EAT-2 overexpression enhances many functions of human innate immune cells
Keywords: chemokines, cytokines, dendritic cells, EAT-2 adaptor, immune modulation, innate immunity, monocytes, NK cells, SLAM receptors
Abstract
The signaling lymphocytic activation molecule (SLAM) receptor-associated adaptor Ewing’s sarcoma-associated transcript-2 (EAT-2) is primarily expressed in innate immune cells including dendritic cells (DCs), macrophages and NK cells. A recent human HIV vaccine study confirmed that EAT-2 expression was associated with the enhanced immunogenicity induced by the MRKAd5/HIV vaccine. We previously harnessed the capability of EAT-2 to modulate signaling mediated by SLAM receptors and demonstrated that by incorporating EAT-2 expression into vaccines, one could enhance innate and adaptive immune responses in mice, even in the face of pre-existing immunity to the vaccine vectors. Herein, we investigated the innate immune responses of human cells exposed to EAT-2-over-expressing vaccines. Our results demonstrate that EAT-2 over-expression can significantly alter the kinetics of critical pro-inflammatory cytokine and chemokine responses elaborated by human PBMCs. In addition, enhanced DC maturation and increased monocyte phagocytosis were observed in EAT-2-transduced human cells. We also found that EAT-2 over-expression improved antigen presentation by human cells. Moreover, EAT-2 over-expression increased the anti-tumor activity of human NK cells against K562 tumor cell targets. Many of these responses were extinguished with use of an EAT-2 variant carrying a mutant SH2 domain (R31Q), suggesting a critical role for the interaction between EAT-2 and SLAM receptors in mediating these responses. In conclusion, these results provide evidence that EAT-2 interacts with key components of multiple arms of the human innate immune system, and that this role highlights the potential for targeting EAT-2 functions so as to improve a number of human immunotherapeutic approaches, including vaccine development.
Introduction
The innate immune system relies heavily on a variety of transmembrane, intracellular or secreted pattern-recognition receptors (PRRs), each of which is vital for recognition of specific molecular structures found on or within potentially infectious agents, such as viruses or microbes (1, 2). Upon encounter with an appropriate pathogen-associated molecular pattern (PAMP), activation of respective PRRs triggers signaling pathways that regulate the transcription of pro-inflammatory cytokine and chemokine genes, as well as other innate immune defense responses—responses that also help shape subsequent, antigen-specific adaptive immune responses (3, 4). For example, the innate immune system also has a key role in initiating and orchestrating the adaptive immune responses to antigens presented during vaccinations (5). These facts suggest that specific modulation of innate immunity during vaccination may allow for the development of improved therapeutic and preventative vaccines. This approach is but one example of numerous immunotherapeutic strategies currently being devised for the treatment of a great number of human conditions, both acquired and genetic (6–11).
The use of vaccine adjuvants has been a traditional way of activating innate immunity so as to amplify the magnitude of T- and/or B-cell adaptive immune responses to antigens expressed by or present within vaccine formulations (11–13). Vaccine adjuvants are represented by different classes of compounds including microbial products, mineral salts, emulsions, nucleic acids, viral vectors and liposomes (6). By improving vaccine interactions with the innate immune system, adjuvants have been found to not only improve the kinetics of initial innate immune responses (jump-start innate immunity) but also increase the generation of immunological memory, alter the breadth of the adaptive immune response and provide specific types of immune responses, such as Th1 versus Th2 or CD8+ versus CD4+ T-cell responses (14).
Triggering innate immune receptor signaling, such as TLRs, RIG-like receptors (RLRs) or NOD-like receptors (NLRs), by use of specific agonists or over-expressing innate immune receptor-associated adaptors (e.g. MyD88 or TRIF) has been shown to induce pro-inflammatory cytokine and chemokine responses, with a result being augmentation of the adaptive immune responses elicited towards target antigens (15).
Another important family of immunoreceptors that play a critical role in innate and adaptive immune regulation is the signaling lymphocytic activation molecule (SLAM) family of receptors (16). SLAM receptors serve as costimulatory molecules that regulate intracellular signaling pathways that govern the function of several innate and adaptive immune cells (17). The SLAM family is currently composed of six distinct innate and adaptive immune cell-specific members, including SLAM (CD150), 2B4 (CD244), Ly9, CD84, NTB-A (natural killer-T- and B-cell antigen; Ly108 in the mouse) and CRACC (CD2-like receptor activating cytotoxic cells, also called CD319 and Cs-1) (18, 19). All SLAM members except 2B4, which interacts with CD48, are self-ligands that initiate intracellular signaling via recruitment of the SLAM-associated protein (SAP) family of adaptors, which includes three members: SAP, Ewing’s sarcoma-associated transcript-2 (EAT-2) and EAT-2-related transducer (ERT; present in rodents only) (19). These adaptors associate with high affinity and specificity to immunoreceptor tyrosine-based switch motifs (ITSMs) present in the cytoplasmic domains of the SLAM family of receptors (17).
We have previously demonstrated that manipulating SLAM signaling by over-expressing the EAT-2 adaptor from a recombinant adenovirus serotype 5 (rAd5)-based vaccine platform enhances the early robust activation of innate immune responses and consequently dramatically improves the induction of antigen-specific adaptive immune responses by the vaccine platform in animals, even in the presence of high levels of anti-Ad5-specific immunity (20–23).
In this study, we set out to investigate what, if any, impact EAT-2 over-expression may have on human innate immune cell responses, a key step towards translating this form of immunomodulation into the human clinical realm. Our data demonstrate that Ad-mediated expression of EAT-2 (rAd5-EAT2) activates signaling cascades that induce the production of several pro-inflammatory cytokines and chemokines from human PBMCs. In addition, we observed enhanced phagocytic activity of monocytes derived from rAd5-EAT2-infected human PBMCs. Moreover, improved dendritic cell (DC) maturation was achieved following Ad-mediated EAT-2 over-expression, as characterized by increased expression of CD80, CD83 and CD86 costimulatory molecules as well as enhanced HLA-DR expression on DCs. Furthermore, the enhanced innate immune responses by EAT-2 translated into more potent NK cell tumoricidal responses. Collectively, our results demonstrate that immunomodulatory strategies that specifically attempt to harness the functions of the immunomodulatory protein EAT-2 for use in humans can be demonstrated to parallel improvements previously noted in animal studies. Specifically, vaccines that over-express EAT-2 have improved efficacy in human cells via a mechanism that is associated with an early activation of several arms of the human innate immune system. This appears to be primarily mediated through the interaction between the SH2 domain of EAT-2 and the ITSMs of SLAM receptors, since use of a mutant form of the EAT-2 adaptor, EAT-2(R31Q), in these same experiments significantly reduced the innate immune responses of human PBMCs. Inclusion of EAT-2 targeting strategies may be a promising approach for use in a number of human immunotherapeutic strategies targeting a number of human diseases, including infectious diseases and cancers.
Methods
Vector construction
The rAd5-EAT2, rAd5-Null, rAd5-EAT2(R31Q) mutant and rAd5-GFP viruses were purified as previously described (20–23). Ad5 vectors expressing the murine EAT-2 were utilized in these studies. Briefly, the open reading frame of the transgene was excised from a plasmid using primers flanked by specific restriction endonucleases (NEB, Ipswich, MA, USA) and sub-cloned into the pShuttle-CMV vector, which contained a CMV promoter/enhancer element and SV40 polyA (24). The resulting pShuttle-CMV-transgene plasmid was linearized with PmeI restriction enzyme and homologously recombined with the pAdEasyI plasmid, containing most of the Ad5 vector genome yielding pAd5 transgene expressing replication-deficient [E1-, E3-] vectors. HEK293 cells were transfected with PacI linearized plasmid and viable virus was obtained and amplified after several rounds of expanding infection. rAd5 viruses were purified using a cesium chloride (CsCl) gradient as previously described (25). Direct sequencing and restriction enzyme mapping were carried out to confirm the integrity of the transgene sequence. All viruses were found to be replication-competent adenovirus (RCA)-free by both RCA PCR (E1 region amplification) and direct sequencing methods as previously described (26).
