Abstract
Background. In cystic fibrosis (CF) patients, chronic lung infection and inflammation due to Pseudomonas aeruginosa contribute to the decline of lung function. The increased prevalence of multidrug resistance among bacteria and the adverse effects of antiinflammatory agents highlight the need for alternative therapeutic approaches that should be tested in a relevant animal model.
Methods. Gut-corrected CF and non-CF mice were chronically infected with a multidrug-resistant P. aeruginosa strain and treated with the long pentraxin PTX3. Body weight, bacterial count, inflammation, and lung pathology were evaluated after 12 days. PTX3 localization in CF sputum specimens was analyzed by immunofluorescence.
Results. Chronic P. aeruginosa infection developed similarly in CF and non-CF mice but differed in terms of the inflammatory response. Leukocyte recruitment in the airways, cytokine levels, and chemokine levels were significantly higher in CF mice, compared with non-CF mice. PTX3 treatment, which facilitates phagocytosis of pathogens, reduced P. aeruginosa colonization and restored airway inflammation in CF mice to levels observed in non-CF mice. The presence of PTX3 in CF sputum, in leukocytes, or bound to P. aeruginosa macrocolonies, as well as previous data on PTX3 polymorphisms in colonized CF patients, confirm the relevance of this molecule.
Conclusions. These findings represent a step forward in demonstrating the therapeutic potential of PTX3 in CF.
Keywords: Respiratory Tract Infections, Pseudomonas Infection, inflammation, Mouse Model, Cystic Fibrosis, PTX3
Lung disease in cystic fibrosis (CF) is characterized by exaggerated neutrophil recruitment and by chronic infection, most notably by Pseudomonas aeruginosa, that leads to lung damage, worsening of lung function, and premature death [1, 2]. Compared with normal individuals, airways fluids of patients with CF show an increased number of neutrophils and increased levels of proinflammatory cytokines, including tumor necrosis factor α, interleukin 6, interleukin 8, and leukotriene B4, but decreased levels of the antiinflammatory cytokine interleukin 10 [3, 4]. Early infection of the lungs with P. aeruginosa is transient, but once it is established, most patients become chronically colonized with multidrug-resistant bacteria that are difficult to eradicate. Treatment of CF-associated lung disease with prolonged and intensive antibiotic therapy and antiinflammatory drugs has been successful in increasing survival and improving the quality of life of CF patients, but the disease remains lethal by early adulthood. Concerns about potential adverse effects have limited the use of antiinflammatory therapies in CF [5, 6], and an increase in bacterial antibiotic resistance has led to an urgent need for new therapeutic strategies for managing CF patients.
Molecules that target the pathogen directly or that selectively enhance and/or alter host defense mechanisms are attractive candidates for therapeutic development [7]. The long pentraxin PTX3 is a soluble pattern-recognition receptor involved in pathogen recognition and resistance facilitating phagocytosis, activating the complement pathway, and orienting the adaptive immune response [8, 9]. PTX3 is produced and released by a variety of cell types and also has a regulatory role in inflammation by modulating leukocyte recruitment [10]. PTX3 deficiency has been correlated with increased susceptibility to P. aeruginosa lung infection [11], and PTX3 genetic variations have been shown to affect the risk of P. aeruginosa airway colonization in CF patients [12]. Moreover, recombinant PTX3 promoted reduction of the bacterial load and limited the inflammatory response in C57Bl/6 mice that developed P. aeruginosa chronic lung infection [13]. These encouraging results for PTX3 support further development in translational application.
Preclinical evaluation of novel therapies ideally should be performed in models that most closely mimic the course of human disease. Although mouse models for CF have been provided, they do not completely mimic the pathology seen in CF lung disease [14]. In addition to concerns regarding murine lung physiology, long-term chronic P. aeruginosa infection is difficult to establish in CF mice [15]. The animal models commonly used to evaluate antiinflammatory and antibiotic candidates relevant for CF patients included in vivo acute and chronic infections established using agar beads. These models were short-term studies lacking the chronic stage of bronchopulmonary infection and may not represent the clinical status of CF patients. When the animal model diverges substantially from the human condition, considerable uncertainty remains regarding the likelihood of therapeutic success.
In previous studies, we mimicked progressive bronchopulmonary infection in C57Bl/6 mice by exposure of airways to multidrug-resistant P. aeruginosa clinical strains obtained from patients with CF [15]. We embedded P. aeruginosa into agar beads to simulate chronic infection. In this study, we characterized the long-term response of gut-corrected CF mice to a multidrug-resistant P. aeruginosa clinical strain and used this model to test the efficacy of recombinant human PTX3 in preventing/eradicating lung colonization and inflammation.
METHODS
Bacterial Strains
The clinical P. aeruginosa strain RP73, isolated at the late stage of chronic infection from sputum of a CF patient [16], was used. The strain was cultured in trypticase soy broth and plated on Pseudomonas isolation agar or trypticase soy agar plates at 37°C.
Recombinant PTX3
Recombinant human PTX3 was purified from CHO cells constitutively expressing the proteins, as described elsewhere [17, 18]. The purity of recombinant proteins was assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis, followed by silver staining. Recombinant PTX3 contained 0.125 endotoxin units/mL, as checked by the Limulus amebocyte lysate assay.
Phagocytosis Assay in Whole Blood and Killing Assay
The phagocytosis assay was performed as described previously [13] and detailed in the Supplementary Materials. The killing assay was performed as detailed in the Supplementary Materials.
