Abstract
We evaluated the efficacy of recombinant human thyroid-stimulating hormone (rhTSH) versus thyroid hormone withdrawal (THW) prior to radioiodine remnant ablation (RRA) in thyroid cancer. A systematic search of MEDLINE, EMBASE, the Cochrane Library, and SCOPUS was performed. Randomized controlled trials that compared ablation success between rhTSH and THW at 6 to 12 months following RRA were included in this study. Six trials with a total of 1,660 patients were included. When ablation success was defined as a thyroglobulin (Tg) cutoff of 1 ng/mL (risk ratio, 0.99; 95% confidence interval, 0.96-1.03) or a Tg cutoff of 1 ng/mL plus imaging modality (RR 0.97; 0.90-1.05), the results of rhTSH and THW were similar. There were no significant differences when ablation success was defined as a Tg cutoff of 2 ng/mL (RR 1.03; 0.95-1.11) or a Tg cutoff of 2 ng/mL plus imaging modality (RR 1.02; 0.95-1.09). When a negative 131I-whole body scan was used solely as the definition of ablation success, the effects of rhTSH and THW were not significantly different (RR 0.97; 0.93-1.02). Therefore, ablation success rates are comparable when RRA is prepared by either rhTSH or THW.
Keywords: Thyroid Neoplasms, Thyrotropin Alfa
INTRODUCTION
The global incidence of thyroid cancer has increased sharply since the mid-1990s in both males and females (1). There is no effective neoadjuvant therapy or alternative therapy, other than surgery, for thyroid cancer of any stage (2). After near-total or total thyroidectomy, radioiodine is administered to destroy any microscopic deposits of thyroid carcinoma as well as any remaining normal thyroid tissue (3). Radioiodine remnant ablation (RRA) is performed following thyroid-stimulating hormone (TSH) elevation and a low-iodine diet (4).
TSH elevation can be achieved by two approaches, thyroid hormone withdrawal (THW) and injection of recombinant human TSH (rhTSH). The standard protocol for THW includes stopping levothyroxine (LT4) and switching to liothyronine (LT3) for 2-4 weeks followed by withdrawal of LT3 for 2 weeks, or discontinuation of LT4 for 2-3 weeks without any use of LT3 (4). rhTSH is produced using a molecular biology technique, and can elevate serum TSH levels immediately after intramuscular injection. Initially, rhTSH was used as an adjunctive diagnostic tool for serum thyroglobulin (Tg) in patient follow-up; however, a randomized clinical trial (RCT) by Pacini et al. (5) led to its approval for pretherapeutic stimulation by the European Medicines Agency (http://www.ema.europa.eu) in 2005, and by the United States Food and Drug Administration (http://www.fda.gov) in 2007.
However, the extent of surgery, the need for RRA, and the optimal RRA dose necessary for ablation success remain controversial subjects. Furthermore, there is uncertainty over the efficacy of rhTSH versus THW prior to RRA in differentiated thyroid cancer (DTC). To our knowledge only one meta-analysis has compared rhTSH with THW (6); this was published in 2010 and analyzed two RCTs (5, 7). Therefore, we performed a meta-analysis of RCTs to assess ablation success rates using rhTSH and THW in patients with DTC. The primary outcome was the success of ablation using both methods.
MATERIALS AND METHODS
Data search and study selection
A systematic search for RCTs from MEDLINE (inception to August 2013), EMBASE (inception to August 2013), the Cochrane Library (inception to August 2013), and SCOPUS (inception to August 2013) was performed using the keywords: 'recombinant human thyroid hormone stimulating hormone', 'thyroid hormone withdrawal', and 'thyroid cancer'. The primary analysis included those RCTs comparing ablation success at 6 to 12 months after RRA between patients preparing for RRA by rhTSH vs THW. Manual searches included scanning of reference lists for relevant studies and eligible articles. Only articles written in English were included in this study. Two of the authors conducted the search independently, and any disagreements were resolved by discussion.
