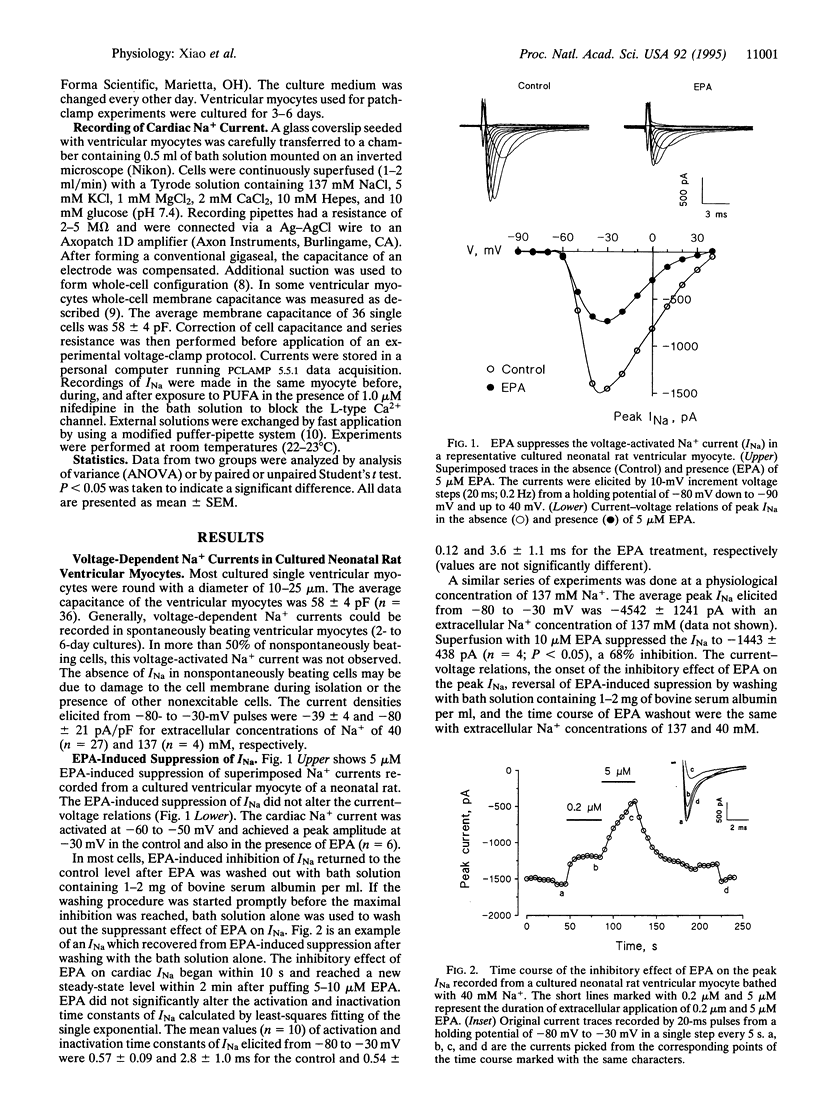
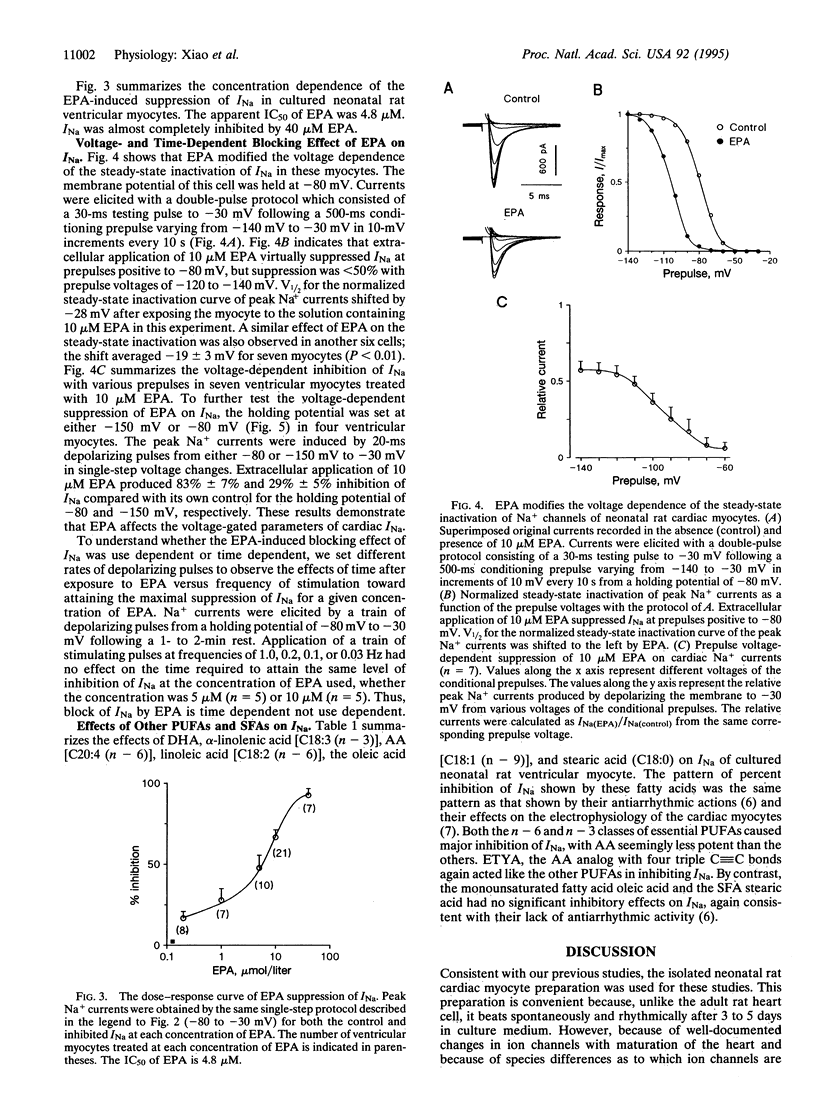
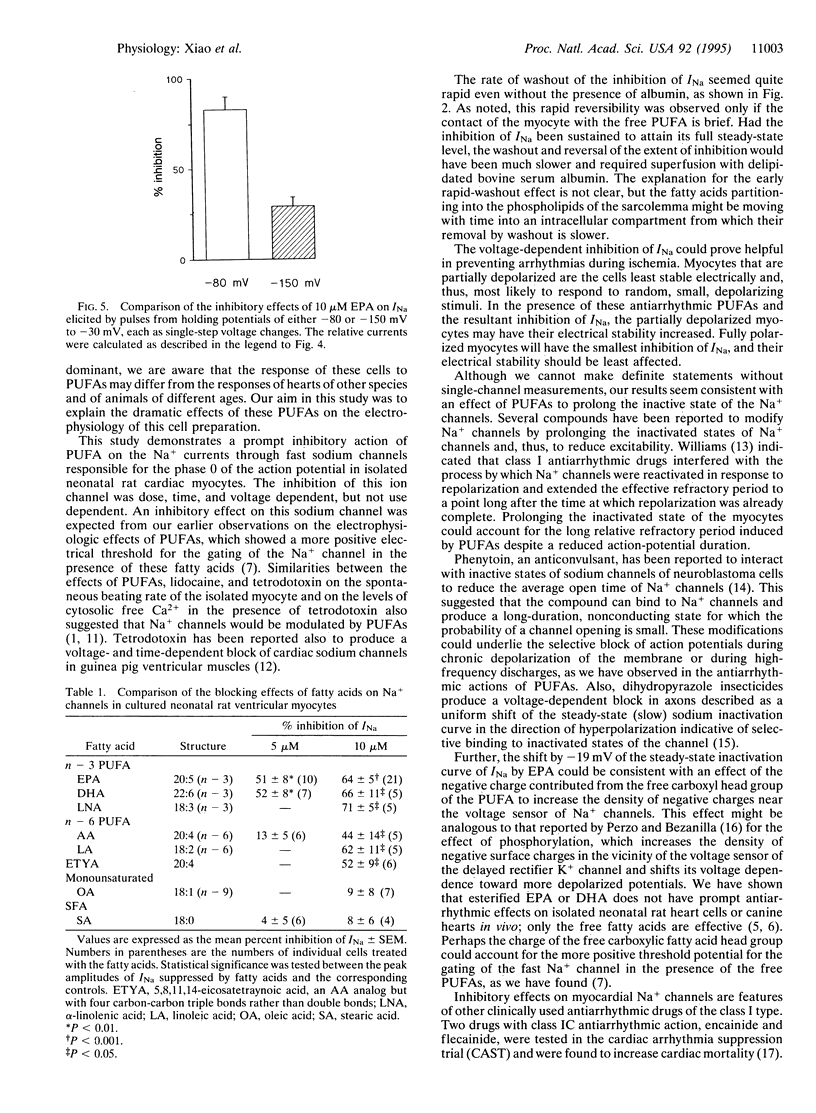
Abstract
Recent evidence indicates that polyunsaturated long-chain fatty acids (PUFAs) prevent lethal ischemia-induced cardiac arrhythmias in animals and probably in humans. To increase understanding of the mechanism(s) of this phenomenon, the effects of PUFAs on Na+ currents were assessed by the whole-cell patch-clamp technique in cultured neonatal rat ventricular myocytes. Extracellular application of the free 5,8,11,14,17-eicosapentaenoic acid (EPA) produced a concentration-dependent suppression of ventricular, voltage-activated Na+ currents (INa). After cardiac myocytes were treated with 5 or 10 microM EPA, the peak INa (elicited by a single-step voltage change with pulses from -80 to -30 mV) was decreased by 51% +/- 8% (P < 0.01; n = 10) and 64% +/- 5% (P < 0.001; n = 21), respectively, within 2 min. Likewise, the same concentrations of 4,7,10,16,19-docosahexaenoic acid produced the same inhibition of INa. By contrast, 5 and 10 microM arachidonic acid (AA) caused less inhibition of INa, but both n - 6 and n - 3 PUFAs inhibited INa significantly. A monounsaturated fatty acid and a saturated fatty acid did not. After washing out EPA, INa returned to the control level. Raising the concentration of EPA to 40 microM completely blocked INa. The IC50 of EPA was 4.8 microM. The inhibition of this Na+ channel was found to be dose and time, but not use dependent. Also, the EPA-induced inhibition of INa was voltage dependent, since 10 microM EPA produced 83% +/- 7% and 29% +/- 5% inhibition of INa elicited by pulses from -80 to -30 mV and from -150 to -30 mV, respectively, in single-step voltage changes. A concentration of 10 microM EPA shifted the steady-state inactivation curve of INa by -19 +/- 3 mV (n = 7; P < 0.01). These effects of PUFAs on INa may be important for their antiarrhythmic effect in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billman G. E., Hallaq H., Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr M. L., Fehily A. M., Gilbert J. F., Rogers S., Holliday R. M., Sweetnam P. M., Elwood P. C., Deadman N. M. