Abstract
The universal temperature dependence of metabolic rates has been used to predict how ocean biology will respond to ocean warming. Determining the temperature sensitivity of phytoplankton metabolism and growth is of special importance because this group of organisms is responsible for nearly half of global primary production, sustains most marine food webs, and contributes to regulate the exchange of CO2 between the ocean and the atmosphere. Phytoplankton growth rates increase with temperature under optimal growth conditions in the laboratory, but it is unclear whether the same degree of temperature dependence exists in nature, where resources are often limiting. Here we use concurrent measurements of phytoplankton biomass and carbon fixation rates in polar, temperate and tropical regions to determine the role of temperature and resource supply in controlling the large-scale variability of in situ metabolic rates. We identify a biogeographic pattern in phytoplankton metabolic rates, which increase from the oligotrophic subtropical gyres to temperate regions and then coastal waters. Variability in phytoplankton growth is driven by changes in resource supply and appears to be independent of seawater temperature. The lack of temperature sensitivity of realized phytoplankton growth is consistent with the limited applicability of Arrhenius enzymatic kinetics when substrate concentrations are low. Our results suggest that, due to widespread resource limitation in the ocean, the direct effect of sea surface warming upon phytoplankton growth and productivity may be smaller than anticipated.
Introduction
Temperature governs the metabolism of all organisms. Within favourable thermal ranges, there exists a positive, often exponential, increase in metabolic rate as temperature rises [1], [2]. On-going and predicted ocean warming [3] prompts the question of how phytoplankton photosynthetic activity and growth may respond to increasing sea surface temperatures [4]–[6]. This response will be relevant for the functioning of the Earth system as a whole, because phytoplankton sustain most marine food webs and, being responsible for nearly half of the global primary production [7], contribute to control the exchange of CO2 and other radiatively active gases between the ocean and the atmosphere [8], [9].
Laboratory work with cultures growing under optimal conditions has shown that the maximum growth rates of phytoplankton, and thus their maximum rate of biomass-specific production, increase exponentially with temperature with a Q 10 of approximately 2 within the tolerable temperature range [10]–[13]. However, it is not clear whether phytoplankton assemblages in nature show the same degree of temperature dependence in their realized rates of metabolic activity and growth. An analysis of in vitro oxygen evolution data across the global ocean suggests that phytoplankton photosynthesis does increase with temperature and, furthermore, that the degree of temperature dependence is similar to that predicted by the metabolic theory of ecology [14]. Similarly, an eco-evolutionary model predicts that phytoplankton living in tropical regions sustain faster growth rates than their counterparts living in temperate and polar regions [6]. These studies, however, did not consider the role of resource supply, which is included in applications of the metabolic theory of ecology [1], [15] and limits phytoplankton production and growth in most of the open ocean [16]. Nutrient limitation has been shown to reduce the temperature sensitivity of carbon fixation by phytoplankton [17]–[19]. If resource limitation attenuates the temperature dependence of metabolic rates, different regions where phytoplankton experience different degrees of resource limitation may show contrasting productivity responses to warming, and models that use laboratory-based values of Q 10 are likely to overestimate the direct effects of temperature upon algal growth.
Understanding the variability and controlling factors of phytoplankton growth rates is key to predict the response of ocean biology to external forcings such as climate variability [20]. However, in spite of decades of observation [10], [20]–[23], a general picture of phytoplankton growth variation over broad spatial scales in the ocean remains elusive. Here, we use concurrent data of phytoplankton carbon biomass and photosynthetic carbon fixation, determined in coastal and open-ocean waters of polar, temperate and tropical biomes, to investigate the large-scale variability of phytoplankton metabolic rates, represented by the biomass-specific rate of carbon fixation [10], [24]. Our analysis allows us to characterize biogeographic patterns in phytoplankton growth and to assess the relative role of temperature and resource availability in the control of marine primary productivity.
Materials and Methods
Data acquisition
We compiled data on phytoplankton carbon biomass concentration and primary production rate that were determined concurrently in surface assemblages of coastal and open-ocean regions. Coastal observations (number of sampling visits, n = 26) were conducted throughout the year at a central station (depth = 40 m) in the Ría de Vigo (NW Iberian Peninsula), a highly productive embayment subject to frequent upwelling events, particularly in spring and summer [25], [26]. Open-ocean stations (n = 38) were visited in April-May 1996 and September-October 1996 along the Atlantic Meridional Transect, which crosses temperate, subtropical and tropical regions in the north and south Atlantic Ocean [27]. The data from the AMT stations were grouped according to their latitude into north temperate (35–49°N), south temperate (35–48°S), north oligotrophic (20–31°N), south oligotrophic (10–34°S) and equatorial and Mauritanian upwelling (5°S–20°N). Additional data (n = 8) were obtained from the Soiree (Southern Ocean Iron Release Experiment) study, conducted in open-ocean, polar waters of the Australasian-Pacific Southern Ocean [28]–[30]. To minimise the effects of vertical variability in irradiance upon phytoplankton photosynthetic activity, the present analysis uses only data from surface samples (0–5 m in coastal waters, 0–20 m in open-ocean waters).
Phytoplankton biomass and production
Phytoplankton carbon biomass was estimated from measurements on fixed samples of cell abundance and biovolume, determined with flow cytometry for the picophytoplankton and with an inverted microscope for the nano- and micro-phytoplankton, following protocols that are detailed elsewhere [26], [27], [30]. Biovolume data were transformed into carbon biomass by applying appropriate conversion factors. Primary production rate was determined with the 14C-uptake technique using on-deck incubations which simulated in situ temperature and irradiance conditions [26], [27], [29]. Phytoplankton biomass turnover rates (d−1), equivalent to intrinsic growth rates [10], [24], were calculated by dividing the daily carbon fixation rate by phytoplankton carbon biomass.
