Abstract
Background
Adherence to the Mediterranean diet (MD) is associated with reduced morbidity and mortality due to cardiovascular disease. However, how the MD exerts its effects is not fully known.
Aim
To assess the 12-month effects of two enhanced MDs compared to a low-fat diet on inflammatory biomarkers related to atherosclerosis and plaque vulnerability in a subcohort of the PREDIMED (Prevención con Dieta Mediterránea) study.
Methods
A total of 164 participants at high risk for cardiovascular disease were randomized into three diet groups: MD supplemented with 50mL/d of extra virgin olive oil (MD+EVOO) or 30 g/d of nuts (MD+Nuts) and a low-fat diet. Changes in classical cardiovascular risk factors, inflammatory biomarkers of atherosclerosis and plaque vulnerability were measured after 12 months of intervention.
Results
Compared to participants in the low-fat diet group, those receiving MD+EVOO and MD+Nuts showed a higher decrease in systolic (6mmHg) and diastolic (3mmHg) blood pressure (P = 0.02; both), as well as a reduction of 10% and 8% in LDL-cholesterol (P = 0.04), respectively. Patients in the MD+Nuts group showed a significant reduction of 34% in CD40 expression on monocyte surface compared to low-fat diet patients (P = 0.03). In addition, inflammatory biomarkers related to plaque instability such as C-reactive protein and interleukin-6 were reduced by 45% and 35% and 95% and 90% in the MD+EVOO and MD+Nuts groups, respectively (P<0.05; all) compared to the low-fat diet group. Likewise, sICAM and P-selectin were also reduced by 50% and 27%, respectively in the MD+EVOO group (P = 0.04) and P-selectin by 19% in MD+Nuts group (P = 0.04) compared to the low-fat diet group.
Conclusions
Adherence to the MD is associated with an increase in serum markers of atheroma plaque stability which may explain, at least in part, the protective role of MD against ischemic heart disease.
Trial Registration
Introduction
Atherosclerosis, the pathological substratum of coronary heart disease (CHD), is a low-grade chronic inflammatory disease of the vascular wall initiated by the accumulation of cholesterol-laden inflammatory cells (monocytes and T-lymphocytes) in the subendothelial space in a self-perpetuating process that leads to the formation of atheroma plaques, the hallmark of the disease [1]. Inflammatory mediators such as adhesion molecules (selectins, integrins) and interleukins (e.g., IL-6, IL-1β, IL-18) participate in this process. In some instances, the atheroma plaque becomes unstable, leading to cap rupture and ensuing thrombosis that occludes the artery and finally, induces cardiovascular events such myocardial infarction or stroke [2]. Some of these inflammatory mediators (e.g., C-reactive protein [CRP], IL-6, intercellular adhesion molecule-1 [ICAM-1], vascular cell adhesion molecule-1 [VCAM-1]) have been considered as useful predictive markers of atherosclerosis [3], whereas other biomarkers (matrix metalloprotease-9 and IL-18) have been associated with plaque vulnerability [4].
The PREDIMED (Prevención con Dieta Mediterránea) study is the first randomized clinical trial designed to assess the beneficial effects of the MD on the primary prevention of cardiovascular diseases in elderly subjects at high cardiovascular risk. Up to now the PREDIMED study has demonstrated that adherence to the MD is associated with a reduced incidence of diabetes [5]–[6], the metabolic syndrome [7], hypertension [8], and better control of other cardiovascular risk factors [9]–[10]. In fact, Estruch et al [11] have recently reported that a MD intervention reduces the incidence of cardiovascular events by 30% in subjects at high cardiovascular risk. However, improvement in classical cardiovascular risk factors associated with the MD intervention could not fully explain the protective effect of the MD against CHD [11]. Some authors have suggested that, at least in the short term, MD reduces oxidative stress [12], vascular inflammation [13]–[14], and endothelial dysfunction [15], all of which are related to atheroma plaque formation. Thus, in a previous study we have observed lower serum concentrations of VCAM-1, ICAM-1, E- and P-selectin, CRP and IL-6, as well as a down-regulation of adhesion molecules on T-lymphocyte and monocyte surfaces after 3 months of MD intervention [10], [14], [16]. However, whether this effect is maintained in the long run is unknown. Moreover, little is known about the effect of the MD on markers of plaque vulnerability. Therefore, we embarked on a study to assess 1-y changes in inflammatory biomarkers of atherosclerosis as well as markers of plaque instability in a free-living population with high risk of CHD participating in the PREDIMED study.
Methods
The protocol for this trial and supporting CONSORT checklist are available as supporting information; see Checklist S1 and Protocol S1.
Subjects and Design
The PREDIMED study is a parallel-group, multicenter, randomized, controlled 5-year clinical trial aimed to assess the effects of the MD on the primary prevention of cardiovascular disease (CVD) (http://www.predimed.es) [17]. Recruitment took place between October 2003 and January 2009, and the 7447 participants were randomly assigned to one of three interventions (two Mediterranean diets enriched with extra virgin olive oil (EVOO) or mixed nuts, and a control low-fat diet). The design, methodology and eligibility criteria for the PREDIMED study have been previously described [10]–[11], [17]. Briefly, we recruited men aged 55 to 80 years and women aged 60 to 80 years with no previously documented CVD. They were eligible if they had type 2 diabetes, or 3 or more major cardiovascular risk factors (hypertension, high plasma LDL-cholesterol, low plasma HDL-cholesterol, overweight or obesity, current smoking, or a family history of premature coronary heart disease). At baseline and after 12 months of follow-up, the participants filled out a 137-item validated food frequency questionnaire (FFQ), a 14-item questionnaire assessing adherence to the MD and the Minnesota leisure-time physical activity questionnaire. We also recorded medication use, measured anthropometric parameters and blood pressure, and collected fasting blood and a spot urine samples, as described previously [14].
In the current study we screened 193 consecutive potential participants from October 2003 to November 2004 in a primary care center associated with the Hospital Clínic of Barcelona, Spain, but 29 did not fulfill the inclusion criteria. Thus, 164 were finnaly included in this substudy. Main outcome measures were 12-month changes in classical cardiovascular risk factors, and inflammatory markers predictive of atherosclerosis or related to plaque instability.
