Abstract
Chemokines were initially identified as bioactive substances, which control the trafficking of inflammatory cells including granulocytes and monocytes/macrophages. Moreover, chemokines have profound impacts on other types of cells associated with inflammatory responses, such as endothelial cells and fibroblasts. These observations would implicate chemokines as master regulators in various inflammatory responses. Subsequent studies have further revealed that chemokines can regulate the movement of a wide variety of immune cells including lymphocytes, natural killer cells, and dendritic cells in both physiological and pathological conditions. These features endow chemokines with crucial roles in immune responses. Furthermore, increasing evidence points to the vital effects of several chemokines on the proliferative and invasive properties of cancer cells. It is widely acknowledged that cancer develops and progresses to invade and metastasize in continuous interaction with noncancerous cells present in cancer tissues, such as macrophages, lymphocytes, fibroblasts, and endothelial cells. The capacity of chemokines to regulate both cancerous and noncancerous cells highlights their crucial roles in cancer development and progression. Here, we will discuss the roles of chemokines in carcinogenesis and the possibility of chemokine targeting therapy for the treatment of cancer.
1. Introduction
Chemokines are heparin-binding proteins with 4 cysteine residues in the conserved positions [1]. Two intermolecular disulfide bonds are formed between the first and third cysteines and between the second and fourth cysteines. These bonds lead to the formation of triple-stranded β-sheet structures, while the carboxyl-terminal region forms a α-helix form [2]. This accounts for their similar three-dimensional structure despite their low overall sequence similarities. Chemokines exert their biological activities by binding their corresponding receptors, which belong to G-protein coupled receptor (GPCR) with 7-span transmembrane portions [1]. Thus, the target cell specificity of each chemokine is determined by the expression pattern of its cognate receptor (Table 1). Moreover, chemokines can bind to proteoglycans and glycosaminoglycans with a high avidity, because the carboxyl-terminal region is capable of binding heparin. Consequently, most chemokines are produced as secretory proteins, but upon their secretion, they are immobilized on endothelium cells and/or in extracellular matrix by interacting with proteoglycans and glycosaminoglycans [2]. The immobilization facilitates the generation of a concentration gradient, which is important for inducing the target cells to migrate in a directed way.
Table 1.
The human chemokine system.
| Chemokine receptor | Chemokines | Receptor expression in | ||
|---|---|---|---|---|
| Leukocytes | Epithelium | Endothelium | ||
| CXCR1 | CXCL6, 8 | PMN | + | − |
| CXCR2 | CXCL1, 2, 3, 5, 6, 7, 8 | PMN | + | + |
| CXCR3 | CXCL4, 9, 10, 11 | Th1, NK | − | + |
| CXCR4 | CXCL12 | Widespread | + | + |
| CXCR5 | CXCL13 | B | − | − |
| CXCR6 | CXCL16 | Activated T | + | − |
| CXCR7 (ACKR3) | CXCL12, CXCL11 | Widespread | + | + |
| Unknown | CXCL14 (acts on monocytes) | |||
| CCR1 | CCL3, 4, 5, 7, 14, 15, 16, 23 | Mo, Mϕ, iDC, NK | + | + |
| CCR2 | CCL2, 7, 8, 12, 13 | Mo, Mϕ, iDC, NK activated T, B |
+ | + |
| CCR3 | CCL5, 7, 11, 13, 15, 24, 26, 28 | Eo, Ba, Th2 | − | + |
| CCR4 | CCL2, 3, 5, 17, 22 | iDC, Th2, NK, T, Mϕ | − | − |
| CCR5 | CCL3, 4, 5, 8 | Mo, Mϕ, NK, Th1 activated T |
+ | − |
| CCR6 | CCL20 | iDC, activated T, B | + | − |
| CCR7 | CCL19, 21 | mDC, Mϕ, naïve T activated T |
+ | − |
| CCR8 | CCL1, 4, 17 | Mo, iDC, Th2, Treg | − | − |
| CCR9 | CCL25 | T | + | − |
| CCR10 | CCL27, 28 | Activated T, Treg | + | − |
| Unknown | CCL18 (acts on mDC and naïve T) | |||
| CX3CR1 | CX3CL1 | Mo, iDC, NK, Th1 | + | − |
| XCR1 | XCL1, 2 | T, NK | − | − |
| Miscellaneous | Scavenger receptors for chemokines | |||
| Duffy antigen (ACKR1) | CCL2, 5, 11, 13, 14 | |||
| CXCL1, 2, 3, 7, 8 | ||||
| D6 (ACKR2) | CCL2, 3, 4, 5, 7, 8, 12 | |||
| CCL13, 14, 17, 22 | ||||
| CCRRL1 (ACKR4) | CCL19, CCL21, CCL25 | |||
Leukocyte anonyms are as follows. Ba: basophil, Eo: eosinophil, iDC: immature dendritic cell, mDC: mature dendritic cell, Mo: monocyte, Mϕ: macrophage, NK: natural killer cell, Th1: type I helper T cell, Th2: type II helper T cell, and Treg: regulatory T cell.
Chemokines are structurally divided into 4 subgroups, namely, CXC, CC, CX3C, and C [1]. The first 2 cysteines are separated by 1 and 3 amino acids in CXC and CX3C chemokines, respectively, while the first 2 cysteines are adjacent in CC chemokines. The C chemokines lacks the second and fourth cysteines [1]. The CXC chemokines are further grouped based on the presence or the absence of a 3-amino acid sequence, glutamic acid-leucine-arginine (the ELR motif), immediately preceding the CXC sequence [3]. Chemokines can be functionally classified as inflammatory, homeostatic, or both, based on their expression patterns [4]. Various types of inflammatory stimuli induce abundantly the expression of inflammatory chemokines to induce the infiltration of inflammatory cells such as granulocytes and monocytes/macrophages. Representative inflammatory chemokines are CXC chemokines with ELR motif and CCL2. On the contrary, homeostatic chemokines are expressed constitutively in specific tissues or cells. They have a crucial role in organogenesis of various organs including lymph nodes, arising from their key roles in stem cell migration. Moreover, most homeostatic chemokines can control the movement of lymphocytes and dendritic cells and eventually adaptive immunity.
The human and mouse genomes contain over 44 and 38 different chemokine genes, respectively [5]. There is a difference in gene numbers with some ambiguities of orthologous relationship between the human and mouse chemokine family. These observations would indicate species-specific expansions and contractions in chemokine genes, resulting from their rapid evolution. A prominent difference is found in one major chemokine, CXCL8, and its receptors, CXCR1 and CXCR2. Mice and rats do not possess a homolog of the CXCL8/IL-8 gene, which is present in other species including humans, rabbits, cats, and dogs [5]. Moreover, the CXCR1 and CXCR2 genes encode functional receptor proteins in humans, whereas there still remains a question on the presence of functional CXCR1 gene in mice or rats [6]. Furthermore, humans and mice exhibit different expression patterns also in other chemokine receptors such as CCR1 [7]. These observations should be taken into consideration when the findings obtained with mouse models are extrapolated into human conditions.
Here, we will review the potential roles of chemokines in tumor development and progression by focusing on their effects on noncancerous and cancerous cells. We will further discuss the potential of chemokine targeting therapy for cancer treatment.
2. Effects on Noncancerous Cells
2.1. Leukocytes
Since the first description by Virchow more than a century ago, it is widely acknowledged that leukocytes are present in both the tumor areas and the tumor-supporting stroma [8]. Moreover, leukocytes might account for up to 50% of the tumor mass, the most predominant subset being macrophages. Tumor-associated macrophages (TAMs) are derived mostly from circulating monocytes which are attracted into tumor sites by locally produced chemotactic factors, such as CCL2, CCL5, CCL7, CCL8, and CXCL12, and macrophage colony stimulating factor (M-CSF) [8]. Among these chemotactic factors, CCL2 is presumed to play an important role in TAM recruitment [8, 9]. Repeated dextran sodium sulfate (DSS) solution ingestion causes the development of multiple colonic tumors in mice, which received a prior administration of azoxymethane (AOM). The resultant colonic tumors contain a large number of monocytes/macrophages expressing cyclooxygenase (COX)-2, an enzyme crucially involved in colon carcinogenesis [10]. Abundant CCL2 is detected in colon tissues, and CCL2 blockade reduces the infiltration of CCR2-positive COX-2 expressing monocytes/macrophages and eventually colonic tumor development and progression [10].
TAMs produce various growth factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) in addition to prostaglandin [8, 9]. Monocytes are recruited by CCL2 to pulmonary metastatic sites of murine breast cancer and promote the extravasation of tumor cells, a necessary step for metastasis, in a process that requires monocyte-derived VEGF [11]. Moreover, TAMs exhibit the properties of M2-polarized macrophages and are capable of producing immunosuppressive molecules including IL-10, transforming growth factor (TGF)-β, and arginase [12]. These properties endow TAMs with an immunosuppressive capacity. Thus, TAMs can promote tumor progression through the production of growth factors and the suppression of antitumor immunity (Figure 1).
