Abstract
Purpose
The addition of 4 % O2 and 10 % N2O to the CO2 pneumoperitoneum (PP), together with slight cooling and humidification (conditioning), contributes to reducing adhesions by preventing mesothelial damage. We investigated the effect of peritoneal damage during laparoscopy on tumor implantation.
Methods
In Experiment 1, different tumor cell concentrations were injected into control mice without PP and into mice with 60-min dry CO2PP (mesothelial damage). In Experiment 2, tumor cells were injected into control mice (group I) and in mice with mesothelial damage (group II). In groups III to VI, mesothelial damage was decreased by adding humidification, humidification + 10 % N2O, humidification + 10 % N2O + 4 % O2, and conditioning, respectively.
Results
In Experiment 1, the tumors increased with the number of cells injected and with mesothelial damage in the abdominal cavity (p = 0.018) and abdominal wall (p < 0.0001). Experiment 2 confirmed that 60 min of dry CO2PP increased the number of tumors in the abdominal cavity and wall (p = 0.026 and p = 0.003, respectively). The number of tumors was decreased in the abdominal cavity by conditioning (p = 0.030) and in the abdominal wall using humidified CO2 (p = 0.032) or conditioning (p = 0.026).
Conclusions
Tumor implantation was enhanced by peritoneal damage (60 min of dry CO2PP and desiccation), but this was prevented by conditioning. If confirmed in humans, conditioning would become important for oncologic surgery.
Keywords: Laparoscopy, Pneumoperitoneum, Mouse model, Tumor implantation, Conditioning of the abdominal cavity, Humidified gas
Introduction
Laparoscopy has become the gold standard of treatment for benign conditions. Its benefits over laparotomy include better cosmesis, less pain, faster recovery of bowel function, and shorter hospitalization. Less immunologic depression [1, 2] is also well documented but its significance remains unclear. However, its suitability for cancer surgery remains a subject of debate because of concerns about tumor metastasis and growth. In addition to these advantages of laparoscopic surgery, image magnification techniques have improved our visualization of metastatic or recurrent disease and allowed for better dissection in challenging areas such as the paravesical and pararectal spaces, with limited bleeding from small vessels because of pressure from the pneumoperitoneum (PP) [3]. Furthermore, faster recovery and no large abdominal incision permit earlier postoperative chemotherapy or radiation, with fewer radiation complications from bowel adhesions [3]. The potential disadvantages are a risk of port site metastasis (PSM) and increased tumor spread. Port site metastasis was suggested to be caused by “the chimney effect” and the aerosolization of cancer cells through leaks around the trocars [4]. The retraction and bulging of mesothelial cells, exposing the underlying basal lamina, caused by CO2 PP raised concerns about the attachment of tumor cells [5]. Mathew et al. observed more dissemination of radiolabeled adenocarcinoma cells during laparoscopy with CO2 PP than with gasless laparoscopy [6]. Champault et al. [7] identified intact cells in the gas that escaped during laparoscopy in six of nine patients. However, current evidence on the role of aerosolization in the development of PSM is inconclusive since other authors have shown that the aerosolization of cancer cells is not responsible for PSM. Whelan et al. [8] were unable to demonstrate aerosol formation in any of the in vivo or in vitro models. In a rat model injected with CC531 cells, Wittich et al. [9] concluded that aerosolization is not a relevant factor in the pathogenesis of the PSM. Similarly, Iwanaka et al. [10] found that CO2 PP was not essential for the development of PSM.
The effects of PP on mesothelial cells and the role of the entire peritoneal cavity as a cofactor in adhesion formation have become well established over the last decade. Non-humidified CO2 PP causes desiccation at the peritoneal layer [11] producing mesothelial cell bulging up, intercellular clefts increase in size, and the underlying basal lamina becomes visible [12]. In a laparoscopic mouse model, we demonstrated that the driving mechanism was acute inflammation in the entire peritoneal cavity [13]. This acute inflammation is the net result of detrimental and beneficial factors. The duration of mesothelial hypoxia caused by CO2 PP [14], the duration of hyperoxia [15], the severity of desiccation [16], mechanical trauma [17], and bleeding [18] have all been identified as detrimental factors, whereas the addition of 4 % O2 [15] and 10 % nitrous oxide (N2O) to the CO2 PP [18], the use of humidified gas [16, 19], and a lower PP temperature [20, 21] have all been identified as beneficial factors. Although the relationship between acute inflammation and peritoneal cell retraction and bulging, which exposes the basal membrane, is demonstrated only for the duration of CO2 pneumoperitoneum, we assume that the same holds true for other factors investigated for adhesion formation and for CO2 resorption.
