Abstract
Acid-sensing ion channels (ASICs) belong to the family of the epithelial sodium channel/degenerin (ENaC/DEG) and are activated by extracellular protons. They are widely distributed within both the central and peripheral nervous systems. ASICs were modified by the activation of γ-aminobutyric acid receptors (GABAA), a ligand-gated chloride channels, in hippocampal neurons. In contrast, the activity of GABAA receptors were also modulated by extracellular pH. However so far, the mechanisms underlying this intermodulation remain obscure. We hypothesized that these two receptors-GABAA receptors and ASICs channels might form a novel protein complex and functionally interact with each other. In the study reported here, we found that ASICs were modified by the activation of GABAA receptors either in HEK293 cells following transient co-transfection of GABAA and ASIC1a or in primary cultured dorsal root ganglia (DRG) neurons. Conversely, activation of ASIC1a also modifies the GABAA receptor-channel kinetics. Immunoassays showed that both GABAA and ASIC1a proteins were co-immunoprecipitated mutually either in HEK293 cells co-transfected with GABAA and ASIC1a or in primary cultured DRG neurons. Our results indicate that putative GABAA and ASIC1a channels functionally interact with each other, possibly via an inter-molecular association by forming a novel protein complex.
Introduction
Acid-sensing ion channels (ASICs) belong to the family of the epithelial sodium channel/degenerin (ENaC/DEG) and are activated by extracellular protons [1]. They are widely distributed within both the central and peripheral nervous systems [2]. The activation of ASICs by protons induces sodium and/or calcium influx, giving rise to depolarization and evoking action potentials in neurons [3].Acid-sensing ion channels(ASICs) are associated with various physiological and pathophysiological functions including regulation of synaptic plasticity [4], perception of pain [5], ischemic death of neurons [6] and the termination of seizures [7]. ASICs were modified by the activation of γ-aminobutyric acid receptors (GABAA), a ligand-gated chloride channels, in hippocampal neurons [8]. In contrast, the activity of GABAA receptors were also modulated by extracellular pH [9]–[11]. However, the mechanisms underlying this intermodulation remain .unclear. Megan et. al. identified the α β subunit TM2 residue mediating proton modulation of GABAA receptors [12], [13]. Huang et. al. reported that external protons regulated GABAA receptor function by direct or allosteric interaction with the GABA binding site [14]. But whether there was a direct binding site for proton within the GABAA receptor was so far unknown. We hypothesized that GABAA receptors and ASICs channels might form a novel protein complex and functionally interact with each other. In the study reported here, we found that ASICs were modified by the activation of GABAA receptors either in HEK293 cells following transient co-transfection of GABAA and ASIC1a or in primary cultured dorsal root ganglia (DRG) neurons. Conversely, activation of ASIC1a also modifies the current kinetics of GABAA current. Immunoassays showed that both GABAA and ASIC1a proteins were co-immunoprecipitated mutually either in HEK293 cells following transient co-transfection of GABAA and ASIC1a or in primary cultured DRG neurons. Our results indicate that putative GABAA and ASIC1a channels functionally interact with each other, possibly via an inter-molecular association by forming a novel protein complex. ASIC1a is specifically located in DRG neurons and function as a pain sensor, thus the interaction of GABAA and ASIC1a may contribute to pain sensation.