Hydrography data
Vertical profiles of temperature and salinity were obtained with a CTD probe attached to a rosette sampling system. Density was calculated from temperature and salinity by using the standard UNESCO equation. The concentration of dissolved nitrate in the euphotic layer was determined by segmented-flow analysis using an automatic analyser and standard colorimetric protocols [26], [27], [31]. The detection limit of this analysis (0.05 µmol L−1) was sufficient to measure nitrate concentration at the base of the euphotic layer, which was always >0.2 µmol L−1. The depth of the euphotic layer was determined from vertical profiles of irradiance obtained with a PAR Li-Cor sensor.
Index of resource supply
We computed a resource supply index (RSI) that takes into account the concentration of nitrate at the base of the euphotic zone (NO3 [1%PAR]), the density difference between the surface and the base of the euphotic zone (Δσt), the depth of the euphotic zone, defined as the 1% PAR level (1%PARz), and the depth of the upper mixed layer (UMLz), defined as the first depth at which σt is 0.125 units higher than the surface value [32]:
| (1) |
The first term in Eq. 1 reflects the fact that nutrient transport to the upper layer increases with increasing nutrient concentration below, but also that this transport becomes progressively more limited as vertical stratification intensifies. The second term in Eq. 1 serves to differentiate conditions in which phytoplankton are confined to a shallow upper mixed layer, relative to the depth of the euphotic zone, and thus experience relatively high average irradiances, from conditions in which intense mixing results in mixed layers whose depth equals or exceeds the penetration of irradiance into the water column, potentially resulting in light limitation of phytoplankton growth. Our index of resource supply is not intended to provide an accurate estimate of local-scale resource availability, but is used here to depict broad differences between regions with widely contrasting hydrographic regimes. RSI was not calculated for the stations sampled in the Soiree study, where, in addition to macronutrients and irradiance, iron was a limiting factor for phytoplankton production and growth [28].
Statistical analyses
Pearson's r was calculated to assess the existence of linear dependence between variables. We applied reduced major-axis regression to determine the linear relationship between log10-transformed variables. Ninety-five percent confidence intervals for the regression parameters were calculated by bootstrapping over cases (2,000 repetitions). The non-parametric Mann-Whitney U test was used to compare means between groups of stations.
Results
Phytoplankton biomass and production
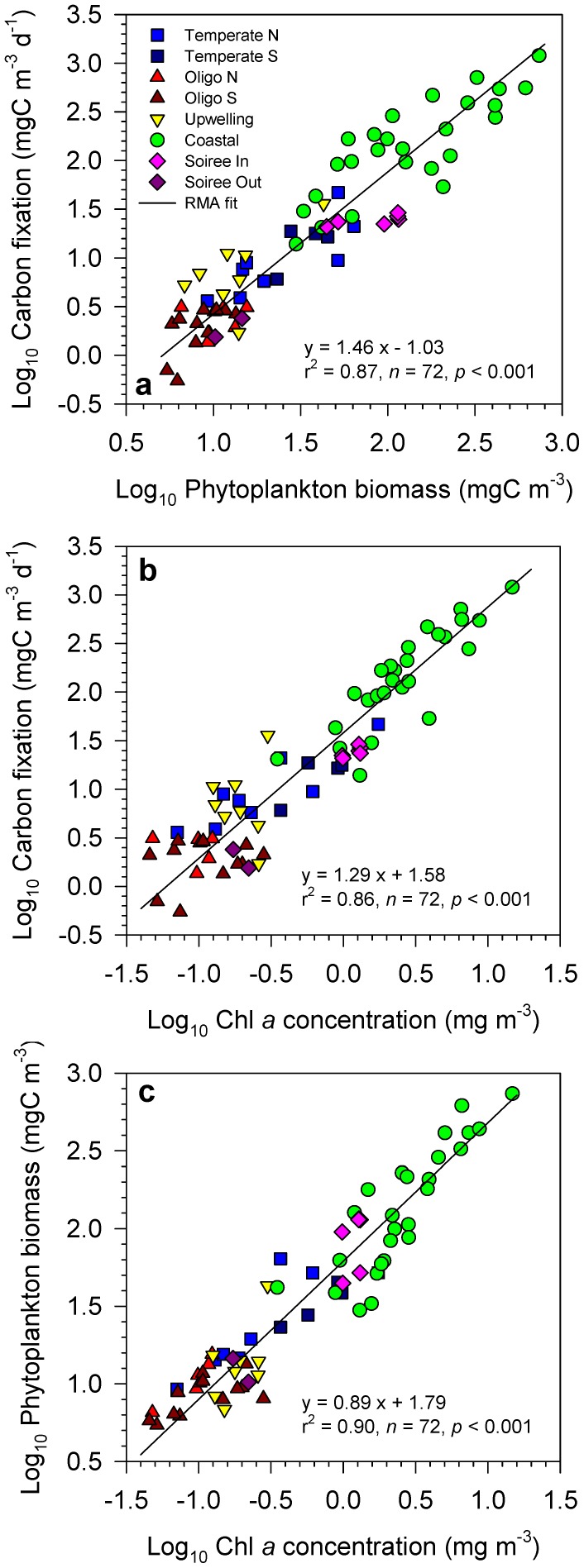
Across all studied regions, which showed widely contrasting hydrographic properties (Table 1), carbon fixation rates covaried closely with phytoplankton standing stocks, represented either by carbon biomass (Fig. 1a) or by chlorophyll a concentration (chl a) (Fig. 1b). The range of variability in phytoplankton production rates was >3 orders of magnitude, compared with approximately 2 orders of magnitude for phytoplankton carbon biomass and chl a. The key feature of these relationships is that in both cases the slope of the fitted line is significantly higher than 1, taking a value of 1.46 (95% confidence interval: 1.35, 1.58) in the production-carbon biomass regression and 1.29 (1.19, 1.39) in the production-chl a regression. This result implies that, as phytoplankton standing stocks increase, primary production rates increase at a faster rate. From oligotrophic regions to coastal waters, mean phytoplankton chl a and biomass increased by a factor of 20–30, whereas primary production increased by a factor of >100 (Table 2).