Isolation of human PBMCs
Human PBMCs were isolated from fresh buffy coat material (Stanford Blood Center, Palo Alto, CA, USA) with standard techniques using either Histopaque 1077 (Sigma, St Louis, MO, USA) or Lympholyte H (Cedarlane Diagnostics, Ontario, Canada) according to the manufacturer’s protocol. Briefly, human buffy coat material was diluted 1:3 (or 1:1) in PBS, layered onto Histopaque 1077 (or Lympholyte H) and centrifuged at room temperature for 30min at 400 × g. Cells sedimenting at the interface were removed and washed (centrifuged for 10min at 200 × g) extensively with PBS to remove remaining platelets. Isolated PBMCs were quantified, diluted and cultured (at 3–5×106 cells ml−1) in RPMI1640 supplemented with either 2 or 5% fetal bovine serum (FBS) and sub-optimal concentrations of hIL-2 (5ng ml−1) and hIL-18 (10ng ml−1).
Cell staining and flow cytometry
PBMCs were harvested and 5×106 cells were initially stained with purified mouse anti-human CD16/CD32 Fcγ block, followed by PE-Cy7-CD83, FITC-CD86, APC-HLA-DR, APC-Cy7-CD1a, Alexa Fluor 700-CD14, Pacific blue-CD80, PE-NKp44, APC-CD56 and APC-Cy7-CD3 (all at 4 µg ml−1; BD Biosciences, San Diego, CA, USA). Cells were incubated on ice with the appropriate antibodies for 45min, washed, sorted for data collection using a BD LSR II instrument and analyzed using FlowJo software (Tree Star, San Carlos, CA, USA). For detecting intracellular levels of EAT-2 or EAT-2(R31Q), human PBMCs were infected with EAT-2- or EAT-2(R31Q)-expressing Ads and EAT-2 expression was evaluated at 48h post-infection by Alexa Fluor 488 (Invitrogen)-conjugated EAT-2 antibody (sc-21572; Santa Cruz biotechnology, Inc.), as previously described (23).
To identify human immune cells transduced by Ad-mediated EAT-2 or GFP gene transduction, human PBMCs (2×106) were infected with multiplicity of infection (MOI) of 10000 of Ad5-EAT-2 or Ad5-GFP for 48h and were then stained with the following antibodies: APC-Cy7-CD14, PE-CD1a, Alexa Fluor 700-CD8a, V450-CD4, APC-CD65 and PerCpCy5.5-CD3 (all at 4 µg ml−1; BD Biosciences). Human PBMCs were then fixed with 2% formaldehyde, permeabilized with saponin and intracellularly stained with FITC-labeled EAT-2 antibody and finally subjected to flow cytometry. For Ad-GFP transduction, cells were surface stained with the above antibodies and directly subjected to flow cytometry. The percentages of Ad-EAT-2 or Ad5-GFP-transduced cells (FITC+) were calculated using FlowJo software.
Phagocytosis analysis
The phagocytic activity of monocytes was assessed in PBMC cultures infected with MOI of 10000 of rAd5-EAT2 or Ad controls by measuring the amount of uptake of latex beads coated with FITC-labeled rabbit IgG into cells using a phagocytosis assay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s protocol. Briefly, PBMCs were cultured in 12-well plates containing RPMI1640 supplemented with 2% FBS, penicillin–streptomycin, hIL-2 (5ng ml−1) and hIL-18 (10ng ml−1). Cells were then either mock infected or infected with rAd5-EAT2, rAd5-EAT2(R31Q) or rAd5-Null vectors at 10000 MOI. Media were changed and cells were treated with latex beads at 48h post-infection and cultured at 37°C. Uptake of the beads into cells was evaluated by FACS at 24h post-bead application, after which the cells were washed twice with FACS buffer and stained with Alexa-700-conjugated CD14 antibody (6 µg ml−1) for 1h. Data were collected using a BD LSR II flow cytometer and analyzed using FlowJo software.
Cell-based antigen presentation assay (infection and flow cytometry staining)
HeLa cells, stably expressing H-2Kb human HLA-B27 (HeLa-Kb-B27), were described previously (27). HeLa-Kb-B27 cells (3×105 cells per well in 6-well plates) were infected with MOI of 1000 of rAd5-EAT2, rAd5-EAT2(R31Q) or rAd5-GFP vectors for 48h. Surface expression of HLA-B27 in virally infected cells was measured by flow cytometry, exactly as described (28). Briefly, at 48h post-transfection, cells were incubated with HLA-B27-specific, ME1, or W6/32 (specific for intact HLA-B, -A, -C, -E, -G complexes (29)) monoclonal antibodies (from murine hybridoma supernatant) for 30min on ice, washed and subsequently stained with secondary antibody Cy5-AffiniPure Goat Anti-Mouse IgG from Jackson Immunoresearch Laboratories (West Grove, PA, USA) for 30min on ice (1:200 dilution). Samples were analyzed on a BD LSR II flow cytometer and analyzed using FlowJo software.
Cytokine and chemokine analysis
A human 27-plex multiplex-based assay was used to determine cytokine/chemokine concentrations in selected spent media, collected from human PBMC cultures per the manufacturer’s instructions (Bio-Rad, Hercules, CA, USA) via Luminex 100 technology (Luminex, Austin, TX, USA) essentially as previously described (30).
Assessment of PBMC cytotoxicity against K562 tumor cells
The cytotoxicity activity of treated PBMCs was assessed using a flow-based assay, summarized as follows. Seventy-two hours following rAd5-EAT2 or control Ad infection, human PBMCs (effector cells) were harvested and co-cultured for an additional 48h with CFSE-labeled K562 cells (target cells) at an effector to target cell (E:T) ratio of 10:1. CFSE labeling was performed as previously described (30). After co-culture, cells were harvested, washed twice and stained with a viability stain (ViViD) exactly as previously described (30). Data were collected on a BD LSRII flow cytometer and analyzed using FlowJo software. For propidium iodide (PI) staining, cells were stained with PI for 2min prior to data acquisition.
Statistical analysis
Statistically significant differences in cytokine induction, ELISA and FACS studies were determined using one-way ANOVA with a Student–Newman–Keuls post-hoc test (P value < 0.05). Graphs in this paper are presented as mean of the average ± SD. GraphPad Prism software was utilized for all statistical analysis.