Results
Activation of GABAA receptors inhibits ASIC1a currents in HEK293 cells
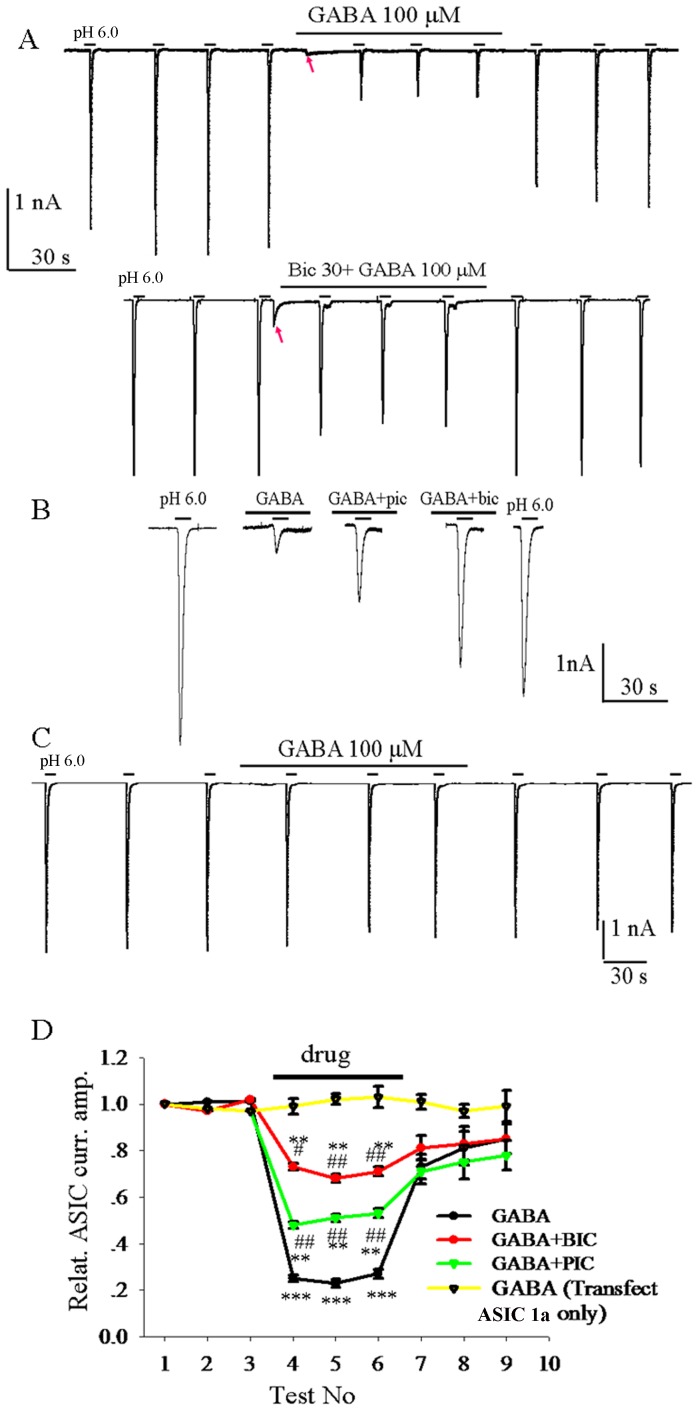
We used a whole-cell voltage-clamp configuration to record ASIC currents in HEK293 cells co-transfected with GABAA receptor subunits (α1 and β2) and ASIC1a in response to repeated application of a pH 6 solution. The peak amplitude of whole-cell ASIC currents (evoked with pH 6 solution) in HEK293 cells was stable, averaging 2.51±0.37 nA (n = 38).Under our recording conditions the responses to GABA (at 100 µM) were small relative to ASICs currents (230±19 pA, n = 27) due to the small driving force on chloride at −60 mV(Figure 1A). Application of GABA reversibly inhibited ASIC currents (Figure 1A), which was largely abolished by application of a GABAA receptors antagonist (either bicuculline or picrotoxin) (Figure 1B). To further confirm this phenomenon, we investigated whether GABA affected ASIC1a currents in HEK293 cells transfected with ASIC1a cDNA only. The result showed that GABA had no any effect on ASIC currents (Figure 1C). To clarify whether this inhibition is pH-dependent, we tested the effect of GABA on ASIC currents evoked by lowered pH (≤3.5). In general, the current evoked with pH 3.5 solution comprised of fast transient component and followed sustained component. Our results show that activation of GABAA receptors also attenuated the peak current amplitude but enhanced the sustained current evoked with pH 3.5 solution, such effect was eliminated when GABAAR was blocked or HEK293 cells was transfected with ASIC1a cDNA only (Figure 2). These results suggested that activation of GABAA receptors strongly regulates ASIC1a currents.
Figure 1. Activation of GABAA receptors reversibly inhibits ASIC1a currents.
A, ASIC1a were activated by pH 6.0 solution repetitively in HEK293 cells co-transfected with GABAA receptor subunits (α1 and β2) and ASIC1a. GABA (100 μM) reversibly attenuated ASIC1a currents. Red arrow indicates the current activated by GABA. B, co-application of bicuculline (BIC, 30 μM) or of picrotoxin (PIC, 100 μM) with GABA largerly abolished the GABA-induced inhibition of ASICs. C, GABA had no effect on ASIC1a currents in HEK293 cells transfected with cDNA of ASIC1a only. D, statistic graph shows relative ASIC currents that were affected by GABA but reversed by antagonists of GABAA receptors. n = 6, ***, p<0.001, T-test, before vs. after drug; ###, p<0.001, one-way ANOVA, GABA plus GABA antagonists vs. GABA alone.