Table 1. Physical and chemical properties of the studied regions.
| Location | Temp. | NO3 [1%PAR] | Δσt | 1%PARz | UMLz |
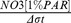
|
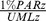
|
| Temperate N (n = 8) | 15.9±3.5 | 6.7±1.6 | 0.6±0.5 | 68±23 | 64±35 | 28±29 | 1.4±0.8 |
| Temperate S (n = 4) | 13.4±4.5 | 9.8±4.2 | 0.4±0.2 | 57±22 | 50±15 | 33±26 | 1.2±0.3 |
| Oligotrophic N (n = 5) | 22.3±2.3 | 1.9±1.5 | 0.8±0.5 | 115±14 | 47±13 | 2.8±1.5 | 2.6±0.8 |
| Oligotrophic S (n = 13) | 24.4±2.9 | 0.9±1.2 | 0.9±0.6 | 126±24 | 59±22 | 1.1±1.5 | 2.6±1.4 |
| Upwelling (n = 8) | 27.3±1.4 | 14.0±2.1 | 3.2±0.6 | 83±13 | 29±12 | 4.4±0.9 | 3.3±1.6 |
| Coastal (n = 26) | 15.3±2.0 | 6.8±3.2 | 0.8±0.6 | 20±4 | 7±4 | 11.5±7.6 | 3.9±2.2 |
| Soiree In (n = 6) | 2.8±0.1 | 23.2±1.1 | 0.02±0.01 | 47±1 | 72±3 | - | - |
| Soiree Out (n = 2) | 2.7±0.0 | 25.4±0.4 | 0.23±0.02 | 74±8 | 69±2 | - | - |
Mean (± standard deviation) values of sea surface temperature (°C, Temp.), nitrate concentration at the base of the euphotic layer (NO3 [1%PAR], µmol L−1), density difference between the surface and the base of the euphotic layer (Δσt, kg m−3), euphotic layer depth (1%PARz, m), and upper mixed layer depth (UMLz, m) for each location. For the regions where the resource supply index was calculated, the mean values of NO3 [1%PAR] divided by Δσt and 1%PARz divided by UMLz are also given. n is the number of measurements conducted at each location. Nitrate concentration data for the Soiree study correspond to 40 m depth [31].
Figure 1. Phytoplankton chlorophyll a, biomass and production.
Scatterplots showing the log-log relationship between (a) primary production and phytoplankton carbon biomass, (b) primary production and chlorophyll a concentration and (c) phytoplankton carbon biomass and chlorophyll a concentration. Different symbols indicate the sampling region as defined in the Methods section. ‘Soiree In’ and ‘Soiree Out’ refer to stations located inside and outside, respectively, the iron-fertilized patch during the Soiree study. Data were fitted to a linear model using reduced major axis regression.
Table 2. Phytoplankton properties at the studied regions.
| Location | Chl a | Phyto C | C:Chl a | P |
| Temperate N (n = 8) | 0.44±0.56 | 30±22 | 99±42 | 13±15 |
| Temperate S (n = 4) | 0.71±0.29 | 34±10 | 50±10 | 15±6 |
| Temperate S (n = 4) | 0.71±0.29 | 34±10 | 50±10 | 15±6 |
| Oligotrophic N (n = 5) | 0.10±0.03 | 11±3 | 117±15 | 2.5±0.8 |
| Oligotrophic S (n = 13) | 0.13±0.07 | 9±2 | 83±31 | 2.1±0.8 |
| Upwelling (n = 8) | 0.20±0.07 | 16±11 | 77±36 | 10±11 |
| Coastal (n = 26) | 3.5±3.2 | 202±187 | 58±28 | 248±271 |
| Soiree In (n = 6) | 1.19±0.16 | 89±33 | 75±25 | 25±3 |
| Soiree Out (n = 2) | 0.20±0.03 | 12±3 | 65±27 | 2.0±0.6 |
Mean (± standard deviation) surface values of chlorophyll a concentration (Chl a, mg m−3), phytoplankton carbon biomass (Phyto C, mgC m−3), phytoplankton carbon to chlorophyll a ratio (C:Chl a), and rate of primary production (P, mgC m−3 d−1) in each location. n is the number of stations visited at each location.
The relationship between chl a and phytoplankton carbon (Fig. 1c) was remarkably robust, considering the uncertainties involved in estimating biomass from biovolume and the fact that the samples were analysed by several independent laboratories. The slope of the log-log regression between phytoplankton carbon and chl a (0.89; 95% CI: 0.83, 0.94) was significantly lower than 1, indicating that the C:Chl a ratio tends to decrease as phytoplankton standing stocks increase from open-ocean oligotrophic to open-ocean temperate and then coastal waters (Table 2). This pattern likely reflects the fact that in temperate and coastal waters phytoplankton are acclimated to lower mean irradiances, which results in increased chl a cellular content.
Temperature and phytoplankton growth
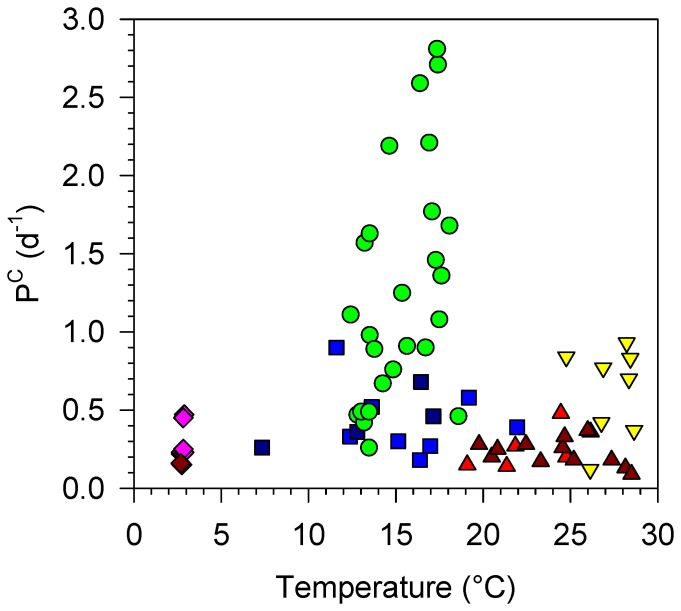
We found no overall relationship between phytoplankton growth rate, represented by the biomass turnover rate (PC), and temperature (Pearson's r = −0.045, p>0.3, n = 72) (Fig. 2). The highest PC values (>1 d−1) were measured in coastal waters, despite the fact that their temperature was 10–15°C lower than that of subtropical and tropical oligotrophic regions (Table 1). Some of the coldest and the warmest waters analysed had similarly low PC values (<0.4 d−1). During the Soiree study, PC inside the iron-fertilised patch took higher values than in surrounding waters with the same temperature. The stations within the Atlantic upwelling region tended to have higher PC values than oligotrophic stations with similar temperatures.
