Abstract
Purpose
Human longevity results from a number of factors, including genetic background, favorable environmental, social factors and chance. In this study, we aimed to elucidate the association of human longevity with genetic variations in several major candidate genes in a Han Chinese population.
Methods
A case-control association study of 1015 long-lived individuals (aged 90 years or older) and 1725 younger controls (30–70 years old) was undertaken. Rs2075650 in TOMM40 was firstly genotyped using the ABI SNaPshot method in an initial cohort consisted of 597 unrelated long-lived individuals and 1275 younger controls enrolled from Sichuan. Secondly, eighteen tag single-nucleotide polymorphisms (SNPs) in the PVRL2-TOMM40-APOE locus were genotyped for extensive study in the same cohort. Finally, 5 associated SNPs were genotyped in a replication cohort including 418 older individuals and 450 younger controls. The genotype and allele frequencies were evaluated using the χ2 tests. The linkage disequilibrium (LD) block structure was examined using the program Haploview.
Results
The case-control study of rs2075650 in TOMM40 showed significant difference in allele frequencies between cases and controls (P = 0.006) in an initial study. Of the 18 SNPs genotyped, rs405509 in APOE and another three SNPs (rs12978931, rs519825 and rs395908) in the PVRL2 gene also showed significant association with human longevity in extensive study in the same cohort. Rs2075650 in TOMM40, rs405509 in APOE and rs519825 in PVRL2 showed a significant association with human longevity in a replication cohort.
Conclusion
These results suggested that PVRL2, TOMM40 and APOE might be associated with human longevity. However, further research is needed to identify the causal variants and determine which of these genes are involved in the progress of human longevity.
Introduction
Human longevity is considered a multi-factorial phenotype [1]. Worldwide human populations have shown an increase in mean life expectancy in the past two centuries [2], [3]. This is mainly due to environmental factors such as improved hygiene, nutrition and health care. The large variation in healthy lifespan among the elderly has prompted research into the determinants of aging and lifespan regulation. Longevity and healthy aging associated genetic research may provide further insights into the mechanisms of aging [4]–[7]. The genetic component has been shown to become stronger with increasing age of the individuals [8], [9]. The genetic contribution to human lifespan variation was estimated at 25–30% in twin studies [8], [10], [11]. The most prominent genetic influence is observed in families in which the capacity to attain a long lifespan clusters [12]–[14]. Epidemiological data indicate the presence of a strong familiar component of longevity that is largely determined by genetics and progeric syndromes of accelerated aging have known genetic causes [15]. Very long life, to beyond the age of 90 years, appears to have an even stronger genetic basis [16], which explains why centenarians and near-centenarians tend to cluster in families. Exceptional longevity can be reached with a low degree of age-related disability [17], [18], raising the question whether protective mechanisms against disease exist in long-lived subjects.
People who survived with long life (centenarians, octogenarians and nonagenarians) are characterized by marked delay or escape from age-related diseases, therefore analysis of genes that modulate susceptibility to age related diseases in these populations may provide insights into the human longevity [4]. Candidate genes for longevity encode proteins engaged in different biological processes including lipoprotein metabolism and inflammatory processes [1]. Several genes by far have repeatedly been associated with human longevity, such as APOE, FOXO3A and AKT1 [19]–[26].
Therefore, the present study was carried out to replicate and extend previous findings by testing the association of candidate genes previously reported to contain lifespan associated polymorphisms (including rs2075650 in TOMM40 and rs405509 in APOE), so that we could investigate the association of human longevity with genetic variations in major candidate genes in the Han Chinese population.
Materials and Methods
Subjects
In this study, the samples comprised of 2740 participants including 1015 individuals (mean age: 93.1±5.7 years, range: 90–105 years old) and 1725 younger controls (mean age: 57.3±10.3 years, range: 30–70 years old) ( Table 1 ). They composed of initial cohort (597 older individuals and 1275 younger controls) from Sichuan Province and replication cohort (418 older individuals and 450 younger controls) from Nantong city in Jiangsu Province. They were all of Han Chinese ethnicity. This study was approved by the Institutional Review Boards of the Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital. Written informed consents were obtained from all subjects prior to the studies.
Table 1. Samples used for analysis.
| Characteristics of the genotyped samples used for analysis | Cases | Controls |
| Initial cohort (Rs2075650 and another 18 SNPs) | 597 | 1275 |
| Mean age (year) * | 92.9±6.5 | 55.3±11.3 |
| Age range (year) | 90–105 | 30–70 |
| Replication cohort (5 SNPs) | 418 | 450 |
| Mean age (year) * | 93.3±5.8 | 58.3±9.7 |
| Age range (year) | 90–105 | 30–70 |
*The age when the cases and controls were recruited. ±: standard deviation;
Selection of candidate genes and SNPs
Rs2075650 in TOMM40 and another 18 tag SNPs in the PVRL2-TOMM40-APOE locus were chosen based on comprehensive literature/data base searches in different candidate longevity genes. Tag SNPs were identified according to the HapMap Phase II+III (Feb. 2009) of CHB database (Han Chinese in Beijing, China) and analyzed by using the Haploview software (version 4.2).
