Abstract
Background
Accumulating evidence suggests that hypoglycaemic agents influence lung cancer risk in patients with diabetes. It remains to be fully elucidated whether conventional hypoglycaemic agents (metformin, sulfonylureas, thiazolidinediones [TZDs] or insulin) affect lung cancer incidence in patients with diabetes.
Methods
We performed a meta-analysis using EMBASE, MEDLINE and Web of Science to search randomised controlled trials (RCTs), cohort studies, and case-control studies published up to October 2013 that assessed the effects of metformin, sulfonylurea, TZDs or insulin on lung cancer risk in subjects with diabetes. Fixed and random effects meta-analysis models were used, and the effect size was expressed as a summary odds ratio (OR) with 95% confidence intervals (CI). The Grades of Research, Assessment, Development and Evaluation (GRADE) approach was applied to define the quality of the evidence.
Results
Analysis of 15 studies (11 cohort studies, 2 case-control studies, and 2 RCTs) showed that metformin use was associated with a 15% reduction in risk of lung cancer (OR 0.85, 95% CI 0.77 to 0.92), but this finding was not supported by sub-analysis of smoking-adjusted studies (OR 0.84, 95% CI 0.61 to 1.06). Moreover, sulfonylurea or TZDs use was not associated with increased or decreased lung cancer risk, respectively (OR 1.10, 95% CI 0.93 to 1.26), (OR 0.86, 95% CI 0.70 to 1.02). Higher lung cancer risk was related to insulin (OR 1.23, 95% CI 1.10 to 1.35). However, all data from RCTs failed to demonstrate a statistically significant effect.
Conclusions
This analysis demonstrated that metformin use may reduce lung cancer risk in patients with diabetes but not in a smoking-adjusted subgroup and that insulin use may be associated with an increased lung cancer risk in subjects with diabetes.
Introduction
Lung cancer is the leading cause of cancer-related death both in the USA and around the world [1]. Diabetes is a rising common problem in many countries worldwide [2]. Diabetes has been established as an independent risk factor for lung cancer [3].Increasing evidence has shown that conventional glucose-lowering drugs such as insulin, insulin sensitisers and secretagogues, may influence the risk of cancer. Metformin exerts an anticancer effect by both insulin-dependent and insulin-independent mechanisms [4]. Thiazolidinediones (TZDs), synthetic peroxisome proliferator-activated receptor gamma (PPARγ) ligands, suppress cancer cell proliferation through the interplay between apoptosis and autophagy [5] [6] [7]. However, sulfonylureas, as insulin secretagogues, can promote cell proliferation and seem to have oncogenic effects [8]. Several observational studies have suggested that the use of metformin and TZDs is associated with a decreased risk of lung cancer compared with other glucose-lowering drugs [9] [10] [11]. In contrast, others have shown a non-significant protective effect on lung cancer [12] [13]. Likewise, insulin and insulin secretagogues have been shown to be related to higher lung cancer incidence and cancer-related mortality [14] [15]. But others have shown no harmful or even protective effects [16] [17]. Although there have been some systematic reviews on the relevant subject, some results of previous systematic reviews remain inconsistent [18], [19]. Some other earlier systematic reviews do not specialize in lung cancer or are limited by small study sizes [20] [21].
To investigate the relationship between the use of glucose-lowering drug (metformin, TZDs, sulfonylureas, and insulin) and lung cancer risk in patients with diabetes, we conducted a meta-analysis of existing randomised controlled trials (RCTs) and observational studies.
Materials and Methods
Literature search
We carried out a computerised search of published research studies in the Medline, Embase and Web of Science databases by using the following search terms: “metformin OR thiazolidinediones OR insulin therapy OR sulfonylurea compounds OR hypoglycemic agents” AND “diabetes” AND “neoplasms” combined with “risk”. An upper publication date limit of October, 2013 was used, but no lower date limit was applied. All English language publications were considered.
Selection criteria
All potentially relevant studies were retrieved and assessed for inclusion according to the following criteria: (1) study must have evaluated lung cancer risk in patients with diabetes on the basis of type of hypoglycaemic agent (metformin, sulfonylureas, rosiglitazone, pioglitazone, insulin); (2) study design must have been RCT, case–control or cohort; (3) study must have reported the hazard ratio (HR) or odds ratio (OR); and (4) population consisted of adult patients. The observational studies not adjusted for any confounder or duplicate publications of studies in the same population were excluded. When the same patient population appeared in several publications, only the most recent or comprehensive study was selected. Disagreements were resolved by discussion.
Data extraction and assessment of quality
Data was extracted from all selected studies by two reviewers working independently, using a standardised form to ensure capture of all relevant information. The following data were collected from each study: first author's name, publication date, country, study design (case-control, cohort, or RCT), time period, mean follow-up time, outcome assessment, type of diabetes, total subjects, lung cancer cases, ratio of each glucose-lowering drug. Where available, adjusted OR or HR values were analysed. If data from any of the above categories were not reported in the primary study, items were treated as ‘‘not available’’. For all analyses, the control group was composed of patients with diabetes not exposed to medication of interest. We did not require a minimum number of patients for a study to be included in our meta-analysis.
Two reviewers assessed the risk of bias in observational studies according to the Newcastle – Ottawa Scale which included selection, comparability of studies groups, and ascertainment of exposure or outcome [22]. The overall maximum score was 9 points. The two reviewers applied the Cochrane Collaboration' s tool to evaluate the risk of bias of randomised trials [23]. This tool based on randomisation, blinding allocation concealment procedures and loss to follow up. Disagreements were resolved by discussion and consensus.
Risk of bias across studies
The Grades of Research, Assessment, Development and Evaluation (GRADE) framework was used to determine quality of evidence for each meta-analysis. Each meta-analysis could receive a recommendation with four levels of evidence quality, ranging from very low to high [24]. Meta-analysis of RCTs was graded as high quality, however they could be downgraded because of factors such as design limitations, indirectness, inconsistency, imprecision and publication bias. Evidences from observational studies were classified as low quality by default, but they also could be downgraded by the factors as above or upgraded due to large magnitude of effect, potential confounding and dose-response relationship.