Figure 2. Temperature and phytoplankton growth.
Carbon-specific production rate (PC) plotted against temperature for all stations. Symbols as in Fig. 2.
Resource supply and phytoplankton growth
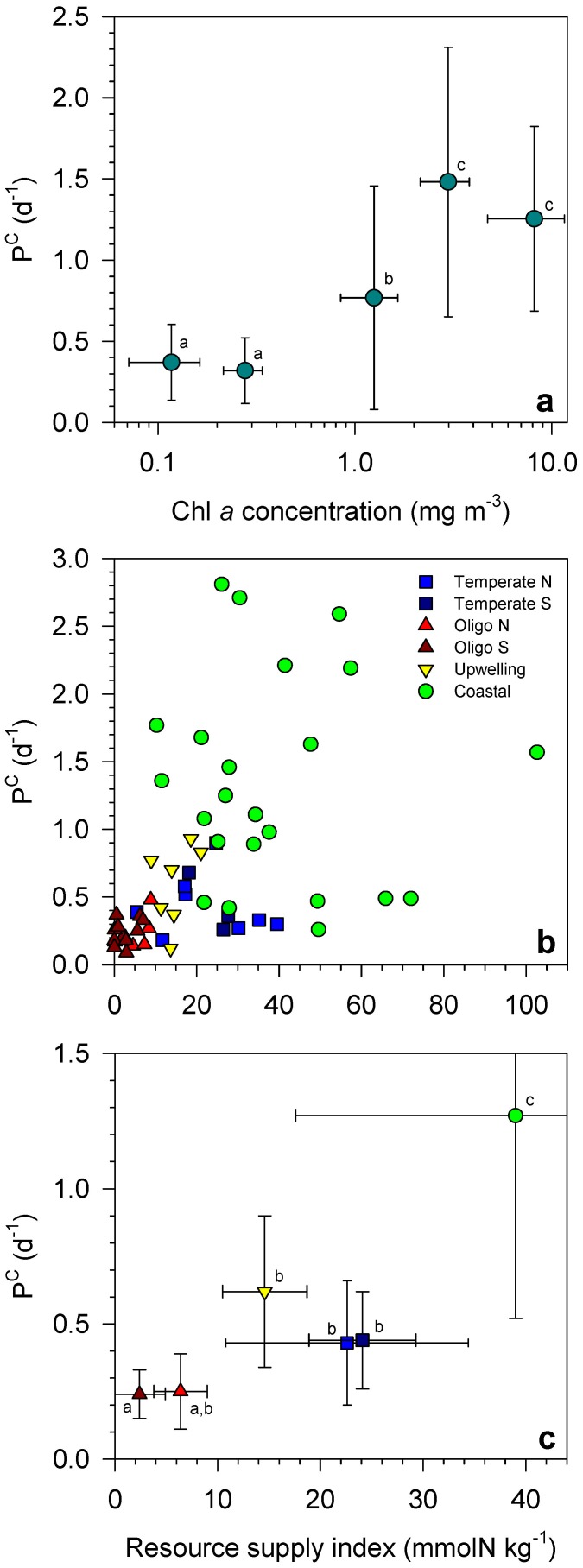
PC increased with chl a (Fig. 3a), suggesting that an enhanced supply of resources, necessary to sustain larger standing stocks, leads to faster phytoplankton growth. Groups of samples with mean chl a above 2 mg m−3 had mean PC values higher than 1.2 d−1, whereas samples with mean chl a below 0.2 mg m−3 showed PC values below 0.4 d−1. These differences were statistically significant (Mann-Whitney's U test, p<0.05). The role of resource supply in controlling phytoplankton biomass turnover was confirmed by the positive correlation between the resource supply index (RSI) and PC (Pearson's r = 0.45, p<0.001, n = 59; Fig. 3b). RSI was calculated as a function of nitrate concentration at the base of the euphotic layer, the density difference between sea surface and the base of the euphotic layer, and the depths of the upper mixed layer and the euphotic zone (see Material and Methods). RSI varied widely, reflecting large across-system differences in nutrient concentration and water column stratification (Table 1). The oligotrophic regions showed low RSI values (<10 mmolN kg−1) (Fig. 3c), mainly due to their low nitrate concentration at the base of the euphotic layer (Table 1). Higher nitrate concentration in the temperate regions resulted in RSI values above 20 mmolN kg−1. The highest RSI values were determined in the Ría de Vigo, where high nitrate concentrations at the base of the euphotic layer co-occur with upper mixed layers that are shallow relative to the depth of the euphotic layer (Table 1). Despite having similar nitrate concentration at the base of the euphotic zone, the temperate regions had lower RSI values than the coastal waters (Fig. 3c), because the former were characterized by deeper mixed layers relative to the depth of the euphotic zone. Finally, RSI was lower in the upwelling region than in the coastal waters of Ría de Vigo, even though deep nitrate concentration was higher in the former, because of the strong thermal stratification, associated with the upwelling, that resulted in a relatively large density gradient (Table 1).
Figure 3. Resources and phytoplankton growth.