Figure 1.
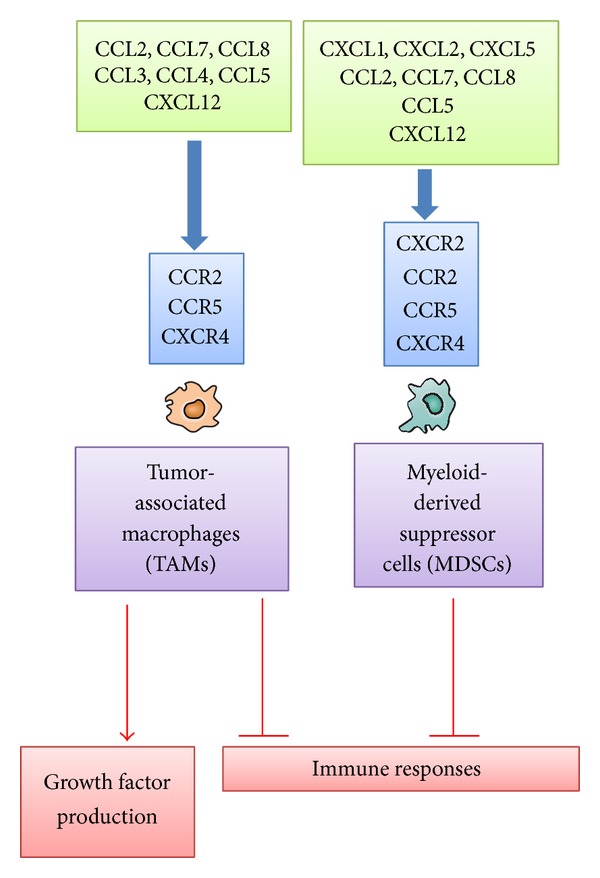
Chemokines acting on TAMs and MDSCs.
Tumor tissues contain myeloid-derived suppressor cells (MDSCs), which can suppress adaptive immunity. MDSCs are characterized by the coexpression of the myeloid-cell lineage differentiation antigen Gr-1 and CD11b in mice, while they are defined as CD14−CD11b+ cells or as cells that express the common myeloid marker CD33 but lack the expression of mature myeloid and lymphoid markers in humans [13]. MDSCs contain abundantly immunosuppressive enzymes, arginase 1 and inducible NO synthetase (iNOS), and produce immunosuppressive cytokines such as TGF-β1 and IL-10, thereby inhibiting the T-cell response [13]. CCL2 recruits MDSCs in several types of mouse cancer, including Lewis lung carcinoma, meth A sarcoma, melanoma, and lymphoma [14]. However, CCR2 deficiency results in the conversion of the MDSC phenotype from macrophage lineage to neutrophil lineage without affecting tumor growth [15]. MDSCs, particularly granulocytic ones, express CXCR2 and are plentifully present in colonic tumors developed after the combined treatment of AOM and DSS [16]. CXCR2 ligands, such as CXCL1, CXCL2, and CXCL5, are present abundantly in the colon tumor tissues and the loss of CXCR2 dramatically suppresses tumorigenesis through inhibiting MDSC infiltration [16] (Figure 1).
Regulatory T (Treg) cells have a crucial role in the maintenance of immunological self-tolerance [17]. A large number of Treg cells often infiltrate into tumors and systemic removal of Treg cells enhances natural as well as vaccine-induced antitumor T-cell immunity. Treg cells express CCR4, and its ligand, CCL22, regulates intratumoral Treg infiltration in various tumors [17]. Hypoxia induces the expression of another chemokine, CCL28, in tumor sites. CCL28 promotes angiogenesis and recruits Treg cells, thereby also propagating immune tolerance [18] (Figure 2).
Figure 2.
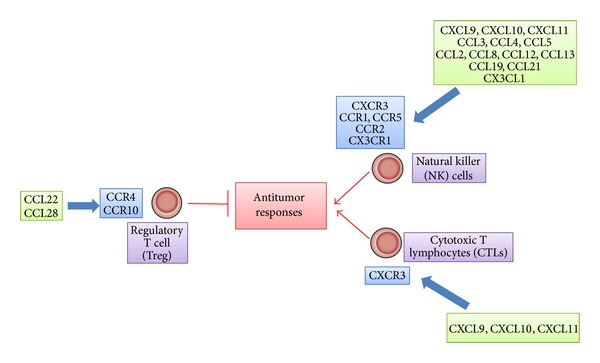
Effects of chemokines on antitumor responses.
Bindea and colleagues demonstrated that T follicular helper (Tfh) cells and B cells infiltrated tumor sites of human colorectal cancer patients, along with tumor progression [19]. Moreover, the numbers of B cells were associated with prolonged survival. Furthermore, when colon cancer cells were endoscopically injected into the colon submucosa, CXCL13 injection reduced tumor formation, whereas the deficiency in CXCR5 gene, a receptor for CXCL13, accentuated tumor formation [19]. Thus, the CXCL13/CXCR5 axis might be pivotal factors for the Tfh/B cell infiltration into tumor sites and subsequent tumor formation.
Antitumor responses are attributable to tumor infiltrating lymphocytes (TILs), particularly cytotoxic T lymphocytes (CTLs) [20]. CTLs can specifically recognize tumor-associated antigens (TAAs) and attack tumor cells in humans as well as in mice [21] (Figure 2). In this process, antigen-presenting cells can deliver TAAs and prime TAA-specific T cells. Dendritic cells (DCs) are professional antigen-presenting cells and can express on their cell surface major histocompatibility complex (MHC) class I and II molecules and costimulatory molecules, all of which assist in T-cell activation [21]. Immature DCs are distributed in almost every peripheral tissue and express several chemokine receptors including CCR1, CCR2, CCR4, CCR5, CCR6, CCR8, and CXCR4, with a high capacity to endocytose various materials [22]. DCs capture exogenous and endogenous antigens including tumor cell-derived antigens in periphery (Figure 3). When DCs capture antigens in the presence of inflammatory stimuli such as toll-like receptor-mediated signals, they change to a mature state and lose endocytosis ability. They start to express a limited set of chemokine receptors, CCR7 and CXCR4, and migrate into the T-cell areas of regional lymph nodes via afferent lymphatic venules under the guidance of chemokines [23] (Figure 3). Indeed, the appearance of apoptotic tumor cells induces the migration of dendritic cells to the draining lymph nodes and eventually generates a specific cytotoxic T lymphocyte population in the draining lymph nodes by utilizing the CCL3-CCR5/CCR1 axis [24]. On the contrary, DCs fail to express costimulatory molecules and to present antigens efficiently if they capture antigens in the absence of inflammatory cues. Mature DCs exhibit enhanced expression of costimulatory molecules and process the antigens into the peptides. The resultant peptides are presented to T cells in conjunction with MHC molecules, in the regional lymph nodes, to induce primary immune responses [23] (Figure 3).
Figure 3.
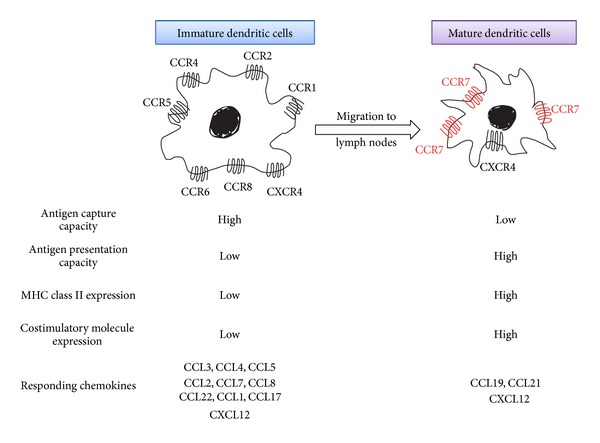
Chemokines acting on dendritic cells at different maturation stages.
Once generated in the regional lymph nodes, TAA-specific CTLs should migrate to tumor sites to kill tumor cells. Numerous clinical studies have indicated that the intratumoral presence of CD3+ or CD8+ lymphocytes has a positive prognostic influence on survival [25]. Several chemokines can regulate the migration of TILs, particularly CTLs, into tumor sites. CXCR3 is deemed to be a major chemokine receptor expressed by TILs. In a mouse model, increased expression of ligands for CXCR3, CXCL9, and CXCL10 can elicit antitumor response accompanied with an enhanced infiltration of CD4+ and CD8+ lymphocytes [26]. Consistently with this observation, in human gastric and colorectal cancer, TILs express CXCR3 (Figure 2) [27–29]. Moreover, high levels of CXCL9 and CXCL10 are produced by stromal cells, mainly macrophages [28]. CD8+ TILs also express another chemokine receptor, CCR5 [27, 29]. Concomitantly, CD8+ TIL numbers correlate well with the expression of CCL5, a ligand for CCR5, by tumor tissues [29]. TILs express additional chemokine receptor, CX3CR1, and the expression of its ligand, CX3CL1, is elevated in tumor cells in colorectal cancer tissues [30]. Furthermore, the expression level of CXCL16 also correlates with CD4+ and CD8+ TIL as well as numbers with a better prognosis although cells expressing CXCR6, a receptor for CXCL16, are not identified [31]. Thus, CXCL9, CXCL10, CXCL16, CCL5, and CX3CL1 can be used to efficiently mobilize CTLs from regional lymph nodes to tumor tissues with an objective to enhance CTL-mediated tumor destruction.