Diets
After two screening visits with the dietician, participants who fullilled inclusion criteria signed an informed consent and were randomily assigned in a 1∶1∶1 ratio to one of three dietary interventions (MD+EVOO, MD+Nuts or low-fat diet). Randomization was performed centrally by means of a computer-generated random-number sequence. All of the participants had a face-to-face interview with the dietician and a group session at the baseline visit and quarterly thereafter. The dietary intervention in the three treatment groups was delivered by the same dietician as described [11]. The group sessions were organized separately for each of the three intervention groups. In each session the 14-point score of adherence to the MD was the main tool to change dietary habits for the two MDs, and a similar 9-point score was used in the participants of the low-fat diet group. The focus in the MD groups was to change portion sizes and the frequency of intake of the different foods and to modify cooking methods towards the traditional MD of Mediterranean countries in the sixties. Thus, participants in both MD groups were recommended to increase the intake of vegetables (≥2 servings/d), fresh fruit (≥3 servings/d), legumes, nuts, fish or seafood (≥3 servings/wk), and the use of olive oil for cooking and dressings, as previously described [11]. The focus in the control group was to reduce all types of fat, with particular emphasis on recommending the consumption of lean meats, low-fat dairy products, cereals, potatoes, pasta, rice, fruits and vegetables. All participants were provided with descriptions of seasonal foods, shopping lists, weekly meal plans and cooking recipes, according to the their intervention group. Olive oil and nut industry companies supplied EVOO (50 mL/d) or 30 g/d of walnuts, almonds and halzelnuts free of charge to the respective MD groups, whereas those in the control group received small nonfood gifts. The fatty acid composition of the EVOO and nuts used in the trial is described elsewhere [10]. No total calorie restriction was advised, nor was physical activity promoted. From the beginning of the study all participants were recommended to not use multivitamin or antioxidant supplements.
Ethics Statement
All participants provided informed consent. Participants had signed the informed Consent. The Institutional Review Board (IRB) of the Hospital Clinic (Barcelona, Spain) accredited by the US Department of Health and Human Services (DHHS) update for Federal wide Assurance for the Protection of Human Subjects for International (Non-US) Institutions #00000738 approved the study protocol on July, 16, 2002. The protocol was also approved by the ethical review board of our hospital.
Clinical and Laboratory Measurements
Trained personnel measured weight and height using calibrated scales and a wall-mounted stadiometer, respectively; waist circumference was determined midway between the lowest rib and the iliac crest using an anthropometric tape, and blood pressure (BP) was measured in triplicate with a validated semiautomatic oscillometer (Omron HEM-705CP) [10]–[11]. Samples of serum, EDTA-plasma, and urine were coded and stored at −80°C until assay. A technician blinded to group allocation processed peripheral blood mononuclear cells (PBMCs) on the same day of blood extraction. PBMCs were isolated from whole blood by Ficoll-Hypaque (Lymphoprep, Axis-Shield PoC AC) density-gradient. The expression of adhesion molecules on the surface of PBMCs was analyzed via double direct immunofluorescence with the use of commercial monoclonal antibodies following the manufacturer’s instructions. The adhesion molecules analyzed were: anti-CD49d (Cytogmos), anti-CD11a and anti-CD11b (Bender Medsystems), anti-CD40, anti-CD14 and anti-CD2 monoclonal antibodies (Caltag). Cell counts (5000 events for T-lymphocytes and 2000 for monocytes) and fluorescence analysis were performed in a FACSCalibur Flow Cytometer (Becton-Dickinson) using CellQuest software. Results are expressed as mean fluorescence intensity (MFI) in arbitrary units.
ELISAs were performed in baseline and 1-year samples at the end of the study period in thawed plasma with commercial kits for soluble (s) E- and P-selectin, sVCAM-1, sICAM-1, IL-18, IL-6 (BLK and PelkinElmer Elast Amplification System), IL-10 and tissular inhibitor of metalloproteases-1 (TIMP-1) (R&D Systems), MMP-9 (Amersham), and transforming growth factor beta 1 (TGF-β1) (R&D Systems). A technician blinded to group allocation processed the ELISA kits.
Additional serum analytes determined included fasting glucose and immunoreactive insulin, total cholesterol, triglycerides, HDL and LDL-cholesterol, and high-sensitivity CRP, as described elsewhere [10]–[11]. In a random sample of 56 participants (34%) we measured urinary tyrosol and hydroxytyrosol levels (as a measure of adherence to EVOO consumption recommendations) and the plasma α-linolenic acid (ALA) proportion (as a measure of adherence to walnut consumption recommendations), as reported previously [10]. For all laboratory methods, the intra- and inter-assay variation coefficients ranged from 1.8 to 8.9% and from 0.9 to 9.9%, respectively.
Statistical Analyses
For a parallel design, the sample size was determined with the ENE 3.0 statistical program (GlaxoSmithKline, Brentford, United Kingdom) assuming a maximum loss of 10% of participants. To detect a mean difference of 10 MFI units on the expression of monocyte CD49d with a conservative standard deviation (SD) of 10, 20 subjects would be needed to complete the study (α risk = 0.05, power = 0.9). The monocyte expression of CD49d was considered the primary outcome and used to determine the sample size, but changes in all endpoints were of equal interest in this study.
We used descriptive statistics with the mean ± SD for the baseline characteristics of the participants. Categorical variables are expressed as percentages. Variables with a skewed distribution (Kolmogorov) were transformed to their natural logarithm for analysis. One-factor analysis of variance or chi-square tests, as appropriate, were used to determine differences in baseline characteristics among the three study groups. Changes in all outcomes were assessed with repeated-measures analysis of variance for the two factors, diet and time, and their interactions. Significant interactions were analyzed by the simple effects test with multiple contrasts of Bonferroni. Within- and between-group differences are expressed as means and 95% confidence intervals (CI).
The relationship between monounsaturated acid (MUFA) intake and inflammatory markers was determined by partial correlation analysis. All statistical tests were two-tailed, with significance set at P<0.05. Analyses were performed using the SPSS, version 18.0 (SPSS Inc., Chicago, IL).