Mouse Model of Chronic Lung Infection
Male and female congenic gut-corrected CFTR-deficient mice B6.129P2-Cftrtm1UNCTgN(FABPCFTR) and heterozygotes, aged 8–12 weeks, were obtained from Case Western Reserve University [19]. Mice were infected with 2 × 106 colony-forming units (CFU) of the RP73 P. aeruginosa strain, embedded in agar beads, as previously described [15, 20]. After injection, mice were treated once per day for 12 days with recombinant PTX3 (10 μg/mouse intraperitoneally) or with sterile saline and were monitored daily for body weight. Twelve days after infection, lung CFU, bronchoalveolar lavage (BAL) fluid cell count, protein content, and myeoloperoxidase (MPO) activity were analyzed as previously described [13]. Murine cytokines and chemokines were measured using Luminex-based technology according to the manufacturer's instructions. Additional details are reported in the Supplementary Materials.
Animal studies were conducted according to protocols approved by the San Raffaele Scientific Institute and Istituto Clinico Humanitas (Milan, Italy) and the Case Western Reserve University Institutional Animal Care and Use Committee (Cleveland, OH).
Histopathologic and Immunofluorescence Analysis
After 12 days of infection, lungs were removed en bloc, fixed in 4% paraformaldehyde–phosphate-buffered saline, and processed for paraffin embedding. Longitudinal sections of 5 μm taken at regular intervals were obtained using a microtome from the proximal, medial, and distal lung regions and stained with hematoxylin and eosin and Alcian blue/periodic acid–Schiff (AB/PAS) for detection of mucopolysaccharides, according to standard procedures. To grade lung involvement, inflammation and mucous secretory cell metaplasia were evaluated as described in the Supplementary Materials.
Localization of P. aeruginosa was performed by indirect immunofluorescence, using a rabbit antiserum specific for P. aeruginosa [21] and Texas red–labeled goat anti-rabbit immunoglobulin G (IgG).
Sputum Samples
Sputum samples were collected by spontaneous expectoration from 10 CF and 5 non-CF patients attending the CF Center at Azienda Ospedaliera. Research on the biologic samples has been approved by the ethics committee of the CF Center at Azienda Ospedaliera of Verona. Samples were processed as described in the Supplementary Materials. Immunofluorescence was performed using rabbit anti-human PTX3 and Texas red–labeled goat anti-rabbit IgG.
Statistical Analysis
Because of the small sample size and the nonnormal distribution of variables, we used a nonparametric approach for all analyses in this article. Data are presented as medians and interquartile ranges. The Mann–Whitney U test was used to compare changes in body weight, CFU, cells, MPO activity, total protein levels, cytokine levels, and histologic measurements between CF, CF-PTX3, and non-CF groups. Tests are considered statistically significant when the significance level is ≤.05.
RESULTS
Survival, Body Weight, and P. aeruginosa Chronic Airway Infection of CFTR-Deficient Mice and Effect of PTX3 Treatment
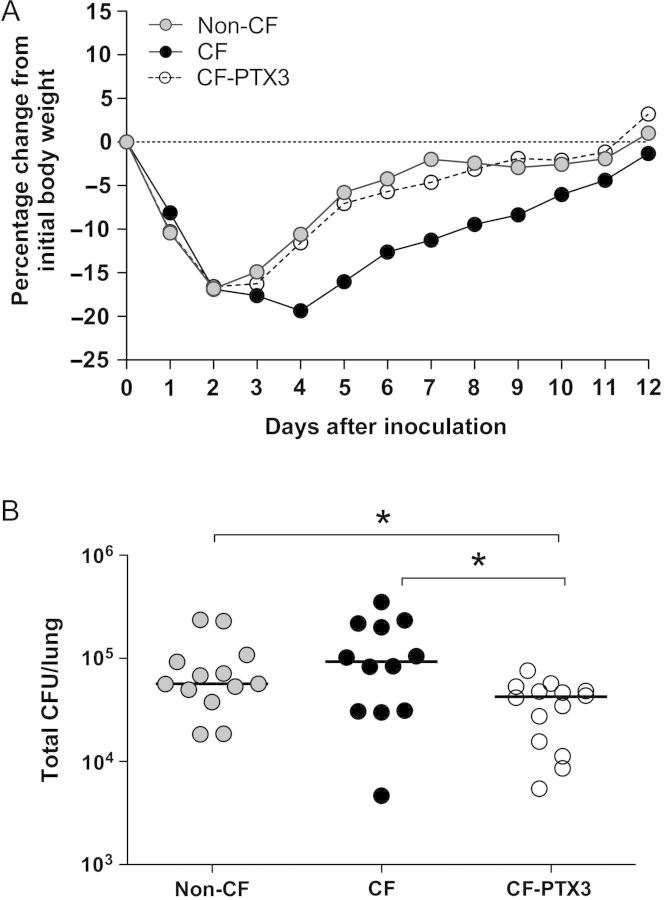
To mimic long-term severe infection in CF patients, Cftrtm1UNCTgN(FABPCFTR) mice (the CF group) and congenic heterozygous mice (the non-CF group) were challenged with 2 × 106 CFU of the multidrug-resistant P. aeruginosa RP73 clinical strain embedded in agar beads by intratracheal inoculation. Next, starting from the day of infection, one group of CF mice (the CF-PTX3 group) was treated daily with 10 μg/mouse of recombinant PTX3, which shares 92% identity with murine ptx3 [8] and has been shown to be active in murine models of infections [13, 11, 22, 23]. Mortality was low and occurred within the first 3 days of infection, when the P. aeruginosa load reaches its peak (Supplementary Table 1). All remaining Pseudomonas-infected mice survived for 12 days after inoculation. The effect of P. aeruginosa infection on CF and non-CF mice and the effect of PTX3 treatment on cumulative weight gain over the 12-day treatment period is shown in Figure 1A. There was no difference between non-CF, CF, and CF-PTX3 mice during the first 2 days after infection, when the major initial decrease in body weight was observed. Thereafter, non-CF and CF-PTX3 mice gained weight better than CF mice. Furthermore, non-CF and CF-PTX3 mice regained the initial weight loss, while CF mice still had not recovered completely.