Natural killer (NK) cells are unconventional lymphocytes, which can in vitro kill a broad range of tumor cells of both hematopoietic and nonhematopoietic origin by utilizing perforin and secreting interferon (IFN)-γ [32]. Moreover, in vivo, mouse NK cells can eliminate many transplantable and spontaneous tumors. NK cells express distinct sets of chemokine receptors (Table 1). NK cells migrate to lymph nodes mainly by utilizing CXCR3, while their migration to the inflamed tissues including tumor sites involves CCR1, CCR2, CCR5, CXCR3, and CX3CR1 (Figure 2) [33]. Thus, the ligands for these receptors can regulate NK cell trafficking and augment their functions. However, in colorectal tumor tissues, NK cells are scarce despite a significant lymphocyte infiltration, even in the presence of high levels of CXCL9, CXL10, CCL3, CCL4, CCL5, and CX3CL1 [34]. Thus, NK cell migration into tumor tissues may be impaired early in the course of tumor development by the mechanism that does not affect CTL trafficking.
We recently succeeded in causing chronic myelogenous leukemia (CML)- like pathology by direct transplantation of BCR-ABL gene-transduced leukemia initiating cells (LICs) into the bone marrow cavity of nonirradiated mice [35]. We further observed that BCR-ABL+lineage−c-kit− immature leukemia cells produced high levels of CCL3, which promoted the development of CML. Conversely, the ablation of CCL3 gene in LICs dramatically inhibited the development of CML and concomitantly reduced recurrence after the cessation of a short-term tyrosine kinase inhibitor treatment. Moreover, normal hematopoietic stem/progenitor cells (HSPCs) can directly impede the maintenance of LICs in bone marrow in the absence of CCL3 signal. These observations would indicate that leukemia cell-derived CCL3 expels normal HSPCs from bone marrow to make spaces for leukemia cells to survive [35].
2.2. Endothelial Cells
Neovascularization is crucial for tumor growth, progression, and metastasis [36]. The ELR-motif-positive CXC chemokines, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8, directly promote the migration and proliferation of endothelial cells and eventually neovascularization, mainly by interacting with CXCR2 but not with CXCR1 [37]. CXCL12 is not an ELR-positive CXC chemokine but has a potent angiogenic activity [38]. Three CC chemokines, CCL2, CCL11, and CCL16, have also been implicated in tumor neovascularization [39–41]. CCR2, a specific receptor for CCL2, is expressed by endothelial cells and CCL2 exerts its angiogenic activity in a membrane type 1- (MT1-) matrix metalloproteinase- (MMP-) dependent manner [39] (Figure 4).
Figure 4.
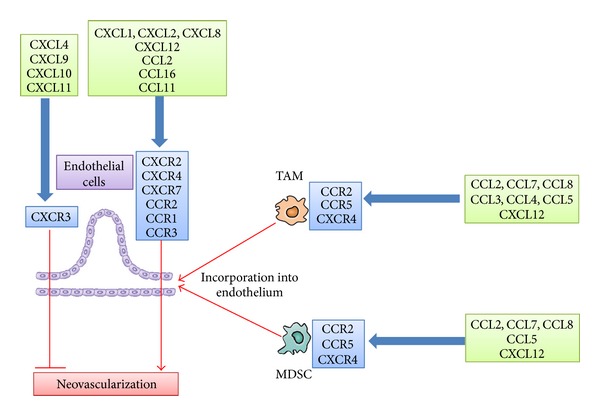
Chemokines acting on tumor neovascularization.
CXCL4 and interferon-inducible ELR-negative CXC chemokines such as CXCL9, CXCL10, and CXCL11 inhibit the angiogenesis induced by ELR-motif-positive CXC chemokines, VEGF, and bFGF [42, 43] by interacting with a common receptor, CXCR3 (Figure 4). Moreover, the Duffy antigen has been shown to suppress the angiogenic effects of the ELR-motif-positive CXC chemokines, as it can sequester the ELR-motif-positive CXC chemokines without eliciting any intracellular signals [44]. Thus, the balance between proangiogenic and antiangiogenic chemokines may determine the degree of tumor neovascularization.
TAMs and MDSCs contributed to tumor angiogenesis by producing a wide variety of angiogenic factors such as VEGF, TGF-β, CXCL8, platelet-derived growth factor (PDGF), and MMPs such as MMP-2 and MMP-9 [8, 9]. Several chemokines, particularly CCL2, can induce tumor angiogenesis by attracting TAMs and MDSCs. Moreover, recruited TAMs and MDSCs can acquire endothelial cell phenotypes and be incorporated into the newly formed vascular structure (Figure 4) [45]. Endothelial cell-derived ELR-motif-positive CXC chemokines, especially CXCL6, induce angiogenesis in gastrointestinal cancer by recruiting neutrophils [46].
2.3. Fibroblasts and Other Cells
Fibroblasts present in tumor tissues are designated as cancer-associated fibroblasts (CAFs). CAFs can produce tumor-promoting molecules such as TGF-β, FGF, hepatocyte growth factor (HGF), and EGF [47]. The proposed cellular sources of CAFs include locally resident fibroblasts, cells undergoing epithelial-mesenchymal transition (EMT), cells undergoing endothelial-mesenchymal transition (EndMT), or bone marrow- (BM-) derived mesenchymal stem cells (MSCs) [48]. In a mouse gastric cancer model, it is estimated that BM-derived MSCs can contribute as much as 25% to the CAF population [49]. On the contrary, in a mouse lung metastasis model, BM-derived cells do not significantly contribute to fibroblast accumulation in lungs [50].
In a mouse gastric cancer model, CXCL12 expression was found to be enhanced together with enhanced fibrosis in tumor sites [49]. Because MSCs express CXCR4 and CXCR7 and migrate to their ligand, CXCL12, the CXC12-CXCR4/CXCR7 axis may regulate the accumulation of fibroblasts and consequent fibrosis development [48]. Moreover, CAFs present in various types of cancer produce CXCL12, and the produced CXCL12 promotes the angiogenic, proliferative, and migratory properties of tumor cells. CAFs produce CCL2, CCL5, CCL7, CXCL8, and CXCL14 and these chemokines promote tumor progression mainly by enhancing the motility of tumor cells [48].
In a mouse lung metastasis model, HGF-expressing fibroblasts were found to be increased in the lungs [50]. Lung fibroblasts express CCR5, and genetic deletion of CCR5 or its ligand, CCL3, attenuates intrapulmonary lung metastasis formation along with reduced fibroblast accumulation and HGF expression [50]. We recently observed that fibroblast accumulation was required for full-blown progression of chronic colitis-associated colon cancer, in addition to inflammatory cell infiltration. Moreover, fibroblast accumulation in colon tissues was regulated by the CCL3-CCR5 axis.
When activated, hepatic stellate cells (HSCs) express a marker of myofibroblasts, α-smooth muscle actin (α-SMA). α-SMA-positive HSCs secrete CXCL12 similarly as CAFs do [51]. Moreover, in a mouse liver metastasis model, tumor-derived CCL2 was found to induce α-SMA-positive HSCs to accumulate at tumor sites and to express MMP-2. Genetic ablation of CCR2 markedly attenuates tumor formation with reduced HSC accumulation and MMP-2 expression [52]. Thus, CCR2-mediated signals may regulate the trafficking and functions of HSCs, thereby inducing liver metastasis.
3. Direct Effects on Cancerous Cells
The tyrosine kinase, RET, is a prototypic transforming oncogene in human papillary thyroid carcinoma and induces the expression of several chemokines including CCL2, CCL20, ELR-motif-positive CXC chemokines, and CXCL12, and CXCR4 [53]. The CXCR4 gene can further be transactivated by the activation of hypoxia-inducible factor- (HIF-) 1α, arising either from loss of the von-Hippel-Lindau tumor suppressor (VHL) or due to the hypoxic conditions observed frequently in tumor tissues [54]. Moreover, components of the Ras-Raf signaling pathway can activate NF-κB [55], which can lead to enhanced expression of chemokines, including CXCL1, CXCL8, and CCL2 [56]. Thus, even in the absence of direct oncogene activation, NF-κB and HIF activation can induce the expression of chemokines and their receptors, in tumor tissues.