Results
EAT-2 induces pro-inflammatory cytokine and chemokine production from human PBMCs via its SH2 domain
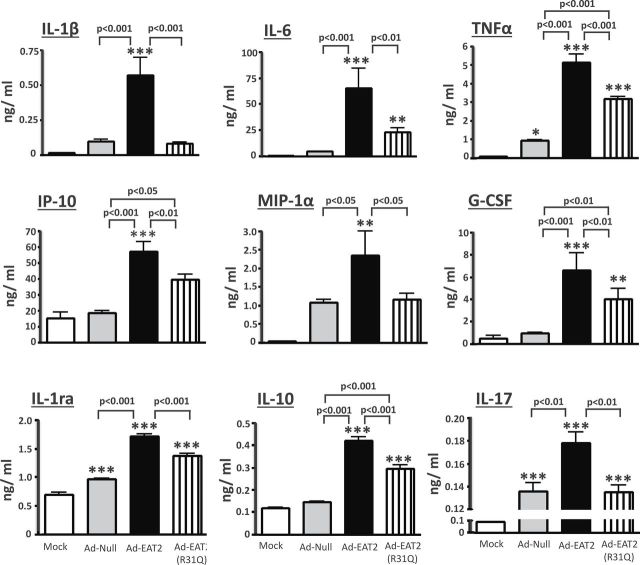
We have previously demonstrated that the efficacy of rAd5-based vaccines is enhanced when expressing the SLAM family of receptors adaptor EAT-2 (20, 22, 23, 30). The enhanced efficacy of EAT-2-augmented vaccines was correlated with increased innate immune responses inclusive of an enhanced production of pro-inflammatory cytokines and chemokines in various strains of mice (20, 22, 23, 30). It is widely appreciated that rAd5 vectors can transduce human innate immune cells (31), so we utilized this vector to test our hypothesis that modulation of EAT-2 functions within human cells could significantly impact upon human innate immune responses. To first determine an optimal MOI, viral particles per cell, for Ad5 vectors in these studies, we analyzed Ad5 vector transduction of human PBMCs by fluorescent microscopy. GFP fluorescence was quantified using the ImageJ software (US National Institutes of Health, Bethesda, MD, USA). Utilizing an Ad5 vector expressing a tracking antigen (GFP), we confirmed that Ad5 vectors can efficiently transduce human PBMCs (Supplementary Figure 1, available at International Immunology Online; data not shown). At both 24 and 48 hpi, similar transduction efficiency was observed in cells infected with rAd5-GFP at MOI (based on OD260 viral particle enumeration) of 10000, 25000 and 50000 (Supplementary Figure 1, available at International Immunology Online); however, the cell viability was significantly improved in cells infected at an MOI of 10000, as compared with cells infected at MOIs of 25000 and 50000 (data not shown). Therefore, an MOI of 10000 (based on OD260 particle enumeration) was selected for all subsequent studies. Previously published data indicated a high homology between murine and human EAT-2 proteins (32). It has also been confirmed that human EAT-2 activates human NK cells via recruitment of phospholipase C (PLC)-γ to the phosphorylated tyrosine 127 (Y127) located into the C-terminal tail of human and murine EAT-2 (33). Human PBMCs infected with murine EAT2-expressing Ad5 vectors or a control Ad5 vector, rAd5-Null (no transgene), indeed revealed that rAd5-EAT2 infection of these cells induced a more robust production of cytokines and chemokines, as compared to rAd5-Null-infected cells (Fig. 1). Specifically, EAT-2 over-expression significantly induced higher levels of IL-1β (~6-fold, P < 0.001), IL-6 (~8-fold, P < 0.001), IP-10 (~6-fold, P < 0.001), TNFα (~6-fold, P < 0.001), G-CSF (~8-fold, P < 0.001), IL-17 (~1-fold, P < 0.01), IL-1rα (~1-fold, P < 0.001), MIP-1α (~2-fold, P < 0.05) and IL-10 (~2-fold, P < 0.001), as compared with rAd5-Null-infected cells (Fig. 1). We also observed significant (P < 0.001) increases of IFNγ, GM-CSF, MIP-1β and IL-13 in human PBMCs infected with rAd5-EAT2; however, no statistically significant differences were observed between rAd5-EAT2- and the control-infected cells (Supplementary Figure 2, available at International Immunology Online). We also confirmed the induction of IL-1β by rAd5-EAT2 utilizing a human IL-1β ELISA assay. We observed significantly (P < 0.001) increased production of IL-1β in media derived from rAd5-EAT2-infected cells, as compared with the rAd5-Null-infected controls (Supplementary Figure 3, available at International Immunology Online).
Fig. 1.
Production of pro-inflammatory cytokines and chemokines from rAd5-EAT2-infected human PBMCs. Human PBMCs were isolated from fresh buffy coat material and were either mock infected or infected with MOI of 10000 with rAd5-EAT2, rAd5-EAT2(R31Q) or rAd5-Null. Culture media were collected at 72h post Ad infection and were analyzed for cytokine and chemokine levels using a 27-plex multiplexed bead array-based quantitative system. Samples were plated in quadruplicates. Data are representative of at least three independent experiments from three different blood donors with similar results. The bars represent mean ± SD. Statistical analysis was completed using a one way ANOVA with a Student–Newman–Keuls post-hoc test. P <0.05 was deemed a statistically significant difference. *P < 0.05, **P < 0.01, ***P < 0.001, statistically significant from mock-treated cells.
Several biochemical and mechanistic studies have demonstrated that SAP adaptors, similar to EAT-2, regulate SLAM-initiated signaling in immune cells via association of their SH2 domain to the phosphorylated ITSMs of SLAM receptors (34). We have recently confirmed that mutating the EAT-2 SH2 domain abrogates EAT-2-mediated early activation of murine innate immune cells and significantly reduces EAT-2-mediated enhancement of cellular immune responses during vaccination (23). The involvement of the EAT-2 SH2 domain in EAT-2 functions prompted us to investigate a possible general role for this domain in EAT-2’s ability to enhance pro-inflammatory cytokine and chemokine responses. Indeed, our studies utilizing a control vector expressing the EAT-2(R31Q) mutant resulted in near complete loss of the activation function of EAT-2 in human PBMCs, suggesting a critical role for SH2 domain-mediated signaling in EAT-2 regulation of the induction of pro-inflammatory cytokines and chemokines from human PBMCs (Fig. 1).
Similarly, utilization of a different assay, ELISA, for supernatant derived from human PBMCs of the rAd-EAT-2(R31Q)-infected cells revealed significantly reduced (P < 0.001) production of IL-1β, as compared with the respective media derived from rAd-EAT-2-infected human PBMCs (Supplementary Figure 3, available at International Immunology Online).
Human innate and adaptive immune cell transduction by the rAd5-EAT2 vector
To determine what human immune cell types may be transduced by the Ad vectors expressing EAT-2 (and potentially be responsible for the enhanced cytokine responses noted in Fig. 1), we analyzed Ad vector transduction of several important classes of innate immune cells found in the human PBMCs by flow cytometry. Utilizing an Ad vector expressing a tracking antigen (GFP), we confirmed that under these experimental conditions, Ad vectors can transduce CD56+ NK cells (6%), CD14+ monocytes (54.6%), CD1a+ DCs (9.33%), as well low levels of CD4+ (3.48%) and CD8+ (5.5%) T cells upon infection of human PBMCs (Fig. 2). Interestingly, we found that the highest subset of human immune cells that were transduced by rAd5-GFP vectors were the CD14+ monocytes, a subset of innate immune cells shown to play a primary role in mediating pro-inflammatory cytokine and chemokine responses (35, 36). Additionally, we confirmed that after identical infection of human PBMCs with the rAd5-EAT2 vector, EAT-2 expression was occurring in same subset of the human immune cells (Fig. 3). Consistent with previously published results (17), we observed significant expression of EAT-2 in CD56+ human NK cells (Fig. 3A). Importantly, our data revealed that levels of EAT-2 expression in NK cells were significantly increased (from ~59 to 82%) following Ad-mediated expression of EAT-2 (Fig. 3A). To confirm that the rAd5-EAT2(R31Q) vector expresses stable protein levels of EAT-2, we performed similar flow cytometry analysis to validate the expression of EAT-2 protein following rAd5-EAT2 or rAd5-EAT2(R31Q) infection. Consistent with our previously published data (23), at 48h post-infection (hpi), similar levels of EAT-2 protein were detected in both rAd5-EAT2- and rAd5-EAT2(R31Q)-infected human immune cells (Fig. 3A–C). We were not able to detect significant transduction levels of human CD4+ and CD8+ T cells by the rAd5-EAT2 vector (data not shown).
Fig. 2.