Results
Participant Characteristics
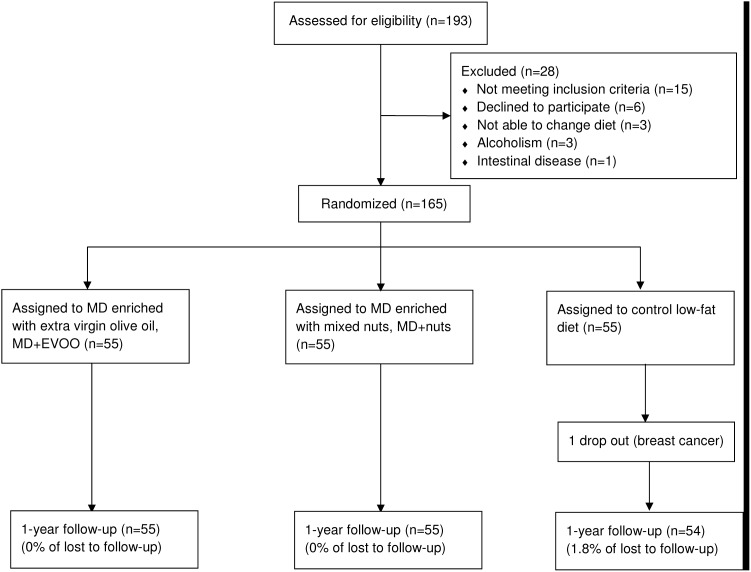
Of the 164 participants finnally included, 55, 55 and 54 were randomized to a MD supplemented with virgin olive oil, a MD supplemented with nuts, and a low-fat control diet, respectively. The retention rates for 1 year follow-up were 100%, 100% and 98.2%, respectively ( Figure 1 ). Table 1 shows the baseline characteristics of these 164 participants, of whom none were lost to follow-up. The groups were well balanced regarding demographic characteristics, adiposity and cardiovascular risk factors. The medication taken and occupation levels were also similar in the three groups. Drug regimens did not appreciably change during the 12-month follow-up.
Figure 1. Flowchart of study participants.
The diagram includes detailed information on the excluded participants. Abbreviations: MD, Mediterranean diet.
Table 1. Baseline characteristics of the participants.1 .
| MD+EVOO (n = 55) | MD+nuts (n = 55) | Low-fat diet (n = 54) | |
| Age (years) | 68.1±6 | 67.6±6 | 67.4±6 |
| Men (%) | 24 (43.6) | 31 (56.4) | 22 (40.7) |
| Family history of CHD (%) | 16 (29.1) | 9 (16.4) | 13 (24.1) |
| Current smokers (%) | 10 (18.2) | 11 (20) | 11 (20.4) |
| BMI (kg/m2) | 27.9±3.4 | 27.8±3.1 | 28.5±3.7 |
| BMI≥25 kg/m (%) | 47 (85.5) | 45 (81.8) | 44 (81.5) |
| Type 2 diabetes (%) | 46 (83.6) | 44 (80) | 37 (68.5) |
| Hypertension (%) | 39 (70.9) | 29 (52.7) | 37 (68.5) |
| Dyslipidemia (%) | 32 (58.2) | 34 (61.8) | 38 (70.4) |
| Medications (%) | |||
| ACE inhibitors | 11 (20) | 12 (21.8) | 13 (24.1) |
| Diuretics | 13 (23.6) | 6 (10.9) | 14 (25.9) |
| Other antihypertensive agents | 10 (18.2) | 8 (14.5) | 9 (16.7) |
| Statins | 17 (31) | 14 (25.5) | 10 (18.5) |
| Other-lipid-lowering agents | 4 (7.3) | 2 (3.6) | 6 (11.1) |
| Insulin | 3 (5.5) | 7 (12.7) | 3 (5.6) |
| Oral hypoglycemic drugs | 30 (54.5) | 25 (45.5) | 29 (53.7) |
| Aspirin or antiplatelet drugs | 10 (18.2) | 8 (14.5) | 5 (9.3) |
| NSAIDS | 6 (10.9) | 10 (18.2) | 8 (14.8) |
Values are mean ± SD or n (%). ACE, angiotensin converting enzyme; BMI, body mass index; CHD, coronary heart disease; EVOO, extra virgin olive oil; MD+EVOO, Mediterranean diet supplemented with extra virgin olive oil; MD+Nuts, Mediterranean diet supplemented with nuts; NSAIDS, Non-steroidal antiinflammatory drugs.
Cardiovascular Risk Factors
The baseline and 12-month values for the classical cardiovascular risk factors are shown in Table 2 . The MD+EVOO and MD+Nuts groups showed a mean reduction in systolic BP of 6 mmHg (P = 0.02; both) and in diastolic BP of around 3 mmHg (P = 0.02; both), of 6% and 7% (P = 0.04), respectively, in total-cholesterol, of 10% and 8% (P = 0.04), respectively, in LDL-cholesterol, and of 9% and 5% (P = 0.01), respectively in the cholesterol/HDL-cholesterol ratio. Both MDs showed a decrease in the waist perimeter (P<0.05; both) of the participants from baseline.
Table 2. Changes in adiposity, blood pressure and cardiovascular risk factors.