Data extraction and quality assessment
Two authors independently extracted data from the publications and recorded the following information: first author, year of publication, country, the number of patients and centers enrolled in the study, TNM staging, dose of RRA, protocols, follow-up time, methods of TSH stimulation at follow-up, the Tg cutoff value, and criteria for ablation success. With regard to the outcome data, the risk ratios (RRs) were calculated from the number of cases in which ablation was successful in each group. Patients with potentially inferring levels of anti-Tg antibodies were excluded from each study, while calculating RRs. Two authors reviewed each publication according to the Cochrane risk of bias assessment tool (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting) (8).
Statistical analysis
Data from each study were analyzed using Review Manager (RevMan, Version 5.2, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). For categorical data, the risk of an outcome was defined as the number of patients in whom ablation was successful compared to the total number of patients. We reported outcome measures as RRs and 95% confidence intervals (CIs), using a fixed-effect model according to Mantel and Haenszel, and presented the results as forest plots. We assessed the heterogeneity of the studies using the chi-square test of heterogeneity; I2>50% was considered significant heterogeneity, as described by Higgins et al. (9). P values <0.05 were considered to indicate statistical significance. Funnel plots were used to assess publication bias graphically (10).
RESULTS
Study characteristics
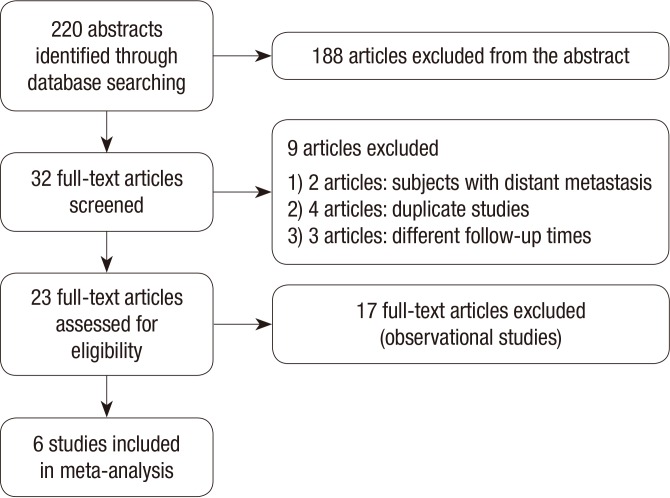
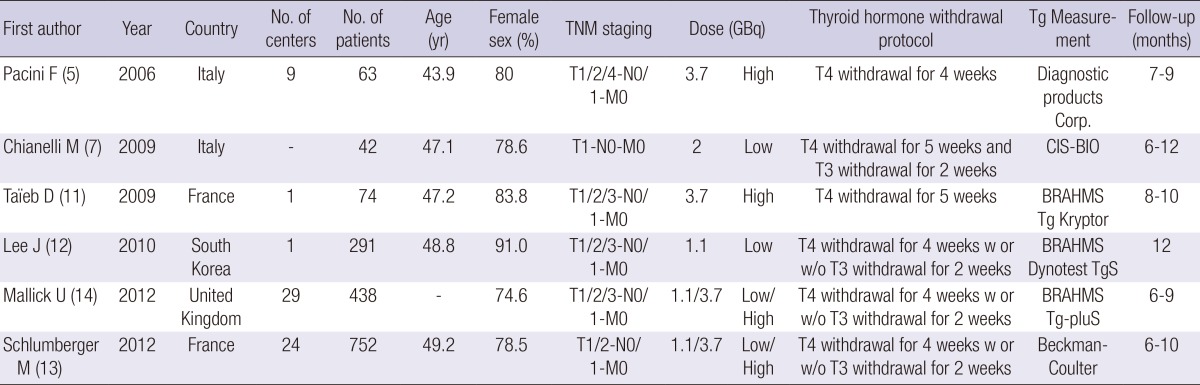
The details of the study selection process are depicted as a flow chart (Fig. 1). In total, 220 articles were identified in MEDLINE, EMBASE, the Cochrane Library, and SCOPUS, of which 32 were analyzed. After applying the inclusion criteria, six studies remained after excluding studies with subjects with distant metastasis (n=2), duplicate studies (n=4), studies with different follow-up times (n=3), and observational studies (n=17). Therefore, data