Extraction and quantification of genomic DNA
Venous blood from each subject was drawn and collected in an EDTA tube. Genomic DNA was extracted from peripheral blood with a QIAamp DNA Blood Maxi Kit (Qiagen), and was fluorometrically quantified with Quant-iT PicoGreen reagent (Invitrogen) according to the manufacturer's protocol.
SNP genotyping
To determine if any polymorphic variant is associated with human longevity between the long-lived individuals and younger controls, we firstly carried out the case-control study of rs2075650 in TOMM40 in an initial cohort from Sichuan ( Table 1 ) using the ABI SNaPshot method. Secondly, 18 tag SNPs in and around the TOMM40 and APOE locus covering the common genetic variation were followed to be genotyped for extensive study in the initial cohort. Finally, associated SNPs from extensive study were genotyped in a replication cohort.
SNP analysis was performed on the ABI 3130 Genetic Analyzer (Applied Biosystems, CA, USA). In brief, the polymerase chain reactions (10 µL final volume) contained 50 ng of genomic DNA, 1 µL of each primer (10 pmol/µL), 1 µL of 10 buffer (Takara Bio Inc., Shiga, Japan), 0.8 µL of deoxyribonucleotide triphosphates (2 mmol/L; Takara Bio Inc.), 0.4 µL MgCl2 (2.5 mmol/L; Takara Bio Inc.), and 0.1 µL of ExTaq polymerase (5 U/µL; Takara Bio Inc.). The product was then processed according to the ABI SNaPshot protocol using primers designed for fluorescent dideoxy nucleotide termination. The SNPs reported in this manuscript have a genotyping success rate of >98% and accuracy as judged by random re-genotyping of 5% of the samples in the cohort by sequencing analysis.
Statistical analysis
A standard χ2 test with a 1-degree-of-freedom (df) was used to assess the Hardy-Weinberg equilibrium (HWE) and the differences of allele frequencies for each SNP between the case and control group. Odds ratios (ORs) with 95 percent confidence intervals (CIs) were assessed for the risk allele of each SNP based on a multiplicative model. For the genotypes, we tested a series of genetic models including additive, dominant/recessive for the SNPs with a p value of <0.05 of allelic, trend test by using unconditional logistic regression with adjustment for gender. For multiple correction, the P values were corrected as combined P*5. All statistical analyses were performed by using the software SPSS 15.0 (SPSS Inc., Chicago, IL). The linkage disequilibrium (LD) block structure was examined using the program Haploview (version 4.2, Broad Institute, Cambridge, MA) [27]. The D' values and r 2 values for all pairs of SNPs were calculated, and the haplotype blocks were estimated using the program haploview.
Conditional Analysis of PVRL2-TOMM40-APOE locus
Conditional analyses of the PVRL2-TOMM40-APOE locus were completed at rs12978931 (PVRL2), rs519825 (PVRL2), rs395908 (PVRL2), rs2075650 (TOMM40) and rs405509 (APOE) in long-lived cases and younger controls. The allelic dosage of each SNP was individually included as an additional covariate in the logistic regression model. Starting with the major allele of one conditional SNP, we performed the likelihood ratio test for independence of the other SNPs to determine the significance of the difference between the alternated SNP model versus unalternated model and see the independent effect on risk of human longevity.
Results
Case-control study of rs2075650 in TOMM40 in an initial cohort
Rs2075650 in TOMM40 was firstly genotyped in an initial cohort consisted of 597 unrelated long-lived individuals and 1275 younger controls enrolled from Sichuan. The case-control study of rs2075650 in TOMM40 showed significant difference in allele frequencies (The MAF = 0.070 for cases and MAF = 0.098 for controls, OR = 1.430(95% CI 1.10–1.85), P = 0.006) between cases and controls in initial study. SNP rs2075650 is located in the intron of the TOMM40 gene at chromosome 19q13.32 but it is a strong proxy of the SNPs that define the APOE alleles [21], and has been shown a significant association longevity [14], [21], [23], [28].
Allelic association study of SNPs in and around TOMM40 in the initial cohort
Eeighteen tag SNPs in and around the TOMM40 and APOE locus covering the common genetic variation recurrently regarded as candidates for human longevity were genotyped in the initial cohort for extensive study. The TOMM40 gene located next to APOE locus, which is a very important genetic factor influencing longevity [1], [23], [25], as reported as a recent GWAS in Dutch Leiden Longevity Study independently confirmed the APOE longevity association [21]. Therefore, we conducted SNP genotyping in and around the TOMM40 gene to identify novel SNPs associated with human longevity in Chinese population. 18 SNPs, spanning about 45 kb (45.365–45.410 Mb), were selected from a genomic region including four genes ( Figure 1A ) and genotyped for extensive study in the same cohort ( Table 2 ). Among them, rs405509 in APOE and three SNPs (rs12978931, rs519825 and rs395908) in PVRL2 showed significant association with human longevity (OR = 1.180(95% CI 1.02–1.37), P = 0.027 for rs405509; OR = 1.152 (95% CI 1.00–1.32), P = 0.0445 for rs12978931; OR = 0.805(95% CI 0.65–1.00), P = 0.048 for rs395908; OR = 1.283(95% CI 1.05–1.57), P = 0.01577 for rs519825), whereas another 14 SNPs did not show significant differences between cases and controls in the cohort ( Table 2 ). In order to not miss the real association SNPs, we have chosen these five SNPs with P value less than 0.05 but not 0.0026 (multiple correction, 0.05/19 = 0.0026) to the replication study.