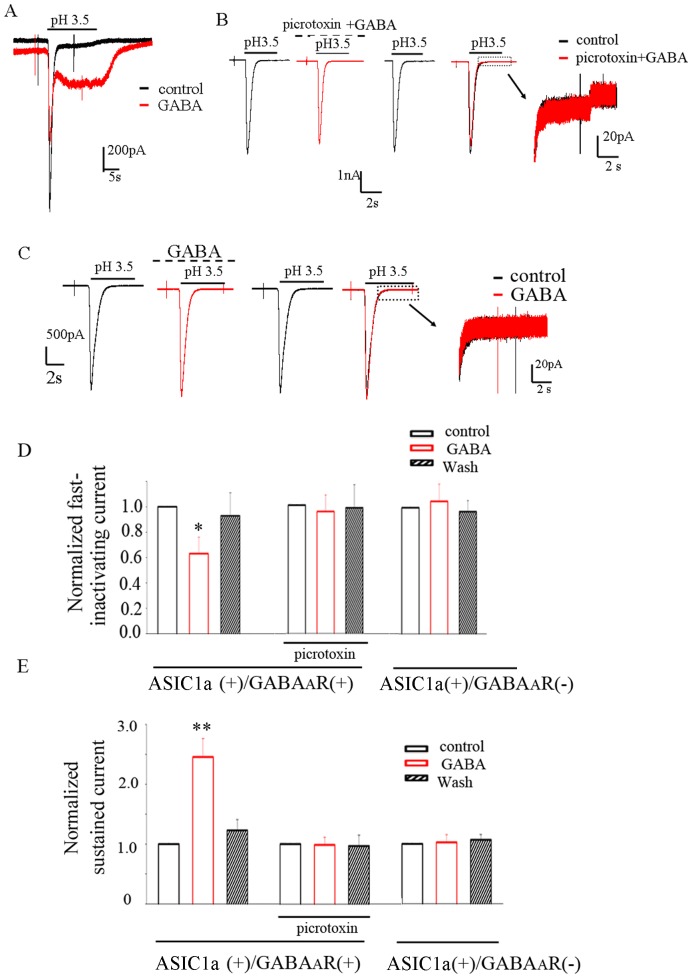
Figure 2. Activation of GABAA receptors attenuated the peak current amplitude and enhanced the sustained current of ASIC1a.
A, Example traces of a fast-inactivating transient current and a sustained current of ASIC1a activated by pH 3.5. GABA (100 μM) attenuated a fast-inactivating transient current and enhanced the sustained current of ASIC1a in HEK293 cells co-transfected with GABAA receptor subunits (α1 and β2) and ASIC1a, which can totally abolished by co-application of picrotoxin (100 μM) with GABA. ASIC1a current traces were superimposed to the right (inset) (B). C, GABA had no effect on ASIC1a currents in HEK293 cells transfected with ASIC1a cDNA only. ASIC1a current traces were superimposed to the right (inset).
Activation of ASIC1a modifies the current kinetics of GABAA current
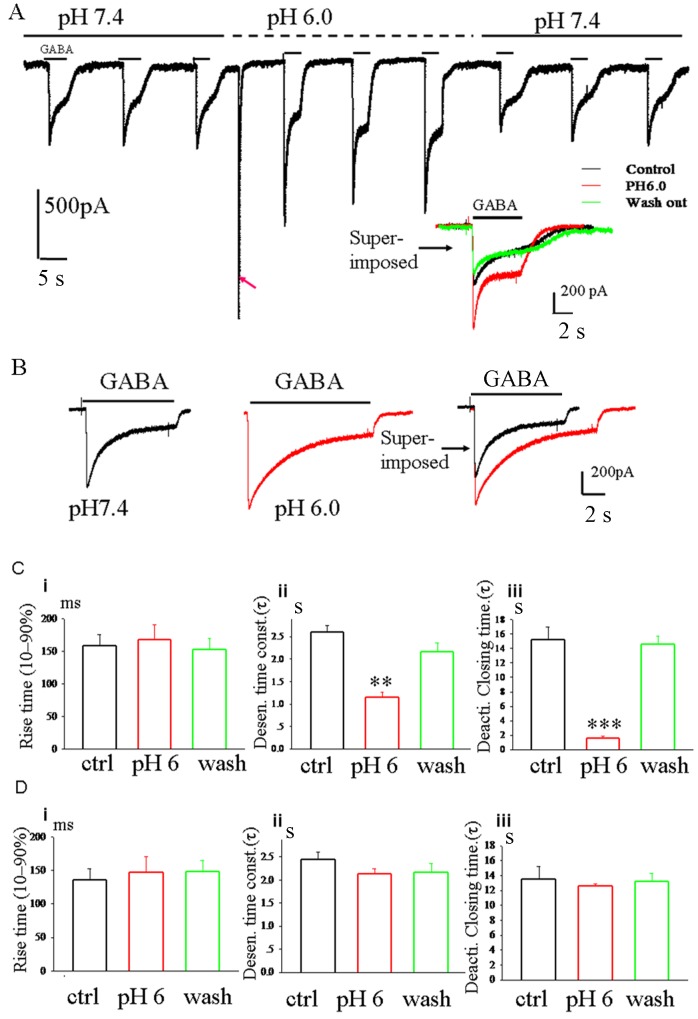
Activation of ASIC1a reversibly altered the overall shape of GABAA currents in HEK293 cells co-transfected with GABAA receptor subunits (α1 and β2) and ASIC1a. Activation of ASIC1a had multiple effects on the GABAA currents, not only was the peak amplitude of the ASIC current enhanced, but also the kinetics of the GABAA currents were altered. Although activation of ASIC1a did not change the rise time (10%–90%) for the GABAA currents, the time for desensitization or deactivation of GABAA currents were markedly decreased when the pH of the extracelluar solution was decreased from 7.4 to 6. After washout, the time for desensitization and deactivation was totally recovered (Figure 3A, n = 12). To exclude the direct role of proton on GABAA currents, we transfected HEK293 cells with GABAA receptor subunits only and did not obtain any current response to pH 6 solution although the peak amplitude of GABAA currents was also altered (Figure 3B, n = 12).These data indicate that the functions of GABAA receptors are modified by ASIC1a.
Figure 3. Activation of ASIC1a modifies the current kinetics of GABAA current.