| MD+EVOO (n = 55) | MD+Nuts (n = 55) | Low-fat diet (n = 54) | ||||||
| Mean | P3 | Mean | P3 | Mean | P3 | Pint 4 | ||
| Weight (Kg) | Baseline1 | 75.5±1.6 | 76.9±1.6 | 75.3±1.7 | 0.70 | |||
| 1y.1 | 74.9±1.6 | 76.7±1.7 | 75.0±1.7 | |||||
| Mean changes2 | −0.6 (−1.4 to 0.1) | 0.09 | −0.2 (−1.0 to 0.5) | 0.54 | −0.3 (−1.1 to 0.5) | 0.44 | ||
| BMI (Kg/m2) | Baseline | 29.2±0.5 | 28.9±0.5 | 29.3±0.5 | 0.85 | |||
| 1y. | 29.1±0.5 | 28.8±0.5 | 29.2±0.6 | |||||
| Mean changes | −0.1 (−0.4 to 0.2) | 0.99 | −0.1 (−0.4 to 0.2) | 0.90 | −0.1 (−0.5 to 0.2) | 0.44 | ||
| Waist circumference (cm) | Baseline | 102±1.3 | 102±1.3 | 100±1.4 | 0.09 | |||
| 1y. | 98.6±1.4 | 99.2±1.4 | 99.4±1.5 | |||||
| Mean changes | −3.2 (−4.6 to −1.7) | <0.001 | −2.8 (−4.3 to −1.4) | <0.001 | −0.6 (−2.1 to 0.9) | 0.42 | ||
| Systolic blood pressure (mmHg) | Baseline | 152±2.6 | 148±2.6 | 153±2.7 | 0.02 | |||
| 1y. | 146±2.6 | 141±2.5 | 155±2.7 | |||||
| Mean changes | −6.0 (−10.1 to −2.0)a | 0.004 | −6.4 (−10.5 to −2.4)a | 0.002 | 2.2 (−2.1 to 6.5) | 0.32 | ||
| Diastolic blood pressure (mmHg) | Baseline | 85.0±1.3 | 85.1±1.3 | 86.8±1.4 | 0.02 | |||
| 1y. | 81.8±1.2 | 82.5±1.2 | 88.4±1.3 | |||||
| Mean changes | −3.2 (−5.4 to −0.9)a | 0.07 | −2.6 (−4.9 to −0.4)a | 0.02 | 1.6 (−0.8 to 4.0) | 0.20 | ||
| Glucose (mg/dL) | Baseline | 130±5.5 | 127±5.6 | 132±5.6 | 0.83 | |||
| 1y. | 131±5.5 | 127±5.5 | 129±5.5 | |||||
| Mean changes | 1.2 (−6.8 to 9.2) | 0.77 | −0.2 (−8.3 to 7.9) | 0.96 | −2.9 (−11.1 to 5.3) | 0.49 | ||
| Glycated hemoglobin (mg/dL) | Baseline | 6.1±0.2 | 6.0±0.2 | 6.1±0.3 | 0.90 | |||
| 1y. | 6.3±0.2 | 6.1±0.2 | 6.0±0.2 | |||||
| Mean changes | 0.2 (−0.1 to 0.5) | 0.23 | 0.1 (−0.2 to 0.3) | 0.66 | −0.1 (−0.4 to 0.2) | 0.45 | ||
| Triglycerides (mg/dL) | Baseline | 147±11.1 | 138±11.1 | 148±11.5 | 0.80 | |||
| 1y. | 143±8.9 | 135±8.9 | 133±9.2 | |||||
| Mean changes | −4.2 (−24.0 to 15.5) | 0.67 | −2.9 (−22.6 to 16.9) | 0.77 | −15.5 (−36.0 to 5.0) | 0.14 | ||
| Total-cholesterol (mg/dL) | Baseline | 228±4.6 | 225±4.6 | 208±4.7 | 0.04 | |||
| 1y. | 214±4.5 | 209±4.5 | 207±4.6 | |||||
| Mean changes | −13.5 (−23.0 to −4.1)a | 0.005 | −15.7 (−25.1 to −6.3)a | 0.001 | 0.1 (−9.4 to 9.6) | 0.98 | ||
| HDL-Cholesterol (mg/dL) | Baseline | 54.5±1.6 | 53.8±1.7 | 55.6±1.7 | 0.47 | |||
| 1y. | 56.6±1.7 | 52.6±1.7 | 53.2±1.8 | |||||
| Mean changes | 2.1 (−0.2 to 4.4) | 0.07 | −1.2 (−3.5 to 1.1) | 0.31 | −2.4 (−2.8 to −2.0) | 0.74 | ||
| LDL-Cholesterol (mg/dL) | Baseline | 145±3.9 | 144±3.9 | 128±4.0 | 0.04 | |||
| 1y. | 130±4.0 | 132±4.0 | 124±4.0 | |||||
| Mean changes | −14.4 (−21.1 to −7.7)a | <0.001 | −11.7 (−18.4 to −5.0)a | 0.001 | −3.6 (−10.4 to 3.2) | 0.56 | ||
| Cholesterol: HDL-Cholesterol ratio | Baseline | 4.4±0.1 | 4.3±0.1 | 3.8±0.1 | 0.01 | |||
| 1y. | 4.0±0.1 | 4.1±0.1 | 3.9±0.1 | |||||
| Mean changes | −0.4 (−0.6 to −0.2)a | <0.001 | −0.2 (−0.5 to −0.03)a | 0.03 | 0.05 (−0.2 to 0.3) | 0.83 | ||
Data analyzed by repeated-measures 2-factor ANOVA (simple-effect analysis by Bonferroni’s multiple contrast).
Values are mean ± SD.
Mean differences (95% CI).
P: Significant differences (P<0.05) between before and after the intervention.
Pint: comparison between measures obtained before and after intervention and among the 3 diet groups, P<0.05.
MD+EVOO or MD+nuts vs. low fat-diet are significantly different, P<0.05. EVOO, extra virgin olive oil; MD+EVOO, Mediterranean diet supplemented with extra virgin olive oil; MD+Nuts, Mediterranean diet supplemented with nuts. BMI, body mass index.
Adhesion Molecule and CD40 Expression in PBMC
As shown in Table 3 , after 12 months of intervention the MD+EVOO group showed a decrease in CD11a (P<0.001), CD49d (P<0.004) and CD40 (P<0.001) in peripheral T-lymphocytes. In addition, MD+EVOO showed decreased CD11a, CD11b, CD49d and CD40 (P<0.001; all) in circulating monocytes.
Table 3. Changes in adhesion molecule expression in circulating T- lymphocytes and monocytes.