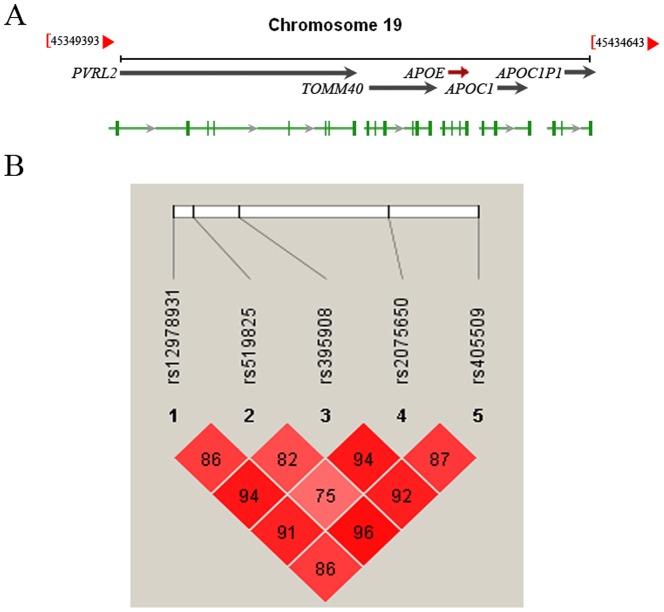
Figure 1. Five SNPs in the genomic region of PVRL2-TOMM40-APOE locus.
A. Genomic region of PVRL2-TOMM40-APOE locus associated with human longevity, horizontal arrows indicate the transcriptional orientations of individual genes. B. Pairwise LD among five SNPs in and surrounding the TOMM40 gene in the replication study. The LD spans the region including the PVRL2, TOMM40 and APOE, a distance of 45 kb. Linkage disequilibrium was measured by the D′ statistic using the data from all subjects. A D′ value of 100 indicates a complete LD between 2 markers, and a D′ value of 0 indicates a complete linkage equilibrium. Haploview version 4.2 software was used for the analysis.
Table 2. Extensive study for the association of SNPs in the vicinity of TOMM40-APOE region.
| SNP ID | Position (bp)a | Gene | Alleleb | Genotype countse | minor allele frequency | OR (95% CI) | Allelic P | ||
| Cases | Controls | Cases | Controls | ||||||
| rs1871046 | 50043777 | PVRL2 | G/A | 19/204/374 | 45/434/796 | 0.203 | 0.205 | 0.983(0.83–1.17) | 0.843 |
| rs12978931 | 50055540 | PVRL2 | G/A | 151/283/158 | 271/628/376 | 0.494 | 0.459 | 1.152 (1.00–1.32) | 0.0445 |
| rs519825 | 50058619 | PVRL2 | G/A | 14/143/440 | 18/258/999 | 0.143 | 0.115 | 1.283(1.05–1.57) | 0.01577 |
| rs419010 | 50060160 | PVRL2 | A/G | 261/253/82 | 489/607/179 | 0.350 | 0.378 | 1.131(0.98–1.31) | 0.091 |
| rs4803766 | 50063008 | PVRL2 | A/G | 72/279/246 | 159/600/514 | 0.354 | 0.361 | 0.972(0.84–1.12) | 0.708 |
| rs11879589 | 50065116 | PVRL2 | A/G | 0/21/576 | 0/27/1248 | 0.018 | 0.011 | 1.673(0.94–2.97) | 0.07601 |
| rs395908 | 50065405 | PVRL2 | T/C | 463/121/13 | 1026/239/10 | 0.123 | 0.102 | 0.805(0.65–1.00) | 0.048 |
| rs519113 | 50068124 | PVRL2 | C/G | 8/109/480 | 23/246/1006 | 0.105 | 0.115 | 0.904(0.72–1.13) | 0.3734 |
| rs6857 | 50084094 | PVRL2 | A/G | 0/55/542 | 0/130/1145 | 0.046 | 0.051 | 0.899(0.65–1.24) | 0.518 |
| rs10119 | 50098513 | TOMM40 | T/C | 593/4/0 | 1267/8/0 | 0.003 | 0.003 | 0.936(0.28–3.12) | 0.914 |
| rs449647 | 50100404 | APOE | A/T | 21/203/368 | 53/421/786 | 0.207 | 0.209 | 1.013(0.85–1.20) | 0.767 |
| rs405509 | 50100676 | APOE | C/A | 63/277/257 | 112/545/618 | 0.338 | 0.302 | 1.180(1.02–1.37) | 0.027 |
| rs440446 | 50101007 | APOE | G/C | 23/184/380 | 49/433/789 | 0.196 | 0.209 | 1.083(0.912–1.289) | 0.509 |
| rs769448 | 50101419 | APOE | T/C | 0/39/554 | 0/91/1183 | 0.033 | 0.036 | 1.089(0.744–1.595) | 0.660 |
| rs769449 | 50101842 | APOE | A/G | 4/109/484 | 6/213/1051 | 0.098 | 0.089 | 0.895(0.707–1.131) | 0.620 |
| rs7412 | 50103919 | APOE | T/C | 9/112/470 | 21/273/970 | 0.110 | 0.125 | 1.152(0.927–1.431) | 0.405 |
| rs429358 | 50103781 | APOE | C/T | 7/90/491 | 12/230/1033 | 0.088 | 0.10 | 0.877(0.69–1.11) | 0.2821 |
