway in lung cancer: problems and pitfalls. Ther Adv Med Oncol. 2012;4:51–60. doi: 10.1177/1758834011427576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camidge DR, Dziadziuszko R, Hirsch FR. The rationale and development of therapeutic insulin-like growth factor axis inhibition for lung and other cancers. Clin Lung Cancer. 2009;10:262–272. doi: 10.3816/CLC.2009.n.037. [DOI] [PubMed] [Google Scholar]
- 30.Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 31.Burgers AM, Biermasz NR, Schoones JW, et al. Meta-analysis and dose-response metaregression: circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab. 2011;96:2912–2920. doi: 10.1210/jc.2011-1377. [DOI] [PubMed] [Google Scholar]
- 32.Bauer TW, Somcio RJ, Fan F, et al. Regulatory role of c-Met in insulin-like growth factor-I receptor-mediated migration and invasion of human pancreatic carcinoma cells. Mol Cancer Ther. 2006;5:1676–1682. doi: 10.1158/1535-7163.MCT-05-0175. [DOI] [PubMed] [Google Scholar]
- 33.Bauer TW, Liu W, Fan F, et al. Targeting of urokinase plasminogen activator receptor in human pancreatic carcinoma cells inhibits c-Met- and insulin-like growth factor-I receptor-mediated migration and invasion and orthotopic tumor growth in mice. Cancer Res. 2005;65:7775–7781. doi: 10.1158/0008-5472.CAN-05-0946. [DOI] [PubMed] [Google Scholar]
- 34.Bauer TW, Fan F, Liu W, et al. Insulinlike growth factor-I-mediated migration and invasion of human colon carcinoma cells requires activation of c-Met and urokinase plasminogen activator receptor. Ann Surg. 2005;241:748–756. doi: 10.1097/01.sla.0000160699.59061.92. discussion 56-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price JA, Kovach SJ, Johnson T, et al. Insulin-like growth factor I is a comitogen for hepatocyte growth factor in a rat model of hepatocellular carcinoma. Hepatology. 2002;36:1089–1097. doi: 10.1053/jhep.2002.36158. [DOI] [PubMed] [Google Scholar]
- 36.Eyal O, Naffaa LN, Elder DA. A case of macroprolactinoma and elevated insulin-like growth factor-I in a young boy. Acta Paediatr. 2005;94:1852–1854. doi: 10.1111/j.1651-2227.2005.tb01869.x. [DOI] [PubMed] [Google Scholar]
- 37.Laron Z. The growth hormone-prolactin relationship: a neglected issue. Pediatr Endocrinol Rev. 2011;9:546–548. [PubMed] [Google Scholar]
- 38.Campbell GS, Argetsinger LS, Ihle JN, et al. Activation of JAK2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc Natl Acad Sci USA. 1994;91:5232–5236. doi: 10.1073/pnas.91.12.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao J, Hughes JP, Auperin B, et al. Interactions among Janus kinases and the prolactin (PRL) receptor in the regulation of a PRL response element. Mol E0ndocrinol. 1996;10:847–856. doi: 10.1210/mend.10.7.8813725. [DOI] [PubMed] [Google Scholar]
- 40.Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell. 2012;150:1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]