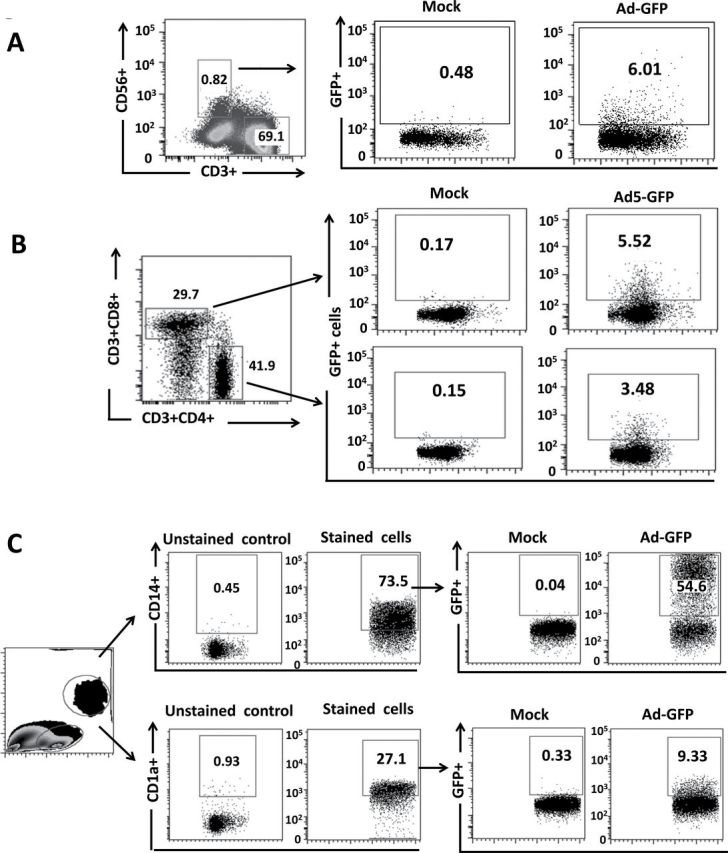
Transduction of human innate and adaptive immune cells by GFP-expressing rAd5 vector. Human PBMCs were isolated from fresh buffy coat material and were either mock infected or infected with MOI of 10000 with rAd5-GFP. FACS analysis for GFP expression at 48h following rAd5-GFP infection of human NK cells (A), CD4+ and CD8+ T cells (B), CD14+ monocytes and CD1a+ cells (C) is shown. Data were collected using a BD LSRII flow cytometer and analyzed using FlowJo software.
Fig. 3.
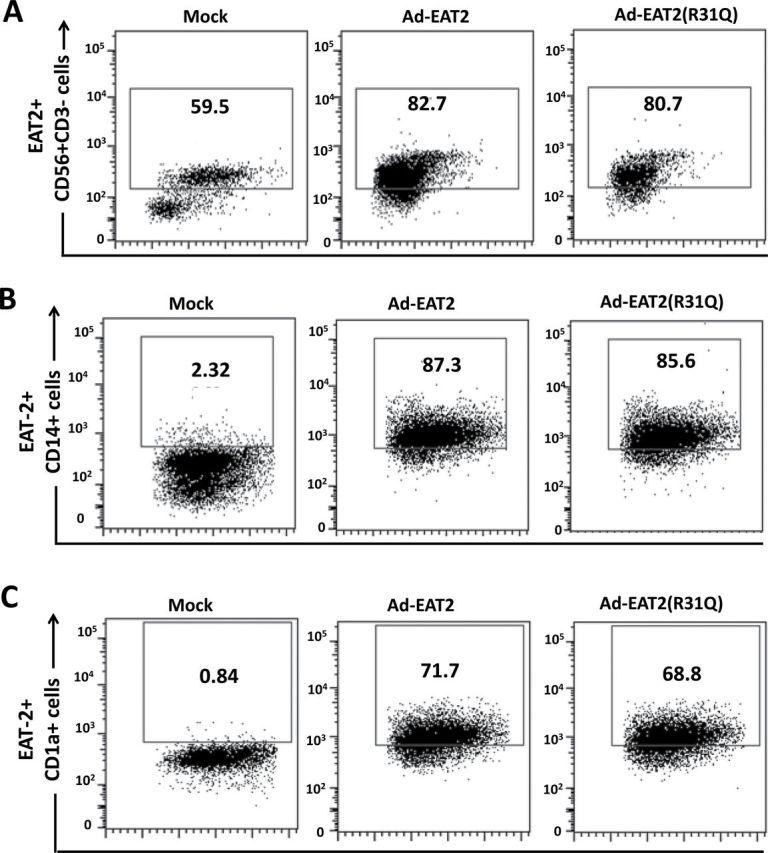
Intracellular staining analysis for EAT-2 expression in human immune cells following rAd5-EAT2 infection. Human PBMCs were isolated from fresh buffy coat material and were either mock infected or infected with MOI of 10000 with rAd5-EAT2 or rAd5-EAT2(R31Q). Intracellular staining analysis for EAT-2 expression at 48h following Ad infection of human NK cells (A), CD14+ monocytes (B) and CD1a+ DCs (C) is shown. Data were collected using a BD LSRII flow cytometer and analyzed using FlowJo software.
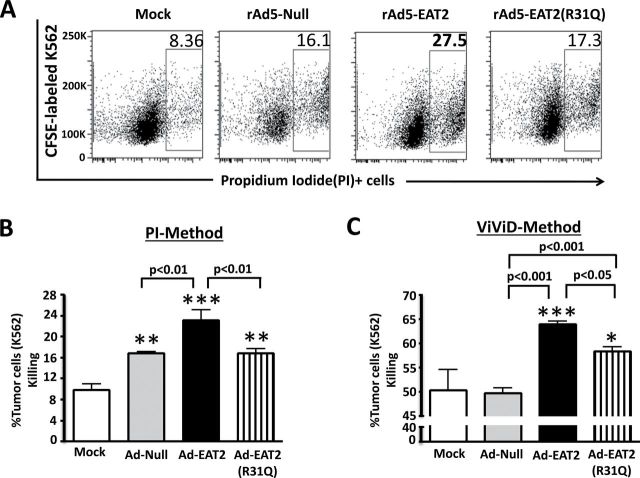
EAT-2 over-expression enhances the cytolytic activity of human PBMCs
It is well documented that induction of pro-inflammatory cytokine and chemokine responses triggers the activation of innate immune cells that play key roles in the regulation of the adaptive immune response (37). We have shown previously that EAT-2 over-expression enhances activation of murine NK cells in vivo (23, 30). Since EAT-2 can bind to all SLAM receptors that have been shown to regulate NK cell cytotoxicity (CRACC, NTB-A and 2B4), we wished to investigate if EAT-2 over-expression enhances human NK cell immune responses. Human PBMCs infected with rAd5-EAT2 or control vectors were co-cultured with the acute myelocytic leukemia cell line, K562, at an effector to target cell ratio of 1:10 and FACS analysis was performed. At 48 hpi, K562 cell viability was evaluated by a flow-based cytotoxicity assay using PI staining, as previously described (38). Interestingly, the cytolytic activity of rAd5-EAT2-treated human PBMCs was significantly (P < 0.01) increased, as compared with rAd5-Null-treated cells (Fig. 4A and B). In contrast to these improved cytolytic responses, we observed significantly (P < 0.01) reduced cytotoxicity when utilizing the control, rAd5-EAT2(R31Q), vector (Fig. 4A and B). We also observed similar results when the K562 killing was evaluated by an alternative method, utilizing the viability dye ViViD (Fig. 4C). The expression levels of the NK cytotoxicity receptor NKp44 correlated with these results as our data revealed that EAT-2 over-expression significantly (P < 0.05) enhanced NKp44 expression on human NK cells (defined as, CD56+, CD3−), as compared with cells infected with the rAd5 controls (Supplementary Figure 4, available at International Immunology Online).
Fig. 4.