It seems logical that mesothelial cell retraction would facilitate tumor implantation. Thus, we conducted this study to confirm whether tumor cell implantation, like adhesion formation, increases with factors causing acute inflammation and mesothelial damage/retraction. We also evaluated whether the same factors that reduce acute inflammation inhibit tumor cell implantation.
Methods
Tumor cell line
CT-26 (colon adenocarcinoma), a syngeneic cell line from BALB/c mice, was used. Cells were cultured in complete medium (RPMI-1640 medium supplemented with 10 % FCS, 1 % penicillin/streptomycin and 1 % l-glutamine) at 37 °C in a humidified environment of 5 % CO2 until they reached 90–95 % confluence. On the day of tumor injection, cells were washed with PBS and detached with Versene buffer (0.48 mM EDTA in PBS). Subsequently, following dispersion in complete medium, cells were counted, and diluted to obtain the number of cells needed for injection in 1.0 ml of RPMI-1640 medium without any supplement. The viability of injected cells was evaluated by trypan blue exclusion (over 90 %) before i.p. injection, while the remaining cells were cultured with complete medium for 3 days to demonstrate attachment and growth.
The laparoscopic mouse model
The laparoscopic mouse model was validated for adhesion formation. BALB/c mice were used because of the important PP-enhanced adhesions, as inbred strains have less variability [22]. The experimental conditions were strictly controlled, as established previously. Mice were anesthetized with i.p. 0.08 mg/g pentobarbital (T0). Exactly 10 min later (T10), the preparation was started, with shaving, positioning on the operating table, and intubation with a 20-gauge catheter. Mice were ventilated with a Mouse Ventilator (MiniVent, Type 845, Hugo Sachs Elektronik-Harvard Apparatus GmbH, March-Hugstetten, Germany) using a tidal volume of 250 μl at 160 strokes/min (to prevent hypercarbia/acidosis especially during PP [23] with humidified room air to prevent cooling) [21].
Laparoscopic surgery was standardized. A midline incision was performed caudal to the xyphoides at T20 and a 2-mm endoscope with a 3.3-mm external sheath for insufflation (Karl Storz, Tuttlingen, Germany) was introduced into the abdominal cavity. The incision was closed gas-tight around the endoscope to prevent leakage.
PP was created at 15 mmHg insufflation pressure using the Thermoflator Plus (Karl Storz) and a water valve to damp pressure changes [14]. Humidification was achieved using the Storz Humidifier (204320 33, Karl Storz). The body temperature of the mice was strictly controlled by correct timing of the preparation and surgical procedures, keeping everything set up in a chamber at 37 °C. Desiccation was induced with a controlled flow of gas through the abdominal cavity, at 23 ml/min using a 26-gauge needle at a pressure of 15 mmHg.
Adhesion formation in the laparoscopic mouse model
These experiments on tumor implantation were conducted under established experimental conditions with known effects on adhesion formation. Adhesions at opposing surgical lesions are known to be enhanced by 60 min of CO2 PP, by desiccation (using a 23 ml/min flow of dry CO2, obtained by placing a 26-gauge needle in the abdomen under 15 mmHg pressure) and a higher body temperature (at 37 °C induced in a heated chamber on a homeothermic pillow) [16, 21]. Adhesions were reduced by adding 10 % N2O to the CO2 [18], the mechanism of which is unknown, although it is the single most effective treatment, and by adding 4 % of oxygen to prevent the mesothelial hypoxia of pure CO2 [15], at a lower body temperature of 32 °C [16, 21], and finally, by preventing desiccation using humidified gas [16, 19].
Animals
Sixty-six female BALB/cJRj mice, 9- to 10-week old, weighing 20 g were kept under standard laboratory conditions and fed a standard laboratory diet with free access to food and water. The study was approved by the Institutional Review Animal Care Committee.