A, The representative current traces recorded from HEK293 cells co-transfected with GABAA receptor subunits (α1 and β2) and ASIC1a. ASIC1a activated by pH 6 reversibly altered the overall shape of GABAA currents. GABAA current traces were superimposed to the right. Red arrow indicates the current activated by pH 6 solution. B, pH 6 solution changed the peak current amplitude but not the shape of GABAA currents in HEK293 cells transfected with cDNA of GABAA receptor subunits only. C (cotransfected with both plasmids) and D (transfected with cDNA of GABAA receptor subunits), bar graph showing the summarized data of rise time of activation (10–90%) (i), desensitization time constant (ii) and deactivation time (iii) of GABAA currents in the presence of pH 7.4 or pH 6. ***, paired t-test, p<0.001, pH6 group vs. control group, n = 12.
Co-immunoprecipitation of ASIC1a and GABAA proteins in transfected HEK293 cells and primary cultured neurons
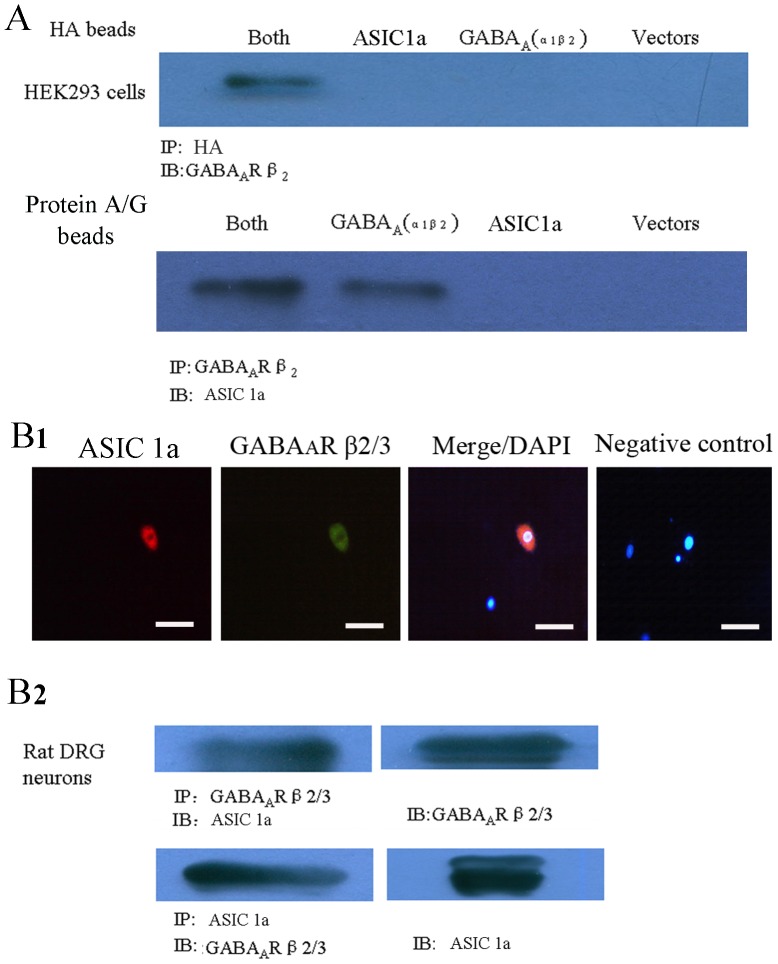
To investigate the underlying mechanisms of interregulation of ASIC1a and GABAA proteins, we transiently co-transfected ASIC1a and GABAAR in HEK293 cells. Due to endogenous expression of ASIC1a in HEK293 cells, we transfected ASIC1a with HA tag. In Co-IP experiments, anti-HA magnetic beads are used for the immunoprecipitation of specific HA-tagged proteins expressed in HEK293 cells. Our results showed that GABAA specifically co-precipitated with ASIC1a only in cells co-transfected with ASIC1a and GABAA, which was confirmed by reversed Co-IP using antibodies to GABAAR β2 (Figure 4 A). ASIC1a endogenously expressed in HEK-293 cells [15]. Indeed, in our studies, we found that endogenous ASIC1a also co-precipitated with GABAAR in HEK293 cells transfect with GABAAR α1β2 subunits. It is well known that DRG neurons expressed both ASIC1a and GABAAR. To examine the possible co-expression of endogenous ASIC1a and GABAAR, primary rat DRG neurons were incubated with specific anti-GABAAR and anti-ASIC1a antibodies. The merged image indicates that ASIC1a and GABAAR are co-segregated with each other (Figure 4 B1). To further investigate a possible association between ASIC1a and GABAAR proteins, GABAAR was immunoprecipitated from rat DRG lysates with a polyclonal anti-GABAAR β2/3 antibody. The immunoprecipitated samples were probed with ASIC1a antibody. Conversely, the total DRG lysates was precipitated with ASIC1a antibody and then probed with GABAAR β2/3 antibody (Figure 4 B2). Taken together, our results showed that ASIC1a and GABAA proteins co-immunoprecipitated each other.