| MD+EVOO (n = 55) | MD+Nuts (n = 55) | Low-fat diet (n = 54) | ||||||
| Mean | P3 | Mean | P3 | Mean | P3 | Pint 4 | ||
| T-LYMPHOCYTES | ||||||||
| CD11a | Baseline1 | 132±4.7 | 137±5.2 | 121±5.2 | 0.26 | |||
| 1y.1 | 107±5.3 | 107±6.0 | 103±6.0 | |||||
| Mean changes2 | −24.4 (−36.0 to −13.0) | <0.001 | −29.9 (−43.1 to −16.7) | <0.001 | −18.3 (−31.2 to −5.2) | 0.006 | ||
| CD49d | Baseline | 48.3±1.1 | 39.0±1.1 | 34.8±1.1 | 0.33 | |||
| 1y. | 36.7±1.1 | 41.0±1.1 | 39.9±1.1 | |||||
| Mean changes | −11.7 (−16.1 to −7.2) | 0.004 | 2.0 (−0.7 to 4.7) | 0.54 | 5.1 (3.1 to 7.1) | 0.14 | ||
| CD40 | Baseline | 47.5±1.1 | 55.3±1.1 | 44.2±1.1 | 0.20 | |||
| 1y. | 36.7±1.1 | 42.0±1.1 | 38.6±1.1 | |||||
| Mean changes | −11.0 (−12.6 to −9.3) | 0.001 | −13.5 (−15.5 to −11.4) | 0.001 | −5.6 (−6.5 to −4.8) | 0.09 | ||
| MONOCYTES | ||||||||
| CD11a | Baseline | 85.0±4.2 | 80.1±4.4 | 78.8±4.2 | 0.41 | |||
| 1y. | 57.3±2.0 | 56.8±2.1 | 53.9±2.1 | |||||
| Mean changes | −27.7 (−36.1 to −19.5) | <0.001 | −23.3 (−32.0 to −14.6) | <0.001 | −24.9 (−33.3 to −6.6) | <0.001 | ||
| CD11b | Baseline | 44.7±2.1 | 47.3±2.2 | 43.9±2.2 | 0.38 | |||
| 1y. | 34.6±1.3 | 36.5±1.3 | 35.1±1.3 | |||||
| Mean changes | −10.1 (−14.6 to −5.5) | <0.001 | −10.8 (−15.6 to −6.1) | <0.001 | −8.8 (−13.6 to −4.1) | <0.001 | ||
| CD49d | Baseline | 35.2±1.1 | 39.0±1.1 | 35.0±1.1 | 0.50 | |||
| 1y. | 27.2±1.1 | 29.2±1.1 | 30.7±1.1 | |||||
| Mean changes | −8.0 (−9.4 to −6.5) | <0.001 | −9.8 (−11.6 to −8.1) | <0.001 | −4.3 (−5.3 to −3.2) | 0.06 | ||
| CD40 | Baseline | 36.1±2.9 | 46.5±2.9 | 35.2±2.9 | 0.03 | |||
| 1y. | 26.3±1.9 | 30.9±1.9 | 31.1±1.9 | |||||
| Mean changes | −9.8 (−15.0 to −4.6)b | <0.001 | −15.6 (−20.9 to −10.2)a ,b | <0.001 | −4.1 (−9.4 to 1.2) | 0.13 | ||
Data analyzed by repeated-measures 2-factor ANOVA (simple-effect analysis by Bonferroni’s multiple contrast).
Values are mean ± SD.
Mean differences (95% CI).
P: Significant differences (P<0.05) between before and after the intervention.
Pint: comparison between measures obtained before and after intervention and among the 3 diet groups, P<0.05.
MD+EVOO or MD+nuts vs. low fat-diet and bMD+EVOO vs. MD+nuts are significantly different, P<0.05. EVOO, extra virgin olive oil; MD+EVOO, Mediterranean diet supplemented with extra virgin olive oil; MD+Nuts, Mediterranean diet supplemented with nuts.
On the other hand, the MD+Nuts group showed a significant decrease in CD11a and CD40 of (P<0.00; all) in T- lymphocytes. For monocytes, the MD+Nuts group showed a significant decrease in CD11a, CD11b, CD49d and CD40 (P<0.001; all).
Finally, the low-fat diet only showed a significant decrease in CD11a in T-lymphocytes and a decrease in CD11a and CD11b expression in circulating monocytes (P<0.001; all).
Circulating Markers of Plaque Instability and other Inflammatory Biomarkers
Baseline plasma concentrations of inflammatory biomarkers were similar among groups. As can be observed in Table 4 , after a 12-month intervention the participants allocated to the MD+EVOO showed a decrease in sVCAM-1 (P<0.02), sICAM-1 (P<0.001) and sP-selectin (P<0.001) concentrations. Furthermore, the MD+Nuts group showed a decrease in sVCAM-1 (P<0.001) and sE-selectin (P<0.002) and sP-selectin of (P<0.007). By contrast, the serum concentration of sICAM-1 was significantly increased (P<0.02) in the control group.
Table 4. Changes in the expression of circulating markers of plaque instability and other inflammatory biomarkers.
| MD+EVOO (n = 55) | MD+Nuts (n = 55) | Low-fat diet (n = 54) | ||||||
| Mean | P3 | Mean | P3 | Mean | P3 | Pint 4 | ||
| sVCAM (ng/mL) | Baseline1 | 872±47.0 | 935±49.2 | 776±48.6 | 0.30 | |||
| 1y.1 | 734±44.9 | 727±47.1 | 720±46.5 | |||||
| Mean changes2 | −138 (−251 to −25.2) | 0.02 | −208 (−327 to −89.6) | 0.001 | −55.6 (−173 to 61.5) | 0.35 | ||
| sICAM (ng/mL) | Baseline | 437±27.3 | 394±23.3 | 369±24.0 | 0.04 | |||
| 1y. | 217±22.0 | 364±18.8 | 431±19.2 | |||||
| Mean changes | −220 (−273 to −166)b | <0.001 | −30.3 (−76.1 to 15.5)b | 0.20 | 62.3 (15.5 to 109)b | 0.01 | ||
| sE-SEL (ng/mL) | Baseline | 28.6±2.5 | 33.0±2.6 | 32.3±2.6 | 0.55 | |||
| 1y. | 26.9±2.4 | 28.3±2.5 | 30.1±2.5 | |||||
| Mean changes | −1.7 (−4.5 to 1.2) | 0.26 | −4.7 (−7.7 to −1.7) | 0.003 | −2.2 (−5.3 to 0.9) | 0.16 | ||
| sP-SEL (ng/mL) | Baseline | 91.4±9.3 | 87.6±9.4 | 50.0±10.5 | 0.04 | |||
| 1y. | 66.5±8.3 | 70.8±8.4 | 51.1±9.3 | |||||
| Mean changes | −25.0 (−32.3 to −17.6)a | <0.001 | −16.8 (−24.3 to −9.4)a | <0.001 | 1.1 (−7.1 to 9.4) | 0.78 | ||
| IL-6 (pg/mL) | Baseline | 0.7±0.1 | 0.9±0.1 | 0.7±0.1 | 0.04 | |||
| 1y. | 0.4±0.1 | 0.5±0.1 | 1.0±0.1 | |||||
| Mean changes | −0.3 (−0.9 to 0.3)a | <0.001 | −0.4 (−1.0 to 0.2)a | <0.001 | 0.3 (−1.1 to 1.7) | <0.001 | ||
| CRP (mg/mL) | Baseline | 3.8±1.1 | 3.5±1.1 | 3.6±1.1 | 0.04 | |||
| 1y. | 1.9±1.1 | 2.1±1.1 | 3.3±1.1 | |||||
| Mean changes | −1.9 (−2.4 to −1.6)a | <0.001 | −1.4 (−2.1 to −0.7)a | <0.001 | −0.3 (−1.3 to 0.8) | 0.46 | ||