| rs4420638 | 50114786 | APOC1 | G/A | 3/104/489 | 8/266/1011 | 0.092 | 0.107 | 0.851(0.67–1.07) | 0.176 |
Genomic positions are according to NCBI build 36;
Minor allele/major allele;
The genotype counts are presented as homozygote/heterozygote/wildtype;
Replication study of the five associated SNPs in a replication cohort
Furthermore, the five associated SNPs based on extensive study including three SNPs in the PVRL2 gene, rs2075650 in TOMM40 and rs405509 in APOE were genotyped in a replication cohort ( Table 3 ). Our results showed the three SNPs (rs519825 in PVRL2, rs2075650 in TOMM40 and rs405509 in APOE) are significantly associated with human longevity in the replication cohort (allelic P = 0.022 for rs519825, P = 0.042 for rs405509 and P = 0.0030 for rs2075650) ( Table 3 ). Combined P values of the three SNPs are 0.00082, 0.0006 and 0.002, respectively; corrected P values of them are 0.04, 0.03 and 0.01.
Table 3. Replication study for the association of the associated SNPs in a replication cohort.
| SNP ID | Position (bp)a | Gene | Alleleb | Genotype countsc | minor allele frequency | OR (95% CI) | Allelic P d | Combined cohorte | CorrectedP f | ||
| Cases | Controls | Cases | Controls | ||||||||
| rs12978931 | 50055540 | PVRL2 | G/A | 105/197/113 | 96/238/110 | 0.490 | 0.484 | 1.025(0.85–1.24) | 0.800 | 0.053 | 0.265 |
| rs519825 | 50058619 | PVRL2 | G/A | 10/103/301 | 8/84/356 | 0.149 | 0.112 | 1.389(1.05–1.84) | 0.022 | 0.00082 | 0.004 |
| rs395908 | 50065405 | PVRL2 | T/C | 322/79/8 | 358/84/6 | 0.114 | 0.107 | 0.928(0.69–1.25) | 0.628 | 0.056 | 0.28 |
| rs2075650 | 50087459 | TOMM40 | G/A | 366/45/4 | 362/81/6 | 0.064 | 0.104 | 1.693(1.19–2.41) | 0.0030 | 0.0006 | 0.003 |
| rs405509 | 50100676 | APOE | C/A | 44/196/171 | 37/195/217 | 0.345 | 0.300 | 1.234(1.00–1.51) | 0.042 | 0.002 | 0.01 |
Genomic positions are according to NCBI build 36;
Minor allele/major allele;
The genotype counts are presented as homozygote/heterozygote/wildtype;
Allelic P value has been adjusted for sex;
Data from different study cohorts (initial cohort and replication cohort) were combined using Mantel-Haenszel models with fixed effects;
P values after multiple correction (combined P*5).
We also tested the association of these 5 SNPs by using recessive and dominant models. Since 3 statistical models were used in the replication study, the p-value for significant observation should be 0.5/(5 SNP*3 models) = 0.0033. Only the SNP rs2075650 in the TOMM40 had a significant association with human longevity of dominant model (P = 0.0000398, OR = 0.63 (0.51–0.79), Table 4 ). Another two SNPs (rs12978931 and rs395908) in the PVRL2 gene showed a trend of association with human longevity of recessive model (P = 0.015 and 0.012, respectively; OR = 1.26 (95% CI 1.05–1.51) and 2.27 (95% CI 1.18–4.38), respectively; Table 4 ).