Figure 4. Co-immunoprecipitation of ASIC1a and GABAA proteins in transfected HEK293 cells and DRG neurons.
A. Co-immunoprecipitation of ASIC1a and GABAA proteins in transfected HEK293 cells. GABAA specifically co-precipitates with ASIC1a in cells co-transfected with ASIC1a and GABAA (both). n = 3. B1. DRG neurons were double-labeled with anti-ASIC1a and -GABAAR antibodies. ASIC1a (red in left panel) as well as GABAAR (green in middle panel) localized to the apical membrane of DRG neurons. The merge (right) of ASIC1a and GABAAR images indicates that ASIC1a co-segregates with GABAAR in the apical membrane in neurons (yellow). Nuclei were identified by DAPI staining (blue). Scale bars equal 50 µm. B2. GABAA precipitates with ASIC 1a in primary cultured DRG neurons. DRG neurons lysates were immunoprecipitated (IP) with anti-GABAA β2/3 subunits polyclonal antibody in 8% SDS-PAGE gel, and the blot was then probed with anti- ASIC 1a polyclonal antibody (IB). In turn, ASIC 1a co-immunoprecipitates with GABAA. Cell lysates were immunoprecipitated with ASIC 1a antibody in 6% SDS-PAGE gel, and the blot was probed with GABAA β2/3 antibody. These experiments were repeated three times with identical results.
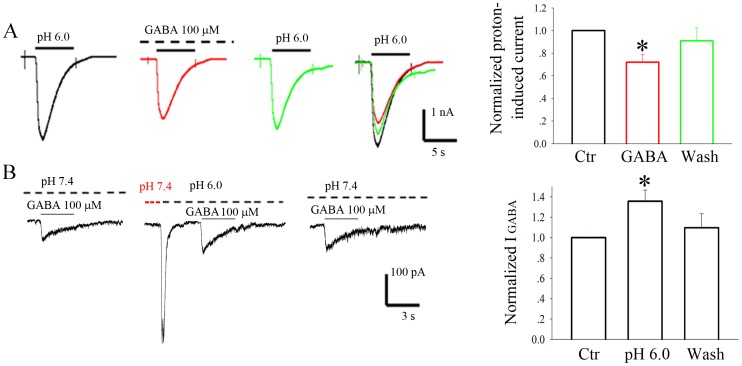
Interregulation of GABAA receptors and ASIC1a in DRG neurons
Both GABAA receptors and ASIC1a channels colocalized in rat DRG neurons. To examine the interaction of endogenous ASIC1a and GABAA receptors in primary cultured rat DRG neurons, we used a whole-cell voltage-clamp configuration to record ASIC currents or GABAA currents in DRG neurons in response to application of a pH 6 solution or 100 µM GABA. GABA (100 μM) reversibly attenuated acid-evoked currents (Figure 5A, n = 12). Conversely, GABA induced current was enhanced in the presence of pH 6.0 solution (Figure 5B, n = 7).