| IL-18 (pg/mL) | Baseline | 139±14.3 | 131±14.5 | 103±14.6 | 0.18 | |||
| 1y. | 137±13.1 | 112±13.2 | 101±13.4 | |||||
| Mean changes | −1.8 (−13.8 to 10.2) | 0.76 | −18.6 (6.4 to 30.7) | 0.003 | −1.3 (−13.5 to 11.0) | 0.84 | ||
| IL-10 (pg/mL) | Baseline | 1.4±1.1 | 1.3±1.1 | 1.2±1.1 | 0.40 | |||
| 1y. | 1.5±1.1 | 1.4±1.1 | 1.3±1.1 | |||||
| Mean changes | 0.05 (−0.2 to 0.3) | 0.62 | 0.05 (−0.2 to 0.3) | 0.60 | 0.1 (−0.1 to 0.3) | 0.29 | ||
| IL-18/IL-10 ratio | Baseline | 31.9±4.0 | 17.0±4.1 | 20.6±4.0 | 0.02 | |||
| 1y. | 17.2±3.4 | 7.9±3.5 | 19.0±3.4 | |||||
| Mean changes | −14.7 (−23.1 to −6.2) | 0.001 | −9.1 (−18.0 to −0.3) | 0.04 | −1.6 (−10.1 to 6.9) | 0.71 | ||
| MMP-9 (ng/mL) | Baseline | 7.7±1.2 | 7.9±1.2 | 6.2±1.2 | 0.78 | |||
| 1y. | 10.0±1.2 | 10.4±1.2 | 10.5±1.2 | |||||
| Mean changes | 2.3 (0.9 to 3.8) | 0.13 | 2.5 (1.1 to 3.8) | 0.11 | 4.3 (1.2 to 7.3) | 0.003 | ||
| TIMP-1 (ng/mL) | Baseline | 143±6.7 | 146±7.3 | 144±7.2 | 0.94 | |||
| 1y. | 146±7.4 | 144±8.2 | 152±8.2 | |||||
| Mean changes | 2.7 (−8.7 to 14.0) | 0.64 | −2.4 (−14.9 to 10.1) | 0.71 | 7.5 (−4.7 to 19.8) | 0.23 | ||
| MMP-9/TIMP-1 ratio | Baseline | 0.06±1.2 | 0.06±1.2 | 0.04±1.2 | 0.60 | |||
| 1y. | 0.08±1.2 | 0.08±1.2 | 0.07±1.2 | |||||
| Mean changes | 0.02 (0.01 to 0.03) | 0.125 | 0.02 (0.01 to 0.03) | 0.15 | 0.03 (0.01 to 0.06) | 0.03 | ||
| TGF-β1 (pg/mL) | Baseline | 40.2±2.3 | 46.7±2.4 | 43.0±2.5 | 0.11 | |||
| 1y. | 44.5±2.1 | 49.3±2.2 | 49.0±2.3 | |||||
| Mean changes | 4.3 (−0.4 to 9.0) | 0.08 | 2.6 (−2.2 to 7.5) | 0.30 | 5.9 (0.9 to 11.0) | 0.02 | ||
Data analyzed by repeated-measures 2-factor ANOVA (simple-effect analysis by Bonferroni’s multiple contrast).
Values are mean ± SD.
Mean differences (95% CI).
P: Significant differences (P<0.05) between before and after the intervention.
Pint: comparison between measures obtained before and after intervention and among the 3 diet groups, P<0.05.
MD+EVOO or MD+nuts vs. low fat-diet are significantly different, P<0.05.
All the groups differed, P<0.05. EVOO, extra virgin olive oil; MD+EVOO, Mediterranean diet supplemented with extra virgin olive oil; MD+Nuts, Mediterranean diet supplemented with nuts.
Other molecules related to plaque instability ( Table 4 ), such as CRP and IL-6 (P<0.001; both) and the IL-18/IL-10 ratio (P≤0.04) decreased in the MD+EVOO and MD+nuts groups. Furthermore, in the MD+nuts group, IL-18 concentration also decreased (P<0.003). Finally, the control group showed a significant increase in IL-6 (P<0.001), MMP-9 (P<0.003) and TGF-β1 (P<0.02) levels. The same group, showed an increase, albeit no significant, in the MMP-9/TIMP-1 ratio (P<0.003) and TIMP-1, although the changes were not significant.
None of the groups showed significant Pearson correlation coefficients between MUFA intake and any of the inflammatory marker concentrations.
Changes in Food and Nutrient Intake during Follow-up
The self-reported dietary habits of the participants prior to starting the study were similar among the three groups. Baseline diets were high in fiber, total fat and MUFA because of a high baseline consumption of olive oil and marine n3 fatty acids due to frequent fish intake (Appendix S1 and S2). The saturated fatty acid (SFA) and ω3-polyunsaturated fatty acid (PUFA) content of the diets was relatively low.
Dietary intervention resulted in favorable changes in food consumption, mainly in the MD groups (Appendix S1). Accordimgly, after 12 months these subjects showed a significant increase in adherence to the MD pattern (P<0.001).
Appendix S2 shows changes in baseline energy and nutrient intake after the 12-month intervention. At 12 months, the participants in the MD+EVOO group increased the average urine concentration of the phenolic compound tyrosol 8.0 ng/mL (P<0.02) from a baseline value of 61.0 ng/mL, and hydroxytyrosol increased 39.0 ng/mL (P<0.04) from a baseline value of 205 ng/mL, while the plasma content of ALA increased 0.14 mol% (P<0.01) from a baseline value of 0.3 mol% in subjects assigned to the MD+Nuts group. These three parameters were used as an objective measure of compliance in the MD intervention diet groups.
Discussion
Higher adherence to a MD intervention supplemented with EVOO or nuts, for at least 12 months, were associated with a significant decrease in inflammatory markers related to atheroma plaque formation and plaque instability, in addition to a reduction in BP and plasma LDL-cholesterol concentration [18]–[19]. All of these mechanisms may explain, at least in part, the lower incidence of CHD, stroke and mortality in subjects at high risk of CVD following a Mediterranean diet as has been recently demonstrated by the PREDIMED study [11].
In the long term, a decrease of 10 or 5 mmHg in systolic or diastolic BP, respectively, is associated with a 40% and 30% reduction in the risk of stroke or myocardial infarction, respectively [20]. Moreover, a 10% reduction in plasma cholesterol concentrations has been associated with a 20% reduction in CHD risk [21].