Table 4. Genotype Analysis of 5 SNPs by recessive and dominant models.
| Gene | SNP | Position | Genotype | Case n(%) | Control n(%) | P_rec * | OR (95% CI)** | P_dom * | OR (95% CI)** |
| PVRL2 | rs12978931 | 50055540 | GG | 256 | 367 | 0.015 | 1.26(1.05, 1.51) | 0.444 | 1.07(0.90, 1.27) |
| GA | 480 | 866 | |||||||
| AA | 271 | 486 | |||||||
| PVRL2 | rs519825 | 50058619 | GG | 24 | 26 | 0.103 | 1.59(0.91, 2.78) | 0.001 | 1.34(1.12, 1.61) |
| GA | 246 | 342 | |||||||
| AA | 741 | 1355 | |||||||
| PVRL2 | rs395908 | 50065405 | CC | 21 | 16 | 0.012 | 2.27(1.18, 4.38) | 0.152 | 1.15(0.95, 1.39) |
| CT | 200 | 323 | |||||||
| TT | 785 | 1384 | |||||||
| TOMM40 | rs2075650 | 50087459 | AA | 7 | 16 | 0.513 | 0.74 (0.30, 1.81) | 3.98E-05 | 0.63 (0.51, 0.79) |
| AG | 123 | 310 | |||||||
| GG | 882 | 1398 | |||||||
| APOE | rs405509 | 50100676 | CC | 107 | 175 | 0.275 | 1.16 (0.89, 1.48) | 0.028 | 1.19 (1.02, 1.39) |
| CA | 473 | 822 | |||||||
| AA | 428 | 875 |
*P_rec, the P value of recessive model, P_dom, the P value of dominant model;
**OR, the odds ratio of homozygote; CI, Confidence Interval.
Conditional, Disequilibrium and Haplotype Association Analysis of PVRL2-TOMM40-APOE locus
To verify our results, we took the three significant association SNPs (PVRL2 rs519825, TOMM40 rs2075650 and APOE rs405509) and sequentially conditioned on the minor allele of each variant. Results of the conditional analysis showed that rs2075650 was significant associated SNP with longevity in the PVRL2-TOMM40-APOE locus ( Table 5 ). The analysis of linkage disequilibrium (LD) and haplotype block structure showed these SNPs were in the same LD block with each other ( Figure 1B ). Risk haplotype AACGA generated from these five SNPs proved to be significantly different between the cases and controls (P = 3.75×10−9, Table 6 ).
Table 5. Conditional analysis of the PVRL2-TOMM40-APOE locus in a replication cohort.
| SNP ID | Position (bp)a | Gene | Alleleb | Conditional on PVRL2 rs519825c | Conditional on TOMM40 rs2075650c | Conditional on APOE rs405509c | |||
| P | OR | P | OR | P | OR | ||||
| rs12978931 | 50055540 | PVRL2 | G/A | 0.80 | 0.97(0.77–1.21) | 0.14 | 0.77(0.62–0.95) | 0.50 | 0.88(0.63–1.25) |
| rs519825 | 50058619 | PVRL2 | G/A | - | - | 0.77 | 1.04(0.77–1.40) | 6.95E-04 | |
| rs395908 | 50065405 | PVRL2 | T/C | 0.68 | 1.07(0.77–1.47) | 0.39 | 1.12(0.84–1.23) | 0.98 | 0.99(0.7–1.4) |
| rs2075650 | 50087459 | TOMM40 | G/A | 0.0000156 | 2.41(1.60–3.64) | - | - | 7.84E-06 | 2.60(1.69–4.0) |
| rs405509 | 50100676 | APOE | C/A | 0.15 | 1.21(0.93–1.58) | 0.79 | 0.97(0.78–1.20) | - | - |
Genomic positions are according to NCBI build 36;
Minor allele/major allele;
The results of association testing of the PVRL2-TOMM40-APOE locus when the allelic dosage of rs519825, rs2075650 or rs405509 was included in the regression model.
Table 6. The haplotype association with human longevity in the replication cohort at PVRL2-TOMM40-APOE locus.
| Haplotypea | Frequency | Case, Control Frequencies | Chi Square | P Value |
| H1:AATGA | 0.394 | 0.403, 0.384 | 0.691 | 0.4058 |
| H2:GATGC | 0.178 | 0.179, 0.177 | 0.018 | 0.8927 |
| H3:GATGA | 0.178 | 0.189, 0.165 | 1.684 | 0.1944 |
| H4:GGTGC | 0.116 | 0.107, 0.126 | 1.559 | 0.2118 |
| H5:AACAA | 0.077 | 0.101, 0.050 | 15.62 | 7.74×10−5 |
| H6:AACGA | 0.028 | 0.006, 0.053 | 34.846 | 3.57×10−9 |
| H7:AATGC | 0.014 | 0.009, 0.019 | 2.945 | 0.0861 |
The haplotypes were generated from SNPs rs12978931, rs519825, rs395908, rs2075650 and rs405509.
Discussion
In the present study, we investigated genetic variations contributing to longevity and observed that rs519825 in PVRL2, a 45 kb-distance with the neighbouring gene APOE, is associated with human longevity. These three SNPs, including rs2075650 in TOMM40 and rs405509 in APOE, are located in at chromosome 19q13.32 in the APOE locus ( Figure 1 ), which has shown consistent evidence for the association with longevity [1], [21], [23], [25]. The strength of this study is that, by case-control study, we have replicated the previously reported association of the APOE locus with longevity [1], [25] as the major locus. In addition, we found that rs519825 in PVRL2, around TOMM40 and APOE, and rs405509 in APOE were associated with human longevity in the Han Chinese population.