Figure 5. Interregulation of ASIC1a and GABAA receptors in DRG neurons.
A. Example traces of ASICs current activated by pH 6.0 solution in DRG neurons. GABA (100 μM) reversibly attenuated acid-evoked currents (n = 12). B. The representative current traces of GABA induced currents in DRG neurons. The pH 6.0 solution induced an inward current (enlarge inset). GABA-induced current was enhanced in the presence of pH 6.0 solution (n = 7).
Discussion
In the study reported here, activation of GABAA receptors strongly attenuates the peak current amplitude of ASIC currents in transfected HEK 293 cells. Conversely, Activation of ASIC1a modifies the current kinetics of GABAA current. These modifications included enhancement of the peak amplitude of GABAA current and slowing of channel kinetics. Similar effects were observed in primary cultured DRG neurons. Furthermore, ASIC1a is co-segregated with GABAA proteins in either transfected HEK 293 cells or rat DRG neurons, a finding verified by the immunoblotting assays. Our overall conclusion is that ASIC1a and GABAA interregulated each other through a conformation-dependent protein-protein interaction.
Interregulation of ASIC1a and GABAA receptors through conformation-dependent protein-protein interaction
Modification of ASIC1a by GABAA receptors occurred rapidly, and when the activation of GABAA chloride channels were blocked by pharmacological blockade or genetic loss, the modifications were eliminated or largely reduced. The ASIC currents also recovered rapidly (Figure 1). This study suggests that ASIC1a current is modifed directly by activation of GABAA receptors. In 2011, Cheng et. al. found that ASICs were reversibly inhibited by activation of GABA receptors in murine hippocampal neurons and such inhibition of ASICs required opening of the chloride channels. Therefore these authors speculate that a conformation-dependent interaction might occur between GABA receptors and ASICs [16]. Our present studies further demonstrated that these two receptors form a novel protein complex by performing the Co-IP experiments. These two receptors physically couple together and interact with each other that may depend on their conformation change. However, so far we can not exclude the possibility that an intermediate protein or regulator might participate in this receptor-receptor interaction.
Furthermore, in our present studies, we also demonstrated that pH 6 extracelluar solution largely decreased the time for desensitization or deactivation of GABAA currents in HEK 293 cells co-transfected with ASIC1a and GABAA, but not in HEK293 cells transfected with GABAA cDNA only, suggesting that these two receptors combine with each other and regulate each other. External protons regulate GABAA receptor function by direct or allosteric interaction with the GABA binding site [14]. So far, we can not exclude the possibility that GABAA receptor may have a proton binding site. In summary, upon binding of GABA, the GABAA receptors undergo a conformational change and then modify current kinetics of ASIC1a by allosteric interaction with the proton binding site. Conversely, when ASIC1a is activated, the ASIC1a channels undergo a conformational change, then such change is converted to GABAA receptors. Our results indicate that putative GABAA receptors and ASIC1a channels phyically couple and functionally interact with each other, possibly via an inter-molecular association.
Physiological and pathophysiological implications of the interaction between ASIC 1a and GABAA receptors
of all ASICs, ASIC1a appears to play a prominent role in determining current amplitude and also affects the kinetics of H+-gated current [4], [17]–[19]. In CNS neurons, ASIC1a has been shown to be involved in synaptic plasticity, learning and memory [4], [20], and in acidosis-mediated, glutamate-independent neuronal injury [6], [21]. ASIC1a is expressed throughout the brain, with prominent expression in areas that receive rich synaptic input [17], [20], [22], [23]. Moreover, ASIC1a have a higher expression in GABAergic interneurons than that in the principal neurons [7], [24]. Given that the GABAA receptors are the predominant inhibitory ionotropic receptors in the CNS, the interaction between ASIC1a and GABAA receptors may occur at numerous locations and could be involved in a number of brain functions. Furthermore, ASIC1a is specifically located in DRG neurons and functions as a pain sensor, thus the interaction of GABAA and ASIC1a may contribute to pain sensation.