Figure 1.
Cumulative weight gain and Pseudomonas aeruginosa load in mice with cystic fibrosis that received placebo (CF mice), mice without cystic fibrosis that received placebo (non-CF mice), and mice with cystic fibrosis that received PTX3 (CF-PTX3 mice). A, Median daily weight gain over the 12-day period in which vehicle or PTX3 was administered. CF-PTX3 mice gained more weight than CF mice and, like non-CF mice, regained the initial weight lost. Data are pooled from 2 independent experiments (non-CF, n = 17; CF, n = 18; and CF-PTX3, n = 19). B, Total lung colony-forming units (CFU) were counted after 12 days in CF, non-CF, and CF-PTX3 mice. Dots represent CFU per lung in individual mice, and horizontal lines represent median values reported in log scale (non-CF, n = 13; CF, n = 12; CF-PTX3, n = 14). Data are pooled from 2 independent experiments. *P < .05 and **P < .01, by the Mann–Whitney U test.
The P. aeruginosa load in lungs of CF and non-CF mice and the effect of PTX3 treatment is shown in Figure 1B. After an initial inoculum of 2 × 106 CFU/mouse, at the end of the 12-day period all mice had lung colonization by P. aeruginosa RP73, demonstrating the persistence of chronic infection (Supplementary Table 1). No significant difference was observed in CFU per lung recovered after 12 days of infection between CF and non-CF mice, revealing the capacity of P. aeruginosa RP73 to persist within murine lung regardless of the CF genetic background (Supplementary Table 1). A significant reduction in CFU was observed in lungs of CF-PTX3 mice, compared with lungs of CF mice (P = .027) and non-CF mice (P = .013). This suggests that PTX3 improved airway clearance of P. aeruginosa to a level even below that observed in non-CF mice.
Exaggerated Inflammatory Response to Chronic P. aeruginosa Infection Among CFTR-Deficient Mice Is Attenuated by PTX3 Treatment
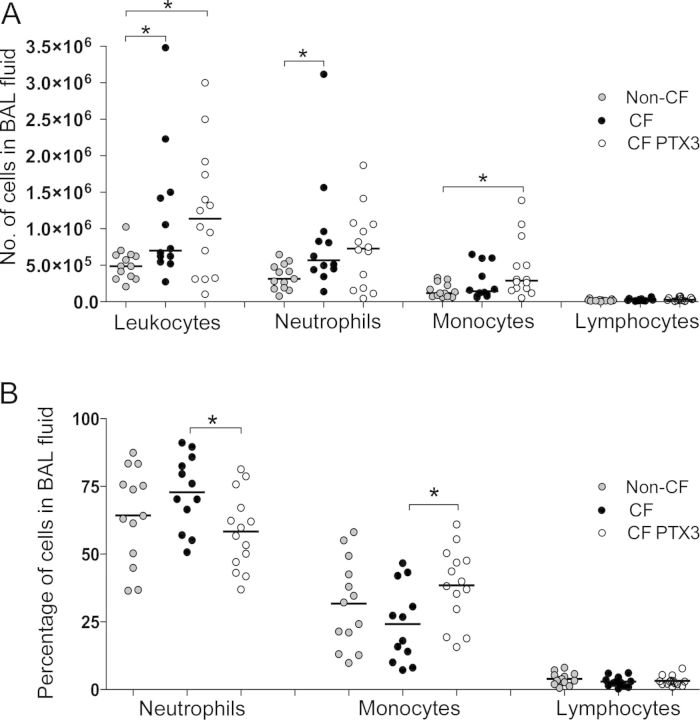
The inflammatory response of Pseudomonas-infected mice in terms of leukocyte recruitment in the airways, vascular permeability, and local cytokine production was investigated. After 12 days of infection, we observed an exaggerated inflammatory response in CF mice as compared to non-CF mice (Figure 2 and Supplementary Table 1), although the same P. aeruginosa load was found in CF mice and non-CF mice (Figure 1B). Indeed, our results showed that BAL fluid from CF mice had significantly more leukocytes than BAL fluid from non-CF mice (P = .021) (Figure 2A). In particular, we observed a significant increase in neutrophil levels for CF mice, compared with non-CF mice (P = .014), but monocyte levels and lymphocyte levels were not modified. After treatment with PTX3, the percentage of neutrophils was significantly reduced (P = .016) and monocyte levels were increased in CF-PTX3 mice as compared to CF mice (P = .016) (Figure 2B).
Figure 2.
Lung inflammatory response after chronic Pseudomonas aeruginosa infection in mice with cystic fibrosis that received placebo (CF mice), mice without cystic fibrosis that received placebo (non-CF mice), and mice with cystic fibrosis that received PTX3 (CF-PTX3 mice). The number (A) and percentage (B) of total leukocytes and of neutrophils, monocytes, and lymphocytes recruited in the airways were determined in bronchoalveolar lavage (BAL) fluid from mice after 12 days of chronic P. aeruginosa lung infection. Dots represent measurements for individual mice, and horizontal lines represent median values. Data are pooled from 2 independent experiments (non-CF, n = 13; CF, n = 12; CF-PTX3, n = 14). P < .05 and **P < .01, by the Mann–Whitney U test.