from six trials totaling 1,660 patients were analyzed. Table 1 summarizes the characteristics of each study. The trials were designed to compare the efficacies of rhTSH and THW prior to RRA. Two studies (5, 11) included patients treated with high RRA doses (3.7 GBq), two studies (7, 12) with low RRA doses (1.1 or 2 GBq), and two studies (13, 14) with low (1.1 GBq) and high (3.7 GBq) RRA doses. Follow-up with stimulated Tg, 131I-WBS, or ultrasonography (US) was performed 6 to 12 months after RRA. Patients with potentially interfering levels of anti-Tg antibodies were excluded in five studies included in this study. Schlumberger et al. (13) presented both data including anti-Tg antibodies and excluding anti-Tg antibodies. Urine iodine levels were measured in two studies (5, 11), which showed no sigificant difference between rhTSH and THW groups. Although urine iodine levels were not measured in other studies, THW protocols were similar among studies (Table 1). Based on the definitions from the Cochrane risk of bias assessment tool, all trials used random assignment and attempted to conceal allocation. However, blinding study participants from knowledge of which intervention a participant received could not be done in these RCTs. All trials were judged to have a low or unclear risk of bias in blinding of outcome assessment, incomplete outcome data, and selective reporting.
Fig. 1.
Flow chart of the selection process.
Table 1.
Characteristics of the studies selected for analysis
Ablation success
Criteria for ablation success differed among studies in terms of Tg cutoff values, 131I-whole body scan (WBS), and US findings (Table 2). Negative 131I-WBS was defined as no detectable uptake in one of the studies (7) and as visible uptake <0.1% in four of the other studies (5, 11, 12, 14). Tg was measured after stimulation of TSH either by rhTSH injection or by THW; however, the cutoff values used differed among the studies.
Table 2.
Follow-up and criteria for ablation success
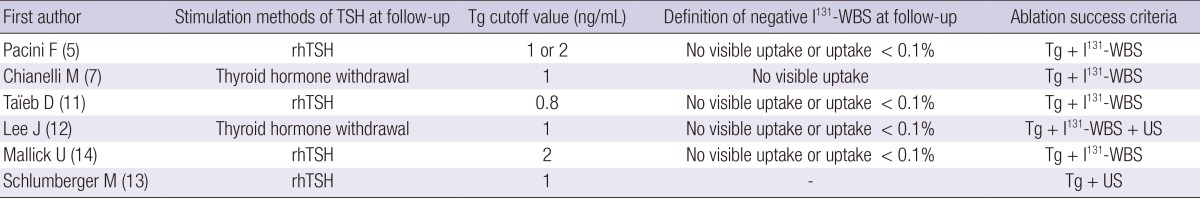
rhTSH, recombinant human thyroid stimulating hormone; Tg, thyroglobulin; WBS, whole-body scan; US, ultrasonography.