Materials and Methods
Transfection of HEK293
HEK 293 cells were cultured in DMEM (HyClone) supplemented with 10% fetal bovine serum (HyClone), 1% penicillin/streptomycin at 37°C in a 5% CO2 incubator. Cells were transfected with pcDNA3.0 constructors encoding ASIC1a and/or GABAAR α1β2, using Lipofectamine TM 2000 (Invitrogen) according to the manufacturer's instructions. All recordings were made 24 to 48 h after transfection in the GFP-positive cells.
DRG Cell Isolation and Culture
All the animal experiments were approved by the Medical Ethics Committee of Shandong University (number ECAESDUSM 2012029). Adult Wistar rats were euthanized by cervical dislocation and the entire spinal columns were removed. Bilateral DRGs were collected and washed twice with L-15 medium (Gibco, Gaithersburg, MD). They were then incubated in 10 ml L-15 medium containing 10 mg collagenase type 1 (Sigma, St. Louis, MO) and 0.25 ml 0.25% Trypsin (HyClone, Thermo scientific, USA) at 37°C for 50 min. DRGs were removed from the enzyme solution, centrifuged for 5 min at 1,000 revolutions/min, washed twice with L-15 medium, and transferred to 2 ml L-15 medium containing 10% FBS. The ganglia were triturated with a suction pipe for 3-min and then centrifuged for 50 seconds at 1,000 revolutions/min. Supernatants were placed into 35 mm diameter Petri dishes. The cells were then cultured at 37°C in a 5% CO2 incubator (Thermo Forma, Hamilton, NJ, USA). In our study, we chose freshly isolated neurons from rat DRGs in the range of 15–30 µm diameter to test the effect of GABA on acid-evoked currents or pH on GABA-induced current.
Electrophysiological Recordings
Whole-cell voltage-clamp and current-clamp recordings were performed at room temperature (22–25°C) using a computer amplifier (Multiclamp 700B; Axon, New York, NY, USA) and a Digidata (1440A; Axon). Patch pipettes were filled with intracellular solution including (in mM): KCl 140, MgCl2 2.5, HEPES 10, EGTA 11 and Na2ATP 5 with pH adjusted to 7.2 using KOH. Cells were bathed in extracellular saline containing (in mM): NaCl 150, KCl 5, CaCl2 2.5, MgCl2 2, HEPES 10, D-glucose 10 with pH adjusted to 7.4 using NaOH. The resistance of the recording pipettes was in the range of 5–8 MΩ. The series resistance was compensated for 70–80% after establishing a whole-cell configuration. The membrane potential was held at −60 mV throughout the recordings unless otherwise specified. Current-clamp recordings were obtained by switching to current-clamp mode after a stable whole-cell configuration was formed in the voltage-clamp mode. In this experiment, only cells with a stable resting membrane potential (less than −50 mV) were used. Signals were filtered at 4 kHz and then digitized at 10 kHz. The data were analyzed with the pCLAMP 10 acquisition software (Axon Instruments, CA, USA).
Co-immunoprecipitation (Co-IP)
For Co-IP in human embryonic kidney 293 (HEK293) cells, Lipofectamine 2000 (Invitrogen) was used to transiently transfect ASIC1a with HA tag and/or GABAAR α1β2 following the manufacturer's instructions. Cell protein was purified for ASIC1a and GABAAR expression 36 h post-transfection. HEK293 cells were lysed in 1 ml of lysis buffer (1% Triton X-100, 50 mM Tris buffer pH 7.5) with a freshly added protein inhibitor mixture tablet. For Co-IP, the protein complexes were immunoprecipitated by anti-HA beads agarose (Sigma). After incubating, the beads were pelleted and washed three times in lysis buffer (1% Triton X-100, 50 mM Tris buffer pH 7.5), and the samples were loaded and run in 8% SDS-PAGE gel. The precipitates were transferred to a PVDF membrane and immunoblotted by anti-GABAAR β2 antibody (Millipore). The blots were developed by the enhanced chemiluminescence kit. Rat DRG or HEK293 cells were lysed in lysis buffer (1% Triton X-100, 50 mM Tris buffer pH 7.5) and a mixture of protease inhibitors. Anti-ASIC1a antibody or anti-GABAAR β2/3 antibody was immunoprecipitated with protein A/G-agarose beads (Santa Cruz Biotechnology). The protein complexes were immunoprecipitated with antibody cross-linked protein A/G-agarose beads. After incubating the lysates with cross-linked antibody, the beads were pelleted and washed three times in lysis buffer (1% Triton X-100, 50 mM Tris buffer pH 7.5), and the samples were loaded and run in 8% SDS-PAGE gel. The precipitates were transferred to a PVDF membrane and immunoblotted by anti-GABAAR β2/3 antibody (Millipore) or anti-ASIC1 antibody (Sigma). The blots were developed by the enhanced chemiluminescence kit.