Statistical analysis
This meta-analysis was conducted according to Cochrane Handbook and PRISMA guidelines (Checklist S1) [25] [26]. Adjusted OR or HR with 95% confidence intervals (CIs) was calculated to determine the assessment of risk of lung cancer in patients with diabetes on the basis of the type of glucose-lowering drug. Since the frequency was relatively low (less than 1%) [27], the OR in case-control studies was considered approximations of HR in cohort studies [28]. OR or HR indicated statistically significant at the p<0.05 level if the 95% CI did not include the value one. The heterogeneity across the included studies was assessed using the chi squared (χ2)-based Q test [29]. A P value less than 0.1 for the Q test indicated a lack of heterogeneity between studies, and indicated that the pooled OR estimate of each study should be calculated by the Mantel–Haenszel fixed-effects model. Otherwise, the random-effects model was used [30], [31]. Subgroup analyses were then carried out by study design, location and whether the study was adjusted for smoking or other glucose-lowering drugs. Due to significant differences in the design of observational studies and post-hoc analysis of RCTs, data from these RCTs were analysed and presented separately. Potential publication bias was estimated by the funnel plot, in which the standard error of log (OR) of each study was plotted against its log (OR). An asymmetric plot suggested a possible publication bias. Funnel plot asymmetry was assessed by Egger's linear regression test. The significance of the intercept was determined by the t test, as suggested by Egger and Smith [32] (P<0.05 was representative of a statistically significant publication bias). All calculations were carried out with the STATA version 12.0 statistical software package (Stata Corp., College Station, TX, USA).
This was a literature-based study, and ethics approval was not required.
Results
Flow of included studies
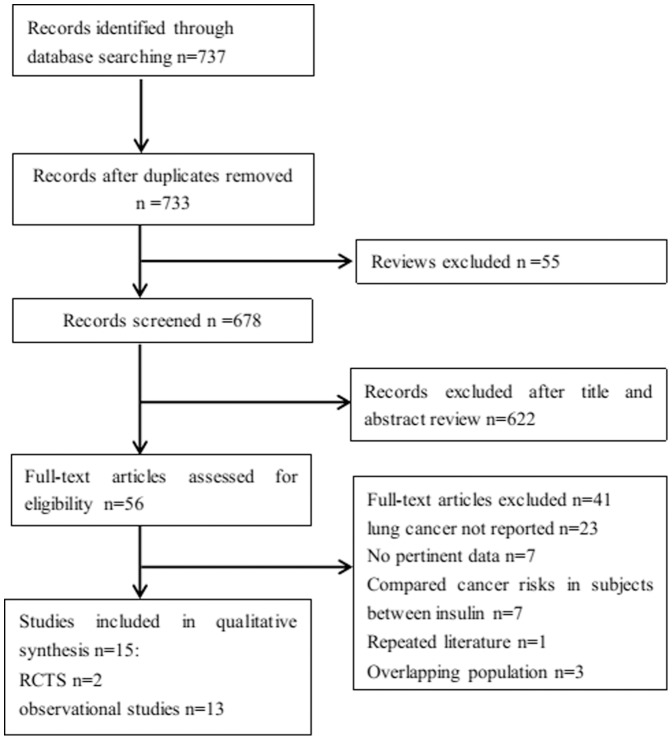
A total of 18 potentially relevant studies were identified by the initial computerised search. There were five Taiwanese studies from the same cohort, and hence, one of these was included in the analysis for metformin, sulfonylureas and insulin, while another was analysed for TZDs. Two studies were from the UK-based General Practice Research Database, one of which was analysed for metformin and the other for sulfonylureas and insulin. Hence, the remaining 15 studies fulfilled the inclusion criteria and were included in meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [27], [33], [34], [35], [36]. There were 11 cohort studies, 2 case-control studies, and 2 RCTs, which were included in one publication (Figure 1).
Figure 1. Flow chart demonstrating the progression of the trials in the review.
The flowchart of selecting procedure and the exclusive reason of studies are summarized.
Study characteristics
Fifteen published studies reporting 21,089 cases of lung cancer in 2,072,425 patients with diabetes met the inclusion criteria and were ultimately analysed. Table 1 presents the main characteristics of these studies. All the patients in the included studies were on multiple glucose-lowering drugs for management of diabetes, and the comparators for the estimation of OR were determined on exposure to drug of interest and non-exposure to the same glucose-lowering drug. Most studies adjusted for the following confounders: age, sex, ethnicity, smoking, chronic lung disease, body mass index (BMI), glycosylated hemoglobin (HbA1C), diabetes duration, and other glucose-lowering drugs (Table 2). The quality of all the studies included was moderate to high. Table S1 showed the risk of bias assessment in RCTs in more detail.
Table 1. Characteristics of included studies assessing glucose-lowering drugs and lung cancer risk in patients with diabetes.