EAT-2 over-expression enhances the cytolytic activity of human NK cells. Human PBMCs isolated from fresh buffy coat material were either mock infected or infected with MOI of 10000 with rAd5-EAT2, rAd5-EAT2(R31Q) or rAd5-Null for 72h and then co-cultured with CFSE-labeled K562 cells for an additional 48h at an E:T ratio of 1:10. Viability of K562 cells was assessed by either PI (A and B) or ViViD (C). Data are representative of two (A) or three (B) independent experiments from three different blood donors with similar results. Samples were plated in quadruplicates. Bars represent mean ± SD. Statistical analysis was completed using a one way ANOVA with a Student–Newman–Keuls post-hoc test. P <0.05 was deemed a statistically significant difference. *P < 0.05, **P < 0.01, ***P < 0.001, statistically significant from mock-treated cells.
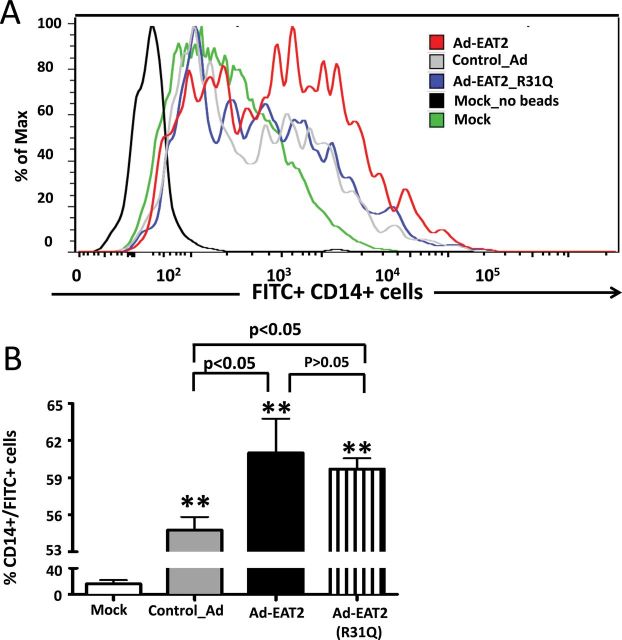
EAT-2 over-expression increases the phagocytic activity of human monocytes
We next investigated the impact that EAT-2 over-expression has on a different cell type of the innate immune system, human monocytes, an essential component of the human innate immune system that are known to participate in pathogen recognition and the production of several pro-inflammatory cytokines and chemokines (35, 36). The functional properties of rAd5-EAT2-infected human monocytes (CD14+ cells) were evaluated through use of a fluorescent phagocytosis assay, as previously described (38). Human PBMCs were infected with the rAd5-EAT2 or control rAd5 vectors and the uptake of FITC-labeled IgG-coated latex beads by these cells was evaluated by flow cytometry, serving as an index of cellular phagocytic activity levels. Mock-treated PBMC-derived monocytes (CD14+ cells) had moderate phagocytic abilities in regard to internalizing FITC-labeled beads (Fig. 5), with rAd5 infection significantly (P < 0.01) enhancing the uptake by CD14+ human monocytes (Fig. 5). Interestingly, monocytes derived from rAd5-EAT2-infected PBMCs have significantly (P < 0.05) greater phagocytic activity, as compared with cells infected with the rAd5-Null controls (Fig. 5). The enhanced phagocytic activity of rAd5-EAT2-infected monocytes trended towards reduced levels when the identical experiment was performed utilizing the rAd5 expressing the EAT-2(R31Q) mutant. However, no statistically significant differences were observed under these experimental conditions (Fig. 5), suggesting that EAT-2 might regulate human monocyte phagocytosis in a mechanism that does not require interaction with the ITSMs of SLAM receptors.
Fig. 5.
Monocytes derived from rAd5-EAT2-infected human PBMCs exhibit enhanced phagocytosis capabilities. Human PBMCs were isolated from fresh buffy coat material and were either mock infected or infected with MOI of 10000 with rAd5-EAT2, rAd5-EAT2(R31Q) or rAd5-Null. Cells were then incubated with FITC+ latex beads and analyzed by FACS as described in Methods. Data are representative of two independent experiments with similar results. Samples were plated in quadruplicates. Bars represent mean ± SD. Statistical analysis was completed using a one way ANOVA with a Student–Newman–Keuls post-hoc test. P <0.05 was deemed a statistically significant difference. **P < 0.01, statistically significant from mock-treated cells.
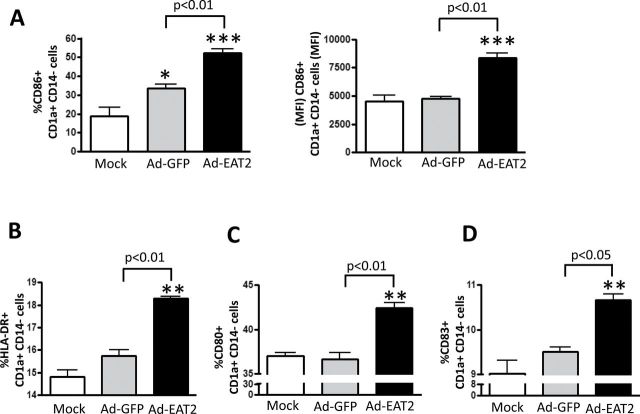
EAT-2 over-expression enhances human DC activation and maturation
The ability of EAT-2 to enhance human DC maturation was evaluated. To investigate if EAT-2 over-expression enhances human DC (CD1a+, CD14−) maturation, human PBMCs were infected with rAd5-EAT2 or rAd5 controls and maturation of DCs was evaluated by flow cytometry. Our results revealed that Ads, in general, induce minor activation of human DCs, as confirmed by the presence of increased percentages of CD86-expressing DCs in rAd5 control-infected PBMCs (Fig. 6A). Importantly, consistent with our previous in vitro and in vivo results in murine systems (21, 23), infecting human PBMCs with rAds expressing EAT-2 increased the expression levels of CD86 (P < 0.01), HLA-DR (P < 0.01), CD80 (P < 0.01) and CD83 (P < 0.05) in human DCs, as compared with the rAd5-treated controls (Fig. 6A–D), suggesting that triggering signaling of the SLAM family of receptors by EAT-2 functions to also enhance human DC maturational status.
Fig. 6.
EAT-2 over-expression enhances human DC maturation. Human PBMCs were isolated from fresh buffy coat material and were either mock infected or infected with MOI of 10000 with rAd5-EAT2 or rAd5-Null. At 72 hpi, cells were stained for surface expression of CD86 (A), HLA-DR (B), CD80 (C) and CD83 (D) and FACS analysis was performed. Data are representative of at least three independent experiments from three different blood donors with similar results. Samples were plated in quadruplicates. Bars represent mean ± SD. Statistical analysis was completed using a one way ANOVA with a Student–Newman–Keuls post-hoc test. P <0.05 was deemed a statistically significant difference. *P < 0.05, **P < 0.01, ***P < 0.001, statistically significant from mock-treated cells.