Experimental design
In all experiments, cells were injected intraperitoneally exactly 80 min after the initiation of anesthesia (T80) and after desufflation of the PP if appropriated, in 1 ml of RPMI-1640 medium without any supplement. The first experiment was designed as a dose-finding curve to estimate the number of cells to be used in further experiments, and to confirm the increase in tumor implantation following 60 min of CO2 PP together with desiccation. The three control groups comprised mice with a BT of 37 °C, which were mechanically ventilated, not subjected to surgery, and were injected with 0.1, 0.3, and 1 × 106 CT-26 cells without PP (groups I, II, and III, respectively). The three experimental groups were subjected to the same conditions, but following 60 min of PP with dry CO2 and a flow of 23 ml/min (desiccation; groups IV, V, and VI, respectively). These six groups comprised five mice per group.
In the second experiment, 0.3 × 106 CT-26 cells were used. Groups I and II, like those in the first experiment, comprised mice with a BT of 37 °C in a control group without PP (group I) and following 60 min of CO2 PP with dry gas and desiccation (group II). The other groups were chosen to evaluate the effect of factors known to be beneficial for preventing adhesion formation. In group III, the CO2 was humidified; in group IV, besides humidification, 10 % of N2O was added; in group V, 4 % O2 was also added (humidified 86 % CO2 +10 % N2O +4 % O2); and in group VI, mice were cooled to 32 °C [21]. These six groups comprised six mice per group.
All experiments were performed using block randomization by days. Therefore, a block of animals comprised one animal from each group, which was always operated on, on the same day, avoiding day-to-day variability. Within a block, experiments were performed in random order, varying each day. After 1 week, the total tumors, being those in the abdominal cavity, the abdominal wall, and the bowel mesentery were quantified as explained below.
Tumor implantation quantification
After 1 week, mice were euthanized with an overdose of anesthesia and tumors were scored blindly and separately by two observers. Abdominal cavities were opened with a xyphopubic vertical midline incision and, without magnification, the number of visible tumors, larger than 1 mm in diameter, were counted. Most tumors were found growing in the abdominal wall and the bowel mesentery. Secondary places were the fat of the pancreas, and the fat tissue close to the bladder, kidney, liver, bowel, cecum, and diaphragm. Tumors were quantified everywhere but especially in the abdominal wall and the bowel mesentery. Figures. 1 and 3 show the tumors in the abdominal cavity, while Figs. 2 and 4 show the tumors in the abdominal wall.
Fig. 1.
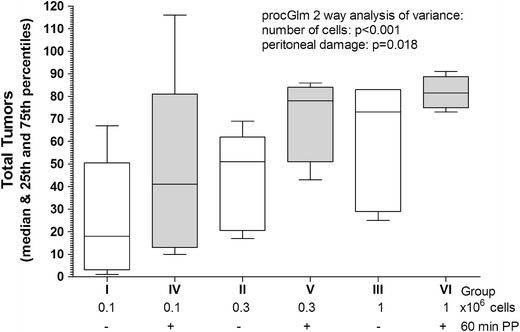
Tumor implantation in the abdominal cavity after laparoscopic surgery using the CT-26 cell line. Implantation in the abdominal cavity (box plots) of CT-26 tumor cells, injected i.p. as 0.1, 0.3, and 1 × 106 in control mice (no surgery and no pneumoperitoneum; groups: I, II, and III, respectively; open bars) or after 60 min of pneumoperitoneum with non-humidified CO2; groups IV, V, and VI, respectively; dark bars). Implantation increased with the number of cells (p < 0.001) and with peritoneal damage (p = 0.018)
Fig. 3.
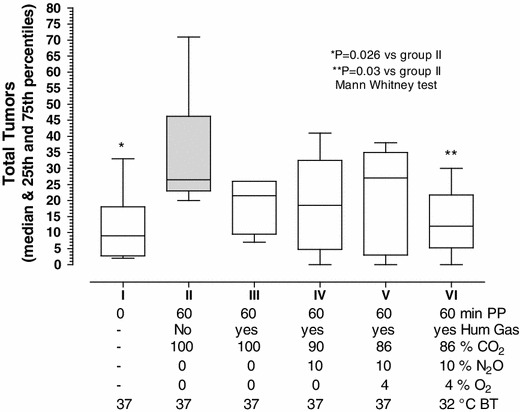
Effect of peritoneal cavity conditioning on tumor implantation in the abdominal cavity. Effect of peritoneal cavity conditioning on the implantation of CT-26 tumor cells (0.3 × 106) injected i.p. in mice not subjected to surgery or pneumoperitoneum (group I) or after 60 min of pneumoperitoneum with non-humidified CO2 (group II; dark bar). To the latter group, we sequentially added beneficial factors such as humidification (group III), 10 % N2O (group IV), 4 % O2 (group V), and cooling the body temperature to 32 °C (group VI). The body temperature was 37 °C in all other groups. Tumor implantation in the abdominal cavity increased with peritoneal damage (p = 0.026) and decreased with conditioning (p = 0.03)
Fig. 2.