The term “cellular senescence” has been used to denote a stable and long-term loss of proliferative capacity, despite continued viability and metabolic activity [57]. Activation of oncogenes, particularly Ras, induces cellular senescence which is designated as oncogene-induced senescence (OIS). OIS serves as a potent barrier against oncogenic transformation by suppressing the unscheduled proliferation of early neoplastic cells [57]. Moreover, cells undergoing OIS secrete CXCR2-binding chemokines and IL-6 through the activation of 2 proinflammatory transcription factors, C/EBP-β and NF-κB [58, 59]. CXCL8/IL-8 specifically colocalizes with arrested p16INK4A-positive epithelium in human colon adenomas [58]. Furthermore, the reduction of CXCR2 expression on tumor cells alleviates OIS and diminishes the DNA-damage response, while ectopic expression of CXCR2 results in premature senescence via a p53-dependent mechanism [59]. Thus, CXCR2-binding chemokines can promote the arrest of cellular growth and eventually delay the early phase of tumorigenesis (Figure 5).
Figure 5.
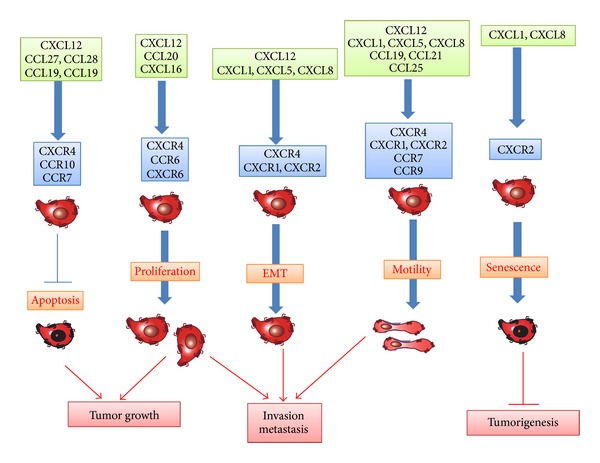
Effects of chemokines on tumor cells.
EMT is indispensable for embryogenesis [60]. EMT induces epithelial cells to lose the expression of components of cell polarity, such as E-cadherin, and reciprocally to express mesenchymal components of the cytoskeleton and to eventually acquire the motility and scattering properties. EMT is presumed to be associated with the capacity of tumor cells to invade and metastasize [60]. Prolonged exposure to TGF-β induces rat hepatocellular carcinoma cells to exhibit a mesenchymal phenotype, and a higher migratory and invasive capacity, coincident with increased CXCR4 expression, and these phenotypic changes are reduced by a CXCR4 antagonist [61]. Likewise, the expression of CXCL8 and its receptor, CXCR1, is induced in a human colon cancer cell line undergoing TGF-β-driven EMT [62]. EMT in cancer cells can be initiated by overexpression of several transcription factors, including Twist, Snail, and Brachyury [60]. Overexpression of Brachyury causes EMT in human pancreatic cancer cell lines, along with enhanced expression of CXCL8, CCL5, and CXCL1, while the inhibition of CXCL8 signaling pathway abrogates Brachyury-induced EMT phenotypes and invasive capacity of cells [63]. These observations suggest the crucial roles of several chemokines in EMT, an indispensable step for the malignant progression of cancer (Figure 5).
Chemokine receptor engagement can also activate the mitogen-activated protein (MAP)/Erk kinase pathway [64], leading to gene expression and cell proliferation (Figure 5). Human gastric cancer cell lines possess CXCR1 and CXCR2 and express epidermal growth factor (EGF) receptor, MMP-9, and VEGF, in response to CXCL8 [65]. The same pathway is utilized to promote proliferation of esophageal cancer cells [66] and melanoma cells [67].
In addition to the CXCL8 axis, several chemokines have impacts on proliferation and survival of tumor cells. CCR6 and CXCR6 promote the proliferation of colorectal cancer cells [68] and prostate cancer cells [69], respectively. Moreover, CCR10 activation in melanoma causes phosphatidylinositol-3-kinase- (PI3K-) mediated protection of tumor cells from apoptosis [70]. Similar observations are obtained on CCR7 activation in squamous cell carcinoma of the head and neck [71] (Figure 1). On the contrary, CCR5 blockade can enhance the proliferation of breast cancer cells, which express wild-type p53 [72]. Furthermore, disease-free survival is shorter in breast cancer patients bearing the CCR5Δ32 allele with a premature stop codon than in CCR5 wild-type patients; this holds true only in wild-type p53-expressing tumors [72]. Likewise, human hepatocellular carcinoma (HCC) cells express CCR1 and its ligands can affect the functions of human HCC cell lines [73].
CXCR4 is the most commonly detected chemokine receptor in tumor cells [38]. CXCR4 has an important role in the proliferation of various cancer cells including ovarian, glioma, melanoma, lung, renal, and thyroid cancer cells [38, 74]. The CXCL12/CXCR4 axis delivers surviving signals to hepatocellular carcinoma cells, ovarian carcinoma cells, and chronic leukemia cells, while CXCR4 blockade induces the apoptosis of these malignant cells [38, 61, 75] (Figure 5). CXCL12 expression correlates well with lower apoptosis in human myelodysplastic syndrome [76].
Chemokines can regulate the migration of tumor cells. CXCR4-expressing cells can migrate in vitro towards CXCL12 [77]. CCR7, CCR9, CXCR1, and CXCR2 are also detected in tumor cells and their ligands can induce the chemotaxis of the corresponding receptor-expressing cells [78, 79]. These chemokines can serve as inducers of invasion within the primary tumor and dissemination to distant organs (Figure 5). Moreover, human pancreatic ductal adenocarcinoma (PDAC) cells express CX3CR1 and CX3CL1 abundantly and the CX3CR1/CX3CL1 axis can regulate intraneural invasion of PDAC [80]. Adult T-cell leukemia (ATL) cells express frequently CCR4 and can migrate in vitro to CCL17 and CCL22, ligands for CCR4 [81]. The CCL17/CCL22-CCR4 axis may account for the frequent infiltration of ATL into skin and lymph nodes, where CCL17 and CCL22 are abundantly expressed.
Circulating tumor cells (CTCs) are presumed to be a source of metastasizing tumor cells, but they can also colonize at their original site [82]. This process, which is called as tumor self-seeding, can accelerate tumor growth and angiogenesis. CTCs produce ELR-motif-positive CXC chemokines including CXCL8 and CXCL1, and these chemokines eventually promote self-seeding [82].
Several models have been proposed to explain the molecular mechanisms underlying the enhancement and regulation of metastasis by the chemokines. Specific chemokine receptor-expressing tumor cells may migrate to organs with high expression levels of the corresponding chemokines along a concentration gradient [77]. This hypothesis may explain the tissue tropism observed in certain types of cancer, but there is little evidence to indicate the presence of chemokine concentration gradients between primary and metastatic sites. A transcellular CCR7 ligand gradient can be created when cancer cells produce CCR7 ligands under flow conditions and the resultant gradient can be the basis of lymphatic metastasis [83]. Thus, cancer cells themselves may actively promote their own metastasis and tropism by producing chemokines. Another plausible explanation is that the arrival of tumor cells in a specific organ is passive and that chemokine receptor expression provides tumor cells with an advantage to survive and grow in a different ligand-rich metastatic microenvironment [84]. Moreover, CXCL12 and a CCR7 ligand, CCL21, can induce the resistance of cancer cells to anoikis, which is a major hindrance to the metastatic spread of various types of cancer, by regulating proapoptotic Bmf and antiapoptotic Bcl-xL proteins [85]. Thus, chemokines may accelerate metastasis by promoting tumor cell proliferation or preventing tumor cell death.
Thus, chemokines can prevent tumorigenesis in the early phase by inducing cellular senescence while they can also promote invasion and metastasis by inducing EMT and enhancing the motility and survival of tumor cells (Figure 5).
4. Potential of Chemokine Targeting Therapy as Cancer Treatment
4.1. Chemokine-Mediated Enhancement in Tumor Immunity
The establishment of tumor immunity is a multistage process: migration of DCs to tumor sites, capture of TAAs by DCs, migration of DCs to regional lymph nodes, antigen presentation to effector cells by DCs in regional lymph nodes, and migration of effector cells to tumor sites (Figure 6) [21, 22]. Chemokines have profound impacts on tumor immunity, particularly migration steps.
Figure 6.
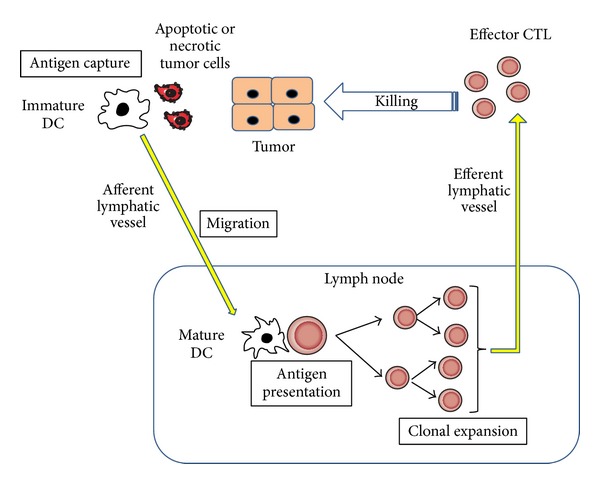
Tumor immunity generation process.