EAT-2 over-expression enhances antigen presentation by MHC class I molecules
Previous reports have shown that activation of innate immune receptors, such as TLRs and NLRs, inducec critical signals regulating antigen presentation functions of antigen-presenting cells (39, 40). Therefore, we investigated if EAT-2 over-expression enhances MHC class I-mediated antigen presentation by human cells. For this, an elegant HLA-Kb-B27 antigen-presentation system was utilized, as previously described (27). HLA-Kb-B27 cells were infected with MOI of 1000 viral particles per cell of rAd5-EAT2 or control Ads and surface expression of HLA-B27 on the infected cells was measured by flow cytometry at 48 hpi. Utilizing ME1, an HLA-B27-specific antibody, we found that infection with rAd5-EAT2 results in significantly (P < 0.001) enhanced antigen presentation on human cells [as measured by increased number of HLA-B27 molecules on the cell surface, mean fluorescence intensity (MFI)] as compared with cells infected with the rAd5-Null controls (Fig. 7A). We also utilized a different antibody, W6/32, that measures the surface expression of several HLA types including HLA-A, HLA-B, HLA-C, HLA-E and HLA-G. We again observed significantly (P < 0.01) increased surface expression of several HLAs in rAd5-EAT2-infected human cells, as compared with cells infected with the rAd5-Null controls (Fig. 7B). Similar statistically significant results were also observed in rAd5-EAT2-infected cells when cells were infected with a different amount (MOI of 5000) and analyzed at both 24 and 48 hpi (data not shown).
Fig. 7.
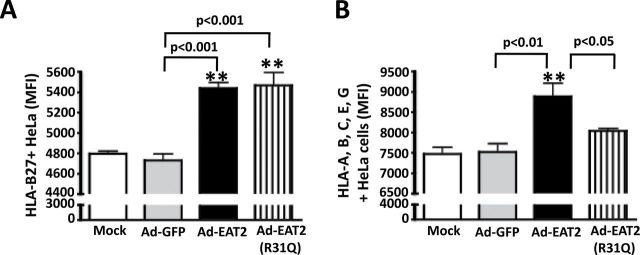
Increased antigen presentation of HeLa-Kb-B27 in rAd5-EAT2-infected cells. HeLa-Kb-B27 cells were either uninfected or infected with MOI of 1000 with rAd5-EAT2, rAd5-EAT2(R31Q) or rAd5-Null. At 24 hpi, cells were harvested and FACS analysis was performed. (A) Surface expression (MFI) of intact HLA-B27, measured by staining with ME1 antibody. (B) Surface expression of HLA-B, -A, -C, -E, -G, measured by staining with W6/32 antibody. Data are representative of four independent experiments with similar results. Samples were plated in quadruplicates. Bars represent mean ± SD. Statistical analysis was completed using a one way ANOVA with a Student–Newman–Keuls post-hoc test. P <0.05 was deemed a statistically significant difference. **P < 0.01, statistically significant from mock-treated cells.
Discussion
Accumulating evidence indicates that the SLAM family of receptors and their associated family of adaptors function to critically facilitate the regulatory and effector functions of both the innate and adaptive arms of the immune system (17, 41). The SLAM family of receptors and their associated adaptors are known to perform multiple functions in hematopoietic cells, including roles in regulating cellular costimulation, NK- and CD8+ T-cell-mediated cytotoxicity; cytokine production from macrophages, DCs and NK cells; adhesion between hematopoietic cells; development of innate T lymphocytes; as well as regulating functions of neutrophils and macrophages (42). In previous studies, we have shown that over-expression of EAT-2, a member of the SAP family, significantly alters the function of multiple murine innate immune cells including increased production of several pro-inflammatory cytokines and chemokines, enhanced cytolytic activity of NK cells, increased macrophage phagocytosis and improved DC maturation (20, 22, 23, 30). When this activity was harnessed for use to improve the efficacy of several vaccines, we confirmed that vaccines that specifically targeted EAT-2 functions had improved efficacy to induce antigen-specific adaptive [T effector memory (TEM)-based] immune responses to a variety of difficult antigens in various strains of mice (C57BL/6 and Balb/c) (20, 22, 23, 30). In this study, we have now shown that a human vaccine over-expressing EAT-2 also significantly alters the function of multiple human innate immune cells including increased production of several pro-inflammatory cytokines and chemokines, enhanced cytolytic activity of NK cells, increased monocyte phagocytosis and improved DC maturation. Our data also revealed that EAT-2 over-expression enhances MHC class I molecule-augmented antigen presentation in human cells as well. It is important to note that some of EAT-2-mediated immune responses of human PBMCs, for example induction of IL-17, activation of monocyte phagocytosis and improvement of NK cells cytotoxicity were modestly increased. There could be many reasons for this. It is possible that these responses are indirectly affected by EAT-2 adaptor over-expression, rather being directly responsive to other innate immune cytokines induced following EAT-2 over-expression, such as IL-1β, IL-6 or TNFα. In addition, it is possible that these responses are strongly induced, although at a different time points than the ones that we tested in our analysis. Together, the data strongly support the notion that human disease applications requiring vaccines and/or other immunomodulatory therapeutics may benefit from harnessing EAT-2 functions in these strategies.
Previous reports have demonstrated that the expression levels of SAP adaptors, including EAT-2, quickly diminish during cellular stimulation, such as TCR stimulation (43, 44). Furthermore, it has been demonstrated recently that upon exposure to a TLR4 agonist, human monocytes down-regulate EAT-2 gene expression (45). It is possible that prolonged SLAM signaling occurs by prolonged expression of the EAT-2 adaptor in rAd5-EAT2-infected human PBMCs and is the reason for the induction of the enhanced innate immune responses we observed in the treated human cells.
Previous reports have demonstrated that the function of SLAM family members can be either activation or inhibition based on the availability of SAP adaptors like EAT-2 (17). The activation of the SLAM family of receptors enhances the activation of immune cells that express SAP adaptors, while activation of SLAM receptor signaling in cells that do not express SAP adaptors, such as in X-linked lymphoproliferative patients (mutation of SAP adaptor alone) or in genetically engineered SAP-deficient mice (EAT-2, ERT or SAP single, double or triple knockout mice), results in markedly reduced activation of immune cells (46). Possibly, the abundance of EAT-2 protein in rAd5-EAT2-infected human immune cells prevents or delays the mechanisms that normally transitions the SLAM family of receptors from activating to inhibitory receptors. In addition, previous studies have suggested that downstream of the 2B4 receptor in a 129 mouse background, the EAT-2 adaptor might function as a negative regulator of murine NK cells (47). However, further mechanistic studies utilizing C57BL/6 and pure 129 mouse backgrounds confirmed that downstream of CRACC and 2B4 receptors, EAT-2 adaptor is a positive regulator of NK cells (48, 49). These studies suggest that EAT-2 might have a dual function based on the studied SLAM receptor and the mouse strains that were utilized.
Induction of pro-inflammatory cytokine and chemokine responses and activation of innate immune cells is regulated by several PRRs and their downstream signaling pathways (50). Recognition of PAMPs, by TLRs for example, results in activation of NFκB/AP-1 signaling pathways, leading to production of pro-inflammatory cytokines and chemokines (such as IL-6, IL-12 and TNFα) that coordinate innate immunity and initiate adaptive immune responses to various pathogens (51). In addition, TLR-3, 4, 7, 8 and 9 can activate the IRF3 and/or IRF7 signaling pathways in plasmacytoid DCs during viral infection and result in the induction of multiple genes involved in innate and adaptive immunity, including type I interferons (IFNα and IFNβ) (51). Similarly, the SAP family of adaptors harbor specific sequences that allow them to couple with the SLAM family of receptors to activate biochemical signaling molecules (17). For example, one SAP adaptor has been shown to have a specific sequence within the SH2 domain, arginine 78 (R78) motif, which associates directly with the protein tyrosine kinase FynT (34). This association links the SLAM family of receptors to protein tyrosine phosphorylation signals that results in PI3K and NFκB activation as well as enhanced Akt phosphorylation (52). Similar to SAP, EAT-2 has also been shown to transduce positive signals downstream of the SLAM family of receptors in human and mouse NK cells (48, 53). The activation function of EAT-2 was dependent on phosphorylation of tyrosine residues directly located in their short carboxy-terminal tails (54). Recent data suggest that PLC-γ is recruited to tyrosine 127 in human EAT-2 (33). Furthermore, over-expression and binding studies indicate that EAT-2 directly binds to the catalytic domain of the Src family kinases, Fyn, Hck, Lyn, Lck and Fgr (55). This suggests that the enhanced production of pro-inflammatory cytokines and chemokines and the improved activation of innate immune cells in rAd5-EAT2-infected human PBMCs might be regulated by these and potentially other innate immune signaling pathways responsive to the presence of the EAT-2 adaptor protein.