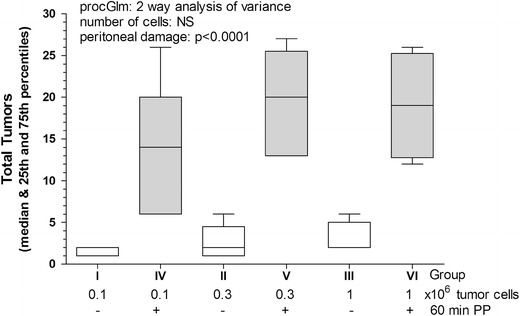
Tumor implantation in the abdominal wall after laparoscopic surgery using the CT-26 cell line. Implantation in the abdominal wall (box plots) of CT-26 tumor cells, injected i.p. as 0.1, 0.3 and 1 × 106, in control mice (no surgery and no pneumoperitoneum, groups I, II, and III, respectively; open bars) or after 60 min of pneumoperitoneum with non-humidified CO2 (groups IV, V, and VI, respectively; dark bars). Tumor implantation increased with the peritoneal damage (p < 0.0001)
Fig. 4.
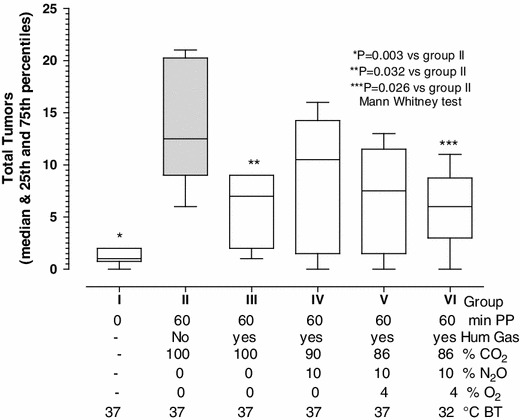
Effect of peritoneal cavity conditioning on tumor implantation in the abdominal wall. Effect of peritoneal cavity conditioning on the implantation of CT-26 tumor cells (0.3 × 106) injected in mice not subjected to surgery or pneumoperitoneum (group I) or after 60 min of pneumoperitoneum with non-humidified CO2 (group II; dark bar). To the latter group, we sequentially added beneficial factors such as humidification (group III), 10 % N2O (group IV), 4 % O2 (group V), and cooling to a body temperature of 32 °C (group VI). The body temperature was 37 °C in all other groups. Tumor implantation in the abdominal wall increased with the peritoneal damage (p = 0.003) and decreased using humidified CO2 (p = 0.032) and conditioning (p = 0.026)
Statistics
In Experiment 1, the effect of mesothelial trauma (dry CO2 PP and desiccation) was evaluated simultaneously for the three tumor concentrations by a two-way analysis of variance (proc GLM) using the SAS System (SAS Institute, Cary, NC, USA). In Experiment 2, statistical differences between groups were evaluated with the Mann–Whitney test using the GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA). All data are expressed as the median, with 25 and 75 % percentiles and minimum and maximum values.
Results
Experiment 1 was designed for dose finding in control mice and in mice with mesothelial damage induced by 60 min of dry CO2 PP and desiccation (23 ml/min). As expected, tumor implantation in the abdominal cavity increased with the number of cells injected (p < 0.001) and with peritoneal damage (p = 0.018; proc GLM two-way analysis of variance; Fig. 1). These effects of tumor implantation were observed equally in the abdominal wall (peritoneal damage p < 0.0001; number of cells, NS; Fig. 2), and in the bowel mesentery (peritoneal damage: NS; number of cells p = 0.007; data not shown), which were analyzed separately.