The MPO activity in the BAL fluid of CF mice was higher than that in the BAL fluid of non-CF mice, but the difference was not statistically significant (P = .054); no difference was observed between CF-PTX3 and CF mice (Supplementary Table 1). In addition, we detected a significant difference in total protein content between CF and non-CF mice (P = .006) and a 1.9-fold reduction of total protein content in CF-PTX3 mice as compared to CF mice.
Cytokine and Chemokine Profiles in the Airways of P. aeruginosa–Infected CFTR-Deficient Mice and Modulation by PTX3 Treatment
To better characterize the airway inflammatory response after 12 days of infection in CF mice in this model and to define the effect of PTX3 treatment, we measured the concentration of a panel of cytokines and chemokines in murine lung homogenates (Table 1). Results show that the levels of proinflammatory cytokines (interleukin 1β [IL-1β] and interleukin 17 [IL-17]) and chemokines (CCL2/MCP-1, CXCL1/KC, and CXCL2/MIP-2) in lung homogenates of CF mice were significantly higher than those in non-CF mice (IL-1β, P = .014; IL-17, P = .012; CCL2/MCP-1, P = .014; KC, P = .012; and CXCL2/MIP-2, P = .021). Treatment with PTX3 significantly reduced IL-17 and CCL2/MCP-1 levels in the lungs of CF mice (IL-17, P = .025; and CCL2/MCP-1, P = .021). Levels of other cytokines, including IL-1β and CCL2/MCP-1, were reduced by at least 2-fold, but differences did not reach statistical significance. IL-6 levels did not differ among the 3 groups at this time point. In contrast, levels of the antiinflammatory cytokine TGFβ-1 did not change in non-CF, CF, or CF-PTX3 mice.
Table 1.
Levels of Cytokines and Chemokines in Lung Homogenates of Pseudomonas aeruginosa–Infected Mice Treated for 12 Days With Vehicle or PTX3
| Variable | Level, pg/mL, Median (IQR) |
Pa |
||||
|---|---|---|---|---|---|---|
| Non-CF | CF | CF-PTX3 | CF vs Non-CF | CF vs CF-PTX3 | Non-CF vs CF-PTX3 | |
| IL-1β | 268.6 (114) | 652.7 (589) | 298.1 (351) | .014 | .068 | .309 |
| IL-17 | 86.8 (44) | 135.3 (67) | 93.3 (54) | .012 | .025 | .554 |
| CCL2/MCP-1 | 40.5 (44) | 314.2 (696) | 102.4 (210) | .014 | .102 | .140 |
| CXCL1/KC | 5.7 (5) | 12.1 (13) | 10.3 (4) | .012 | .171 | .038 |
| CXCL2/MIP-2 | 13.2 (14) | 41 (51) | 9.9 (12) | .021 | .021 | .972 |
| TGF-β1 | 52 (23) | 49.5 (18) | 44.4 (23) | .732 | .447 | .921 |
| INF-γ | ND | ND | ND | |||
| IL-13 | ND | ND | ND | |||
| IL-6 | 112.7 (136) | 122.8 (119) | 143.5 (82) | .447 | .909 | .554 |
| IL-4 | ND | ND | ND | |||
| IL-10 | ND | ND | ND | |||
Abbreviations: CF, mice with cystic fibrosis that received vehicle; CF-PTX3, mice with cystic fibrosis that received PTX3; IL-1β, interleukin 1β; IL-4, interleukin 4; IL-6, interleukin 6; IL-10, interleukin 10; IL-13, interleukin 13; IL-17, interleukin 17; INF-γ, interferon γ; IQR, interquartile range; ND, not detectable; non-CF, mice without cystic fibrosis that received vehicle; TGF-β1, transforming growth factor β1.
a By the nonparametric Mann–Whitney U test.
Histopathologic Lesions in the Airways of P. aeruginosa–Infected CFTR-Deficient Mice and Effect of PTX3 Treatment
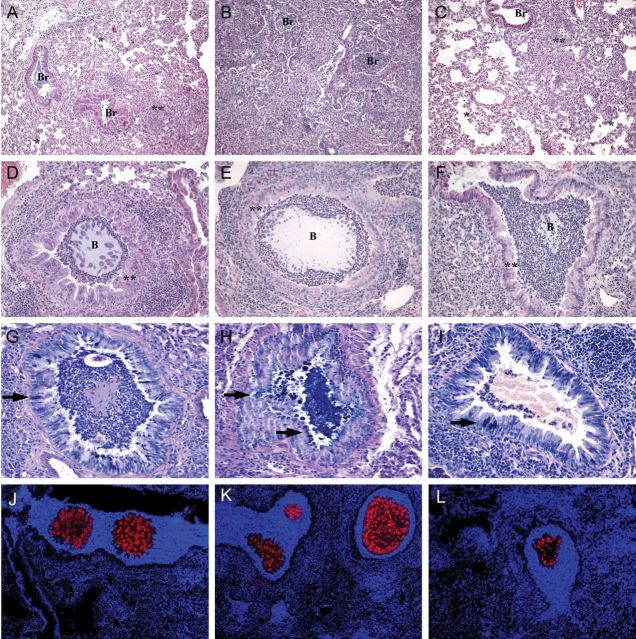
The histopathologic analysis of P. aeruginosa RP73–induced chronic pneumonia indicates that in this model the lung was not totally compromised: the infection was plurifocal and generally involved ≥1 lung lobes, but the other lobes were unaffected or marginally involved. Lung histopathologic analysis showed inflammatory lesions in bronchi and pulmonary parenchyma. The bronchi were filled and surrounded by a massive number of inflammation-associated neutrophils, whereas the parenchyma was infiltrated by macrophages, lymphocytes, and some neutrophils (Figure 3A–C). In the same cases, agar beads were observed in bronchial lumina (Figure 3D–F). Moreover, mucous secretory cell hyperplasia was found (Figure 3G–I). Histologic examination revealed that lesions in CF mice (Figure 3B, 3E, and 3H) were more severe than those in non-CF mice (Figure 3A, 3D, and 3G). Treatment with PTX3 reduced the lesions in CF mice (Figure 3C, 3F, and 3I). As described in Supplementary Table 2, we quantified the percentage of parenchyma involved and infected bronchi, the severity of inflammation, and the severity of mucous hyperplasia. In particular, the numbers of mucous secretory cells were significantly higher in CF mice, compared with CF-PTX3 mice (P = .020). No significant differences were found in the scoring of inflammation and the percentage of parenchyma involved. Immunofluorescence revealed fewer and smaller agar beads with a reduced bacterial load per cell in CF-PTX3 mice (Figure 3L), compared with CF and non-CF mice (Figure 3K and 3J), supporting the quantitative data reported above (Figure 1B).