Immature dendritic cells move to the tumor tissues to phagocytose apoptotic tumor cells, capture tumor-associated antigens (TAAs), and migrate to draining lymph nodes, where DCs present antigens to induce specific CTLs [21–23]. Tumor-infiltrating DCs expressed CCR1 and CCR5, and a ligand for these receptors, CCL3, was abundantly detected in mouse bearing HCC [24]. Mirroring the capacity of CCL3 to mobilize CCR1- or CCR5-expressing immature DCs to peripheral blood from bone marrow [86], systemic administration of CCL3 increased the numbers of DCs in peripheral blood and tumor tissues and concomitantly augmented antitumor effects after radiofrequency ablation of murine HCCs [87]. Thus, CCL3 may be effective to enhance tumor immunity by attracting immature DCs to dying tumor cells.
CCL19 and CCL21, ligands for CCR7, can regulate DC migration to lymph nodes for antigen presentation to naïve T cells, which also utilize CCL19 and/or CCL21 to enter T-cell zone [88], and they can additionally attract NK cells to the lymph node. As a consequence, when CCL21 was injected into a regional lymph node of SV40-transgenic mice that developed bilateral multifocal lung adenocarcinomas, it increased CD4+ and CD8+ lymphocytes as well as DCs at lymph nodes and tumor sites and eventually led to a marked reduction in tumor burdens with enhanced survival [89]. Similar results were also obtained when CCL19 was injected intranodally into SV40-transgenic mice [90].
Low clinical efficacy of DC-based vaccines can be explained by a very limited capacity of ex vivo generated DC to move from the injected sites to draining lymph nodes [91]. In order to circumvent these problems, DCs are genetically modified to express CCL19 and CCL21. Indeed, intratumoral injection of CCL21 gene-modified DCs resulted in more effective tumor growth inhibition than unmodified control DCs [92], together with intratumoral accumulation of DCs and T cells [93]. Moreover, even when CCL21 gene-modified DCs were pulsed with tumor lysates and subsequently injected subcutaneously to tumor-free sites in tumor-bearing mice, it elicited a good antitumor response [92]. These promising preclinical results have led to ongoing phase I clinical trials [94].
Intratumoral administration of CCL21 gene-modified DCs reduced a tumor burden in spontaneous murine lung carcinoma, accompanied with extensive T-cell infiltration and the enhanced IFN-γ, IL-12, CXCL9, and CXCL10 expression [95]. Moreover, in vivo depletion of either CXCL9 or CXCL10 significantly reduced the antitumor efficacy of CCL21 gene-modified DCs, probably because CXCR3 is highly expressed by activated effector CD8+ T cells and Th1-type CD4+ T cells [96]. CXCL10 gene transduction into tumor cells had few effects on in vitro tumor cell proliferation but in vivo elicited a potent T-cell-dependent antitumor response [97]. Likewise, tumor cells expressing CXCL10 induced the infiltration of tumor-specific cytotoxic T cells into the tumor site [98]. Moreover, tumor cells induced these cytotoxic T cells to proliferate and to produce high level of IFN-γ, while CXCL10 expanded these tumor-specific T cells [98]. Similar observations were obtained on gene transduction of another ligand for CXCR3, CXCL11, into tumor cells [99]. Moreover, as T cells rapidly acquire CXCR3 expression upon activation with IL-2 [96], combined strategy of systemic IL-2 with intratumor CXCL9 administration was proven to be more efficacious than either cytokine alone, for augmenting tumor-associated immunity [26]. Thus, CXCR3-binding chemokines can be utilized to redirect the migration of effector T cells to tumor sites.
CCL2 was initially isolated as a factor which can also augment monocyte-mediated tumor cytostatic activity [100]. Indeed, tumor formation was suppressed in vivo but not in vitro when the tumor was genetically engineered to express CCL2 gene [101]. CCL2-expressing cells elicited a predominantly monocytic infiltrate at the site of injection, suggesting the roles of infiltrating monocytes in tumor rejection process [101]. In another tumor models, CCL2 gene transduction into tumor cells retarded tumor growth in vivo by inducing NK infiltration into tumor sites [102], because NK cells express CCR2, a receptor for CCL2 (Table 1). Moreover, NK cell infiltration was associated with elevated Th1 response in tumor sites [103], suggesting that CCL2 can regulate the infiltration and activation of Th1 cells in tumor sites by recruiting and activating NK cells.
Gene therapy using CX3CL1 gene could activate T cells as well as NK cells to exert its antitumor responses [104–106]. Moreover, intratumoral injection of a DNA plasmid coding for a chimeric immunoglobulin combining with CX3CL1 chemokine domain provided strong antitumor activity [107]. The administration of this fusion protein with tumor antigens induced a strong in vivo antigen-specific T-cell proliferation and effector function, accompanied with myeloid DC accumulation [107]. Thus, CX3CL1 can redirect T cells and DCs as well as NK cells, thereby augmenting adaptive immunity to tumor antigens.
In order to enhance the capacity to move to tumor sites by utilizing the chemokine(s) produced by tumor cells, several groups genetically engineered T cells to express the corresponding chemokine receptor. The Reed-Sternberg cells of Hodgkin lymphoma predominantly produce CCL17 and CCL22 [108]. Effector CD8+ T cells lack CCR4, but CCR4 gene-modified CD8+ T cells migrated more efficiently to Hodgkin lymphoma site to inhibit tumor formation [109]. Similarly, CCL2 was highly secreted by malignant pleural mesothelioma cells, and CCR2 gene was further transduced to activate human T cells expressing a chimeric antibody receptor (CAR) directed to mesothelioma tumor antigen, mesothelin (mesoCAR T cells) [110]. The resultant gene-modified T cells exhibited enhanced antitumor responses accompanied with augmented T-cell infiltration into tumor sites, when they were given intravenously [110]. This novel gene therapy technology using a chemokine receptor can effectively enhance the migration of adoptively transferred T cells into tumor sites, where a corresponding chemokine is expressed abundantly.
4.2. Reversal of Suppressor Cell-Mediated Immune Suppression
Tumor immunity can frequently induce immune suppressive mechanisms to reduce the “immunity to self” by the action of several negative immunoregulatory receptors such as cytotoxic T lymphocyte antigen-4 (CTLA-4) and the programmed death receptor-1- (PD-1-) PD ligand-1 (PD-L1) axis. Consequently, the antagonizing monoclonal antibodies to CTLA-4, PD-1, or PD-L1 are effective against various types of cancer even at advanced stages [111, 112]. These observations indicate that reversal of immune suppression can be effective to enhance tumor immunity.
Tumor tissues contain the leukocytes that can diminish tumor immunity. The most predominant subset is TAMs [8, 12]. TAMs can promote tumor progression by inducing angiogenesis and suppression of adaptive and innate antitumor immunity (Figure 1). Circulating monocytes are mostly the precursor of these TAMs and are attracted into tumor sites, by several chemokines, particularly CCL2 [8, 12]. Indeed, systemic delivery of neutralizing anti-CCL2 antibody attenuated tumor burdens in human prostate cancer-bearing mice although its effects on TAMs have not been examined [113]. Moreover, CCL2 blockade reduced CCR2-expressing TAM infiltration and eventually tumor formation in chronic colitis-associated carcinogenesis [10]. Furthermore, CCL2 also recruited monocytes to pulmonary metastatic sites of murine breast cancer to promote the extravasation of tumor cells, a prerequisite step for metastasis, in monocyte-derived VEGF-dependent manner [11]. CCL2 blockade markedly reduced lung metastasis together with reduced monocyte/macrophage infiltration.
Another type of immune suppressor cells is MDSCs with a strong ability to suppress various T-cell functions [13]. CCL2 recruits MDSCs in several types of mouse cancer [14]. Moreover, CCL2-mediated MDSC accumulation can negatively regulate the entry of adoptively transferred activated CD8+ cells into tumor sites [114]. CCR2 deficiency, however, caused conversion of the MDSC phenotype to neutrophil lineage without affecting tumor growth [15], probably because MDSC contains a subset of immature neutrophils [115]. On the contrary, CXCR2 blockade reduced the infiltration of CXCR2-expressing granulocytic MDSCs and eventually tumor growth in chronic colitis-associated colon cancer [16].
Adult T-cell leukemia (ATL) cells are characterized by robust expression of CCR4 and can migrate in vitro to CCL17 and CCL22, ligands for CCR4 [81]. Humanized defucosylated monoclonal antibody to CCR4 has been obtained. The resultant antibody can exert more potent antibody-dependent cytotoxicity (ADCC) [116] and is capable of removing CCR4-expressing ATL cells in peripheral blood and bone marrow mainly by ADCC.