Experiment 2 confirmed that the mesothelial damage induced by 60 min of dry CO2 PP and desiccation (23 ml/min) increased tumor implantation in the abdominal cavity (I vs II: p = 0.0260; Mann–Whitney test; Fig. 3). This implantation was decreased to a level comparable to that of the control group when mesothelial damage was prevented by conditioning, through humidification plus the addition of 10 % N2O, 4 % of O2 and cooling to 32 °C (II vs VI: p = 0.03). All other intermediary steps failed to reach significance; that is, humidification (II vs III), humidification + 10 % N2O (II vs IV), and humidification + 10 % of N2O + 4 % of O2 (II vs V).
When the number of tumors implanted in the abdominal wall was analyzed separately, similar conclusions were made (Fig. 4). Peritoneal damage increased tumor implantation (I vs II, p = 0,0031, Mann–Whitney test), whereas conditioning (humidified CO2 plus 10 % N2O 4 % O2 and cooling), decreased tumor implantation (II vs VI: p = 0,026) to levels comparable to those of the control group (I vs VI: NS). When analyzing the intermediary steps, humidified CO2 reduced tumor implantation significantly (II vs III; 0.0318), whereas the effects of humidification + 10 % of N2O and humidification plus 10 % of N2O + 4 % of O2 failed to reach significance (II vs IV and II vs V). Surprisingly, the number of tumors in the mesentery was not affected by mesothelial damage in either experiment 1 or 2.
Discussion
These results contribute to our understanding of the role of the peritoneal cavity in adhesion formation. Adhesions are enhanced by factors causing acute inflammation, mesothelial damage, and cell retraction. The factors identified that prevent this acute inflammatory reaction and cell retraction are the addition of 10 % N2O and 4 % of O2 to the CO2 PP, together with cooling to 32 °C in the absence of desiccation. The combination of these factors is called peritoneal conditioning. We found that the same damaging factors increase CO2 resorption [24], whereas conditioning can prevent this increase. In this study, as expected, the same damaging factors increased tumor cell implantation but this increase can be prevented by conditioning.
The increase in tumor cell implantation by CO2 PP and desiccation, known to cause mesothelial cell retraction and bulging, confirms previous observations. In a study by Shen et al. [25], 106 of CT-26 were inoculated into the lower abdominal cavity of mice, which were then randomized for PP with CO2 or helium or to a control group. They demonstrated that PP enhances the implantation and growth of free intraperitoneal malignant cells, suggesting that insufflation during PP plays an important role in peritoneal dissemination when free tumor cells are in the abdominal cavity. Moreover, the effect achieved by CO2 was greater than that achieved by helium. In another experiment using a rat model, mammary adenocarcinoma cells were injected into the lower right quadrant of the peritoneal cavity [26]. In comparison with a control group, the insertion of trocars with CO2 PP resulted in greater tumor cell implantation than laparotomy incisions. In another rat model [27], following the i.p. injection of 105 colon cancer cells, tumor growth was significantly increased by both cold and warm CO2 PP in comparison with a control group.
These data demonstrate that the increase in tumor cell implantation by CO2 PP and desiccation can be prevented by a combination of adding 10 % N2O, 4 % O2, cooling, and absence of desiccation, known as peritoneal conditioning. In fact, following conditioning, tumor implantation is comparable to that of control groups, both in the abdominal cavity and the wall. This observation is strikingly similar to that of adhesion formation, which is not surprising considering the underlying mechanism of a dose/trauma-dependent acute inflammation and mesothelial cell retraction, exposing the basal membrane. For adhesion formation, we demonstrated that the single most effective way of preventing mesothelial damage was to add N2O and that O2 had little if any effect, although it was slightly effective when used alone. The second most important factor is cooling and avoiding desiccation; however, the effectiveness of cooling and desiccation on adhesion formation is difficult to demonstrate in our mouse model after the addition of N2O, since so few adhesions remain. These tumor experiments were therefore designed to demonstrate the eventual effect of adding N2O after humidification. We also demonstrated that humidifying the insufflation gas decreased tumor implantation when compared with the control group subjected to dry CO2 PP, although the effect of humidification was not as great as that of full conditioning since it reduced tumor implantation in the wall but not in the whole abdominal cavity. These results underline the importance of preventing desiccation in the context of preventing damage to the mesothelial cells. It is not surprising that the effects are more pronounced in the abdominal wall, which is directly exposed to the PP, than in the mesentery, which is only partially exposed.