Tg cutoff value of 1 ng/mL alone or Tg cutoff value of 1 ng/mL plus imaging modality
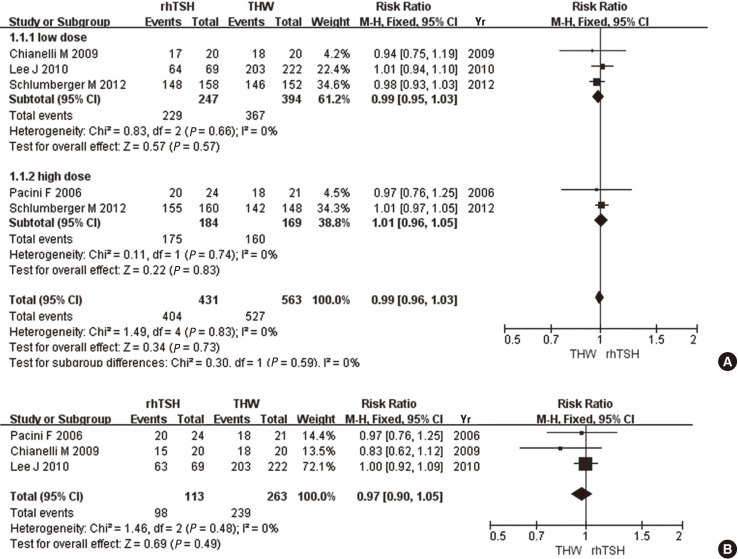
When successful ablation of the remnant thyroid was defined solely as a Tg cutoff value of 1 ng/mL, there was no statistically significant difference in the ablation success rate between studies using a low (RR, 0.99; 95% CI 0.95-1.03, P=0.57) or high (RR, 1.01; 95% CI 0.96-1.05, P=0.83) RRA dose. When studies using low and high doses were combined, the fixed-effect meta-analysis also showed no significant difference in ablation success rates (RR, 0.99; 95% CI 0.96-1.03, P=0.73, I2=0%) (Fig. 2A). When ablation success was defined as a Tg cutoff value of 1 ng/mL plus imaging modality, there was no significant difference in the rate of successful ablation between rhTSH and THW (RR, 0.97; 95% CI 0.90-1.05, P=0.49, I2=0%) (Fig. 2B).
Fig. 2.
Comparison of ablation success, as defined by a Tg cutoff value of 1 ng/mL (A), and by a Tg cutoff value of 1 ng/mL plus imaging modality (B), between recombinant human thyroid stimulating hormone (rhTSH) and thyroid hormone withdrawal (THW).
Tg cutoff value of 2 ng/mL alone or Tg cutoff value of 2 ng/mL plus imaging modality
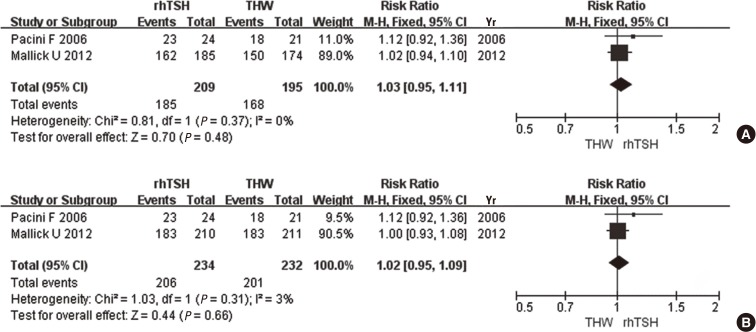
When a Tg cutoff value of 2 ng/mL was adopted as the criterion for ablation success, there was no significant difference in the rate of successful ablation between rhTSH and THW (RR, 1.03; 95% CI 0.95-1.11, P=0.48, I2=0%) (Fig. 3A). When ablation success was defined as a Tg cutoff value of 2 ng/mL plus imaging modality, again there was no significant difference in the rate of successful ablation between the two preparation methods (RR, 1.02; 95% CI 0.95-1.09, P=0.66, I2=3%) (Fig. 3B).
Fig. 3.
Comparison of ablation success, as defined by a Tg cutoff value of 2 ng/mL (A), and by a Tg cutoff value of 2 ng/mL plus imaging modality (B), between recombinant human thyroid stimulating hormone (rhTSH) and thyroid hormone withdrawal (THW).