| Author | Year | Country | Design | Time period | Mean follow-up(years) | Outcome Measures | Type of DM | Study of quality | Total subjects | Lung cancer cases | Metformin (%) | TZDs (%) | Sulfonylurea (%) | Insulin (%) |
| Smiechowski | 2013 | UK | Cohort | 1988–2009 | 5.6 | diagnostic codes | 2 | 8 of 9 | 8572 | 808 | 77 | NA | NA | NA |
| Ruiter | 2012 | Netherlands | Cohort | 1998–2008 | 2.8 for metformin; 4.6 for sulfonylurea | ICD-9 | NA | 7 of 9 | 85,289 | 454 | 61.80 | NA | 38.20 | NA |
| Mazzone | 2012 | USA | case-control | 2001–2011 | NA | direct review of medical record | NA | 6 of 9 | 1014 | 507 | 27.70 | 8.50 | NA | NA |
| Luo | 2012 | US | Cohort | 2005–2010 | 11 | ICD-O | NA | 7 of 9 | 8,154 | 108 | 11.40 | NA | NA | 17.20 |
| Lai | 2012 | Taiwan | Cohort | 2000–2008 | NA | ICD-9 | NA | 8 of 9 | 19,624 | 129 | 84.70 | 20.10 | 83.50 | 65.90 |
| Bodmer | 2012 | U.K. | case-control | 1995–2009 | NA | Diagnostic codes | NA | 7 of 9 | 7203 | 1029 | 45.80 | NA | 48.30 | 15.60 |
| Ferrara | 2011 | U.S.A. | Cohort | 1997–2005 | 2.5 for pioglitazone 3.7 for never used | KPNC Cancer Registry | NA | 8 of 9 | 252,467 | 1637 | 45.30 | 12.60 | 52.80 | 20.40 |
| Libby | 2009 | Scotland, | Cohort | 1994–2003 | NA | ICD-9 ICD-10 | 2 | 8 of 9 | 8170 | 93 | 50 | NA | 36.45 | 10.10 |
| Govindarajan | 2007 | USA, | Cohort | 1997–2004 | NA | ICD-9 | 1, 2 | 7 of 9 | 87,678 | 1,371 | NA | 12.90 | NA | NA |
| Neumann | 2012 | French, | Cohort | 2006–2009 | 3,125 | ICD-10 | NA | 8 of 9 | 1,493,472 | 9,298 | 69.70 | 20.70 | 57.20 | 26.70 |
| Gu | 2013 | China | Cohort | 2001–2010 | 4.1 | ICD-9, ICD-10 | 2 | 9 of 9 | 8,774 | 40 | NA | NA | NA | 51.50 |
| Vallarino | 2013 | US | cohort | 2000–2010 | 2.2 for pioglitazone 1.9 for insulin | ICD-9 | 2 | 8 of 9 | 56,536 | 215 | 68.30 | 37.40 | 34.70 | 31.70 |
| Chang | 2012 | Taiwan | cohort | 2000–2007 | NA | ICD-9 | 2 | 8 of 9 | 26,674 | 5361 | 80.15 | 16.87 | 90.70 | 36.13 |
| ADOPT | 2006 | USA, Europe | RCT | 2000–2006 | 4 | Adverse event review | 2 | NA | 4351 | 18 | 33.40 | 33.10 | 33.50 | NA |
| RECORD | 2009 | Europe, Australia | RCT | 2001–2008 | 5.5 | Adverse event review | 2 | NA | 4447 | 21 | 75.20% | 49.90 | 74.90 | NA |
Abbreviations: RCT: randomised controlled trials; ICD: International Classification of Diseases; NA: available; ADOPT: A Diabetes Outcome Progression Trial; RECORD: Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes.
Table 2. Adjustment variables of included observational studies.
| Study | HR/OR | Adjustment variables |
| Smiechowski | Multivariable adjusted | Diabetes duration, HbA1C, obesity, smoking, excessive alcohol use, previous cancer, COPD, asthma, NSAID, aspirin, statins, other antidiabetic drugs |
| Ruiter | Multivariable adjusted | Age, sex, other medications |
| Mazzone | Multivariable adjusted | Age, sex, smoking, BMI, HbA1c, medication use |
| Luo | Multivariable adjusted | Age, ethnicity, education, smoking, BMI, waist-to-hip ratio, recreational physical activity, alcohol intake, total energy intake, percent calories from fat, total fruit/vegetable intake, history of hormone therapy, different treatment assignments for clinical trials |
| Lai | Multivariable adjusted | Sex, age, pulmonary tuberculosis, COPD, and propensity score |
| Bodmer | Multivariable adjusted | BMI, smoking, each other |
| Ferrara | Multivariable adjusted | Age, sex, year of cohort entry, race, income, smoking, glycemic control, diabetes duration, creatinin, congestive heart failure, glucose-lowering drugs |
| Libby | Multivariable adjusted | Age, sex, smoking, deprivation, BMI, HbA1c, glucose-lowering drugs |
| Govindarajan | Multivariable adjusted | Age, race/ethnicity, BMI, HbA1C, glucose-lowering drugs |
| Neumann | Multivariable adjusted | Age, sex, and glucose-lowering drugs |
| Gu | Multivariable adjusted | Age, sex, smoking, diabetes duration, macrovascular, HbA1c. and glucose lowering agents |
| Vallarino | Multivariable adjusted | Age, sex, tobacco use, use of medications, medical history |
| Chang | Multivariable adjusted | Anti-diabetic agents, chronic lung disease, retinopathy, calcium channel blockers, chronic kidney disease, statins, angiotensin receptor blockers, chronic liver disease. |
Abbreviations: HR: hazard ratio; OR: odds ratio; COPD: chronic obstructive pulmonary disease; NSAID: nonsteroidal antiinflammatory drugs; BMI: body mass index; HbA1C: glycosylated hemoglobin.