The experiment was not powered to detect subtle differences, especially since variability in tumor implantation was much higher than the variability previously observed for adhesion formation. Searching the literature for each individual factor did not reveal data concerning tumor implantation following the addition of oxygen or humidification. Most experiments investigate port site metastasis and have observed that helium is associated with less port site metastasis than pure CO2, N2O or air [25, 26, 28]. However, Hopkins et al. found no difference in tumor spread when using CO2, N2O, or helium [29]. When studying the effect of warm vs. cold CO2 gas on tumor spread, it was found to be less when warm gas was used [27]. This was in contrast to our findings, but we can only speculate about this discrepancy. As described in our “Methods”, we controlled the temperature of the mice, the time between the induction of anesthesia and manipulations, and the ventilation, strictly. We have data only on cooling mice to 26 °C without desiccation, but not on what happens when mice are cooled further. Thus, the apparent conflicting data could be explained by differences between mice and rats, or by the fact that when cold gas was used, the rats were cooled much more, as a result of desiccation if trocar insertion was not leak free. The role of immunology in this will also need investigation. Moreover, our search of the literature revealed no evidence of the use of humidified gas, which could explain the discrepancy.
The findings of this study obviously raise questions about such surgery in humans. Translation to human surgery was started by investigating cooling and desiccation in detail and the results can be summarized as follows [30]: first, to avoid desiccation, cooling has to be done with a third means. If cold humidified gas is used for insufflation, this gas will warm up in the abdomen at 37 °C, making desiccation unavoidable. Second, cooling of the abdominal cavity to 28 °C will not affect core body temperature unless desiccation occurs. We do not know the consequences of cooling to lower temperatures, although data from the mouse model show that the relationship between temperature and adhesions is exponential so that cooling to less than 26 °C would be unlikely to affect the results further. The choice to use 10 % N2O and 4 % O2 was based on mouse experiments [18]. We also recently finalized a randomized control trial in which it was demonstrated in humans that conditioning decreases CO2 resorption and decreases postoperative pain, while being the single most effective means of preventing postoperative adhesions [24].
In conclusion, as described for adhesion formation in mice, laparoscopic surgery with dry CO2 PP increases tumor implantation in the abdominal cavity and wall. Moreover, full conditioning of the abdominal cavity, achieved by humidified CO2 with 10 % N2O and 4 % O2 together with a lower local temperature, reduces tumor implantation in the abdomen and its wall. The clinical implication that full conditioning might inhibit tumor spread in oncologic surgery is suggested by the recent discovery that full conditioning decreases adhesion formation in humans [24].
Acknowledgments
We thank Catherina Luyten, Rieta Van Bree, Lieve Coenegrachts, Petra Stevens (Laboratory of Experimental Gynecology, KUL) and Marleen Craessaerts (University Hospital Leuven). We also thank Eugeen Steurs (Karl Storz) and Nicolò Giacomuzzi-Moore for their continued support. This study was supported by the “Leuven Quality Surgery Fund” (Fisher & Paykel Health Care, Nordic Pharma and eSaturnus NV) and the “Verelst Endometrial Cancer Fund”. We thank Karl Storz Endoscopy for kindly supplying the laparoscopic equipment.
Conflict of interest
Maria Mercedes Binda, Roberta Corona, and Frederic Amant have no conflicts of interest. Philippe Robert Koninckx has a leadership position at EndoSAT.