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989 Sep 30;2(8666):757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- Clarkson C. W., Matsubara T., Hondeghem L. M. Evidence for voltage-dependent block of cardiac sodium channels by tetrodotoxin. J Mol Cell Cardiol. 1988 Dec;20(12):1119–1131. doi: 10.1016/0022-2828(88)90592-5. [DOI] [PubMed] [Google Scholar]
- Duff H. J., Offord J., West J., Catterall W. A. Class I and IV antiarrhythmic drugs and cytosolic calcium regulate mRNA encoding the sodium channel alpha subunit in rat cardiac muscle. Mol Pharmacol. 1992 Oct;42(4):570–574. [PubMed] [Google Scholar]
- Hallaq H., Smith T. W., Leaf A. Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1760–1764. doi: 10.1073/pnas.89.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Honoré E., Barhanin J., Attali B., Lesage F., Lazdunski M. External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1937–1941. doi: 10.1073/pnas.91.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. X., Leaf A. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9886–9890. doi: 10.1073/pnas.91.21.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. X., Xiao Y. F., Leaf A. Free, long-chain, polyunsaturated fatty acids reduce membrane electrical excitability in neonatal rat cardiac myocytes. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3997–4001. doi: 10.1073/pnas.92.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Clapham D. E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989 Jun 9;244(4909):1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Lux H. D., Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987 May;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan P. L., Abeywardena M. Y., Charnock J. S. Influence of dietary lipids on arrhythmias and infarction after coronary artery ligation in rats. Can J Physiol Pharmacol. 1985 Nov;63(11):1411–1417. doi: 10.1139/y85-232. [DOI] [PubMed] [Google Scholar]
- McLennan P. L., Bridle T. M., Abeywardena M. Y., Charnock J. S. Dietary lipid modulation of ventricular fibrillation threshold in the marmoset monkey. Am Heart J. 1992 Jun;123(6):1555–1561. doi: 10.1016/0002-8703(92)90809-a. [DOI] [PubMed] [Google Scholar]