In the current paper, we observed a significant decrease of 6 mmHg in systolic BP and 3 mmHg in diastolic BP in both MD groups [10]. This same trend was observed in plasma total-cholesterol concentrations, with a decrease of 5% for the MD+EVOO and 8% for the MD+Nuts compared to the control group. These declines were also similar to those already observed at 3 months for both MDs [10]. Nonetheless, improvements in BP and the lipid profile can not explain the whole protective action of the MD against atherosclerosis, thereby suggesting the presence of alternative effects.
It is well known that atherosclerosis is a chronic low-grade inflammatory disease of the arterial wall [2]. Thus, modulation of this inflammatory reaction may be another potential way by which the MD protects against atherosclerosis. In the current study, we detected several antiinflammatory effects in the three diets studied, although they were more intense in subjects allocated to the two MD interventions. These subjects showed a higher down-regulation of adhesion molecules in T-lymphocytes and monocytes compared to those in the control group. Moreover, serum concentrations of endothelial soluble cell adhesion molecules, CRP and IL-6 were also lower in subjects following the two MDs compared to control subjects. These results agree with those previously published by Castaner et al [28] and Estruch et al [10], both based in data from PREDIMED trial, in which a sustained traditional MD supplemented with EVOO or nuts may exert health benefits through changes in the transcriptomic response of genes related to cardiovascular risk. Moreover, the IL-18 and IL-18/IL-10 ratio, which are related to ischemic events in the heart and brain [29]–, were decreased after the MD+nuts and both MD interventions, respectively, suggesting a greater stability of atheroma plaque in patients following a MD diet.
This antiinflammatory effect has already been associated with different interventions related to a lower incidence of cardiovascular disease such as a diet rich in fruit, vegetables and olive oil [22], statins [23], moderate alcohol intake [24] or physical activity [25]. In fact, previous studies have reported that some components of the MD such as EVOO or nuts may down-regulate inflammatory markers related to atherosclerosis such as VCAM-1, ICAM-1, E- and P-Selectin, CRP and IL-6 [26]–[27].
When we analyzed the possible mechanisms responsible for the antiinflammatory effect observed in both MD groups, the role of exercise on immunomodulation is probably residual since no changes in physical activity were observed in the three groups. In fact, we only observed a significant increase in physical activity in the low-fat diet group compared to both MD groups, but paradoxically this situation was not associated with a greater antiinflammatory effect as would be expected in this group [25]. Finally, the antiinflammatory effect may be due to a synergistic action among nutrients from key foods of the MD such as EVOO or nuts.
Interestingly, the antiinflammatory effect of the MD seems to be greater and more intense in the mid-term compared to the short-term [10], [14], while the effect on classical cardiovascular risk factors was similar, thereby suggesting that the MD exerts its effects on lipids and blood pressure relatively quickly (at 3 mo), with the maximum effect on systemic inflammatory biomarkers being achieved later (at 1 y). Thus, in the short-term the effect on BP and the lipid profile is higher, whereas in the mid-term the effect on chronic inflammatory response in the arterial wall is more pronounced.
The strengths of our study are its design as a randomized controlled clinical trial, excellent completion rates, good compliance, and the specific inflammatory biomarkers studied, which are involved in different phases of atheroma plaque formation. Finally, there is the question as to the clinical relevance of the antiinflammatory effects of MD. However, the recently published results of PREDIMED [11] showing lower cardiovascular morbility and mortality are sufficiently consistent to reject any doubt about the antiinflammatory effect of the MD. Regarding the limitations, this study was performed in subjects at high cardiovascular risk and therefore, the results may not be generalized to the overall population. Moreover, this study was limited to classical cardiovascular risk factors and inflammatory parameters. Thus, we can not exclude other protective effects on other clinical or biological parameters related to cardiovascular risk such as markers of arterial structure and function or oxidative stress.
In summary, the results of our study suggest that the Mediterranean diet supplemented with EVOO or nuts has a dual effect on the prevention of cardiovascular disease improving classical cardiovascular risk factors and also has an intense antiinflammatory effect. Both of these effects could partially explain the overall beneficial effect of the MD on the primary prevention of cardiovascular disease observed in high risk subjects.
Supporting Information
Consumption of key food items, physical ctivity and 14-point Mediterranean diet score.
(PDF)
Changes in baseline energy and nutrient intake.
(PDF)
Predimed Study: Mediterranean diet in the primary prevention of cardiovascular disease.
(PDF)
CONSORT checklist.
(DOC)
Acknowledgments
The authors thank the participants for their enthusiastic collaboration, the PREDIMED personnel for their excellent assistance and the personnel of all affiliated primary care centers. M.U.-S. would like to thank the “Ramon y Cajal” program from the MINECO and the Fondo Social Europeo.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work has been supported by grants from CIBEROBN, RETICS RD06/0045, Spanish Ministry of Economy and Competitiveness (MEC) (AGL2009-13906-C02-02 and AGL2010-22319-C03-02); Instituto de Salud Carlos III (PI10/01407, G03/140), CIBEROBN, and European Union (FEDER). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hansson GK, Hermansson A (2011) The immune system in atherosclerosis. Nat Immunol 12(3): 204–12. [DOI] [PubMed] [Google Scholar]
- 2. Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R (2010) Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol 30(7): 1282–92. [DOI] [PubMed] [Google Scholar]
- 3. Barbaresko J, Koch M, Schulze MB, Nöthlings U (2013) Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev 71(8): 511–27. [DOI] [PubMed] [Google Scholar]
- 4. Koenig W, Khuseyinova N (2007) Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol 27(1): 15–26. [DOI] [PubMed] [Google Scholar]