131I-Whole body scan
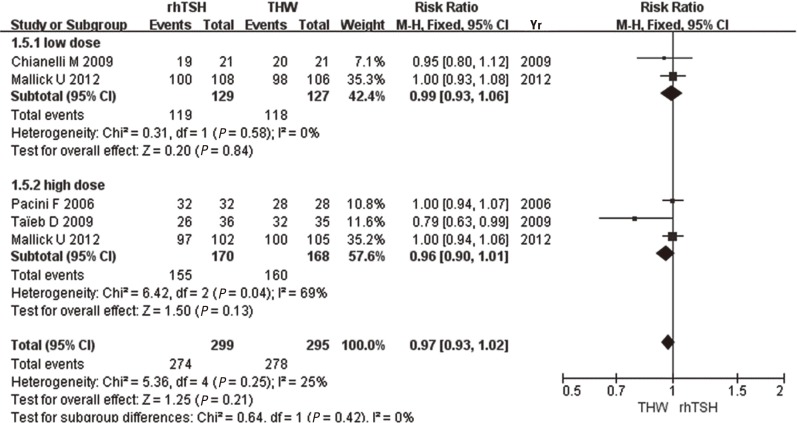
When ablation success was defined as only a negative 131I-WBS, no difference was found between the subgroups using a low (RR, 0.99; 95% CI 0.93-1.06, P=0.84) and high (RR, 0.96; 95% CI 0.90-1.01, P=0.13) RRA dose. Overall, the differences between rhTSH and THW were not statistically significant (RR, 0.97; 0.93-1.02, P=0.21, I2=25%) (Fig. 4).
Fig. 4.
Comparison of ablation success, as defined by the 131I-whole body scan (WBS), between recombinant human thyroid stimulating hormone (rhTSH) and thyroid hormone withdrawal (THW).
DISCUSSION
Specific accumulation of iodine in the thyroid gland was first reported in 1915 and used for treatment of thyroid carcinoma starting in 1942 (15, 16). RRA is the current standard for thyroid cancer treatment, and from an oncological point of view, it may be considered an adjuvant therapy (4). The goals of RRA are clear: first, to destroy residual normal thyroid tissue and, second, to destroy microscopic cancer cells (17).
Iodine accumulation and Tg synthesis are apparent in DTC. Therefore, 131I-WBS can be used to detect thyroid remnants and regional or distant metastases during follow-up visits, and the serum Tg level is a useful marker for monitoring residual or recurrent disease (4). To increase iodine uptake by RRA or 131I-WBS, TSH stimulation must be achieved (18). Since stimulated Tg levels represent the best means of detecting normal or pathological thyroid tissue, Tg levels are measured after TSH stimulation using either rhTSH or THW. Because Tg levels and 131I-WBS are considered complementary, five studies defined ablation success using criteria based on both Tg levels and 131I-WBS (5, 7, 11, 12, 14). However, the Tg cutoff can differ significantly among medical centers and laboratories. In the studies included in this meta-analysis, the Tg cutoff values were as follows: 0.8 (11), 1 (7, 12, 13), or 2 (5, 14). A Tg level <1 ng/mL suggested the absence of disease, while levels >2 ng/mL indicated the possibility of metastatic disease (19).
In this study, to reduce the impact of differences in criteria, we performed a subgroup analysis in which successful ablation was defined according to the following criteria: 1) Tg cutoff value of 1 ng/mL, 2) Tg cutoff value of 1 ng/mL plus imaging modality (131I-WBS and/or US), 3) Tg cutoff value of 2 ng/mL, 4) Tg cutoff value of 2 ng/mL plus imaging modality (131I-WBS and/or US), and 5) 131I-WBS alone. Regardless of the criteria used, the primary outcome was identical: the rate of successful ablation was similar regardless of whether rhTSH or THW was used. According to the guidelines of the American Thyroid Association, RRA is recommended for all patients with distant metastasis, gross extrathyroidal extension, or a tumor size >4 cm, and selectively recommended for patients with lymph node metastases or high-risk features (4). However, few studies have compared the efficacy of rhTSH with THW in high-risk patients. Therefore, only RCTs involving patients at low-to-intermediate risk were included in this analysis.
In addition to displaying a similar ablation success rate to THW, other advantages of rhTSH over THW have been suggested. rhTSH preserves a better quality of life (20), since it was developed to avoid hypothyroidism. Symptoms resulting from hypothyroidism include fatigue, cold intolerance, weight gain, constipation, depression, and cardiac and renal problems (12). Lower radiotoxicity has also been associated with the use of rhTSH compared to hypothyroidism (21, 22), possibly due to impairment of renal clearance during hypothyroidism (5). In addition, the total chromosomal translocation rate was higher after RRA with THW than with rhTSH (23). Although the financial cost of rhTSH is high, it can be offset by the benefits of shorter hospital stays and improved job performance (24). Borget et al. showed that rhTSH administration reduced the length of sick leave by 8.1 days, when compared with that of THW (25). Furthermore, rhTSH provides more flexible scheduling of RRA for both the patient and the clinician (26).