Metformin and lung cancer risk
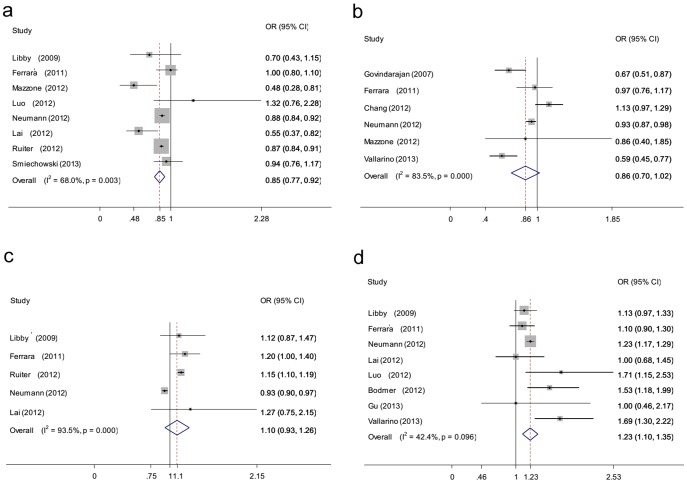
Meta-analysis of all 8 observational studies reported that the metformin use was associated with a statistically significant 15% reduction in lung cancer incidence (OR 0.85, 95% CI 0.77 to 0.92 P = 0.003 for heterogeneity) (Figure 2a) [9], [10], [12], [13], [15], [17], [33], [35]. The summary OR of 7 cohort studies was 0.87 (95% CI 0.81 to 0.93). The summary OR of studies that adjusted for other glucose-lowering drugs was 0.87 (95% CI 0.81 to 0.94). A subgroup analysis of seven Western populations showed that metformin exposure was linked to a 13% reduction in lung cancer risk (OR 0.87, 95% CI 0.81 to 0.94). A subgroup analysis was then carried out on only studies that adjusted for smoking. The relation was not statistically significant (OR 0.84, 95% CI 0.61 to 1.06). Separate pooled post-hoc analysis of two RCTs revealed no significant association between metformin and lung cancer (OR 0.65, 95% CI 0.33 to 1.26; P = 0.22 for heterogeneity) [27]. The evidence was of low or moderate quality. A subgroup analysis of metformin is shown in table 3.
Figure 2. Forest plot of hypoglycaemic agents and the risk of lung cancer in patients with diabetes.
a: metformin; b:thiazolidinediones (TZDs); c:sulfonylureas; d:insulin. The squares and horizontal lines correspond to the study-specific OR and 95% CI. The area of the squares reflects the weight (inverse of the variance). The diamond represents the summary OR and 95% CI.
Table 3. A subgroup analysis of metformin use and lung cancer risk in patients with diabetes.
| Subgroup analysis | N | OR | 95% CI | P for heterogeneity | Quality of evidence (GRADE) |
| RCTs | 2 | 0.65 | 0.33–1.26 | 0.22 | Moderate |
| Observational studies | 8 | 0.85 | 0.77–0.92 | 0.003 | Low |
| Study design | |||||
| Case-control | 1 | - | - | - | |
| Cohort | 7 | 0.87 | 0.81–0.93 | 0.04 | Low |
| Study location | |||||
| Asian | 1 | - | - | - | |
| Western | 7 | 0.87 | 0.81–0.94 | 0.03 | Low |
| Adjusted for smoking | |||||
| Yes | 5 | 0.84 | 0.61–1.06 | 0.008 | Low |
| No | 3 | ||||
| Adjusted for glucose-lowering drug | |||||
| Yes | 7 | 0.87 | 0.81–0.94 | 0.03 | Low |
| No | 1 | - | - | - |
Abbreviations: CI: confidence interval; OR: odds ratio; RCTs, randomised controlled trials.
TZDs and lung cancer risk
Meta-analysis of 6 observational studies that evaluated the risk of lung cancer with TZDs exposure in patients with diabetes showed that the association was not statistically significant (OR 0.86, 95% CI 0.70 to 1.02; P = .00 for heterogeneity) (Figure 2b) [10], [11], [13], [17], [34], [37]. The association was still not significant in the subgroup of five cohort studies (OR 0.86, 95% CI 0.69 to 1.03). However, the result of the Western population showed that TZDs exposure was associated with a 20% reduction in lung cancer risk (OR 0.80, 95% CI 0.62 to 0.98). Separate analysis of two RCTs also did not show an increased or decreased effect of TZDs on lung cancer risk (OR 1.06, 95% CI 0.55 to 2.02; P = 0.42 for heterogeneity) [27]. The quality of evidence was ranging from very low to moderate. A subgroup analysis of TZDs is shown in table 4.
Table 4. A subgroup analysis of thiazolidinediones use and lung cancer risk in patients with diabetes.
| Subgroup analysis | N | OR | 95% CI | P for heterogeneity | Quality of evidence (GRADE) |
| RCTs | 2 | 1.06 | 0.55–2.02 | 0.42 | Moderate |
| Observational studies | 6 | 0.86 | 0.70–1.02 | 0.00 | Low |
| Study design | |||||
| Case-control | 1 | - | - | - | |
| Cohort | 5 | 0.86 | 0.69–1.03 | 0.00 | Low |
| Study location | |||||
| Asian | 1 | - | - | - | |
| Western | 5 | 0.80 | 0.62–0.98 | 0.03 | Low |
| Adjusted for smoking | |||||
| Yes | 3 | 0.79 | 0.47–1.10 | 0.02 | Very low |
| No | 3 | 0.92 | 0.72–1.11 | 0.001 | Very low |
| Adjusted for glucose-lowering drug | |||||
| Yes | 6 | 0.86 | 0.70–1.02 | 0.00 | Low |
| No | 0 | - | - | - |
Abbreviations: CI: confidence interval; OR: odds ratio; RCTs, randomised controlled trials.
Sulfonylureas and lung cancer risk
Sulfonylureas use was not associated with lung cancer risk in patients with diabetes (OR 1.10, 95% CI 0.93 to 1.26; P = 0.000 for heterogeneity) (Figure 2c) [9], [10], [15], [17], [35]. A subgroup analysis of Western populations pointed out that sulfonylurea use did not modify lung cancer risk (OR 1.09, 95% CI 0.92 to 1.25). Moreover, post-hoc analysis of two RCTs also showed no significant effect of sulfonylureas on lung cancer (OR 1.56, 95% CI 0.75 to 3.26; P = 0.57 for heterogeneity) [27]. The evidence was of low or moderate quality. A subgroup analysis of TZDs is shown in table 5.