Carbon-specific production rate (PC) as a function of (a) chlorophyll a concentration (chl a) and (b, c) resource supply index (RSI). PC data in (a) were grouped into the following chl a ranges: 0–0.2, 0.2–0.5, 0.5–2, 2–5 and >5 mg m−3. Bars indicate ±1 standard deviation. Data groups labelled with different letters in (a) and (c) have significantly different PC values (Mann-Whitney U test, p<0.05). The units of RSI derive from the division of nitrate concentration by seawater density. Note that RSI is not a nutrient concentration, but reflects both nutrient and light availability (see Material and Methods for details).
There was an overall positive relationship between PC and RSI across all studied regions (Fig. 3c). The open-ocean temperate and upwelling regions had faster biomass turnover rates than the oligotrophic regions and in turn the coastal waters showed faster growth rates than all other regions. The oligotrophic stations of the south Atlantic gyre, characterized by the lowest RSI values, showed mean PC values that were significantly lower than those determined in the temperate and upwelling open-ocean regions and in the coastal waters (Mann-Whitney's U test, p<0.05). The differences in PC between coastal waters and all other regions were also statistically significant (Mann-Whitney's U test, p<0.05).
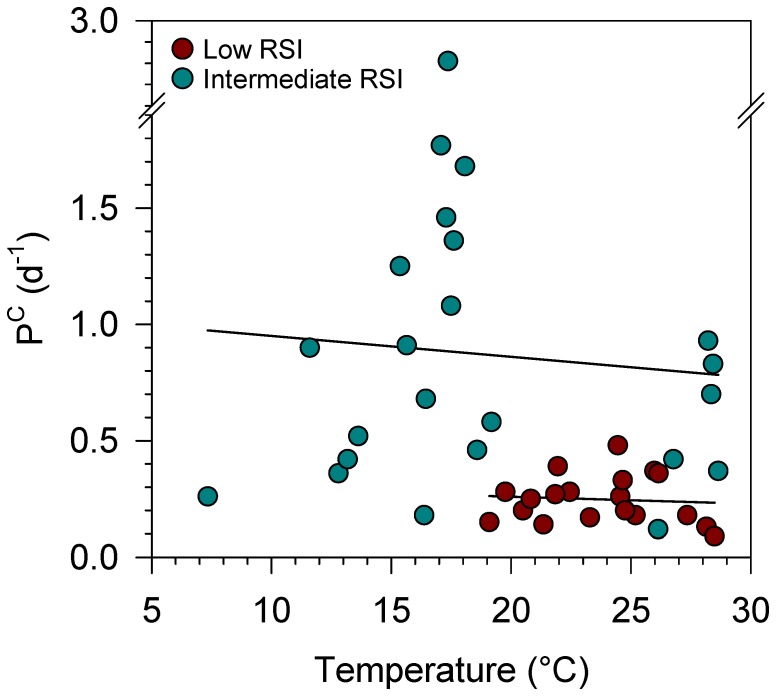
The relationship between temperature and phytoplankton growth rate could be masked due to the fact that most of the variability in PC was due to changes in resource supply. To test this possibility, we plotted temperature against PC in groups of stations which had similar resource supply conditions, as indicated by their RSI values, and covered a temperature range of at least 10°C (Fig. 4). In both low-RSI and intermediate-RSI stations, we found no significant relationship between temperature and PC.
Figure 4. Temperature and phytoplankton growth in samples with different resource availability.
Carbon-specific production rate (PC) is plotted against temperature for oligotrophic stations with low resource supply index (RSI<10 mmolN kg−1) and temperate, upwelling and coastal stations with intermediate RSI (10–20 mmolN kg−1). Both linear regression fits were non-significant (p>0.6).
Discussion
Our results suggest that the large-scale variability of realized mass-specific carbon fixation rates, and hence intrinsic growth rates [33], [34], of marine phytoplankton is not controlled by seawater temperature, but depends primarily on resource supply. Given that under resource-saturated conditions phytoplankton growth does show a clear temperature dependence [10], [13], [35], our observations imply that temperature and resource limitation effects in phytoplankton are not independent. We hypothesise that the lack of effect of temperature upon phytoplankton metabolic rate under resource-limited conditions arises because the applicability of Arrhenius kinetics is limited when substrate concentration are low [36]. The maximum reaction rate (V max) of an enzyme increases with temperature, up to an optimal temperature, due to higher substrate kinetic energy and enhanced collision rate between reactants. However, an increase in temperature also causes reduced ligand binding ability [37], and as a result the Michaelis-Menten constant (K m) of most enzymes also increases with temperature [38]–[40]. Thus, the temperature sensitivities of V max and K m can neutralize each other and give way to Q 10 values near 1 (absence of temperature-dependence) when substrate concentrations are around or below K m [36], [41]. Our hypothesis is consistent with the analysis of Raven and Geider [12], who concluded that nutrient limitation leads to a smaller temperature dependence of growth, compared with nutrient-saturated conditions. Moreover, experimental studies with natural phytoplankton assemblages have shown that under nutrient limitation the temperature dependence of photosynthetic rate is reduced [17] or disappears altogether [18].
The lack of relationship between temperature and phytoplankton growth rate in our data may seem at odds with the results of some experimental studies in open-ocean waters which have shown that phytoplankton metabolism responds to temperature changes [42], [43]. However, these manipulation experiments reflect short-term, transient responses in phytoplankton physiology and therefore do not capture the large acclimation potential of phytoplankton [44]. The present results are also in contrast to the findings of Regaudie-de-Gioux and Duarte [14], who concluded that there is a strong temperature dependence of phytoplankton photosynthesis in the sea. The discrepancy is likely to result from the fact that in their analysis these authors pooled together data obtained throughout the euphotic zone, thus introducing irradiance as a covarying factor. Samples near the base of the euphotic zone, where temperatures are colder than at the surface, will necessarily show lower rates of photosynthesis, not as a result of the lower temperature but as a result of lower irradiance. In addition, photosynthetic rates in the study by Regaudie-de-Gioux and Duarte were normalised by chlorophyll a instead of carbon biomass. However, it is well-established that the cellular content of chlorophyll a increases with decreasing irradiance and increasing nutrient availability [45]–[47]. Therefore, normalising metabolic rates by chlorophyll a results in biased patterns of temperature dependence whenever there is covariation between temperature, nutrient availability and irradiance.