We also demonstrated that EAT-2 over-expression potently enhances IL-1β production from human PBMCs. The activation of the inflammasome, a multicellular complex (56), activates the proteolytic enzyme caspase-1 that processes IL-1β into its mature form (57). So far, four inflammasomes have been identified that activate caspase-1 including NLRP1, NALP3, NLRC4 and AIM2 (58). Since adenovirus vectors have been shown to activate the NALP3 and AIM2 inflammasomes (59, 60), it is possible that in rAd5-EAT2-infected human cells, augmented signaling downstream of SLAM receptors by EAT-2 over-expression functions to amplify Ad-mediated inflammasome activation. In addition, it is possible that EAT-2 directly triggers signaling pathways that activate the caspase-1-activating inflammasomes. Future studies addressing these intriguing hypotheses will be required to verify these notions.
Our studies also found that the phagocytic activity of rAd5-EAT2-infected human monocytes was dramatically enhanced, as compared with cells infected with rAd5 controls. The process of phagocytosis is an effective innate immune strategy used by the host to prevent and eradicate infections (61). Importantly, it has been shown previously that the SLAM family member, SLAM (CD150), enhances macrophage phagocytosis by functioning as a vital regulator in the innate immune defense against Gram-negative bacteria (62). Since the EAT-2 adaptor can bind to all members of the SLAM family, it is possible that the enhanced phagocytosis by monocytes that we observed in rAd5-EAT2-treated human cells is primarily mediated by interactions with this SLAM receptor. Finally, our data also revealed enhanced killing of K562 cells by rAd5-EAT2-infected human PBMCs. Since signaling mediated by the EAT-2 adaptor has been shown to modulate NK cell cytolytic activity levels, it is possible that rAd5-EAT2-transduced NK cells function as more potent cytolytic cells, suggesting justification for the use of rAd5-EAT2 in cancer immunotherapy approaches.
Importantly, a recent human HIV vaccine study utilizing the Merck® Ad5 HIV vaccine vectors (MRKAd5/HIV) confirmed that EAT-2 was among the innate immune response genes that were associated with the enhanced immunogenicity of MRKAd5/HIV (63). These data are consistent with our current and previously published data (20, 22, 23, 30) and suggest that increased expression of EAT-2 functions to increase innate immune responses and orchestrate the downstream adaptive immune responses in human immune cells.
In summary, enhanced cellular immune responses to vaccine antigens may be achievable in humans following methods that alter EAT-2 expression or activity levels. The enhancement of beneficial adaptive immune responses mediated by EAT-2 over-expression has been shown to be regulated by several innate immune mechanisms that involve increased production of pro-inflammatory cytokines and chemokines, enhanced maturation of DCs, enhanced activation of monocytes/macrophages, as well as increased activation and cytotoxicity of NK cells. Our studies suggest that modulation of EAT-2 in human cells, for example when used in vaccines and/or cancer immunotherapy approaches, may result in similar efficacies.
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
Michigan State University Foundation and Osteopathic Heritage Foundation to A.A.; King Abdullah bin Abdulaziz Scholarship, Ministry of Higher Education, Kingdom of Saudi Arabia to Y.A.A; NIH/NIAMS (AR056981).
Supplementary Material
Acknowledgements
We wish to thank Dr Louis King at the Michigan State University Flow Cytometry facility for his assistance in performing Flow Cytometry runs and the Michigan State University Laboratory Animal support facility for their assistance in the humane care and maintenance of the animals utilized in this work.
Conflict of Interest statement: The authors have no conflicting financial interest.
References
- 1. Takeuchi O., Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805. [DOI] [PubMed] [Google Scholar]
- 2. Iwasaki A., Medzhitov R. 2010. Regulation of adaptive immunity by the innate immune system. Science 327:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beutler B. A. 2009. TLRs and innate immunity. Blood. 113:1399–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Medzhitov R., Horng T. 2009. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 9:692. [DOI] [PubMed] [Google Scholar]
- 5. Pulendran B., Ahmed R. 2006. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124:849. [DOI] [PubMed] [Google Scholar]
- 6. Coffman R. L., Sher A., Seder R. A. 2010. Vaccine adjuvants: putting innate immunity to work. Immunity 33:492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marrack P., McKee A. S., Munks M. W. 2009. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 9:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manicassamy S., Pulendran B. 2009. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 21:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Querec T. D., Pulendran B. 2007. Understanding the role of innate immunity in the mechanism of action of the live attenuated Yellow Fever Vaccine 17D. Adv. Exp. Med. Biol. 590:43. [DOI] [PubMed] [Google Scholar]
- 10. Ha S. J., West E. E., Araki K., Smith K. A., Ahmed R. 2008. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunol. Rev. 223:317. [DOI] [PubMed] [Google Scholar]
- 11. Pulendran B. 2004. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol. Rev. 199:227. [DOI] [PubMed] [Google Scholar]
- 12. Alving C. R., Peachman K. K., Rao M., Reed S. G. 2012. Adjuvants for human vaccines. Curr. Opin. Immunol. 24:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwissa M., Kasturi S. P., Pulendran B. 2007. The science of adjuvants. Expert Rev. Vaccines 6:673. [DOI] [PubMed] [Google Scholar]
- 14. Akira S. 2011. Innate immunity and adjuvants. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 366:2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takeshita F., Tanaka T., Matsuda T., et al. 2006. Toll-like receptor adaptor molecules enhance DNA-raised adaptive immune responses against influenza and tumors through activation of innate immunity. J. Virol. 80:6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ma C. S., Nichols K. E., Tangye S. G. 2007. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu. Rev. Immunol. 25:337. [DOI] [PubMed] [Google Scholar]
- 17. Veillette A. 2010. SLAM-family receptors: immune regulators with or without SAP-family adaptors. Cold Spring Harb. Perspect. Biol. 2:a002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Veillette A. 2006. Immune regulation by SLAM family receptors and SAP-related adaptors. Nat. Rev. Immunol. 6:56 [DOI] [PubMed] [Google Scholar]
- 19. Veillette A., Dong Z., Latour S. 2007. Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity 27:698. [DOI] [PubMed] [Google Scholar]
- 20. Schuldt N. J., Aldhamen Y. A., Appledorn D. M., et al. 2011. Vaccine platforms combining circumsporozoite protein and potent immune modulators, rEA or EAT-2, paradoxically result in opposing immune responses PLoS One. 6:e24147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aldhamen Y. A., Seregin S. S., Amalfitano A. 2011. Immune recognition of gene transfer vectors: focus on adenovirus as a paradigm. Front. Immunol. 2:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aldhamen Y. A., Seregin S. S., Kousa Y. A., et al. 2013. Improved cytotoxic T-lymphocyte immune responses to a tumor antigen by vaccines co-expressing the SLAM-associated adaptor EAT-2. Cancer Gene Ther. 20:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aldhamen Y. A., Seregin S. S., Schuldt N. J., et al. 2012. Vaccines expressing the innate immune modulator EAT-2 elicit potent effector memory T lymphocyte responses despite pre-existing vaccine immunity. J. Immunol. 189:1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seregin S. S., Aldhamen Y. A., Appledorn D. M., et al. 2010. Adenovirus capsid-display of the retro-oriented human complement inhibitor DAF reduces Ad vector-triggered immune responses in vitro and in vivo . Blood 116:1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ng P., Graham F. L. 2002. Construction of first-generation adenoviral vectors. Methods Mol. Med. 69:389. [DOI] [PubMed] [Google Scholar]