Table 5. A subgroup analysis of sulfonylureas use and lung cancer risk in patients with diabetes.
| Subgroup analysis | N | OR | 95% CI | P for heterogeneity | Quality of evidence (GRADE) |
| RCTs | 2 | 1.56 | 0.75–3.26 | 0.57 | Moderate |
| Observational studies | 5 | 1.10 | 0.93–1.26 | 0.000 | Low |
| Study design | |||||
| Case-control | 0 | - | - | - | |
| Cohort | 5 | - | - | - | |
| Study location | |||||
| Asian | 1 | - | - | - | |
| Western | 4 | 1.09 | 0.92–1.25 | 0.000 | Low |
| Adjusted for smoking | |||||
| Yes | 2 | 1.18 | 1.01–1.34 | 0.66 | |
| No | 3 | 1.06 | 0.85–1.26 | 0.000 | Low |
| Adjusted for glucose-lowering drug | Moderate | ||||
| Yes | 4 | 1.09 | 0.92–1.25 | 0.000 | Low |
| No | 1 | - | - | - |
Abbreviations: CI: confidence interval; OR: odds ratio; RCTs, randomised controlled trials.
Insulin and lung cancer risk
Insulin use was associated with a statistically significant 22% increase in lung cancer risk in patients with diabetes on meta-analysis of eight observational studies (OR 1.23 95% CI 1.10 to 1.35 P = 0.096 for heterogeneity) (Figure 2d) [9], [10], [14], [16], [17], [33], [35], [37]. The summary OR of cohort studies was 1.22 (95% CI 1.16 to 1.27). The summary OR of studies that adjusted for other glucose-lowering drugs was 1.22 (95% CI 1.17 to 1.27). Further analysis of the Western population indicated that the OR was 1.26 (95% CI 1.12 to 1.39). The result of studies that adjusted for smoking illustrated that insulin still increased lung cancer risk (OR 1.30, 95% CI 1.09 to 1.51). The evidence was of low or moderate quality. A subgroup analysis of insulin is shown in online table 6.
Table 6. A subgroup analysis of insulin use and lung cancer risk in patients with diabetes.
| Subgroup analysis | N | OR | 95% CI | P for heterogeneity | Quality of evidence (GRADE) |
| RCTs | 0 | - | - | - | |
| Observational studies | 8 | 1.23 | 1.10–1.35 | 0.096 | Moderate |
| Study design | |||||
| Case-control | 1 | - | - | - | |
| Cohort | 7 | 1.22 | 1.16–1.27 | 0.13 | Moderate |
| Study location | |||||
| Asian | 2 | 1.00 | 0.65–1.35 | 1 | Low |
| Western | 6 | 1.26 | 1.12–1.39 | 0.06 | Moderate |
| Adjusted for smoking | |||||
| Yes | 6 | 1.30 | 1.09–1.51 | 0.06 | Low |
| No | 2 | 1.23 | 1.17–1.28 | 0.28 | Moderate |
| Adjusted for glucose-lowering drug | |||||
| Yes | 6 | 1.22 | 1.17–1.27 | 0.12 | Moderate |
| No | 2 | 1.26 | 0.74–1.78 | 0.06 | Low |
Abbreviations: CI: confidence interval; OR: odds ratio; RCTs, randomised controlled trials.
Sensitivity analysis
To assess the influence of individual studies on overall outcome, we excluded the studies with the most weight and analyzsed [15] [17]. The conclusions of the main analysis did not change for metformin (OR 0.81, 95% CI 0.67 to 0.95), sulfonylureas (OR 1.09, 95% CI 0.96 to 1.23), TZDs (OR 0.84, 95% CI 0.6 to 1.09) or insulin (OR 1.25, 95% CI 1.06 to 1.44). On replacing this study with similar population-based cohort studies from the same Taiwanese population, where Chang et al. [38] was replaced for sulfonylureas and insulin and where Hsieh et al. [39] was replaced for metformin, there was no significant change in overall association of lung cancer with sulfonylureas (OR 1.05, 95% CI 0.90 to 1.20), insulin (OR 1.36, 95% CI 1.18 to 1.53), or metformin (OR 0.86, 95% CI 0.79 to 0.92).
Publication bias
The shapes of the funnel plots did not show any obvious asymmetry (Figure S1). The quantitative results of Egger's test still did not suggest the presence of any publication bias (P = 0.61 for metformin, P = 0.48 for TZDs, P = 0.69 for sulfonylureas, and P = 0.63 for insulin).
Discussion
Our comprehensive study quantitatively analysed the possible association between glucose-lowering drugs and incidence of lung cancer in individuals with diabetes. We found that, compared to non-use, metformin use was associated with a 15% decrease in the risk of lung cancer in observational studies, but in the subgroup of studies adjusted for smoking, the protective effect disappeared. Moreover, the preventive effect was not seen in RCTs. Insulin use may be associated with 23% increased lung cancer risk, and the effect persisted in studies adjusted for smoking. Use of TZDs or sulfonylureas did not show an increased or decreased risk of lung cancer.
Metformin is the first-choice glucose-lowering drug in type 2 diabetes. The exact molecular mechanisms connecting metformin use to lung cancer are largely unknown. Metformin inhibits the growth of lung cancer cells and induces apoptosis by activating AMP-activated protein kinase (AMPK), JNK/p38 MAPK signaling pathway [40] [41]. Metformin also exerts an effect by AMPK-independent mechanisms such as inactivation of Raf-ERK-Nrf2 signaling or decreasing plasma IGF-I or receptor tyrosine kinase signaling [4] [42]. Besides a tumor cell-specific effect, metformin has a systemic antiproliferative effect by lowering circulating glucose and insulin levels, contributing to tumorigenesis [43]. Although there is experimental evidence for both cancer treatment and chemoprevention with metformin, clinical chemoprevention is complex and epidemiological studies are inconsistent. Noto et al. [20] recently reported a meta-analysis of metformin and cancer risk, and in their subgroup of lung cancer risk, they observed a 33% lower lung cancer risk with metformin use. However, they included three studies, namely two RCTs and one cohort study, and determined the total risk ratio together. Another meta-analysis demonstrated that metformin use reduces all-cause risk in subjects with type 2 diabetes, but there was no analysis with regard to lung cancer [21]. In our study, we included more studies and also found that metformin was associated with a 15% decreased risk of lung cancer in observational studies, This protective potential of metformin use agrees with previous meta-analysis [20]. Interestingly, in a subgroup of only studies adjusted for smoking, the decrease in risk of lung cancer tended toward null, where this agreed with the meta-analysis of two RCTs. The reason is not clear. A recent study showed that metformin delays the onset of tobacco carcinogen-induced lung tumorigenesis in a non-diabetic mouse model, but the laboratory data are insufficient to translate to humans with diabetes [42].