Figure 3.
Histologic lesions after chronic Pseudomonas aeruginosa infection in mice with cystic fibrosis that received placebo (CF mice), mice without cystic fibrosis that received placebo (non-CF mice), and mice with cystic fibrosis that received PTX3 (CF-PTX3 mice). Four mice per group were used for histopathologic examination. The lungs of non-CF mice (A, D, G, and J), CF mice (B, E, H, and K), and CF-PTX3 mice (C, F, I, and L) were stained with hematoxylin and eosin (A–F); with Alcian blue/periodic acid–Schiff, for detection of mucopolysaccharides according to standard procedures (G–I); or with specific antibody against P. aeruginosa, for immunofluorescence (red; J–L; counterstaining was performed with DAPI). CF mice were characterized by massive bronchiolitis (Br) and a huge amount of interstitial/alveolar inflammation; non-CF mice and CF-PTX3 mice had focal inflammation involving the interstitium and alveoli (**), with most of the alveolar spaces spared (*). D–F, Fewer and smaller agar beads (B) were observed in CF-PTX3 mice, compared with CF and non-CF mice. G–I, Representative results of Alcian blue staining, showing that the percentage of mucous secretory cells (arrows) was higher in CF mice, compared with non-CF and CF-PTX3 mice. Bacterial macrocolonies were visible by immunofluorescence (J–L) in beads from non-CF, CF, and CF-PTX3 mice. A reduced bacterial load per cell was observed in CF-PTX3 mice, compared with CF and non-CF mice, supporting the morphologic data reported above. Original magnification: 10 × , for panels A–C; 20 × , for panels D–F and J–L; and 40 × for panels G–I.
PTX3 Facilitates Phagocytosis and Killing of P. aeruginosa in CFTR-Deficient Neutrophils
Next, we addressed the prophagocytic activity of CF neutrophils in comparison to non-CF neutrophils toward P. aeruginosa and the effect of PTX3 treatment [13]. As shown in Figure 4A and Supplementary Table 3, in a phagocytosis assay performed with CFSE-labeled P. aeruginosa RP73 and whole blood, we first observed that phagocytosis was comparable between CF and non-CF neutrophils. Moreover, preopsonization of bacteria with recombinant PTX3 significantly increased internalization by CF neutrophils (P = 0.043).
Figure 4.
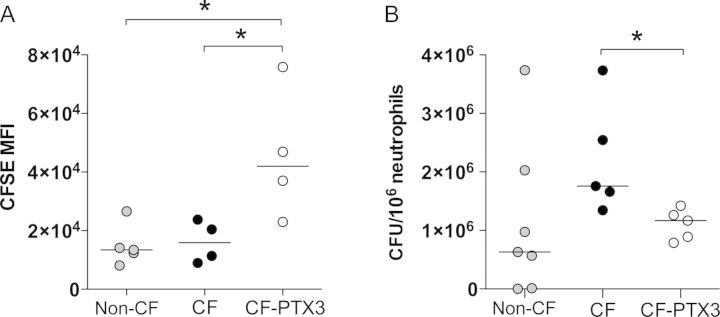
In vitro phagocytosis and killing of Pseudomonas aeruginosa by neutrophils from mice with cystic fibrosis that received placebo (CF mice), mice without cystic fibrosis that received placebo (non-CF mice), and mice with cystic fibrosis that received PTX3 (CF-PTX3 mice). A, In vitro phagocytosis of CFSE-labeled P. aeruginosa RP73 by neutrophils (CD45high, Ly6Ghigh, CD11bhigh cells) was analyzed by fluorescence-activated cell sorting and reported as CFSE mean fluorescence intensity (MFI). Whole blood from CF and non-CF mice (n = 4–5) was used. P. aeruginosa RP73 was preincubated with PTX3 (20 μg/mL) or phosphate-buffered saline (PBS). B, In vitro killing of P. aeruginosa RP73 by neutrophils was analyzed. Bone marrow from CF and non-CF mice was used (n = 5–7). P. aeruginosa RP73 was preincubated with PTX3 (20 μg/mL) or PBS. Data were normalized and expressed as colony-forming units (CFU) per 106 neutrophils. Dots represent individual measurements, and horizontal lines represent median values. Data are pooled from 2 independent experiments. *P < .05, by the Mann–Whitney U test.
Next, we examined whether the prophagocytic effect of the recombinant PTX3 in neutrophils was also correlated with an increased killing activity. As shown in Figure 4B and Supplementary Table 3, killing activity did not reach significance between CF and non-CF murine neutrophils, although a 2.8-fold difference was detected, indicating a potential biologic difference. Bacterial preopsonization with recombinant PTX3 resulted in a significant decrease in the number of surviving bacteria in CF neutrophils (P = 0.016).