References
- 1.Angst E, Hiatt JR, Gloor B, Reber HA, Hines OJ. Laparoscopic surgery for cancer: systematic review and a way forward. J Am Coll Surg. 2010;211:412–423. doi: 10.1016/j.jamcollsurg.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujii K, Izumi K, Sonoda K, Shiraishi N, Adachi Y, Kitano S. Less impaired cell-mediated immune response in the murine peritoneal cavity after CO(2) pneumoperitoneum. Surg Today. 2003;33:833–838. doi: 10.1007/s00595-003-2615-2. [DOI] [PubMed] [Google Scholar]
- 3.Cho JE, Liu C, Gossner G, Nezhat FR. Laparoscopy and gynecologic oncology. Clin Obstet Gynecol. 2009;52:313–326. doi: 10.1097/GRF.0b013e3181b088d2. [DOI] [PubMed] [Google Scholar]
- 4.Are C, Talamini MA. Laparoscopy and malignancy. Laparoendosc Adv Surg Tech A. 2005;15:38–47. doi: 10.1089/lap.2005.15.38. [DOI] [PubMed] [Google Scholar]
- 5.Volz J, Koster S, Spacek Z, Paweletz N. The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumour growth. Cancer. 1999;86:770–774. doi: 10.1002/(SICI)1097-0142(19990901)86:5<770::AID-CNCR11>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Mathew G, Watson DI, Ellis T, De Young N, Rofe AM, Jamieson GG. The effect of laparoscopy on the movement of tumour cells and metastasis to surgical wounds. Surg Endosc. 1997;11:1163–1166. doi: 10.1007/s004649900561. [DOI] [PubMed] [Google Scholar]
- 7.Champault G, Taffinder N, Ziol M, Riskalla H, Catheline JM. Cells are present in the smoke created during laparoscopic surgery. Br J Surg. 1997;84:993–995. doi: 10.1002/bjs.1800840724. [DOI] [PubMed] [Google Scholar]
- 8.Whelan RL, Sellers GJ, Allendorf JD, Laird D, Bessler MD, Nowygrod R, et al. Trocar site recurrence is unlikely to result from aerosolization of tumour cells. Dis Colon Rectum. 1996;39:S7–S13. doi: 10.1007/BF02053799. [DOI] [PubMed] [Google Scholar]
- 9.Wittich P, Marquet RL, Kazemier G, Bonjer HJ. Port-site metastases after CO(2) laparoscopy. Is aerosolization of tumour cells a pivotal factor? Surg Endosc. 2000;14:189–192. doi: 10.1007/s004649900098. [DOI] [PubMed] [Google Scholar]
- 10.Iwanaka T, Arya G, Ziegler MM. Mechanism and prevention of port-site tumour recurrence after laparoscopy in a murine model. J Pediatr Surg. 1998;33:457–461. doi: 10.1016/S0022-3468(98)90088-4. [DOI] [PubMed] [Google Scholar]
- 11.Gray RI, Ott DE, Henderson AC, Cochran SA, Roth EA. Severe local hypothermia from laparoscopic gas evaporative jet cooling: a mechanism to explain clinical observations. JSLS. 1999;3:171–177. [PMC free article] [PubMed] [Google Scholar]
- 12.Volz J, Koster S, Spacek Z, Paweletz N. Characteristic alterations of the peritoneum after carbon dioxide pneumoperitoneum. Surg Endosc. 1999;13:611–614. doi: 10.1007/s004649901052. [DOI] [PubMed] [Google Scholar]
- 13.Corona R, Verguts J, Schonman R, Binda MM, Mailova K, Koninckx PR. Postoperative inflammation in the abdominal cavity increases adhesion formation in a laparoscopic mouse model. Fertil Steril. 2011;15(95):1224–1228. doi: 10.1016/j.fertnstert.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Molinas CR, Mynbaev O, Pauwels A, Novak P, Koninckx PR. Peritoneal mesothelial hypoxia during pneumoperitoneum is a cofactor in adhesion formation in a laparoscopic mouse model. Fertil Steril. 2001;76:560–567. doi: 10.1016/S0015-0282(01)01964-1. [DOI] [PubMed] [Google Scholar]
- 15.Elkelani OA, Binda MM, Molinas CR, Koninckx PR. Effect of adding more than 3% oxygen to carbon dioxide pneumoperitoneum on adhesion formation in a laparoscopic mouse model. Fertil Steril. 2004;82:1616–1622. doi: 10.1016/j.fertnstert.2004.07.933. [DOI] [PubMed] [Google Scholar]