Our observations depict a consistent, broad-scale pattern in phytoplankton growth rates, which increase from oligotrophic gyres to temperate, open-ocean regions and then to coastal, productive waters. This resource-driven pattern is associated with changes in community structure [26], [27], as oligotrophic waters are dominated by small cells (picocyanobacteria and picoeukaryotes) with relatively low biomass turnover rates, whereas the most productive waters are dominated by fast-growing diatoms. Warm temperatures and a tight coupling between zooplankton-mediated nutrient regeneration and phytoplankton uptake could, in principle, result in an absence of nutrient limitation and near-maximal phytoplankton growth rates in the nutrient-impoverished subtropical gyres [20], [21], [23]. This possibility, however, contrasts with the experimental evidence showing physiological stress of the dominant picophotoautotrophs in the oligotrophic Atlantic, which is alleviated upon addition of nitrogen [16], [48], [49]. Our results support the existence of nutrient limitation of both standing stocks and growth rates in the oligotrophic gyres [50], i.e. a situation in which both Liebig's and Blackman's types of limitation apply [16], [51]. This limitation appears to be less acute in temperate open-ocean regions, which are subject to seasonal nutrient injections through vertical mixing, and in the upwelling open-ocean region, where upward water motion enhances the nutrient supply to the euphotic zone. In the coastal, productive waters of Ría de Vigo, high resource supply results in growth rates that are, on average, similar to the maximum growth rates expected given the in situ temperatures. These resource-driven biogeographic patterns imply a predominantly bottom-up control of phytoplankton growth in the ocean.
A previous study [52] has shown that phytoplankton growth rates in coastal, nutrient-rich waters show a degree of temperature dependence comparable to that observed in laboratory cultures, which confirms that temperature sets an upper limit to the maximum growth rates that phytoplankton can achieve under resource-saturated conditions. In addition, nutrient stoichiometry and pigment content have been shown to be temperature-dependent, reflecting intracellular changes in the abundance of macromolecules involved in light harvesting, photochemistry and biosynthesis [12], [53], [54]. Furthermore, inter-species differences in thermal tolerance ranges and optimal temperature for growth [55] contribute to explain changes in species distribution, community structure and associated ecosystem functioning over multiple temporal and spatial scales [56]–[59]. Nevertheless, as far as the large-scale variability in phytoplankton production and growth rates is concerned, temperature seems to be unimportant whereas resource supply plays a crucial role. This result may have important consequences for the modelling of ocean ecosystem responses to climate change. Biogeochemical models typically estimate phytoplankton growth rates as a multiplicative function of nutrient concentration, irradiance, and temperature-dependent maximum growth rate [5], [60], [61]. This formulation assumes that temperature effects on growth are independent of resource supply status, so that for a given condition of resource availability, an increase in temperature will necessarily result in faster phytoplankton growth. In contrast, our observations indicate that resource limitation drastically reduces the temperature dependence of phytoplankton metabolism. Similarly, the growth [62] and extracellular enzymatic activities [63] of natural marine bacterial assemblages show only a very limited response to temperature when resources are in low supply. Hence, the sensitivity of microbial metabolic rates to sea surface warming, particularly in open-ocean, nutrient-limited regions, may be smaller than anticipated [4], [14], [64], [65].
Acknowledgments
We thank D. S. Harbour for the phytoplankton data from the AMT cruises and P. W. Boyd and M. P. Gall for the phytoplankton and productivity data from the SOIREE study. Comments from two anonymous reviewers are also acknowledged.
Funding Statement
This research was funded by the Spanish Ministry of Science and Innovation through research projects ‘Macroecological patterns in marine phytoplankton’ (CTM2008-03699), ‘MALASPINA 2010’ (CSD2008-00077) and ‘Role of turbulence and nutrient supply dynamics on the structure of marine phytoplankton communities (CTM2012-30680). PC and BMC were supported by a Ramón y Cajal-MEC contract. This is contribution 250 of the AMT programme. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85: 1771–1789. [Google Scholar]
- 2. Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293: 2248–2251. [DOI] [PubMed] [Google Scholar]
- 3.Solomon S, Qin D, Manning M, Marquis M, Averyt K, et al, editors (2007) Climate change 2007: the physical science basis. Cambridge (United Kingdom): Cambridge Univ. Press. 996 p.