PPAR-γ is a member of the nuclear receptor superfamily [44]. Once activated, it will preferentially bind to retinoid X receptorα and signal antiproliferative, antiangiogenic, and prodifferentiation pathways in several tissues [5]. In vitro studies have shown that TZDs induce apoptosis and differentiation for potential chemoprevention in non-small-cell lung cancer, but it is not clear whether these mechanisms are relevant in humans [45] [46]. But epidemiological studies have provided varying results, suggesting either a beneficial or neutral effect on lung cancer risk [10] [11] [37]. Colmers et al. [18] observed in their meta-analysis a 9% decreased risk in the lung cancer subgroup among observational studies. Another meta-analysis of Bosetti et al. [19] showed that the TZDs use was not associated with the lung cancer risk, which included two publications for the same population from Taiwan. Our present meta-analysis included one population of the larger sample from Taiwan and another study from the USA [34] [13]. Our updated evidence did not indicate any relevant role of TZDs use on lung cancer risk either in observational studies or in RCTs. However, the association between TZDs and lung cancer risk was pronounced in the Western population when except for a study from Taiwan.
Sulfonylureas seem to promote oncogenesis by increasing insulin secretion, enhancing growth factor-dependent cell proliferation and affecting cell metabolism [8]. Epidemiological evidences in lung cancer are conflicting [10], [17] [15]. A previous meta-analysis suggested that sulfonylurea use significantly increases all-cancer risk in patients with type 2 diabetes [21]. Our overall evidence did not indicate any relevant role of sulfonylurea use in lung cancer risk. The result did not change in the Western population, cohort studies or RCTs. The heterogeneous effects of different sulfonylureas may explain it. Preclinical evidence has also shown that glibenclamide has antitumor activity, besides a role in promoting cancer. The role of glibenclamide as a KATP channel inhibitor and its interaction with reactive oxygen species(ROS) production seem to underlie the proapoptotic and neoangiogenesis effect [47] [48]. The principal mechanism of action of sulfonylureas on lung cancer has not yet been identified, and clinical data also has shown no association of sulfonylureas with lung cancer. There is a need for further clinical studies according to different sulfonylureas if possible.
Insulin and insulin-like growth factor (IGF) signaling in accelerating neoplastic growth is impressive. Insulin is known to stimulate the proliferation of tumor cells both directly and indirectly by acting upon IGF-1 receptors expressed on lung cancer [49]. Therefore, the use of exogenous insulin may further contribute to neoplastic growth of lung cancer. Increasing epidemiologic evidence suggests insulin effect on both the risk and the prognosis of cancer, but the effect is different in different cancer types. A meta-analysis of reported new use of insulin or insulin glargine was associated with an increased risk of pancreatic cancer, but with a decreased risk of colorectal cancer. They also reported that insulin glargine use had no effect on lung cancer. Comparing non-glargine insulins, two meta-analysis failed to confirm an association between insulin glargine and an increased risk of respiratory tract cancer [50] [51]. Our present analysis focused on ever-used insulin and non-used insulin. Our results indicated that compared with non-insulin use, there was a 23% increased risk of lung cancer in patients with diabetes. These findings were confirmed in subgroup analyses of studies adjusted for smoking or adjusted for other anti-diabetic drugs. Further evaluation of different forms of exogenous insulin is required to better understand this possible association with lung cancer.
The strengths of this study were that we conducted an extensive evaluation of the effects of conventional glucose-lowering drugs on modification of lung cancer risk. Some meta-analyses have shown that metformin use reduces while sulfonylurea and insulin use increases overall cancer risk [21] [20] [52]. However, cancer is a heterogeneous disease, and diabetes differs in the direction and magnitude of relation with site-specific cancer [53]. Thus, the glucose-lowering drug effect on lung cancer risk is necessary. Second, most observational studies are cohort studies and we only included those with adjusted risk estimates controlled for potential confounders such as age, sex, BMI, HbA1C, smoking and so on. A cohort study can provide strong evidence in assessing latent or rare outcomes such as lung cancer incidence [54]. Third, we did a multiple subgroup analysis according to study design, adjusting variables such as smoking and other glucose-lowering drugs. Smoking is the most important risk in lung cancer. We tried to account for this by performing a subgroup analysis restricted to those studies that reported OR after adjusting for smoking. Besides, we also performed a subgroup analysis restricted to those studies that reported OR after adjusting for other glucose-lowering drugs, which may have inherent cancer-modifying effects. Additionally, we conducted a sensitivity analysis and found that removing the studies with the most weight did not have a significant impact on the overall ORs [15] [17]. Finally, With regard to publication bias, both the graphical display of funnel plots and the statistical tests did not indicate any major bias.
There were several limitations in our analysis. First, the meta-analysis was based on data mainly from observational studies because there were only two RCTs. These RCTs were not powerful enough to detect a significant association between glucose-lowering drugs and lung cancer risk, and the subjects included in these studies were not systematically screened for lung cancer, which might have introduced some degree of detection bias. Several observational studies included in our analysis may also have had inherent time-related biases [55]. Second, although we chose the adjusted risk ratio from the original paper, all of the studies did not adjust for the same confounders. Third, the individual studies were limited in reporting an association between glucose-lowering drug and specific pathological type of lung cancer risk. Thus we could not do further analysis according to pathology type. Fourth, evidence quality of meta-analyses in our review was ranging from very low to moderate due largely to a small number of RCTs or heterogeneity. Additionally, the included studies showed heterogeneity. Differences in comparison groups, study population and design, and covariates may explain part of the observed differences between studies. To solve the problem, we did a subgroup analysis to decrease the heterogeneity. Finally, some studies included were population-based and did not specify the type of diabetes. We could not do a subgroup analysis according to type of diabetes. However, over 90% of individuals with diabetes in the general population have type 2 diabetes, so it would have little impact on pooled ORs [56].