To date, there is abundant evidence that human longevity is heritable based on twin studies and family studies, and approximately 25% of the overall variation in human lifespan can be attributed to genetic factors [3]–[5], which become more relevant for extreme longevity [6]. The Insulin/IGF-1 pathway has been reported to regulate lifespan extension in organisms ranging from invertebrates to mammals [29]. However, no convincing causal genes have yet been identified and efforts to map loci responsible for variation in human lifespan have limited success. One approach to investigate aging and longevity is to compare frequencies of genetic variants between nonagenarians or centenarians and the general population. In humans, both candidate gene and genome-wide genetic association approaches have been applied in an attempt to identify longevity loci. Multiple genes could mediate the aging process but would have their effects through numerous different patho-physiological processes and diseases that act as intermediate factors on the pathway to death [30], [31]. Therefore, any common variation in genes associated with aging probably has a small effect.
The more consistent evidence obtained by repeated observation in independent cohort studies for association with longevity has so far only been observed for three loci, the APOE locus [1], [25], [32], the FOXO3A locus [33]–[36] and the AKT1 locus [34]. APOE is involved in lipoprotein metabolism and believed to pose an effect on longevity [1]. The genetic origin of the three common variants of the human apolipoprotein E (apoE) protein, known as E2, E3 and E4, has been related to a number of age-related diseases, including Alzheimer disease, as well as to healthy aging and longevity. The APOE ε4 haplotype is by far the most validated genetic variation and has repeatedly been associated with human longevity [19]–[25], [37]. The best known longevity variant rs2075650 in TOMM40/APOE has been showed to be associated with human longevity, it reached irrefutable genome wide significance and replicated in the independent cohorts including German, Dutch and Danish cohorts [21], [23], [28]. Schupf et al found that the frequency and likelihood of carrying a G allele in rs2075650 of TOMM40 was lower among offspring in a long life family study (LLFS) in US Caucasian, compared with the likelihood in married-in controls [38]. Therefore, replication of this association in other long-lived cohorts will be needed to elucidate these results.
In our study, we applied a three-stage approach to investigate genetic variations contributing to longevity in a Han Chinese population. We found that three variations in PVRL2 showed significant association with human longevity in initial cohort. Furthermore, the association of rs519825 in PVRL2 could be replicated in another cohort, as well as the rs2075650 in TOMM40 and rs405509 in APOE. Haplotype analysis showed that the three SNPs (rs2075650 in TOMM40, rs405509 in APOE and rs519825 in PVRL2) were in the same LD block. Rs7412 and rs429358 are not significant associated with human longevity in Chinese population in this study, this finding was consistent with previous report shown rs429358 and rs7412 were not significantly different between Super-Seniors and controls [37]. The G allele in rs2075650 of TOMM40 was lower in the long-live individuals than that in the younger controls, which is consistent with previous studies [21], [23], [28], [38]. SNP rs405509 is a A/C variation upstream of the APOE gene, which has been reported to be associated with human lifespan [1], [21], [23], [25]. Rs2075650 is close to and in linkage disequilibrium with the rs429358, the APOE ε4 allele, and the investigators suggested that rs2075650 might not have an independent effect on longevity [21], [23], [28]. In this study, there were lower APOEε4 (rs429358) allele frequencies and higher ApoE ε2 (rs7412) allele frequencies in older group compared with younger cohort. This result suggested that ApoE ε4 carriers have an increased risk for mortality, while ApoE ε2 carriers are protected from diseases, which is consistent with previous studies [25], [39]. However, Yu et al's study [40] showed that rs429358 and rs7412 are not in high linkage disequilibrium with each other, which suggested that they are not necessarily expected to behave the consistent associations. The controversy regarding the association of rs429358 and rs7412 with longevity is possibly due to differences in the study design and population control selection between the studies. These differences of APOE genotype may caused by different ethnic group and influenced by other genetic and environmental factors. Therefore, further study with an increased sample size would be needed in order to conclusively test for variant associations in APOE.
PVRL2 encodes a single-pass type I membrane glycoprotein with two Ig-like C2-type domains and an Ig-like V-type domain. This protein is one of the plasma membrane components of adherents junctions. Expression of PVRL2 has been detected in many organs [41] including brain (http://genecards.ccbb.re.kr/cgi-bin/carddisp.pl?gene=PVRL2) and in several neuronal cell lines [42], [43]. So far, the biological relationship of PVRL2, as well as TOMM40 and APOE with human longevity, have not been well evaluated. Therefore, it is meaningful to explore the role of PVRL2 in influencing human longevity. Further biological studies will be helpful to explore the joint effects of different gene variants in longevity-related pathways and determine which genes are actually involved in the human longevity pathogenesis.
In conclusion, our results replicated the association between human longevity and a known variant in TMM40 near APOE in the mainland Han Chinese population. In addition, we have found another two variants (rs519825 in PVRL2 and rs405509 in APOE) were significantly associated with human longevity. The three SNPs are localized in APOE locus, which is universally recognized as a major disease susceptibility locus for human longevity, suggesting that this locus may contribute to human longevity in the Han Chinese population. Therefore, future exact studies in much larger cohorts to identify the causal variants and functional study are very important to verify whether any of these genes are involved in the development of human longevity.