- 26. Seregin S. S., Aldhamen Y. A., Appledorn D. M., et al. 2011. Use of DAF-displaying adenovirus vectors reduces induction of transgene- and vector-specific adaptive immune responses in mice. Hum. Gene Ther. 22:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. York I. A., Chang S. C., Saric T., et al. 2002. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8-9 residues. Nat. Immunol. 3:1177. [DOI] [PubMed] [Google Scholar]
- 28. Evnouchidou I., Kamal R. P., Seregin S. S., et al. 2011. Cutting edge: coding single nucleotide polymorphisms of endoplasmic reticulum aminopeptidase 1 can affect antigenic peptide generation in vitro by influencing basic enzymatic properties of the enzyme. J. Immunol. 186:1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perosa F., Luccarelli G., Prete M., Favoino E., Ferrone S., Dammacco F. 2003. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J. Immunol. 171:1918. [DOI] [PubMed] [Google Scholar]
- 30. Aldhamen Y. A., Appledorn D. M., Seregin S. S., et al. 2011. Expression of the SLAM family of receptors adapter EAT-2 as a novel strategy for enhancing beneficial immune responses to vaccine antigens. J. Immunol. 186:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adams W. C., Bond E., Havenga M. J., et al. 2009. Adenovirus serotype 5 infects human dendritic cells via a coxsackievirus-adenovirus receptor-independent receptor pathway mediated by lactoferrin and DC-SIGN. J. Gen. Virol. 90(Pt 7):1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morra M., Lu J., Poy F., et al. 2001. Structural basis for the interaction of the free SH2 domain EAT-2 with SLAM receptors in hematopoietic cells. EMBO J. 20:5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clarkson N. G., Brown M. H. 2009. Inhibition and activation by CD244 depends on CD2 and phospholipase C-gamma1. J. Biol. Chem. 284:24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Latour S., Roncagalli R., Chen R., et al. 2003. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat. Cell Biol. 5:149. [DOI] [PubMed] [Google Scholar]
- 35. Serbina N. V., Jia T., Hohl T. M., Pamer E. G. 2008. Monocyte-mediated defense against microbial pathogens. Annu. Rev. Immunol 26:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soudja S. M., Ruiz A. L., Marie J. C., Lauvau G. 2012. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 37:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawai T., Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11:373. [DOI] [PubMed] [Google Scholar]
- 38. Aylsworth C. F., Aldhamen Y. A., Seregin S. S., Godbehere S., Amalfitano A. 2013. Activation of human natural killer cells by the novel innate immune modulator recombinant Eimeria antigen. Hum. Immunol. 74:916. [DOI] [PubMed] [Google Scholar]
- 39. Blander J. M. 2008. Phagocytosis and antigen presentation: a partnership initiated by Toll-like receptors. Ann. Rheum. Dis. 67(Suppl. 3):iii44. [DOI] [PubMed] [Google Scholar]
- 40. Yao Y., Wang Y., Chen F., et al. 2012. NLRC5 regulates MHC class I antigen presentation in host defense against intracellular pathogens. Cell Res. 22:836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma C. S., Deenick E. K. 2011. The role of SAP and SLAM family molecules in the humoral immune response. Ann. N. Y. Acad. Sci. 1217:32. [DOI] [PubMed] [Google Scholar]
- 42. Cannons J. L., Tangye S. G., Schwartzberg P. L. 2011. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 29:665. [DOI] [PubMed] [Google Scholar]
- 43. Qi H., Cannons J. L., Klauschen F., Schwartzberg P. L., Germain R. N. 2008. SAP-controlled T-B cell interactions underlie germinal centre formation Nature. 455:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crotty S., Kersh E. N., Cannons J., Schwartzberg P. L., Ahmed R. 2003. SAP is required for generating long-term humoral immunity. Nature 421:282. [DOI] [PubMed] [Google Scholar]
- 45. Kim J. R., Horton N. C., Mathew S. O., Mathew P. A. 2013. CS1 (SLAMF7) inhibits production of proinflammatory cytokines by activated monocytes. Inflamm. Res. 62:765. [DOI] [PubMed] [Google Scholar]
- 46. Dong Z., Cruz-Munoz M. E., Zhong M. C., Chen R., Latour S., Veillette A. 2009. Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nat. Immunol. 10:973. [DOI] [PubMed] [Google Scholar]
- 47. Roncagalli R., Taylor J. E., Zhang S., et al. 2005. Negative regulation of natural killer cell function by EAT-2, a SAP-related adaptor. Nat. Immunol. 6:1002. [DOI] [PubMed] [Google Scholar]
- 48. Cruz-Munoz M. E., Dong Z., Shi X., Zhang S., Veillette A. 2009. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat. Immunol. 10:297. [DOI] [PubMed] [Google Scholar]
- 49. Wang N., Calpe S., Westcott J., et al. 2010. Cutting edge: the adapters EAT-2A and -2B are positive regulators of CD244- and CD84-dependent NK cell functions in the C57BL/6 mouse. J. Immunol. 185:5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kumar H., Kawai T., Akira S. 2009. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388:621. [DOI] [PubMed] [Google Scholar]
- 51. Lee M. S., Kim Y. J. 2007. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu. Rev. Biochem. 76:447. [DOI] [PubMed] [Google Scholar]
- 52. Veillette A., Dong Z., Pérez-Quintero L. A., Zhong M. C., Cruz-Munoz M. E. 2009. Importance and mechanism of ‘switch’ function of SAP family adapters. Immunol. Rev. 232:229. [DOI] [PubMed] [Google Scholar]
- 53. Tassi I., Colonna M. 2005. The cytotoxicity receptor CRACC (CS-1) recruits EAT-2 and activates the PI3K and phospholipase Cgamma signaling pathways in human NK cells. J. Immunol. 175:7996. [DOI] [PubMed] [Google Scholar]
- 54. Veillette A. 2006. NK cell regulation by SLAM family receptors and SAP-related adapters. Immunol. Rev. 214:22. [DOI] [PubMed] [Google Scholar]
- 55. Calpe S., Erdos E., Liao G., et al. 2006. Identification and characterization of two related murine genes, Eat2a and Eat2b, encoding single SH2-domain adapters. Immunogenetics 58:15. [DOI] [PubMed] [Google Scholar]
- 56. Schroder K., Tschopp J. 2010. The inflammasomes. Cell 140:821. [DOI] [PubMed] [Google Scholar]
- 57. Martinon F., Burns K., Tschopp J. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 10:417. [DOI] [PubMed] [Google Scholar]
- 58. Rathinam V. A., Vanaja S. K., Fitzgerald K. A. 2012. Regulation of inflammasome signaling. Nat. Immunol. 13:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Muruve D. A., Pétrilli V., Zaiss A. K., et al. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 452:103. [DOI] [PubMed] [Google Scholar]
- 60. Barlan A. U., Griffin T. M., McGuire K. A., Wiethoff C. M. 2011. Adenovirus membrane penetration activates the NLRP3 inflammasome. J. Virol. 85:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sarantis H., Grinstein S. 2012. Subversion of phagocytosis for pathogen survival. Cell Host Microbe. 12:419. [DOI] [PubMed] [Google Scholar]
- 62. Calpe S., Wang N., Romero X., et al. 2008. The SLAM and SAP gene families control innate and adaptive immune responses. Adv. Immunol. 97:177. [DOI] [PubMed] [Google Scholar]
- 63. Zak D. E., Andersen-Nissen E., Peterson E. R., et al. 2012. Merck Ad5/HIV induces broad innate immune activation that predicts CD8+ T-cell responses but is attenuated by preexisting Ad5 immunity. Proc. Natl Acad. Sci. U S A 109:E3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.