In conclusion, based on the results of this meta-analysis, metformin use appeared to be associated with a lower risk of lung cancer in diabetic patients, but the association disappeared when the analysis was restricted to the studies adjusted for smoking. Insulin use increased lung cancer risk, while sulfonylureas and TZDs did not significantly have an association with lung cancer risk. However, this observation needs further investigation before the findings can be translated to clinical practice. A definitive, randomised trial is needed to rigorously assess the effects of glucose-lowering drugs on lung cancer incidence in diabetic patients.
Supporting Information
PRISMA Checklist.
(DOC)
Funnel plots of hypoglycaemic agents and the risk of lung cancer in patients with diabetes, a: metformin; b: thiazolidinediones (TZDs); c: sulfonylureas; d: insulin.
(DOCX)
Risk of bias assessment in Randomised controlled trials.
(DOCX)
Funding Statement
The authors have no support or funding to report.
References
- 1. Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63: 11–30. [DOI] [PubMed] [Google Scholar]
- 2. Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, et al. (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378: 31–40. [DOI] [PubMed] [Google Scholar]
- 3. Lee JY, Jeon I, Lee JM, Yoon JM, Park SM (2013) Diabetes mellitus as an independent risk factor for lung cancer: a meta-analysis of observational studies. Eur J Cancer 49: 2411–2423. [DOI] [PubMed] [Google Scholar]
- 4. Do MT, Kim HG, Khanal T, Choi JH, Kim DH, et al. (2013) Metformin inhibits heme oxygenase-1 expression in cancer cells through inactivation of Raf-ERK-Nrf2 signaling and AMPK-independent pathways. Toxicol Appl Pharmacol 271: 229–238. [DOI] [PubMed] [Google Scholar]
- 5. Sertznig P, Seifert M, Tilgen W, Reichrath J (2007) Present concepts and future outlook: function of peroxisome proliferator-activated receptors (PPARs) for pathogenesis, progression, and therapy of cancer. J Cell Physiol 212: 1–12. [DOI] [PubMed] [Google Scholar]
- 6. Zhou J, Zhang W, Liang B, Casimiro MC, Whitaker-Menezes D, et al. (2009) PPARgamma activation induces autophagy in breast cancer cells. Int J Biochem Cell Biol 41: 2334–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shiau CW, Yang CC, Kulp SK, Chen KF, Chen CS, et al. (2005) Thiazolidenediones mediate apoptosis in prostate cancer cells in part through inhibition of Bcl-xL/Bcl-2 functions independently of PPARgamma. Cancer research 65: 1561–1569. [DOI] [PubMed] [Google Scholar]
- 8. Pollak M (2012) The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer 12: 159–169. [DOI] [PubMed] [Google Scholar]
- 9. Lai SW, Liao KF, Chen PC, Tsai PY, Hsieh DP, et al. (2012) Antidiabetes drugs correlate with decreased risk of lung cancer: a population-based observation in Taiwan. Clin Lung Cancer 13: 143–148. [DOI] [PubMed] [Google Scholar]
- 10. Ferrara A, Lewis JD, Quesenberry CP Jr, Peng T, Strom BL, et al. (2011) Cohort study of pioglitazone and cancer incidence in patients with diabetes. Diabetes Care 34: 923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, et al. (2007) Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol 25: 1476–1481. [DOI] [PubMed] [Google Scholar]
- 12. Smiechowski BB, Azoulay L, Yin H, Pollak MN, Suissa S (2013) The use of metformin and the incidence of lung cancer in patients with type 2 diabetes. Diabetes Care 36: 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mazzone PJ, Rai H, Beukemann M, Xu M, Jain A, et al. (2012) The effect of metformin and thiazolidinedione use on lung cancer in diabetics. BMC Cancer 12: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bodmer M, Becker C, Jick SS, Meier CR (2012) Metformin does not alter the risk of lung cancer: a case-control analysis. Lung Cancer 78: 133–137. [DOI] [PubMed] [Google Scholar]
- 15. Ruiter R, Visser LE, van Herk-Sukel MP, Coebergh JW, Haak HR, et al. (2012) Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up study. Diabetes Care 35: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gu Y, Wang C, Zheng Y, Hou X, Mo Y, et al. (2013) Cancer incidence and mortality in patients with type 2 diabetes treated with human insulin: a cohort study in Shanghai. PloS one 8: e53411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, et al. (2012) Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia 55: 1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Colmers IN, Bowker SL, Johnson JA (2012) Thiazolidinedione use and cancer incidence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab 38: 475–484. [DOI] [PubMed] [Google Scholar]
- 19. Bosetti C, Rosato V, Buniato D, Zambon A, La Vecchia C, et al. (2013) Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist 18: 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Noto H, Goto A, Tsujimoto T, Noda M (2012) Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PloS one 7: e33411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thakkar B, Aronis KN, Vamvini MT, Shields K, Mantzoros CS (2013) Metformin and sulfonylureas in relation to cancer risk in type II diabetes patients: a meta-analysis using primary data of published studies. Metabolism 62: 922–934. [DOI] [PubMed] [Google Scholar]
- 22.Wells G, Shea B, O'connell D, Peterson J, Welch V, et al.. (2000) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
- 23. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, et al. (2011) The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, et al. (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64: 383–394. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J (2012) Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0. The Cochrane Collaboration; 2011. Accessed 9/8/2011. Available: http://www.cochrane-handbook.org.