The elevation of TSH by rhTSH may be due to different pathophysiological mechanisms by THW. TSH stimulation increases expression of the sodium/iodide symporter (NIS) (16); thus, the transport of iodide into and through the thyroid gland is tightly regulated by TSH (16, 27, 28). Although both methods appear to elevate serum TSH levels, THW induces a gradual increase in serum TSH for 2 weeks in contrast to the abrupt TSH elevation with the rhTSH method, NIS is usually located in the cytoplasm and moves rapidly to the membrane in response to TSH stimulation (29). TSH also stimulates synthesis of NIS by increasing its transcription (16). The longer duration of TSH stimulation in THW can be advantageous over rhTSH. In addition, several intracellular processes occur after TSH stimulation, such as those involving thyroid peroxidase. In the case of THW, these mechanisms appear to be activated gradually, which enhances the uptake and retention of radioiodines. In the case of rhTSH, such processes cannot be activated due to its short biological half-life. However, the factors regulating NIS activity and the differences between the methods require further clarification (30).
Our study has several limitations. DTC can relapse 10 to 20 yr after initial treatment, which accounts for the need for long-term follow-up (31). Five primary studies of the long-term outcomes of rhTSH and THW were found during the literature database search. The study with the longest follow-up (at least 10 yr) concluded that the outcome of rhTSH is similar to that of THW (32). We included RCTs in which patients were treated with the maximum RRA doses of 3.7 GBq and those evaluating patients without distant metastasis. However, a small number of studies included patients with distant metastasis, those at high risk, or those receiving RRA doses greater than 3.7 GBq. In addition, as various measurement kits were used in each study, there is possibility of differences of TSH and Tg level between studies (33). Also, influences from difference in iodine diets could not be compared in this study (34).
In conclusion, based on this meta-analysis, ablation success rates after preparation for RRA by either rhTSH or THW are comparable. Further studies that include long-term follow-up evaluations are essential.
Footnotes
This work was supported by the National Research Foundation of Korea (NRF) grant for the Global Core Research Center (GCRC) funded by the Korean government (MSIP, Ministry of Science, ICT& Future Planning) No. 2011-0030680.
No potential conflict of interest relevant to this article.
References
- 1.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Lang BH, Law TT. The role of 18F-fluorodeoxyglucose positron emission tomography in thyroid neoplasms. Oncologist. 2011;16:458–466. doi: 10.1634/theoncologist.2010-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins RJ, Schlumberger MJ. The evolving role of (131)I for the treatment of differentiated thyroid carcinoma. J Nucl Med. 2005;46:28S–37S. [PubMed] [Google Scholar]
- 4.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 5.Pacini F, Ladenson PW, Schlumberger M, Driedger A, Luster M, Kloos RT, Sherman S, Haugen B, Corone C, Molinaro E, et al. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. 2006;91:926–932. doi: 10.1210/jc.2005-1651. [DOI] [PubMed] [Google Scholar]