A large number of Treg cells often infiltrate into tumors and systemic removal of Treg cells enhances natural as well as vaccine-induced antitumor T-cell immunity [17]. Treg cells express CCR4 and its ligand, CCL22, mainly regulates intratumoral Treg infiltration in various tumors [17] (Figure 2). Indeed, intratumoral CCL22 expression correlated well with Foxp3 expression in colorectal carcinoma tissues [117]. In line with these observations, anti-CCR4 antibody treatment depletes Tregs and eventually evokes CD8+ T-cell response against TAAs [118]. Furthermore, CCL2 is also involved in Treg accumulation and, as a consequence, anti-CCL2 antibody augmented cancer immunotherapy against non-small cell lung cancer in mice with reduced intratumoral Tregs and increased numbers of intratumoral antigen-specific activated CD8+ cells, when it was administered in combination with a tumor vaccine [119]. These observations illustrate that targeting these chemokines can reduce intratumoral Treg cells, resulting in the enhancement of tumor immunity.
Recently, CCR1-expressing CD34+ immature myeloid cells have been detected in murine intestinal tumors with SMAD4 deficiency [120]. These cells expressed abundantly MMP-9 and MMP-2 and were involved in invasion. Moreover, a CCR1 antagonist suppressed colon cancer liver metastasis by blocking accumulation of CD34+ immature myeloid cells [121].
4.3. Other Strategies of Antitumor Therapy Targeting Chemokines
Neovascularization is crucial for tumor growth, progression, and metastasis [36]. The ELR motif-positive CXC chemokines, CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8, can directly promote the migration and proliferation of endothelial cells and eventually neovascularization [37] (Figure 4). Indeed, the administration of anti-CXCL8 reduced the tumor sizes of human non-small cell lung cancer cells which are injected into severe combined immune deficient (SCID) mice in advance [122]. The reduction in tumor size was associated with a decline in tumor-associated vascular density and was accompanied by a decrease in spontaneous lung metastasis. CXCL12 [123] and three CC chemokines, CCL2, CCL11, and CCL16, have also been implicated in tumor neovascularization [39–41, 45]. However, it still remains elusive on the efficacy of targeting these chemokines for the control of tumor neovascularization.
CXCL4, CXCL9, CXCL10, and CXCL11 inhibit the angiogenesis induced by ELR motif-positive CXC chemokines, VEGF, and bFGF [42, 43]. Targeted expression of CXCL9 or intratumoral CXCL9 administration retarded in vivo tumor growth by inhibiting tumor-derived angiogenesis [26, 124]. Thus, these chemokines can be effective for tumor therapy by inhibiting neovascularization as well as inducing CXCR3-expressing cytotoxic T-cell infiltration.
5. Concluding Remarks
Cancer development and progression are profoundly affected by inflammatory and immune responses. Inflammatory responses consist of leukocyte infiltration, neovascularization, and fibrosis, while immune responses were exerted by immune cells such as lymphocytes and dendritic cells. Chemokines have great impacts on the cells involved in both inflammatory and immune responses. Moreover, several chemokines have direct effects on the proliferative and invasive properties of tumor cells. Consequently, chemokines play crucial roles in tumor development and progression by acting on cancerous and noncancerous cells. However, it is embarrassing that the same chemokine can induce tumor progression as well as protection against a tumor, in a context-dependent manner. Given the multifactorial roles of chemokines in carcinogenesis, the elucidation of their roles will further advance our understanding of the pathophysiological processes of tumor development and progression and will subsequently pave a novel way to develop a novel type of anticancer treatment by targeting chemokines.
Acknowledgment
This work is partly supported by the grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends in Immunology. 2004;25(2):75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annual Review of Pharmacology and Toxicology. 2002;42:469–499. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- 3.Vandercappellen J, van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Letters. 2008;267(2):226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 4.Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–716. doi: 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nomiyama H, Osada N, Yoshie O. The evolution of mammalian chemokine genes. Cytokine and Growth Factor Reviews. 2010;21(4):253–262. doi: 10.1016/j.cytogfr.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Moepps B, Nuesseler E, Braun M, Gierschik P. A homolog of the human chemokine receptor CXCR1 is expressed in the mouse. Molecular Immunology. 2006;43(7):897–914. doi: 10.1016/j.molimm.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 7.Su S-B, Mukaida N, Wang J, Nomura H, Matsushima K. Preparation of specific antagonizing polyclonal antibodies to a C-C chemokine receptor, CCR1 and determination of its distribution of various types of leukocytes. Journal of Leukocyte Biology. 1996;60(5):658–666. doi: 10.1002/jlb.60.5.658. [DOI] [PubMed] [Google Scholar]
- 8.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Letters. 2008;267(2):204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Lazennec G, Richmond A. Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends in Molecular Medicine. 2010;16(3):133–144. doi: 10.1016/j.molmed.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Popivanova BK, Kostadinova FI, Furuichi K, et al. Blockade of a chemokine, CCL2, reduces chronic colitis-associated carcinogenesis in mice. Cancer Research. 2009;69(19):7884–7892. doi: 10.1158/0008-5472.CAN-09-1451. [DOI] [PubMed] [Google Scholar]
- 11.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475(7355):222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang B, Lei Z, Zhao J, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Letters. 2007;252(1):86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Sawanobori Y, Ueha S, Kurachi M, et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111(12):5457–5466. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 16.Katoh H, Wang D, Daikoku T, Sun H, Dey SK, DuBois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24(3):631–644. doi: 10.1016/j.ccr.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. International Journal of Cancer. 2010;127(4):759–767. doi: 10.1002/ijc.25429. [DOI] [PubMed] [Google Scholar]
- 18.Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature. 2011;475(7355):226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 19.Bindea G, Miecnik B, Tosolini M, et al. Spaciotemporal dynamics of intratumoral immune cells reveals the immune landscape in human cancer. Immunity. 2013;39(4):782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Steer HJ, Lake RA, Nowak AK, Robinson BW. Harnessing the immune response to treat cancer. Oncogene. 2010;29(48):6301–6313. doi: 10.1038/onc.2010.437. [DOI] [PubMed] [Google Scholar]
- 21.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunology and Immunotherapy. 2005;54(8):721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nature Reviews Cancer. 2012;12(4):265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sozzani S. Dendritic cell trafficking: more than just chemokines. Cytokine and Growth Factor Reviews. 2005;16(6):581–592. doi: 10.1016/j.cytogfr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Iida N, Nakamoto Y, Baba T, et al. Tumor cell apoptosis induces tumor-specific immunity in a CC chemokine receptor 1- and 5-dependent manner in mice. Journal of Leukocyte Biology. 2008;84(4):1001–1010. doi: 10.1189/jlb.1107791. [DOI] [PubMed] [Google Scholar]
- 25.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. The British Journal of Cancer. 2011;105(1):93–103. doi: 10.1038/bjc.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan J, Burdick MD, Belperio JA, et al. CXCR3/CXCR3 ligand biological axis impairs RENCA tumor growth by a mechanism of immunoangiostasis. Journal of Immunology. 2006;176(3):1456–1464. doi: 10.4049/jimmunol.176.3.1456. [DOI] [PubMed] [Google Scholar]
- 27.Musha H, Ohtani H, Mizoi T, et al. Selective infiltration of CCR5+CXCR3+ T lymphocytes in human colorectal carcinoma. International Journal of Cancer. 2005;116(6):949–956. doi: 10.1002/ijc.21135. [DOI] [PubMed] [Google Scholar]
- 28.Ohtani H, Jin Z, Takegawa S, Nakayama T, Yoshie O. Abundant expression of CXCL9 (MIG) by stromal cells that include dendritic cells and accumulation of CXCR3+ T cells in lymphocyte-rich gastric carcinoma. Journal of Pathology. 2009;217(1):21–31. doi: 10.1002/path.2448. [DOI] [PubMed] [Google Scholar]
- 29.Muthuswamy R, Berk E, Junecko BF, et al. NF-κB hyperactivation in tumor tissues allows tumor-selective reprogramming of the chemokine microenvironment to enhance the recruitment of cytolytic T effector cells. Cancer Research. 2012;72(15):3735–3743. doi: 10.1158/0008-5472.CAN-11-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohta M, Tanaka F, Yamaguchi H, Sadanaga N, Inoue H, Mori M. The high expression of fractalkine results in a better prognosis for colorectal cancer patients. International Journal of Oncology. 2005;26(1):41–47. [PubMed] [Google Scholar]
- 31.Hojo S, Koizumi K, Tsuneyama K, et al. High-level expression of chemokine CXCL16 by tumor cells correlates with a good prognosis and increased tumor-infiltrating lymphocytes in colorectal cancer. Cancer Research. 2007;67(10):4725–4731. doi: 10.1158/0008-5472.CAN-06-3424. [DOI] [PubMed] [Google Scholar]