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 27. Home PD, Kahn SE, Jones NP, Noronha D, Beck-Nielsen H, et al. (2010) Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 53: 1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Greenland S (1987) Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 9: 1–30. [DOI] [PubMed] [Google Scholar]
- 29. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 30. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 31. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 32. Egger M, Smith GD (1998) Bias in location and selection of studies. BMJ 316: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo J, Chlebowski R, Wactawski-Wende J, Schlecht NF, Tinker L, et al. (2012) Diabetes and lung cancer among postmenopausal women. Diabetes Care 35: 1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang CH, Lin JW, Wu LC, Lai MS, Chuang LM, et al. (2012) Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology 55: 1462–1472. [DOI] [PubMed] [Google Scholar]
- 35. Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, et al. (2009) New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 32: 1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schiel R, Muller UA, Braun A, Stein G, Kath R (2005) Risk of malignancies in patients with insulin-treated diabetes mellitus: results of a population-based trial with 10-year follow-up (JEVIN). Eur J Med Res 10: 339–344. [PubMed] [Google Scholar]
- 37. Vallarino C, Perez A, Fusco G, Liang H, Bron M, et al. (2013) Comparing pioglitazone to insulin with respect to cancer, cardiovascular and bone fracture endpoints, using propensity score weights. Clinical Drug Investigation 33: 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chang CH, Lin JW, Wu LC, Lai MS, Chuang LM (2012) Oral insulin secretagogues, insulin, and cancer risk in type 2 diabetes mellitus. J Clin Endocrinol Metab 97: E1170–1175. [DOI] [PubMed] [Google Scholar]
- 39. Hsieh MC, Lee TC, Cheng SM, Tu ST, Yen MH, et al. (2012) The influence of type 2 diabetes and glucose-lowering therapies on cancer risk in the Taiwanese. Exp Diabetes Res 2012: 413782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu N, Gu C, Gu H, Hu H, Han Y, et al. (2011) Metformin induces apoptosis of lung cancer cells through activating JNK/p38 MAPK pathway and GADD153. Neoplasma 58: 482–490. [DOI] [PubMed] [Google Scholar]
- 41. Salani B, Maffioli S, Hamoudane M, Parodi A, Ravera S, et al. (2012) Caveolin-1 is essential for metformin inhibitory effect on IGF1 action in non-small-cell lung cancer cells. FASEB J 26: 788–798. [DOI] [PubMed] [Google Scholar]
- 42. Quinn BJ, Dallos M, Kitagawa H, Kunnumakkara AB, Memmott RM, et al. (2013) Inhibition of lung tumorigenesis by metformin is associated with decreased plasma IGF-I and diminished receptor tyrosine kinase signaling. Cancer Prev Res (Phila) 6: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, et al. (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310: 1642–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Berger J, Moller DE (2002) The mechanisms of action of PPARs. Annu Rev Med 53: 409–435. [DOI] [PubMed] [Google Scholar]
- 45. Bren-Mattison Y, Van Putten V, Chan D, Winn R, Geraci MW, et al. (2005) Peroxisome proliferator-activated receptor-gamma (PPAR(gamma)) inhibits tumorigenesis by reversing the undifferentiated phenotype of metastatic non-small-cell lung cancer cells (NSCLC). Oncogene 24: 1412–1422. [DOI] [PubMed] [Google Scholar]
- 46. Chang TH, Szabo E (2000) Induction of differentiation and apoptosis by ligands of peroxisome proliferator-activated receptor gamma in non-small cell lung cancer. Cancer research 60: 1129–1138. [PubMed] [Google Scholar]
- 47. Qian X, Li J, Ding J, Wang Z, Duan L, et al. (2008) Glibenclamide exerts an antitumor activity through reactive oxygen species-c-jun NH2-terminal kinase pathway in human gastric cancer cell line MGC-803. Biochem Pharmacol 76: 1705–1715. [DOI] [PubMed] [Google Scholar]
- 48. Suzuki Y, Inoue T, Murai M, Suzuki-Karasaki M, Ochiai T, et al. (2012) Depolarization potentiates TRAIL-induced apoptosis in human melanoma cells: role for ATP-sensitive K+ channels and endoplasmic reticulum stress. Int J Oncol 41: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsuta K, Mimae T, Nitta H, Yoshida A, Maeshima AM, et al. (2013) Insulin-like growth factor-1 receptor protein expression and gene copy number alterations in non-small cell lung carcinomas. Hum Pathol 44: 975–982. [DOI] [PubMed] [Google Scholar]
- 50. Tang X, Yang L, He Z, Liu J (2012) Insulin glargine and cancer risk in patients with diabetes: a meta-analysis. PloS one 7: e51814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Colmers IN, Bowker SL, Tjosvold LA, Johnson JA (2012) Insulin use and cancer risk in patients with type 2 diabetes: a systematic review and meta-analysis of observational studies. Diabetes Metab 38: 485–506. [DOI] [PubMed] [Google Scholar]
- 52. Janghorbani M, Dehghani M, Salehi-Marzijarani M (2012) Systematic review and meta-analysis of insulin therapy and risk of cancer. Horm Cancer 3: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johnson JA, Carstensen B, Witte D, Bowker SL, Lipscombe L, et al. (2012) Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia 55: 1607–1618. [DOI] [PubMed] [Google Scholar]
- 54. Golder S, Loke YK, Bland M (2011) Meta-analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Med 8: e1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Suissa S (2008) Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 167: 492–499. [DOI] [PubMed] [Google Scholar]
- 56.Inzucchi SE, Sherwin RS (2005) The prevention of type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 34: 199–219, viii. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)
Funnel plots of hypoglycaemic agents and the risk of lung cancer in patients with diabetes, a: metformin; b: thiazolidinediones (TZDs); c: sulfonylureas; d: insulin.
(DOCX)
Risk of bias assessment in Randomised controlled trials.
(DOCX)