Localization of PTX3 in Sputum Specimens From CF Patients
To explore the relevance of PTX3 in CF patients, we used immunofluorescence with anti-PTX3 antibody to localize PTX3 in patients’ sputum specimens, which contain cells of the immune system, bacteria, and squamous epithelial cells. Microscopic analysis revealed that PTX3 was located intracellularly in cells of the immune system (Figure 5A), mainly neutrophils, or was associated to DNA filaments, possibly neutrophil extracellular traps (Figure 5B), and bound to P. aeruginosa macrocolonies (Figure 5C). In particular, we observed P. aeruginosa macrocolonies surrounded by PTX3. Staining of sputum specimens from uninfected patients with or without CF showed similar localization of PTX3 (data not shown).
Figure 5.
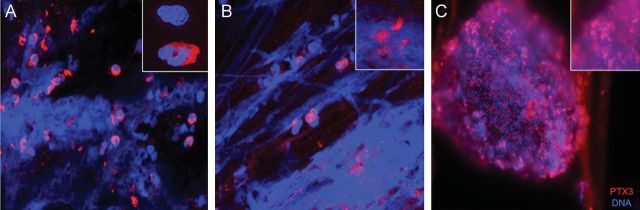
PTX3 localization in sputum specimens from patients with cystic fibrosis (CF). Sputum specimens from 10 CF patients were isolated and characterized for PTX3 localization by indirect immunofluorescence, using a human PTX3 antibody. Texas red–labeled goat anti-rabbit immunoglobulin G was used as secondary antibody; DAPI labeling was used to visualize cell nuclei and extracellular DNA strands. A and B, Localization of PTX3 in cells of the immune system, mainly neutrophils (insets). B, PTX3 entrapped in the actin-DNA filaments (insets). C, PTX3 bound to Pseudomonas aeruginosa macrocolonies (insets). Original magnification: 40 × , for panels A–C; and 100 × , for insets.
These results of PTX3 localization associated with P. aeruginosa macrocolonies in sputum specimens suggest its involvement in the immune response and support the evidence that PTX3 genetic variations affect the risk of P. aeruginosa airway colonization in CF patients.
DISCUSSION
The goal of this preclinical study was to test the response of CF mice to long-term severe P. aeruginosa airway infection and to use this model to test the efficacy of PTX3 treatment. To mimic the progressive bronchopulmonary infection typical of CF patients, we challenged gut-corrected CF mice (Cftrtm1UNCTgN[FABPCFTR]) and their congenic heterozygote mice with a RP73 P. aeruginosa clinical strain isolated after years of colonization from a CF patient and embedded in agar beads [15]. We report, for the first time, the response to long-term severe P. aeruginosa lung infection among CF mice, compared with the response among non-CF mice. Daily measurement of the weight gain was included as index of overall well-being. We observed that, after P. aeruginosa infection, non-CF mice gained weight better than CF mice.
Our results showed that CF mice experienced higher inflammatory mediator and neutrophil levels, compared with their congenic heterozygous mice, at 12 days after chronic lung infections. The concentration of inflammatory mediators, including IL-1ß, IL-17, CCL-2/MCP1, CXCL1/KC, and CXCL2/MIP-2, in CF mice was significantly increased, compared with concentrations in non-CF mice. According to these data, in CF mice as compared to non-CF mice, we observed an increased neutrophil recruitment; increased MPO activity, which is correlated with the total number of neutrophils recruited; and increased protein content in BAL fluid, which is a parameter of vascular leakage. Lesions in CF mice, including those involving mucous secretory cells, were more severe than lesions in non-CF mice. Semiquantitative assessment of histologic samples indicated that CF mice had greater quantities of parenchyma involved and inflammation, compared with non-CF mice, but the difference was not statistically significant, probably because of the focal distribution of tissue injury in lung parenchyma.
Interestingly, numbers of bacteria recovered from the murine airways of mice and immunofluorescence-determined localization of P. aeruginosa cells did not differ between CF and non-CF mice, suggesting that chronic infection in mouse lungs is established regardless of the CF genetic background and that the exaggerated inflammatory response may not be directly associated with the infection. Furthermore, when tested in vitro, CF and non-CF murine neutrophils showed similar phagocytic properties. The killing activity against P. aeruginosa was nearly 3-fold higher for non-CF mice than for CF mice, indicating a potential biologic difference, although statistical significance was not reached in this case. Whether CF mice have a defective ability to eliminate bacteria from the airways and whether inflammation during CF is strictly dependent on infection is still a matter of debate. Previous studies in Cftrtm1UNCTgN(FABPCFTR) mice reported that early bacterial clearance was just as rapid in CF mice as in wild-type mice, despite a prolonged inflammatory response in CF mice [24]. However, other reports on results obtained with mice with a different genetic background (Cftrtm1G551D) showed that these mice had an increased susceptibility to P. aeruginosa infection [25]. Our results showed similar bacterial loads in CF and non-CF mice 12 days after challenge with P. aeruginosa.