- 32.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nature Immunology. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 33.Walzer T, Vivier E. G-protein-coupled receptors in control of natural killer cell migration. Trends in Immunology. 2011;32(10):486–492. doi: 10.1016/j.it.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Halama N, Braun M, Kahlert C, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clinical Cancer Research. 2011;17(4):678–689. doi: 10.1158/1078-0432.CCR-10-2173. [DOI] [PubMed] [Google Scholar]
- 35.Baba T, Naka L, Morishita S, Komatsu N, Hirao A, Mukaida N. MIP-1α/CCL3-mediated maintenance of leukemia initiating cells in the initiation process of chronic leukemia. Journal of Experimental Medicine. 2013;210(12):2661–2673. doi: 10.1084/jem.20130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79(2):185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 37.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of tumor angiogenesis and neovascularization. Experimental Cell Research. 2011;317(5):685–690. doi: 10.1016/j.yexcr.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clinical Cancer Research. 2010;16(11):2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 39.Gálvez BG, Genís L, Matías-Román S, et al. Membrane type 1-matrix metalloproteinase is regulated by chemokines monocyte-chemoattractant protein-1/CCL2 and interleukin-8/CXCL8 in endothelial cells during angiogenesis. The Journal of Biological Chemistry. 2005;280(2):1292–1298. doi: 10.1074/jbc.M408673200. [DOI] [PubMed] [Google Scholar]
- 40.Salcedo R, Young HA, Ponce ML, et al. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. Journal of Immunology. 2001;166(12):7571–7578. doi: 10.4049/jimmunol.166.12.7571. [DOI] [PubMed] [Google Scholar]
- 41.Strasly M, Doronzo G, Cappello P, et al. CCL16 activates an angiogenic program in vascular endothelial cells. Blood. 2004;103(1):40–49. doi: 10.1182/blood-2003-05-1387. [DOI] [PubMed] [Google Scholar]
- 42.Maione TE, Gray GS, Petro J, et al. Inhibition of angiogenesis by recombinant human platelet factor-4 and related peptides. Science. 1990;247(4938):77–79. doi: 10.1126/science.1688470. [DOI] [PubMed] [Google Scholar]
- 43.Romagnani P, Annunziato F, Lasagni L, et al. Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. Journal of Clinical Investigation. 2001;107(1):53–63. doi: 10.1172/JCI9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du J, Luan J, Liu H, et al. Potential role for Duffy antigen chemokine-binding protein in angiogenesis and maintenance of homeostasis in response to stress. Journal of Leukocyte Biology. 2002;71(1):141–153. [PMC free article] [PubMed] [Google Scholar]
- 45.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107(8):1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 46.Gijsbers K, Gouwy M, Struyf S, et al. GCP-2/CXCL6 synergizes with other endothelial cell-derived chemokines in neutrophil mobilization and is associated with angiogenesis in gastrointestinal tumors. Experimental Cell Research. 2005;303(2):331–342. doi: 10.1016/j.yexcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 47.Räsänen K, Vaheri A. Activation of fibroblasts in cancer stroma. Experimental Cell Research. 2010;316(17):2713–2722. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 48.Mishra P, Banerjee D, Ben-Baruch A. Chemokines at the crossroads of tumor-fibroblast interactions that promote malignancy. Journal of Leukocyte Biology. 2011;89(1):31–39. doi: 10.1189/jlb.0310182. [DOI] [PubMed] [Google Scholar]
- 49.Guo X, Oshima H, Kitmura T, Taketo MM, Oshima M. Stromal fibroblasts activated by tumor cells promote angiogenesis in mouse gastric cancer. The Journal of Biological Chemistry. 2008;283(28):19864–19871. doi: 10.1074/jbc.M800798200. [DOI] [PubMed] [Google Scholar]
- 50.Wu Y, Li YY, Matsushima K, Baba T, Mukaida N. CCL3-CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process. Journal of Immunology. 2008;181(9):6384–6393. doi: 10.4049/jimmunol.181.9.6384. [DOI] [PubMed] [Google Scholar]
- 51.Matsusue R, Kubo H, Hisamori S, et al. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Annals of Surgical Oncology. 2009;16(9):2645–2653. doi: 10.1245/s10434-009-0599-x. [DOI] [PubMed] [Google Scholar]
- 52.Yang X, Lu P, Ishida Y, Kuziel WA, Fujii C, Mukaida N. Attenuated liver tumor formation in the absence of CCR2 with a concomitant reduction in the accumulation of hepatic stellate cells, macrophages and neovascularization. International Journal of Cancer. 2006;118(2):335–345. doi: 10.1002/ijc.21371. [DOI] [PubMed] [Google Scholar]
- 53.Borrello MG, Alberti L, Fischer A, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(41):14825–14830. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. Journal of Experimental Medicine. 2003;198(9):1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nature Immunology. 2011;12(8):715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 56.Hold GL, El-Omar EM. Genetic aspects of inflammation and cancer. Biochemical Journal. 2008;410(2):225–235. doi: 10.1042/BJ20071341. [DOI] [PubMed] [Google Scholar]
- 57.Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes and Development. 2010;24(22):2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuilman T, Michaloglou C, Vredeveld LC, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 59.Acosta JC, O’Loghlen A, Banito A, et al. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133(6):1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 60.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 61.Bertran E, Caja L, Navarro E, et al. Role of CXCR4/SDF-1α in the migratory phenotype of hepatoma cells that have undergone epithelial-mesenchymal transition in response to the transforming growth factor-β . Cellular Signalling. 2009;21(11):1595–1606. doi: 10.1016/j.cellsig.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Bates RC, Deleo MJ, III, Mercurio AM. The epithelial-mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1-mediated chemotaxis. Experimental Cell Research. 2004;299(2):315–324. doi: 10.1016/j.yexcr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 63.Fernando RI, Castillo MD, Litzinger M, Hamilton DH, Palena C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Research. 2011;71(15):5296–5306. doi: 10.1158/0008-5472.CAN-11-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allavena P, Germano G, Marchesi F, Mantovani A. Chemokines in cancer related inflammation. Experimental Cell Research. 2011;317(5):664–673. doi: 10.1016/j.yexcr.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 65.Kitadai Y, Haruma K, Mukaida N, et al. Regulation of disease-progression genes in human gastric carcinoma cells by interleukin 8. Clinical Cancer Research. 2000;6(7):2735–2740. [PubMed] [Google Scholar]
- 66.Wang B, Hendricks DT, Wamunyokoli F, Parker MI. A growth-related oncogene/CXC chemokine receptor 2 autocrine loop contributes to cellular proliferation in esophageal cancer. Cancer Research. 2006;66(6):3071–3077. doi: 10.1158/0008-5472.CAN-05-2871. [DOI] [PubMed] [Google Scholar]
- 67.Singh S, Nannuru KC, Sadanandam A, Varney ML, Singh RK. CXCR1 and CXCR2 enhances human melanoma tumourigenesis, growth and invasion. The British Journal of Cancer. 2009;100(10):1638–1646. doi: 10.1038/sj.bjc.6605055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghadjar P, Rubie C, Aebersold DM, Keilholz U. The chemokine CCL20 and its receptor CCR6 in human malignancy with focus on colorectal cancer. International Journal of Cancer. 2009;125(4):741–745. doi: 10.1002/ijc.24468. [DOI] [PubMed] [Google Scholar]
- 69.Darash-Yahana M, Gillespie JW, Hewitt SM, et al. The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS ONE. 2009;4(8) doi: 10.1371/journal.pone.0006695.e6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murakami T, Cardones AR, Finkelstein SE, et al. Immune evasion by murine melanoma mediated through CC chemokine receptor-10. Journal of Experimental Medicine. 2003;198(9):1337–1347. doi: 10.1084/jem.20030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang J, Seethala RR, Zhang Q, et al. Autocrine and paracrine chemokine receptor 7 activation in head and neck cancer: implications for therapy. Journal of the National Cancer Institute. 2008;100(7):502–512. doi: 10.1093/jnci/djn059. [DOI] [PubMed] [Google Scholar]
- 72.Mañes S, Mira E, Colomer R, et al. CCR5 expression influences the progression of human breast cancer in a p53-dependent manner. Journal of Experimental Medicine. 2003;198(9):1381–1389. doi: 10.1084/jem.20030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu P, Nakamoto Y, Nemoto-Sasaki Y, et al. Potential interaction between CCR1 and its ligand, CCL3, induced by endogenously produced interleukin-1 in human hepatomas. The American Journal of Pathology. 2003;162(4):1249–1258. doi: 10.1016/S0002-9440(10)63921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Righi E, Kashiwagi S, Yuan J, et al. CXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancer. Cancer Research. 2011;71(16):5522–5534. doi: 10.1158/0008-5472.CAN-10-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Messmer D, Fecteau J-F, O’Hayre M, Bharati IS, Handel TM, Kipps TJ. Chronic lymphocytic leukemia cells receive RAF-dependent survival signals in response to CXCL12 that are sensitive to inhibition by sorafenib. Blood. 2011;117(3):882–889. doi: 10.1182/blood-2010-04-282400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y, Zhao H, Zhao D, et al. SDF-1/CXCR4 axis in myelodysplastic syndromes: correlation with angiogenesis and apoptosis. Leukemia Research. 2012;36(3):281–286. doi: 10.1016/j.leukres.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 77.Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 78.Amersi FF, Terando AM, Goto Y, et al. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clinical Cancer Research. 2008;14(3):638–645. doi: 10.1158/1078-0432.CCR-07-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clinical Cancer Research. 2008;14(21):6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 80.Marchesi F, Piemonti L, Fedele G, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Research. 2008;68(21):9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 81.Yoshie O, Fujisawa R, Nakayama T, et al. Frequent expression of CCR4 in adult T-cell leukemia and human T-cell leukemia virus type 1-transformed T cells. Blood. 2002;99(5):1505–1511. doi: 10.1182/blood.v99.5.1505. [DOI] [PubMed] [Google Scholar]