Drug Application
All drugs were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Except picrotoxin (dissolved in DMSO), all drugs were initially made up as stock solutions in distilled water and subsequently diluted in the external solution of the cells at a maximum of 1∶1000 to achieve their final working concentrations.
Data Analysis
Data were expressed as mean ± SEM and compared statistically using paired t tests by Sigma Plot 10.0. A p<0.05 was required for the results to be considered statistically significant.
Acknowledgments
We thank Dr. TL Xu from the Institute of Neuroscience and State Key Laboratory of Neuroscience, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, China, for providing plasmids of ASIC1a and Dr. Yu-Tian Wang from University of British Columbia, Vancouver, BC, Canada, for providing plasmids of GABAAR α1 β2.
Funding Statement
This work was supported by grants from the Promotive Research Fund for Excellent Young and Middle-Aged Scientists of Shandong Province (No. BS2010SW029) and the National Natural Science Foundation of China (No. 31171108). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Krishtal OA, Pidoplichko VI (1981) A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience 6: 2599–2601. [DOI] [PubMed] [Google Scholar]
- 2. Krishtal O (2003) The ASICs: signaling molecules? Modulators? Trends Neurosci 26: 477–483. [DOI] [PubMed] [Google Scholar]
- 3. Mamet J, Baron A, Lazdunski M, Voilley N (2002) Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci 22: 10662–10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, et al. (2002) The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 34: 463–477. [DOI] [PubMed] [Google Scholar]
- 5. Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, et al. (2002) A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A 99: 8992–8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, et al. (2004) Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118: 687–698. [DOI] [PubMed] [Google Scholar]
- 7. Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA 3rd, et al. (2008) Seizure termination by acidosis depends on ASIC1a. Nat Neurosci 11: 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen X, Whissell P, Orser BA, MacDonald JF Functional modifications of acid-sensing ion channels by ligand-gated chloride channels. PLoS One 6: e21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou C, Xiao C, Deng C, Hong Ye J (2007) Extracellular proton modulates GABAergic synaptic transmission in rat hippocampal CA3 neurons. Brain Res 1145: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dietrich CJ, Morad M Synaptic acidification enhances GABAA signaling. J Neurosci 30: 16044–16052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robello M, Baldelli P, Cupello A (1994) Modulation by extracellular pH of the activity of GABAA receptors on rat cerebellum granule cells. Neuroscience 61: 833–837. [DOI] [PubMed] [Google Scholar]
- 12. Wilkins ME, Hosie AM, Smart TG (2002) Identification of a beta subunit TM2 residue mediating proton modulation of GABA type A receptors. J Neurosci 22: 5328–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilkins ME, Hosie AM, Smart TG (2005) Proton modulation of recombinant GABA(A) receptors: influence of GABA concentration and the beta subunit TM2-TM3 domain. J Physiol 567: 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang RQ, Chen Z, Dillon GH (2004) Molecular basis for modulation of recombinant alpha1beta2gamma2 GABAA receptors by protons. J Neurophysiol 92: 883–894. [DOI] [PubMed] [Google Scholar]
- 15. Gunthorpe MJ, Smith GD, Davis JB, Randall AD (2001) Characterisation of a human acid-sensing ion channel (hASIC1a) endogenously expressed in HEK293 cells. Pflugers Arch 442: 668–674. [DOI] [PubMed] [Google Scholar]
- 16. Chen X, Whissell P, Orser BA, MacDonald JF (2011) Functional modifications of acid-sensing ion channels by ligand-gated chloride channels. PLoS One 6: e21970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M (1997) A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177. [DOI] [PubMed] [Google Scholar]
- 18. Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ (2004) Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem 279: 18296–18305. [DOI] [PubMed] [Google Scholar]
- 19. Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, et al. (2000) Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem 275: 25116–25121. [DOI] [PubMed] [Google Scholar]
- 20. Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH Jr, et al. (2003) Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci 23: 5496–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ (2004) Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A 101: 6752–6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia-Anoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP (1997) BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci U S A 94: 1459–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alvarez de la Rosa D, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, et al. (2003) Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol 546: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weng JY, Lin YC, Lien CC (2010) Cell type-specific expression of acid-sensing ion channels in hippocampal interneurons. J Neurosci 30: 6548–6558. [DOI] [PMC free article] [PubMed] [Google Scholar]