Treatment of CF mice with recombinant human PTX3 was based on previously performed pharmacokinetic analysis (C. Garlanda, unpublished results) and therapeutic approaches in a murine model of lung aspergillosis [23] and P. aeruginosa infection in C57Bl/6 mice [13]. Early initiation of treatment (ie, on the day of infection) with intraperitoneal administration of PTX3 in Cftrtm1UNCTgN(FABPCFTR) mice significantly decreases the P. aeruginosa load, compared with vehicle. Thus, PTX3-dependent recognition and phagocytosis of pathogens, which was previously demonstrated, is also conserved in the CF environment. Numbers of bacteria recovered from the airways of CF-PTX3 mice were also significantly reduced, compared with numbers in non-CF mice, indicating a clear benefit. PTX3 attenuated the extent of the inflammatory response to chronic endobronchial infection. The concentrations of inflammatory mediators, including IL-1β, IL-17, CCL-2/MCP1, CXCL1/KC, and CXCL2/MIP-2, were decreased in CF-PTX3 mice, compared with CF mice, and differences reached statistical significance for IL-17 and CXCL2/MIP-2. According to these data, we observed a significantly decreased percentage of recruited neutrophils in association with a significantly increased percentage of monocytes and a significantly increased total protein content in BAL fluid from CF-PTX3 mice, compared with CF mice. In addition, PTX3 treatment of CF mice resulted in significantly better weight gain, compared with vehicle, and a decrease in the percentage of hyperplasic mucous secretory cells to the level observed in non-CF mice.
The mechanism by which PTX3 attenuates the infection and inflammatory response in this murine model is not well-defined. Previous studies showed the interplay between PTX3, complement, and FcγR in innate resistance to the fungus Aspergillus fumigatus and to P. aeruginosa [22]. Our in vitro results indicated that preopsonization of P. aeruginosa with recombinant PTX3 significantly increased both phagocytosis and killing by neutrophils in the CF environment, explaining the lower bacterial lung burden detected in CF-PTX3 mice, compared with CF mice. These results suggest that the opsonizing activity of recombinant PTX3 is also preserved in the CF environment. The role of PTX3 in negatively regulating neutrophil recruitment in inflammatory contexts through interaction with P-selectin [10] has not been addressed in this study. However, its contribution to reducing leukocyte recruitment and lung inflammation cannot be excluded.
It was recently proposed that PTX3 is stored in neutrophil granules and rapidly mobilized and secreted on stimulation [26]. PTX3 was also reported to bind fungi and bacterial cells [11]. In this work, we localized PTX3 in sputum from CF patients. Sputum specimens are an accurate indicator of the respiratory tract environment because they contain squamous epithelial cells, a high concentration of bacteria as macrocolonies, and cells of the immune system. In agreement with previous studies, PTX3 was detected in cells of the immune system or was entrapped in the actin-DNA filaments in all patient groups. In addition, PTX3 bound to P. aeruginosa macrocolonies in infected CF patients. Whether endogenous PTX3 exerts a protective role against P. aeruginosa infection in CF patients has not been described. However, previous studies showing increased susceptibility among ptx3-deficient mice to lung infection caused by a laboratory P. aeruginosa strain [11] and genetic evidence that PTX3 gene polymorphisms affect CF-associated P. aeruginosa lung infections [12] suggest that PTX3 is involved in resistance to P. aeruginosa in CF.
It has been reported that the CF environment can reduce or inactivate the activity of natural antimicrobial peptides, such as the β-defensins and the cathelicidin LL-37 [27]. A study of PTX3 in patients with chronic obstructive pulmonary disease showed that, in this condition, the pulmonary interstitial expression of PTX3 is reduced [28]. Whether endogenous PTX3 is susceptible to degradation or inactivation in a CF environment is not known. However, this study suggests a beneficial effect of treatment with exogenous PTX3. Indeed, numerous studies indicated that exogenous peptide administration increases bacterial clearance and host survival following bacterial infection, providing support for the use of this therapeutic strategy. The results presented in this study provide important information with respect to P. aeruginosa chronic infection in CF mice. The evaluation of potential therapies in CF murine models of chronic infection provides the greatest opportunity to optimally translate results obtained in animal models to the clinical care of patients. The encouraging results obtained so far for PTX3 support further development in translational application.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank B. Tümmler (Klinische Forschergruppe, Medizinische Hochschule Hannover, Germany), for supplying the P. aeruginosa RP73 clinical strain; G. Pier, for supplying the rabbit antiserum specific for P. aeruginosa; M. Drumm (Case Western Reserve University, Cystic Fibrosis Animal Core Center), for supplying B6.129P2-Cftrtm1UNCTgN(FABPCFTR) mice; and P. Melotti and B. Assael (Azienda Ospedaliera of Verona, Cystic Fibrosis Center), for collecting the specimens from patients with CF.