- 6.Ma C, Xie J, Liu W, Wang G, Zuo S, Wang X, Wu F. Recombinant human thyrotropin (rhTSH) aided radioiodine treatment for residual or metastatic differentiated thyroid cancer. Cochrane Database Syst Rev. 2010;(11):CD008302. doi: 10.1002/14651858.CD008302.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chianelli M, Todino V, Graziano FM, Panunzi C, Pace D, Guglielmi R, Signore A, Papini E. Low-activity (2.0 GBq; 54 mCi) radioiodine post-surgical remnant ablation in thyroid cancer: comparison between hormone withdrawal and use of rhTSH in low-risk patients. Eur J Endocrinol. 2009;160:431–436. doi: 10.1530/EJE-08-0669. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taïeb D, Sebag F, Cherenko M, Baumstarck-Barrau K, Fortanier C, Farman-Ara B, De Micco C, Vaillant J, Thomas S, Conte-Devolx B, et al. Quality of life changes and clinical outcomes in thyroid cancer patients undergoing radioiodine remnant ablation (RRA) with recombinant human TSH (rhTSH): a randomized controlled study. Clin Endocrinol (Oxf) 2009;71:115–123. doi: 10.1111/j.1365-2265.2008.03424.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Yun MJ, Nam KH, Chung WY, Soh EY, Park CS. Quality of life and effectiveness comparisons of thyroxine withdrawal, triiodothyronine withdrawal, and recombinant thyroid-stimulating hormone administration for low-dose radioiodine remnant ablation of differentiated thyroid carcinoma. Thyroid. 2010;20:173–179. doi: 10.1089/thy.2009.0187. [DOI] [PubMed] [Google Scholar]
- 13.Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, Bardet S, Leenhardt L, Bastie D, Schvartz C, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366:1663–1673. doi: 10.1056/NEJMoa1108586. [DOI] [PubMed] [Google Scholar]
- 14.Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, Nicol A, Clark PM, Farnell K, McCready R, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366:1674–1685. doi: 10.1056/NEJMoa1109589. [DOI] [PubMed] [Google Scholar]
- 15.Sawin CT, Becker DV. Radioiodine and the treatment of hyperthyroidism: the early history. Thyroid. 1997;7:163–176. doi: 10.1089/thy.1997.7.163. [DOI] [PubMed] [Google Scholar]
- 16.Chung JK, Youn HW, Kang JH, Lee HY, Kang KW. Sodium iodide symporter and the radioiodine treatment of thyroid carcinoma. Nucl Med Mol Imaging. 2010;44:4–14. doi: 10.1007/s13139-009-0016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hugo J, Robenshtok E, Grewal R, Larson S, Tuttle RM. Recombinant human thyroid stimulating hormone-assisted radioactive iodine remnant ablation in thyroid cancer patients at intermediate to high risk of recurrence. Thyroid. 2012;22:1007–1015. doi: 10.1089/thy.2012.0183. [DOI] [PubMed] [Google Scholar]
- 18.Coliez R, Tubiana M, Dutreix J, Guelfi J. Results of examination of 85 cases of cancer of the thyroid with radioactive iodine. J Radiol Electrol Arch Electr Medicale. 1951;32:881–895. [PubMed] [Google Scholar]
- 19.Barbaro D, Boni G, Meucci G, Simi U, Lapi P, Orsini P, Pasquini C, Turco A, Mariani G. Recombinant human thyroid-stimulating hormone is effective for radioiodine ablation of post-surgical thyroid remnants. Nucl Med Commun. 2006;27:627–632. doi: 10.1097/00006231-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Haugen BR, Pacini F, Reiners C, Schlumberger M, Ladenson PW, Sherman SI, Cooper DS, Graham KE, Braverman LE, Skarulis MC, et al. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999;84:3877–3885. doi: 10.1210/jcem.84.11.6094. [DOI] [PubMed] [Google Scholar]
- 21.Rosário PW, Borges MA, Purisch S. Preparation with recombinant human thyroid-stimulating hormone for thyroid remnant ablation with 131I is associated with lowered radiotoxicity. J Nucl Med. 2008;49:1776–1782. doi: 10.2967/jnumed.108.050591. [DOI] [PubMed] [Google Scholar]
- 22.Taïeb D, Sebag F, Farman-Ara B, Portal T, Baumstarck-Barrau K, Fortanier C, Bourrelly M, Mancini J, De Micco C, Auquier P, et al. Iodine biokinetics and radioiodine exposure after recombinant human thyrotropin-assisted remnant ablation in comparison with thyroid hormone withdrawal. J Clin Endocrinol Metab. 2010;95:3283–3290. doi: 10.1210/jc.2009-2528. [DOI] [PubMed] [Google Scholar]