- Murnaghan M. F. Effect of fatty acids on the ventricular arrhythmia threshold in the isolated heart of the rabbit. Br J Pharmacol. 1981 Aug;73(4):909–915. doi: 10.1111/j.1476-5381.1981.tb08745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe S., Bogdanov K., Hallaq H., Spurgeon H., Leaf A., Lakatta E. Omega 3 polyunsaturated fatty acid modulates dihydropyridine effects on L-type Ca2+ channels, cytosolic Ca2+, and contraction in adult rat cardiac myocytes. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8832–8836. doi: 10.1073/pnas.91.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo E., Bezanilla F. Phosphorylation affects voltage gating of the delayed rectifier K+ channel by electrostatic interactions. Neuron. 1990 Nov;5(5):685–690. doi: 10.1016/0896-6273(90)90222-2. [DOI] [PubMed] [Google Scholar]
- Quandt F. N. Modification of slow inactivation of single sodium channels by phenytoin in neuroblastoma cells. Mol Pharmacol. 1988 Oct;34(4):557–565. [PubMed] [Google Scholar]
- Salgado V. L. Slow voltage-dependent block of sodium channels in crayfish nerve by dihydropyrazole insecticides. Mol Pharmacol. 1992 Jan;41(1):120–126. [PubMed] [Google Scholar]
- Taouis M., Sheldon R. S., Duff H. J. Upregulation of the rat cardiac sodium channel by in vivo treatment with a class I antiarrhythmic drug. J Clin Invest. 1991 Aug;88(2):375–378. doi: 10.1172/JCI115313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan Williams E. M. A classification of antiarrhythmic actions reassessed after a decade of new drugs. J Clin Pharmacol. 1984 Apr;24(4):129–147. doi: 10.1002/j.1552-4604.1984.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Xiao Y. F., McArdle J. J. Elevated density and altered pharmacologic properties of myocardial calcium current of the spontaneously hypertensive rat. J Hypertens. 1994 Jul;12(7):783–790. [PubMed] [Google Scholar]
- de Lorgeril M., Renaud S., Mamelle N., Salen P., Martin J. L., Monjaud I., Guidollet J., Touboul P., Delaye J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994 Jun 11;343(8911):1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]