Funding Statement
This study was supported by grants from the natural Science Foundation of China (81371048 to B.G., 81241001 and 81070761 to F.L.); the Department of Science and Technology of Sichuan Province (2011JTD0020 to Z.Y. and 2012JQ0023 to Y.S.); the Natural Science Foundation of China (81070718 to H.G.); the Medical key foundation of Jiangsu Province (XK200724 to H.G.); and the Department of Sichuan Provincial Health (130167 to B.G., 130193 to X.L.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Christensen K, Johnson TE, Vaupel JW (2006) The quest for genetic determinants of human longevity: challenges and insights. Nat Rev Genet 7: 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oeppen J, Vaupel JW (2002) Demography. Broken limits to life expectancy. Science 296: 1029–1031. [DOI] [PubMed] [Google Scholar]
- 3. Andersen SL, Sebastiani P, Dworkis DA, Feldman L, Perls TT (2011) Health span approximates life span among many supercentenarians: compression of morbidity at the approximate limit of life span. J Gerontol A Biol Sci Med Sci 67: 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herskind AM, McGue M, Holm NV, Sorensen TI, Harvald B, et al. (1996) The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet 97: 319–323. [DOI] [PubMed] [Google Scholar]
- 5. McGue M, Vaupel JW, Holm N, Harvald B (1993) Longevity is moderately heritable in a sample of Danish twins born 1870-1880. J Gerontol 48: B237–244. [DOI] [PubMed] [Google Scholar]
- 6. Reed T, Dick DM (2003) Heritability and validity of healthy physical aging (wellness) in elderly male twins. Twin Res 6: 227–234. [DOI] [PubMed] [Google Scholar]
- 7. Sebastiani P, Bae H, Sun FX, Andersen SL, Daw EW, et al. (2013) Meta-analysis of genetic variants associated with human exceptional longevity. Aging (Albany NY) 5: 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. v BHJ, Iachine I, Skytthe A, Vaupel JW, McGue M, et al. (2006) Genetic influence on human lifespan and longevity. Hum Genet 119: 312–321. [DOI] [PubMed] [Google Scholar]
- 9. Gogele M, Pattaro C, Fuchsberger C, Minelli C, Pramstaller PP, et al. (2011) Heritability analysis of life span in a semi-isolated population followed across four centuries reveals the presence of pleiotropy between life span and reproduction. J Gerontol A Biol Sci Med Sci 66: 26–37. [DOI] [PubMed] [Google Scholar]
- 10. Gudmundsson H, Gudbjartsson DF, Frigge M, Gulcher JR, Stefansson K (2000) Inheritance of human longevity in Iceland. Eur J Hum Genet 8: 743–749. [DOI] [PubMed] [Google Scholar]
- 11. Skytthe A, Pedersen NL, Kaprio J, Stazi MA, Hjelmborg JV, et al. (2003) Longevity studies in GenomEUtwin. Twin Res 6: 448–454. [DOI] [PubMed] [Google Scholar]
- 12. Perls T, Shea-Drinkwater M, Bowen-Flynn J, Ridge SB, Kang S, et al. (2000) Exceptional familial clustering for extreme longevity in humans. J Am Geriatr Soc 48: 1483–1485. [PubMed] [Google Scholar]
- 13. Schoenmaker M, de Craen AJ, de Meijer PH, Beekman M, Blauw GJ, et al. (2006) Evidence of genetic enrichment for exceptional survival using a family approach: the Leiden Longevity Study. Eur J Hum Genet 14: 79–84. [DOI] [PubMed] [Google Scholar]
- 14. Sebastiani P, Sun FX, Andersen SL, Lee JH, Wojczynski MK, et al. (2013) Families Enriched for Exceptional Longevity also have Increased Health-Span: Findings from the Long Life Family Study. Front Public Health 1: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fossel M (2000) Human aging and progeria. J Pediatr Endocrinol Metab 13 Suppl 6 1477–1481. [DOI] [PubMed] [Google Scholar]
- 16. Perls T, Kunkel LM, Puca AA (2002) The genetics of exceptional human longevity. J Mol Neurosci 19: 233–238. [DOI] [PubMed] [Google Scholar]
- 17. Christensen K, McGue M, Petersen I, Jeune B, Vaupel JW (2008) Exceptional longevity does not result in excessive levels of disability. Proc Natl Acad Sci U S A 105: 13274–13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Terry DF, Sebastiani P, Andersen SL, Perls TT (2008) Disentangling the roles of disability and morbidity in survival to exceptional old age. Arch Intern Med 168: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asada T, Kariya T, Yamagata Z, Kinoshita T, Asaka A (1996) ApoE epsilon 4 allele and cognitive decline in patients with Alzheimer's disease. Neurology 47: 603. [DOI] [PubMed] [Google Scholar]
- 20. Bathum L, Christiansen L, Jeune B, Vaupel J, McGue M, et al. (2006) Apolipoprotein e genotypes: relationship to cognitive functioning, cognitive decline, and survival in nonagenarians. J Am Geriatr Soc 54: 654–658. [DOI] [PubMed] [Google Scholar]