- 23.Frigo A, Dardano A, Danese E, Davì MV, Moghetti P, Colato C, Francia G, Bernardi F, Traino C, Monzani F, et al. Chromosome translocation frequency after radioiodine thyroid remnant ablation: a comparison between recombinant human thyrotropin stimulation and prolonged levothyroxine withdrawal. J Clin Endocrinol Metab. 2009;94:3472–3476. doi: 10.1210/jc.2008-2830. [DOI] [PubMed] [Google Scholar]
- 24.Vallejo Casas JA, Mena Bares LM, Gálvez MA, Marlowe RJ, Latre Romero JM, Martínez-Paredes M. Treatment room length-of-stay and patient throughput with radioiodine thyroid remnant ablation in differentiated thyroid cancer: comparison of thyroid-stimulating hormone stimulation methods. Nucl Med Commun. 2011;32:840–846. doi: 10.1097/MNM.0b013e32834863b0. [DOI] [PubMed] [Google Scholar]
- 25.Borget I, Corone C, Nocaudie M, Allyn M, Iacobelli S, Schlumberger M, De Pouvourville G. Sick leave for follow-up control in thyroid cancer patients: comparison between stimulation with Thyrogen and thyroid hormone withdrawal. Eur J Endocrinol. 2007;156:531–538. doi: 10.1530/EJE-06-0724. [DOI] [PubMed] [Google Scholar]
- 26.Mernagh P, Campbell S, Dietlein M, Luster M, Mazzaferri E, Weston AR. Cost-effectiveness of using recombinant human TSH prior to radioiodine ablation for thyroid cancer, compared with treating patients in a hypothyroid state: the German perspective. Eur J Endocrinol. 2006;155:405–414. doi: 10.1530/eje.1.02223. [DOI] [PubMed] [Google Scholar]
- 27.Dohán O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 28.Kogai T, Taki K, Brent GA. Enhancement of sodium/iodide symporter expression in thyroid and breast cancer. Endocr Relat Cancer. 2006;13:797–826. doi: 10.1677/erc.1.01143. [DOI] [PubMed] [Google Scholar]
- 29.Andros G, Wollman SH. Autoradiographic localization of radioiodide in the thyroid gland of the mouse. Am J Physiol. 1967;213:198–208. doi: 10.1152/ajplegacy.1967.213.1.198. [DOI] [PubMed] [Google Scholar]
- 30.Kogai T, Endo T, Saito T, Miyazaki A, Kawaguchi A, Onaya T. Regulation by thyroid-stimulating hormone of sodium/iodide symporter gene expression and protein levels in FRTL-5 cells. Endocrinology. 1997;138:2227–2232. doi: 10.1210/endo.138.6.5189. [DOI] [PubMed] [Google Scholar]
- 31.Mazzaferri EL. Thyroid remnant 131I ablation for papillary and follicular thyroid carcinoma. Thyroid. 1997;7:265–271. doi: 10.1089/thy.1997.7.265. [DOI] [PubMed] [Google Scholar]
- 32.Molinaro E, Giani C, Agate L, Biagini A, Pieruzzi L, Bianchi F, Brozzi F, Ceccarelli C, Viola D, Piaggi P, et al. Patients with differentiated thyroid cancer who underwent radioiodine thyroid remnant ablation with low-activity 131I after either recombinant human TSH or thyroid hormone therapy withdrawal showed the same outcome after a 10-year follow-up. J Clin Endocrinol Metab. 2013;98:2693–2700. doi: 10.1210/jc.2012-4137. [DOI] [PubMed] [Google Scholar]
- 33.Ahn BC, Lee WK, Jeong SY, Lee SW, Lee J. Estimation of true serum thyroglobulin concentration using simultaneous measurement of serum antithyroglobulin antibody. Int J Endocrinol. 2013;2013:210639. doi: 10.1155/2013/210639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoo ID, Kim SH, Seo YY, Oh JK, O JH, Chung SK. The success rate of initial 131I ablation in differentiated thyroid cancer: comparison between less strict and very strict low iodine diets. Nucl Med Mol Imaging. 2012;46:34–40. doi: 10.1007/s13139-011-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]