- 16.Binda MM, Molinas CR, Hansen P, Koninckx PR. Effect of desiccation and temperature during laparoscopy on adhesion formation in mice. Fertil Steril. 2006;86:166–175. doi: 10.1016/j.fertnstert.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 17.Schonman R, Corona R, Bastidas A, De Cicco C, Koninckx PR. Effect of upper abdomen tissue manipulation on adhesion formation between injured areas in a laparoscopic mouse model. J Minim Invasive Gynecol. 2009;16:307–312. doi: 10.1016/j.jmig.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Corona R, Binda MM, Mailova K, Verguts J, Koninckx PR. Addition of nitrous oxide to the carbon dioxide pneumoperitoneum strongly decreases adhesion formation and the dose-dependent adhesiogenic effect of blood in a laparoscopic mouse model. Fertil Steril. 2013;100:1777–1783. doi: 10.1016/j.fertnstert.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 19.Peng Y, Zheng M, Ye Q, Chen X, Yu B, Liu B. Heated and humidified CO2 prevents hypothermia, peritoneal injury, and intra-abdominal adhesions during prolonged laparoscopic insufflations. J Surg Res. 2009;151:40–47. doi: 10.1016/j.jss.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 20.Binda MM, Koninckx PR. Prevention of adhesion formation in a laparoscopic mouse model should combine local treatment with peritoneal cavity conditioning. Hum Reprod. 2009;24:1473–1479. doi: 10.1093/humrep/dep053. [DOI] [PubMed] [Google Scholar]
- 21.Binda MM, Molinas CR, Mailova K, Koninckx PR. Effect of temperature upon adhesion formation in a laparoscopic mouse model. Hum Reprod. 2004;19:2626–2632. doi: 10.1093/humrep/deh495. [DOI] [PubMed] [Google Scholar]
- 22.Molinas CR, Binda MM, Campo R, Koninckx PR. Adhesion formation and interanimal variability in a laparoscopic mouse model varies with strains. Fertil Steril. 2005;83:1871–1874. doi: 10.1016/j.fertnstert.2004.11.084. [DOI] [PubMed] [Google Scholar]
- 23.Molinas CR, Tjwa M, Vanacker B, Binda MM, Elkelani O, Koninckx PR. Role of CO(2) pneumoperitoneum-induced acidosis in CO(2) pneumoperitoneum-enhanced adhesion formation in mice. Fertil Steril. 2004;81:708–711. doi: 10.1016/j.fertnstert.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Koninckx PR, Corona R, Timmerman D, Verguts J, Adamyan L. Peritoneal full-conditioning reduces postoperative adhesions and pain: a randomised controlled trial in deep endometriosis surgery. J Ovarian Res. 2013;6:90. doi: 10.1186/1757-2215-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen MY, Huang IP, Chen WS, Chang JT, Lin JK. Influence of pneumoperitoneum on tumour growth and pattern of intra-abdominal tumour spreading: in vivo study of a murine model. Hepatogastroenterology. 2008;55:947–951. [PubMed] [Google Scholar]
- 26.Hopkins MP, Dulai RM, Occhino A, Holda S. The effects of carbon dioxide pneumoperitoneum on seeding of tumour in port sites in a rat model. Am J Obstet Gynecol. 1999;181:1329–1333. doi: 10.1016/S0002-9378(99)70372-8. [DOI] [PubMed] [Google Scholar]
- 27.Nduka CC, Puttick M, Coates P, Yong L, Peck D, Darzi A. Intraperitoneal hypothermia during surgery enhances postoperative tumour growth. Surg Endosc. 2002;16:611–615. doi: 10.1007/s00464-001-9055-0. [DOI] [PubMed] [Google Scholar]
- 28.Neuhaus SJ, Ellis T, Rofe AM, Pike GK, Jamieson GG, Watson DI. Tumour implantation following laparoscopy using different insufflation gases. Surg Endosc. 1998;12:1300–1302. doi: 10.1007/s004649900845. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins MP, von Gruenigen V, Haller NA, Holda S. The effect of various insufflation gases on tumour implantation in an animal model. Am J Obstet Gynecol. 2002;187:994–996. doi: 10.1067/mob.2002.126644. [DOI] [PubMed] [Google Scholar]
- 30.Corona R, Verguts J, Koninckx R, Mailova K, Binda MM, Koninckx PR. Intraperitoneal temperature and desiccation during endoscopic surgery. Intraoperative humidification and cooling of the peritoneal cavity can reduce adhesions. Am J Obstet Gynecol. 2011;205:392–397. doi: 10.1016/j.ajog.2011.06.091. [DOI] [PubMed] [Google Scholar]