Financial support. This work was supported by the European Commission (European Research Council project HIIS, and Mucosal Vaccines for Poverty Related Diseases LSHP-CT-2003-503240); Ministero dell'Istruzione, Università e della Ricerca (Project Fund for Investment in Basic Research RBLA039LSF); Telethon (grant GGP05095); and the Italian Cystic Fibrosis Research Foundation (Festa per l'80° compleanno del Presidente Faganelli).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest. 2008;133:489–95. doi: 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- 2.Ratjen F. What's new in CF airway inflammation: an update. Paediatr Respir Rev. 2006;7:S70–2. doi: 10.1016/j.prrv.2006.04.170. [DOI] [PubMed] [Google Scholar]
- 3.Bonfield T, Panuska JR, Konstan MW, et al. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–8. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 4.Bonfield T, Konstan MW, Burfeind P, Panuska JR, Hilliard JB, Berger M. Normal bronchial epithelial cells constitutively produce the anti-inflammatory cytokine interleukin-10, which is downregulated in cystic fibrosis. Am J Respir Cell Mol Biol. 1995;13:257–61. doi: 10.1165/ajrcmb.13.3.7544594. [DOI] [PubMed] [Google Scholar]
- 5.Koehler D, Downey GP, Sweezey NB, Tanswell AK, Hu J. Lung inflammation as a therapeutic target in cystic fibrosis. Am J Respir Cell Mol Biol. 2004;31:377–81. doi: 10.1165/rcmb.2004-0124TR. [DOI] [PubMed] [Google Scholar]
- 6.Lands L, Dauletbaev N. High-dose ibuprofen in cystic fibrosis. Pharmaceuticals. 2010;3:2213–24. doi: 10.3390/ph3072213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mookherjee N, Hancock RE. Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections. Cell Mol Life Sci. 2007;64:922–33. doi: 10.1007/s00018-007-6475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol. 2005;23:337–66. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- 9.Inforzato A, Jaillon S, Moalli F, et al. The long pentraxin PTX3 at the crossroads between innate immunity and tissue remodelling. Tissue Antigens. 2011;4:271–82. doi: 10.1111/j.1399-0039.2011.01645.x. [DOI] [PubMed] [Google Scholar]
- 10.Deban L, Russo RC, Sironi M, et al. Regulation of leukocyte recruitment by the long pentraxin PTX3. Nat Immunol. 2010;11:328–34. doi: 10.1038/ni.1854. [DOI] [PubMed] [Google Scholar]
- 11.Garlanda C, Hirsch E, Bozza S, et al. Non-redundant role of the long pentraxin PTX3 in anti-fungal innate immune response. Nature. 2002;420:182–6. doi: 10.1038/nature01195. [DOI] [PubMed] [Google Scholar]
- 12.Chiarini M, Sabelli C, Melotti P, et al. PTX3 genetic variations affect the risk of Pseudomonas aeruginosa airway colonization in cystic fibrosis patients. Genes Immun. 2010;11:665–70. doi: 10.1038/gene.2010.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moalli F, Paroni M, Véliz Rodriguez T, et al. The therapeutic potential of the humoral pattern recognition molecule PTX3 in chronic lung infection caused by Pseudomonas aeruginosa. J Immunol. 2011;186:5425–34. doi: 10.4049/jimmunol.1002035. [DOI] [PubMed] [Google Scholar]
- 14.Bragonzi A. Murine models of acute and chronic lung infection with cystic fibrosis pathogens. IJMM. 2010;300:584–93. doi: 10.1016/j.ijmm.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Bragonzi A, Paroni M, Nonis A, et al. Pseudomonas aeruginosa microevolution during cystic fibrosis lung infection establishes clones with adapted virulence. AJRCCM. 2009;180:138–45. doi: 10.1164/rccm.200812-1943OC. [DOI] [PubMed] [Google Scholar]
- 16.Bragonzi A, Wiehlmann L, Klockgether J, et al. Sequence diversity of the mucABD locus in Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Microbiology. 2006;152:3261–9. doi: 10.1099/mic.0.29175-0. [DOI] [PubMed] [Google Scholar]
- 17.Bottazzi B, Vouret-Craviari V, Bastone A, et al. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem. 1997;272:32817–23. doi: 10.1074/jbc.272.52.32817. [DOI] [PubMed] [Google Scholar]
- 18.Rivieccio V, Esposito A, Bellofiore P, Palladino P, Sassano M, Colombo M. Verdoliva high-level expression and efficient purification of recombinant human long pentraxin PTX3 in Chinese hamster ovary cells. Protein Expr Purif. 2007;51:49–58. doi: 10.1016/j.pep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 19.van Heeckeren A, Schluchter MD, Drumm ML, Davis PB. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L944–52. doi: 10.1152/ajplung.00387.2003. [DOI] [PubMed] [Google Scholar]
- 20.Bragonzi A, Worlitzsch D, Pier GB, et al. Nonmucoid Pseudomonas aeruginosa expresses alginate in the lungs of patients with cystic fibrosis and in a mouse model. J Infect Dis. 2005;192:410–9. doi: 10.1086/431516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pier G, Thomas DM. Lipopolysaccharide and high-molecular-weight polysaccharide serotypes of Pseudomonas aeruginosa. J Infect Dis. 1982;145:217–23. doi: 10.1093/infdis/145.2.217. [DOI] [PubMed] [Google Scholar]
- 22.Moalli F, Doni A, Deban L, et al. Role of complement and Fc{gamma} receptors in the protective activity of the long pentraxin PTX3 against Aspergillus fumigatus. Blood. 2010;116:5170–80. doi: 10.1182/blood-2009-12-258376. [DOI] [PubMed] [Google Scholar]
- 23.Gaziano R, Bozza S, Bellocchio S, et al. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals. Antimicrob Agents Chemother. 2004;11:4414–21. doi: 10.1128/AAC.48.11.4414-4421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Heeckeren AM, Schluchter M, Xue W, Davis PB. Response to acute lung infection with mucoid Pseudomonas aeruginosa in cystic fibrosis mice. Am J Respir Crit Care Med. 2006;173:288–96. doi: 10.1164/rccm.200506-917OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMorran BJ, Palmer J, Lunn DP, et al. G551D CF mice display an abnormal host response and have impaired clearance of Pseudomonas lung disease. Am J Physiol Lung Cell Mol Physiol. 2001;281:740–7. doi: 10.1152/ajplung.2001.281.3.L740. [DOI] [PubMed] [Google Scholar]
- 26.Jaillon S, Peri G, Delneste Y, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007;204:793–804. doi: 10.1084/jem.20061301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C, Schaller-Bals S, Paul KP, Wahn U, Bals R. Beta-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J Cyst Fibros. 2004;3:45–50. doi: 10.1016/j.jcf.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Geert RMVP, Bracke KR, Pauwels NS, et al. Reduced expression of pentraxin-3 in the lungs of patients with COPD. Am J Respir Crit Care Med. 2010;181:A3887. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.