- 4. Taucher J, Oschlies A (2011) Can we predict the direction of marine primary production change under global warming? Geophys Res Lett 38 10.1029/2010GL045934 [DOI] [Google Scholar]
- 5. Marinov I, Doney SC, Lima ID (2010) Response of ocean phytoplankton community structure to climate change over the 21st century: partitioning the effects of nutrients, temperature and light. Biogeosciences 7: 3941–3959. [Google Scholar]
- 6. Thomas MK, Kremer CT, Klausmeier CA, Litchman E (2012) A global pattern of thermal adaptation in marine phytoplankton. Science 338: 1085–1088. [DOI] [PubMed] [Google Scholar]
- 7. Field C, Behrenfeld M, Randerson J, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281: 237–240. [DOI] [PubMed] [Google Scholar]
- 8. Falkowski PG (2012) The power of plankton. Nature 483: S17–S20. [DOI] [PubMed] [Google Scholar]
- 9. Falkowski P, Barber R, Smetacek V (1998) Biogeochemical controls and feedbacks on ocean primary production. Science 281: 200–206. [DOI] [PubMed] [Google Scholar]
- 10. Eppley RW (1972) Temperature and phytoplankton growth in the sea. Fish Bull 70: 1063–1085. [Google Scholar]
- 11. Goldman JC, Carpenter EJ (1974) A kinetic approach to the effect of temperature on algal growth. Limnol Oceanogr 19: 756–766. [Google Scholar]
- 12. Raven JA, Geider RJ (1988) Temperature and algal growth. New Phytol 110: 441–461. [Google Scholar]
- 13. Bissinger JE, Montagnes DJS, Sharples J, Atkinson D (2008) Predicting marine phytoplankton maximum growth rates from temperature: Improving on the Eppley curve using quantile regression. Limnol Oceanogr 53: 487–493. [Google Scholar]
- 14. Regaudie-de-Gioux A, Duarte CM (2012) Temperature dependence of planktonic metabolism in the ocean. Global Biogeochem Cy 26 10.1029/2010GB003907 [DOI] [Google Scholar]
- 15. Allen AP, Gillooly JF (2009) Towards an integration of ecological stoichiometry and the metabolic theory of ecology to better understand nutrient cycling. Ecol Lett 12: 369–384. [DOI] [PubMed] [Google Scholar]
- 16. Moore CM, Mills MM, Arrigo KR, Berman-Frank I, Bopp L, et al. (2013) Processes and patterns of oceanic nutrient limitation. Nature Geosci 6: 701–710. [Google Scholar]
- 17. Staehr PA, Sand-Jensen K (2006) Seasonal changes in temperature and nutrient control of photosynthesis, respiration and growth of natural phytoplankton communities. Freshwater Biol 51: 249–262. [Google Scholar]
- 18. O'Connor MI, Piehler MF, Leech DM, Anton A, Bruno JF (2009) Warming and resource availability shift food web structure and metabolism. PLoS Biol 7: e1000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tadonléké R (2010) Evidence of warming effects on phytoplankton productivity rates and their dependence on eutrophication status. Limnol Oceanogr 55: 973–982. [Google Scholar]
- 20. Laws EA (2013) Evaluation of in situ phytoplankton growth rates: a synthesis of data from varied approaches. Ann Rev Mar Sci 5: 247–268. [DOI] [PubMed] [Google Scholar]
- 21. Goldman JC, McCarthy JJ, Peavey DG (1979) Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature 279: 210–215. [Google Scholar]
- 22. Furnas MJ (1990) In situ growth rates of marine phytoplankton: Approaches to measurement, community and species growth rates. J Plankton Res 12: 1117–1151. [Google Scholar]
- 23. Banse K (1995) Zooplankton - pivotal role in the control of ocean production. ICES J Mar Sci 52: 265–277. [Google Scholar]
- 24. Kirchman DL (2002) Calculating microbial growth rates from data on production and standing stocks. Mar Ecol Prog Ser 233: 303–306. [Google Scholar]
- 25. Nogueira E, Pérez FF, Ríos AF (1997) Seasonal patterns and long-term trends in an estuarine upwelling ecosystem (Ría de Vigo, NW Spain). Est Coast Shelf Sci 44: 285–300. [Google Scholar]
- 26. Cermeño P, Marañón E, Perez V, Serret P, Fernández E, et al. (2006) Phytoplankton size structure and primary production in a highly dynamic coastal ecosystem (Ria de Vigo, NW-Spain): Seasonal and short-time scale variability. Est Coast Shelf Sci 67: 251–266. [Google Scholar]
- 27. Marañón E, Holligan PM, Varela M, Mouriño B, Bale AJ (2000) Basin-scale variability of phytoplankton biomass, production and growth in the Atlantic Ocean. Deep-Sea Res I 47: 825–857. [Google Scholar]
- 28. Boyd PW, Watson AJ, Law CS, Abraham ER, Trull T, et al. (2000) A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization. Nature 407: 695–702. [DOI] [PubMed] [Google Scholar]
- 29. Gall MP, Strzepek R, Maldonado M, Boyd PW (2001) Phytoplankton processes. Part 2: Rates of primary production and factors controlling algal growth during the Southern Ocean Iron RElease Experiment (SOIREE). Deep-Sea Res II 48: 2571–2590. [Google Scholar]
- 30. Gall MP, Boyd PW, Hall J, Safi KA, Chang H (2001) Phytoplankton processes. Part 1: Community structure during the Southern Ocean Iron RElease Experiment (SOIREE). Deep-Sea Res II 48: 2551–2570. [Google Scholar]
- 31. Frew R, Bowie A, Croot P, Pickmere S (2001) Macronutrient and trace-metal geochemistry of an in situ iron-induced Southern Ocean bloom. Deep-Sea Res II 48: 2467–2481. [Google Scholar]
- 32. Kara AB, Rochford PA, Hurlburt HE (2000) An optimal definition for ocean mixed layer depth. J Geophys Res-Oceans 105: 16803–16821. [Google Scholar]
- 33. Fenchel T (1974) Intrinsic rate of natural increase: the relationship with body size. Oecologia 14: 317–326. [DOI] [PubMed] [Google Scholar]
- 34. Marañón E, Cermeño P, López-Sandoval DC, Rodríguez-Ramos T, Sobrino C, et al. (2013) Unimodal size scaling of phytoplankton growth and the size dependence of nutrient uptake and use. Ecol Lett 16: 371–379. [DOI] [PubMed] [Google Scholar]
- 35. Montagnes DJS, Franklin DJ (2001) Effect of temperature on diatom volume, growth rate, and carbon and nitrogen content: Reconsidering some paradigms. Limnol Oceanogr 46: 2008–2018. [Google Scholar]
- 36. Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440: 165–173. [DOI] [PubMed] [Google Scholar]
- 37. Somero GN (1995) Proteins and temperature. Ann Rev Physiol 57: 43–68. [DOI] [PubMed] [Google Scholar]
- 38.Berry JA, Raison JK (1981) Responses of macrophytes to temperature. In: Lange OL, Nobel PS, Osmond CB, Ziegler H, editors. Physiological Plant Ecology I: Springer Berlin Heidelberg. pp. 277–338.