- 5. Salas-Salvadó J, Bulló M, Babio N, Martínez-González MÁ, Ibarrola-Jurado N, et al. (2011) Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care 34(1): 14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juanola-Falgarona M, Salas-Salvadó J, Estruch R, Portillo MP, Casas R, et al. (2013) Association between dietary phylloquinone intake and peripheral metabolic risk markers related to insulin resistance and diabetes in elderly subjects at high cardiovascular risk. Cardiovasc Diabetol 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Babio N, Ibarrola-Jurado N, Bulló M, Martínez-González MÁ, Wärnberg J, et al. (2013) White blood cell counts as risk markers of developing metabolic syndrome and its components in the predimed study. Plos One 8(3): e58354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toledo E, Delgado-Rodríguez M, Estruch R, Salas-Salvadó J, Corella D, et al. (2009) Low-fat dairy products and blood pressure: follow-up of 2290 older persons at high cardiovascular risk participating in the PREDIMED study. Br J Nutr 101(1): 59–67. [DOI] [PubMed] [Google Scholar]
- 9. Lohse B, Psota T, Estruch R, Zazpe I, Sorli JV, et al. (2010) Eating competence of elderly Spanish adults is associated with a healthy diet and a favorable cardiovascular disease risk profile. J Nutr 140(7): 1322–7. [DOI] [PubMed] [Google Scholar]
- 10. Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, et al. (2006) Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med 145(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 11. Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, et al. (2013) Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 368(14): 1279–90. [DOI] [PubMed] [Google Scholar]
- 12. Mitjavila MT, Fandos M, Salas-Salvadó J, Covas MI, Borrego S, et al. (2013) The Mediterranean diet improves the systemic lipid and DNA oxidative damage in metabolic syndrome individuals. A randomized, controlled, trial. Clin Nutr 32(2): 172–8. [DOI] [PubMed] [Google Scholar]
- 13. Urpi-Sarda M, Casas R, Chiva-Blanch G, Romero-Mamani ES, Valderas-Martínez P, et al. (2012) Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacol Res 65(6): 577–83. [DOI] [PubMed] [Google Scholar]
- 14. Mena MP, Sacanella E, Vazquez-Agell M, Morales M, Fitó M, et al. (2009) Inhibition of circulating immune cell activation: a molecular antiinflammatory effect of the Mediterranean diet. Am J Clin Nutr 89(1): 248–56. [DOI] [PubMed] [Google Scholar]
- 15. Marin C, Ramirez R, Delgado-Lista J, Yubero-Serrano EM, Perez-Martinez P, et al. (2011) Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am J Clin Nutr 93(2): 267–74. [DOI] [PubMed] [Google Scholar]
- 16. Llorente-Cortés V, Estruch R, Mena MP, Ros E, González MA, et al. (2010) Effect of Mediterranean diet on the expression of pro-atherogenic genes in a population at high cardiovascular risk. Atherosclerosis 208(2): 442–50. [DOI] [PubMed] [Google Scholar]
- 17. Martínez-González MÁ, Corella D, Salas-Salvadó J, Ros E, Covas MI, et al. (2012) Cohort profile: design and methods of the PREDIMED study. Int J Epidemiol 41(2): 377–85. [DOI] [PubMed] [Google Scholar]
- 18. Mente A, de Koning L, Shannon HS, Anand SS (2009) A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Inter Med 69(7): 659–69. [DOI] [PubMed] [Google Scholar]
- 19. Sofi F, Abbate R, Gensini GF, Casini A (2010) Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 92(5): 1189–96. [DOI] [PubMed] [Google Scholar]
- 20. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R (2002) Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360(9349): 1903–13. [DOI] [PubMed] [Google Scholar]
- 21. Björck L, Welin C, Rosengren A (2007) Secular trends in lipid-lowering treatment and lipid levels after a first acute myocardial infarction. Vasc Heal Risk Manag 3(6): 1045–51. [PMC free article] [PubMed] [Google Scholar]
- 22. Bendinelli B, Masala G, Saieva C, Salvini S, Calonico C, et al. (2011) Fruit, vegetables, and olive oil and risk of coronary heart disease in Italian women: the EPICOR Study. Am J Clin Nutr 93(2): 275–83. [DOI] [PubMed] [Google Scholar]
- 23. Hu Y, Tong G, Xu W, Pan J, Ryan K, et al. (2009) Anti-inflammatory effects of simvastatin on adipokines in type 2 diabetic patients with carotid atherosclerosis. Diabetes Vasc Dis Res 6(4): 262–8. [DOI] [PubMed] [Google Scholar]
- 24. Chiva-Blanch G, Arranz S, Lamuela-Raventos RM, Estruch R (2013) Effects of Wine, Alcohol and Polyphenols on Cardiovascular Disease Risk Factors: Evidences from Human Studies. Alcohol Alcohol 48(3): 270–7. [DOI] [PubMed] [Google Scholar]
- 25. Hu EA, Toledo E, Diez-Espino J, Estruch R, Corella D, et al. (2013) Lifestyles and risk factors associated with adherence to the Mediterranean diet: a baseline assessment of the PREDIMED trial. PloS One 8(4): e60166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ros E (2009) Nuts and novel biomarkers of cardiovascular disease. Am J Clin Nutr 89(5): 1649S–56S. [DOI] [PubMed] [Google Scholar]
- 27. López-Miranda J, Pérez-Jiménez F, Ros E, De Caterina R, Badimón L, et al. (2008) Olive oil and health: summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr Metab Cardiovasc Dis 20(4): 284–94. [DOI] [PubMed] [Google Scholar]
- 28. Castañer O, Corella D, Covas M-I, Sorlí JV, Subirana I, et al. (2013) In vivo transcriptomic profile after a Mediterranean diet in high-cardiovascular risk patients: a randomized controlled trial. Am J Clin Nutr 98(3): 845–53. [DOI] [PubMed] [Google Scholar]
- 29. Hermus L, Lefrandt JD, Tio RA, Breek J-C, Zeebregts CJ (2010) Carotid plaque formation and serum biomarkers. Atherosclerosis 213(1): 21–9. [DOI] [PubMed] [Google Scholar]
- 30. Tanindi A, Sahinarslan A, Elbeg S, Cemri M (2011) Association of matrix metalloproteinase-1, matrix metalloproteinase-9, tissue inhibitor of matrix metalloproteinase-1, and interleukin-6 with epicardial and myocardial perfusion. Coron Artery Dis 22(4): 253–8. [DOI] [PubMed] [Google Scholar]
- 31. Hansson J, Vasan RS, Ärnlöv J, Ingelsson E, Lind L, et al. (2011) Biomarkers of extracellular matrix metabolism (MMP-9 and TIMP-1) and risk of stroke, myocardial infarction, and cause-specific mortality: cohort study. Plos One 6(1): e16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khan DA, Ansari WM, Khan FA (2011) Pro/anti-inflammatory cytokines in the pathogenesis of premature coronary artery disease. J Interf Cytokine Res 31(7): 561–7. [DOI] [PubMed] [Google Scholar]
- 33. Trøseid M, Seljeflot I, Arnesen H (2010) The role of interleukin-18 in the metabolic syndrome. Cardiovasc Diabetol 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consumption of key food items, physical ctivity and 14-point Mediterranean diet score.
(PDF)
Changes in baseline energy and nutrient intake.
(PDF)
Predimed Study: Mediterranean diet in the primary prevention of cardiovascular disease.
(PDF)
CONSORT checklist.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.