- 82.Kim MY, Oskarsson T, Acharyya S, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139(7):1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 singaling. Cancer Cell. 2007;11(6):526–538. doi: 10.1016/j.ccr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 84.Zhang XH, Wang Q, Gerald W, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16(1):67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kochetkova M, Kumar S, McColl SR. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death and Differentiation. 2009;16(5):664–673. doi: 10.1038/cdd.2008.190. [DOI] [PubMed] [Google Scholar]
- 86.Zhang Y, Yoneyama H, Wang Y, et al. Mobilization of dendritic cell precursors into the circulation by administration of MIP-1 α in mice. Journal of the National Cancer Institute. 2004;96(3):201–209. doi: 10.1093/jnci/djh024. [DOI] [PubMed] [Google Scholar]
- 87.Iida N, Nakamoto Y, Baba T, et al. Antitumor effect after radiofrequency ablation of murine hepatoma is augmented by an active variant of CC chemokine ligand 3/macrophage inflammatory protein-1α . Cancer Research. 2010;70(16):6556–6565. doi: 10.1158/0008-5472.CAN-10-0096. [DOI] [PubMed] [Google Scholar]
- 88.Förster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99(1):23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 89.Sharma S, Stolina M, Zhu L, et al. Secondary lymphoid organ chemokine reduces pulmonary tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Research. 2001;61(17):6406–6412. [PubMed] [Google Scholar]
- 90.Hillinger S, Yang SC, Batra RK, et al. CCL19 reduces tumour burden in a model of advanced lung cancer. The British Journal of Cancer. 2006;94(7):1029–1034. doi: 10.1038/sj.bjc.6603061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chang AE, Redman BG, Whitfield JR, et al. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clinical Cancer Research. 2002;8(4):1021–1032. [PubMed] [Google Scholar]
- 92.Kirk CJ, Hartigan-O’Connor D, Nickoloff BJ, et al. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Research. 2001;61(5):2062–2070. [PubMed] [Google Scholar]
- 93.Kirk CJ, Hartigan-O’Connor D, Mulé JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Research. 2001;61(24):8794–8802. [PubMed] [Google Scholar]
- 94.Baratelli F, Takedatsu H, Hazra S, et al. Pre-clinical characterization of GMP grade CCL21-gene modified dendritic cells for application in a phase I trial in non-small cell lung cancer. Journal of Translational Medicine. 2008;6, article 38 doi: 10.1186/1479-5876-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang SC, Hillinger S, Riedl K, et al. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clinical Cancer Research. 2004;10(8):2891–2901. doi: 10.1158/1078-0432.ccr-03-0380. [DOI] [PubMed] [Google Scholar]
- 96.Groom JR, Luster AD. CXCR3 in T cell function. Experimental Cell Research. 2011;317(5):620–631. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luster AD, Leder P. IP-10, a -C-X-C- chemokine, elicits a potent thymus-dependent antitumor response in vivo . Journal of Experimental Medicine. 1993;178(3):1057–1065. doi: 10.1084/jem.178.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang X, Chu Y, Wang Y, Zhang R, Xiong S. Targeted in vivo expression of IFN-γ-inducible protein 10 induces specific antitumor activity. Journal of Leukocyte Biology. 2006;80(6):1434–1444. doi: 10.1189/jlb.0306212. [DOI] [PubMed] [Google Scholar]
- 99.Hensbergen PJ, Wijnands PG, Schreurs MW, Scheper RJ, Willemze R, Tensen CP. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. Journal of Immunotherapy. 2005;28(4):343–351. doi: 10.1097/01.cji.0000165355.26795.27. [DOI] [PubMed] [Google Scholar]
- 100.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. Journal of Experimental Medicine. 1989;169(4):1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rollins BJ, Sunday ME. Suppression of tumor formation in vivo by expression of the JE gene in malignant cells. Molecular and Cellular Biology. 1991;11(6):3125–3131. doi: 10.1128/mcb.11.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nokihara H, Yanagawa H, Nishioka Y, et al. Natural killer cell-dependent suppression of systemic spread of human lung adenocarcinoma cells by monocyte chemoattractant protein-1 gene transfection in severe combined immunodeficient mice. Cancer Research. 2000;60(24):7002–7007. [PubMed] [Google Scholar]
- 103.Tsuchiyama T, Nakamoto Y, Sakai Y, et al. Prolonged, NK cell-mediated antitumor effects of suicide gene therapy combined with monocyte chemoattractant protein-1 against hepatocellular carcinoma. Journal of Immunology. 2007;178(1):574–583. doi: 10.4049/jimmunol.178.1.574. [DOI] [PubMed] [Google Scholar]
- 104.Lavergne E, Combadière B, Bonduelle O, et al. Fractalkine mediates natural killer-dependent antitumor responses in vivo . Cancer Research. 2003;63(21):7468–7474. [PubMed] [Google Scholar]
- 105.Tang L, Hu HD, Hu P, et al. Gene therapy with CX3CL1/Fractalkine induces antitumor immunity to regress effectively mouse hepatocellular carcinoma. Gene Therapy. 2007;14(16):1226–1234. doi: 10.1038/sj.gt.3302959. [DOI] [PubMed] [Google Scholar]
- 106.Zeng Y, Huebener N, Fest S, et al. Fractalkine (CX3CL1)- and interleukin-2-enriched neuroblastoma microenvironment induces eradication of metastases mediated by T cells and natural killer cells. Cancer Research. 2007;67(5):2331–2338. doi: 10.1158/0008-5472.CAN-06-3041. [DOI] [PubMed] [Google Scholar]
- 107.Iga M, Boissonnas A, Mahé B, Bonduelle O, Combadière C, Combadière B. Single CX3CL1-Ig DNA administration enhances T cell priming in vivo . Vaccine. 2007;25(23):4554–4563. doi: 10.1016/j.vaccine.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 108.van den Berg A, Visser L, Poppema S. High expression of the CC chemokine TARC in Reed-Sternberg cells: a possible explanation for the characteristic T-cell infiltrate in Hodgkin’s lymphoma. The American Journal of Pathology. 1999;154(6):1685–1691. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.di Stasi A, de Angelis B, Rooney CM, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113(25):6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moon EK, Carpenito C, Sun J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clinical Cancer Research. 2011;17(14):4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sarnaik AA, Weber JS. Recent advances using anti-CTLA-4 for the treatment of melanoma. Cancer Journal. 2009;15(3):169–173. doi: 10.1097/PPO.0b013e3181a7450f. [DOI] [PubMed] [Google Scholar]
- 112.Ribas A. Tumor immunotherapy directed at PD-1. The New England Journal of Medicine. 2012;366(26):2517–2519. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 113.Loberg RD, Ying C, Craig M, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo . Cancer Research. 2007;67(19):9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 114.Lesokhin AM, Hohl TM, Kitano S, et al. Monocytic CCR2+ myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Research. 2012;72(4):876–886. doi: 10.1158/0008-5472.CAN-11-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brandau S, Trellakis S, Bruderek K, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. Journal of Leukocyte Biology. 2011;89(2):311–317. doi: 10.1189/jlb.0310162. [DOI] [PubMed] [Google Scholar]
- 116.Ishida T, Ueda R. Antibody therapy for adult T-cell leukemia-lymphoma. International Journal of Hematology. 2011;94(5):443–452. doi: 10.1007/s12185-011-0941-5. [DOI] [PubMed] [Google Scholar]
- 117.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 118.Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor responses in humans. Proceeding of National Academy of Science of the United States of America. 2013;110(44):17945–17950. doi: 10.1073/pnas.1316796110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fridlender ZG, Buchlis G, Kapoor V, et al. CCL2 blockade augments cancer immunotherapy. Cancer Research. 2010;70(1):109–118. doi: 10.1158/0008-5472.CAN-09-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kitamura T, Kometani K, Hashida H, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nature Genetics. 2007;39(4):467–475. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 121.Kitamura T, Fujishita T, Loetscher P, et al. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in amouse model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(29):13063–13068. doi: 10.1073/pnas.1002372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. Journal of Clinical Investigation. 1996;97(12):2792–2802. doi: 10.1172/JCI118734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kryczek I, Wei S, Keller E, Liu R, Zou W. Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis. The American Journal of Physiology—Cell Physiology. 2007;292(3):C987–C995. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- 124.Addison CL, Arenberg DA, Morris SB, et al. The CXC chemokine, monokine induced by interferon-γ, inhibits non-small cell lung carcinoma tumor growth and metastasis. Human Gene Therapy. 2000;11(2):247–261. doi: 10.1089/10430340050015996. [DOI] [PubMed] [Google Scholar]