- 39. Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8: 343–351. [DOI] [PubMed] [Google Scholar]
- 40. Griffith SM, Brewer TG, Steiner JJ (2001) Thermal dependence of the apparent Km of glutathione reductase from three wetland grasses and maize. Ann Bot 87: 599–603. [Google Scholar]
- 41. Davidson EA, Janssens IA, Luo Y (2006) On the variability of respiration in terrestrial ecosystems: moving beyond Q10 . Glob Change Biol 12: 154–164. [Google Scholar]
- 42. Reay DS, Priddle J, Nedwell DB, Whitehouse MJ, Ellis-Evans JC, et al. (2001) Regulation by low temperature of phytoplankton growth and nutrient uptake in the Southern Ocean. Mar Ecol Prog Ser 219: 51–64. [Google Scholar]
- 43. Li WKW (1985) Photosynthetic response to temperature of marine phytoplankton along a latitudinal gradient (16°N to 74°N). Deep-Sea Res A 32: 1381–1391. [Google Scholar]
- 44. Staehr PA, Birkeland MJ (2006) Temperature acclimation of growth, photosynthesis and respiration in two mesophilic phytoplankton species. Phycologia 45: 648–656. [Google Scholar]
- 45. Geider RJ (1987) Light and temperature dependence of the carbon to chlorophyll a ratio in microalgae and cyanobacteria: implications for physiology and growth of phytoplankton. New Phytol 106: 1–34. [Google Scholar]
- 46. Veldhuis MJW, Kraay GW (2004) Phytoplankton in the subtropical Atlantic Ocean: towards a better assessment of biomass and composition. Deep-Sea Res I 51: 507–530. [Google Scholar]
- 47. Behrenfeld MJ, Boss E, Siegel DA, Shea DM (2005) Carbon-based ocean productivity and phytoplankton physiology from space. Glob Biogeochem Cy 19 10.1029/2004GB002299 [DOI] [Google Scholar]
- 48. Moore CM, Mills MM, Langlois R, Milne A, Achterberg EP, et al. (2008) Relative influence of nitrogen and phosphorus availability on phytoplankton physiology and productivity in the oligotrophic sub-tropical North Atlantic Ocean. Limnol Oceanogr 53: 291–305. [Google Scholar]
- 49. Davey M, Tarran GA, Mills MM, Ridame C, Geider RJ, et al. (2008) Nutrient limitation of picophytoplankton photosynthesis and growth in the tropical North Atlantic. Limnol Oceanogr 53: 1722–1733. [Google Scholar]
- 50. Marañón E (2005) Phytoplankton growth rates in the Atlantic subtropical gyres. Limnol Oceanogr 50: 299–310. [Google Scholar]
- 51.Cullen J, Yang X, MacIntyre HL (1992) Nutrient limitation and marine photosynthesis. In: Falkowski PG, editor. Primary Productivity and Biogeochemical Cycles in the Sea. New York and London: Plenum Press. pp. 69–88.
- 52. Banse K (1991) Rates of phytoplankton cell division in the field and in iron enrichment experiments. Limnol Oceanogr 36: 1886–1898. [Google Scholar]
- 53. Rhee G-Y, Gotham IJ (1981) The effect of environmental factors on phytoplankton growth: temperature and the interactions of temperature with nutrient limitation. Limnol Oceanogr 26: 635–648. [Google Scholar]
- 54. Toseland A, Daines SJ, Clark JR, Kirkham A, Strauss J, et al. (2013) The impact of temperature on marine phytoplankton resource allocation and metabolism. Nature Clim Change 3: 979–984. [Google Scholar]
- 55. Boyd PW, Rynearson TA, Armstrong EA, Fu F, Hayashi K, et al. (2013) Marine phytoplankton temperature versus growth responses from polar to tropical waters – outcome of a scientific community-wide study. PLOS ONE 8: e63091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moisan JR, Moisan TA, Abbott MR (2002) Modelling the effect of temperature on the maximum growth rates of phytoplankton populations. Ecol Model 153: 197–215. [Google Scholar]
- 57. Boyd PW, Strzepek R, Fu F, Hutchins DA (2010) Environmental control of open-ocean phytoplankton groups: Now and in the future. Limnol Oceanogr 55: 1353–1376. [Google Scholar]
- 58. Litchman E, Edwards KF, Klausmeier CA, Thomas MK (2012) Phytoplankton niches, traits and eco-evolutionary responses to global environmental change. Mar Ecol Prog Ser 470: 235–248. [Google Scholar]
- 59. Flombaum P, Gallegos JL, Gordillo RA, Rincón J, Zabala LL, et al. (2013) Present and future global distributions of the marine cyanobacteria Prochlorococcus and Synechococcus . Proc Natl Acad Sci USA 110: 9824–9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Doney SC, Glover DM, Najjar RG (1996) A new coupled, one-dimensional biological-physical model for the upper ocean: Applications to the JGOFS Bermuda Atlantic Time-series Study (BATS) site. Deep-Sea Res II 43: 591–624. [Google Scholar]
- 61. Schmittner A, Oschlies A, Giraud X, Eby M, Simmons HL (2005) A global model of the marine ecosystem for long-term simulations: Sensitivity to ocean mixing, buoyancy forcing, particle sinking, and dissolved organic matter cycling. Glob Biogeochem Cy 19 10.1029/2004GB002283 [DOI] [Google Scholar]
- 62. Degerman R, Dinasquet J, Riemann L, Sjostedt de Luna S, Andersson A (2013) Effect of resource availability on bacterial community responses to increased temperature. Aq Microb Ecol 68: 131–142. [Google Scholar]
- 63. Wohlers-Zollner J, Breithaupt P, Walther K, Jurgens K, Riebesell U (2012) Temperature and nutrient stoichiometry interactively modulate organic matter cycling in a pelagic algal-bacterial community. Limnol Oceanogr 2011: 599–610. [Google Scholar]
- 64. López-Urrutia A, San Martin E, Harris RP, Irigoien X (2006) Scaling the metabolic balance of the oceans. Proc Natl Acad Sci USA 103: 8739–8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yvon-Durocher G, Jones JI, Trimmer M, Woodward G, Montoya JM (2010) Warming alters the metabolic balance of ecosystems. P Roy Soc Lond B Bio 365: 2117–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]