- 21. Deelen J, Beekman M, Uh HW, Helmer Q, Kuningas M, et al. (2011) Genome-wide association study identifies a single major locus contributing to survival into old age; the APOE locus revisited. Aging Cell 10: 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerdes LU, Jeune B, Ranberg KA, Nybo H, Vaupel JW (2000) Estimation of apolipoprotein E genotype-specific relative mortality risks from the distribution of genotypes in centenarians and middle-aged men: apolipoprotein E gene is a "frailty gene," not a "longevity gene". Genet Epidemiol 19: 202–210. [DOI] [PubMed] [Google Scholar]
- 23. Nebel A, Kleindorp R, Caliebe A, Nothnagel M, Blanche H, et al. (2011) A genome-wide association study confirms APOE as the major gene influencing survival in long-lived individuals. Mech Ageing Dev 132: 324–330. [DOI] [PubMed] [Google Scholar]
- 24. McKay GJ, Silvestri G, Chakravarthy U, Dasari S, Fritsche LG, et al. (2011) Variations in apolipoprotein E frequency with age in a pooled analysis of a large group of older people. Am J Epidemiol 173: 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schachter F, Faure-Delanef L, Guenot F, Rouger H, Froguel P, et al. (1994) Genetic associations with human longevity at the APOE and ACE loci. Nat Genet 6: 29–32. [DOI] [PubMed] [Google Scholar]
- 26. Li Y, Wang WJ, Cao H, Lu J, Wu C, et al. (2009) Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum Mol Genet 18: 4897–4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21: 263–265. [DOI] [PubMed] [Google Scholar]
- 28. Sebastiani P, Solovieff N, Dewan AT, Walsh KM, Puca A, et al. (2012) Genetic signatures of exceptional longevity in humans. PLoS One 7: e29848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kenyon CJ (2010) The genetics of ageing. Nature 464: 504–512. [DOI] [PubMed] [Google Scholar]
- 30. de Magalhaes JP, Finch CE, Janssens G (2010) Next-generation sequencing in aging research: emerging applications, problems, pitfalls and possible solutions. Ageing Res Rev 9: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao L, Yang F, Xu K, Cao H, Zheng GY, et al. (2012) Common genetic variants of the beta2-adrenergic receptor affect its translational efficiency and are associated with human longevity. Aging Cell 11: 1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soerensen M, Dato S, Tan Q, Thinggaard M, Kleindorp R, et al.. (2012) Evidence from case-control and longitudinal studies supports associations of genetic variation in APOE, CETP, and IL6 with human longevity. Age (Dordr). [DOI] [PMC free article] [PubMed]
- 33. Flachsbart F, Caliebe A, Kleindorp R, Blanche H, von Eller-Eberstein H, et al. (2009) Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci U S A 106: 2700–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, et al. (2009) Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell 8: 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Soerensen M, Dato S, Christensen K, McGue M, Stevnsner T, et al. (2010) Replication of an association of variation in the FOXO3A gene with human longevity using both case-control and longitudinal data. Aging Cell 9: 1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, et al. (2008) FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A 105: 13987–13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tindale LC, Leach S, Ushey K, Daley D, Brooks-Wilson AR (2014) Rare and common variants in the Apolipoprotein E gene in healthy oldest old. Neurobiol Aging 35: : 727 e721–723. [DOI] [PubMed] [Google Scholar]
- 38.Schupf N, Barral S, Perls T, Newman A, Christensen K, et al.. (2012) Apolipoprotein E and familial longevity. Neurobiol Aging. [DOI] [PMC free article] [PubMed]
- 39. Kervinen K, Savolainen MJ, Salokannel J, Hynninen A, Heikkinen J, et al. (1994) Apolipoprotein E and B polymorphisms—longevity factors assessed in nonagenarians. Atherosclerosis 105: 89–95. [DOI] [PubMed] [Google Scholar]
- 40. Yu CE, Seltman H, Peskind ER, Galloway N, Zhou PX, et al. (2007) Comprehensive analysis of APOE and selected proximate markers for late-onset Alzheimer's disease: patterns of linkage disequilibrium and disease/marker association. Genomics 89: 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eberle F, Dubreuil P, Mattei MG, Devilard E, Lopez M (1995) The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene 159: 267–272. [DOI] [PubMed] [Google Scholar]
- 42. Warner MS, Geraghty RJ, Martinez WM, Montgomery RI, Whitbeck JC, et al. (1998) A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246: 179–189. [DOI] [PubMed] [Google Scholar]
- 43. Shukla D, Rowe CL, Dong Y, Racaniello VR, Spear PG (1999) The murine homolog (Mph) of human herpesvirus entry protein B (HveB) mediates entry of pseudorabies virus but not herpes simplex virus types 1 and 2. J Virol 73: 4493–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]