Abstract
OBJECTIVE
Complications from transvaginal mesh placed for prolapse often require operative management. The aim of this study is to describe the outcomes of vaginal mesh removal.
METHODS
A retrospective review of all patients having surgery by the Urogynecology group in the Department of Obstetrics & Gynecology at our institution for a complication of transvaginal mesh placed for prolapse was performed. Demographics, presenting symptoms, surgical procedures, and postoperative symptoms were abstracted. Comparative statistics were performed using the chi-squared or Fisher’s exact test with significance at p<0.05.
RESULTS
Between January 2008 and April 2012, 90 patients had surgery for complications related to vaginal mesh and 84 had follow-up data. The most common presenting signs and symptoms were: mesh exposure 62% (n=56), pain 64% (n=58), and dyspareunia 48% (n=43). During operative management, mesh erosion was encountered unexpectedly in a second area of the vagina in 5% (n=4), in the bladder in 1% (n=1), and in the bowel in 2% (n=2). After vaginal mesh removal, 51% (n=43) had resolution of all presenting symptoms. Mesh exposure was treated successfully in 95% of cases, while pain was only successfully treated in 51% of women.
CONCLUSION
Removal of vaginal mesh is helpful in relieving symptoms of presentation. Patients can be reassured that exposed mesh can almost always be successfully managed surgically, but pain and dyspareunia are only resolved completely in half of cases.
Introduction
Complications from permanent synthetic mesh used in vaginal prolapse repair have been well-documented1–4. A mesh exposure or infection can sometimes be treated with conservative measures such as topical estrogen cream or antibiotics alone. Pain or dyspareunia after a vaginal mesh surgery can sometimes resolve on its own or be successfully treated with physical therapy or other treatments. However, surgical removal of transvaginal mesh may be recommended due to these and other symptoms. With the recent FDA warning regarding transvaginal mesh and the increase in litigation, more patients may be interested in removal of mesh than other more conservative treatments5.
There have been many studies published reporting on the rate of complications after placement of vaginal mesh. However, there have been fewer studies published on outcomes after surgical removal of transvaginal mesh. Therefore, there has been little data to guide the physician when counseling patients about expected outcomes after removal of the vaginal mesh. The aim of this study is to present a description of operative findings, an analysis of our patients’ experience, and the outcomes of vaginal mesh removal.
Materials and Methods
This is a retrospective chart review of all patients undergoing removal of vaginal mesh by the urogynecologists at our institution between January 2008 and April 2012. Approval was obtained by the University of Michigan Institutional Review Board (HUM00038668). Patients that had mesh removal were identified by CPT codes. Those patients that had removal of a midurethral sling only or sacrocolpopexy grafts were excluded, leaving only those patients undergoing removal of vaginally-placed mesh used to treat prolapse. The operative report from the original mesh-placement surgery was obtained from the outside institutions and reviewed as well as reports from any previous mesh removal procedures. A chart review was performed and demographics, medical and surgical histories and presenting signs and symptoms were recorded as well as postoperative symptoms.
Patients were classified as having a chronic pain condition if they had a history of chronic pelvic pain, endometriosis, interstitial cystitis, irritable bowel syndrome, fibromyalgia or vulvodynia that predated mesh placement. If a patient reported pain as a presenting symptom before mesh removal, the improvement after mesh removal was categorized as little to no improvement, moderate improvement or significant improvement/resolution using the patient’s description of improvement. The improvement after mesh removal was categorized as little to none if the phrase used was “no better”, “pain is not improved”, “slightly better” et cetera. Examples of phrases for which a patient was categorized as moderate improvement include “50% better”, “somewhat improved”, and “still with some pain”. Examples of phrases for which a patient was categorized as significant improvement or pain resolution include “much better”, “the patient is almost gone” and “80% improvement”. In instances in which phrases used fell into more than one category, the chart abstractors judged which predominated.
The operative report from the mesh removal procedure at our institution was reviewed. Details of the procedure were recorded including the vaginal compartment from which mesh was removed and whether the mesh removal was “partial” or “all vaginally-accessible”. A mesh removal was categorized as “all vaginally-accessible” if all mesh was removed to the level of the pelvic sidewall, with or without removal of the mesh arms, and “partial” if less mesh was removed.
Mesh excision was performed by making an incision in the vaginal epithelium and sharply dissecting the mesh from the overlying epithelium and underlying connective tissue. The mesh was divided in the midline, the dissection carried as far from the midline as desired, and the mesh removed. Typically, if the procedure was performed for mesh exposure and no other bothersome symptoms, only the mesh involved in the exposure was removed. If the presenting symptom was pain or dyspareunia, or if the patient desired complete mesh removal, as much mesh was removed as possible. Once the mesh was removed, a concomitant prolapse repair or anti-incontinence procedure was performed if needed. The vaginal epithelium was closed in a tension-free manner.
Descriptive statistics were performed. Comparative analyses were done using the chi-squared test or Fisher’s exact test with p<0.05 as significant.
Results
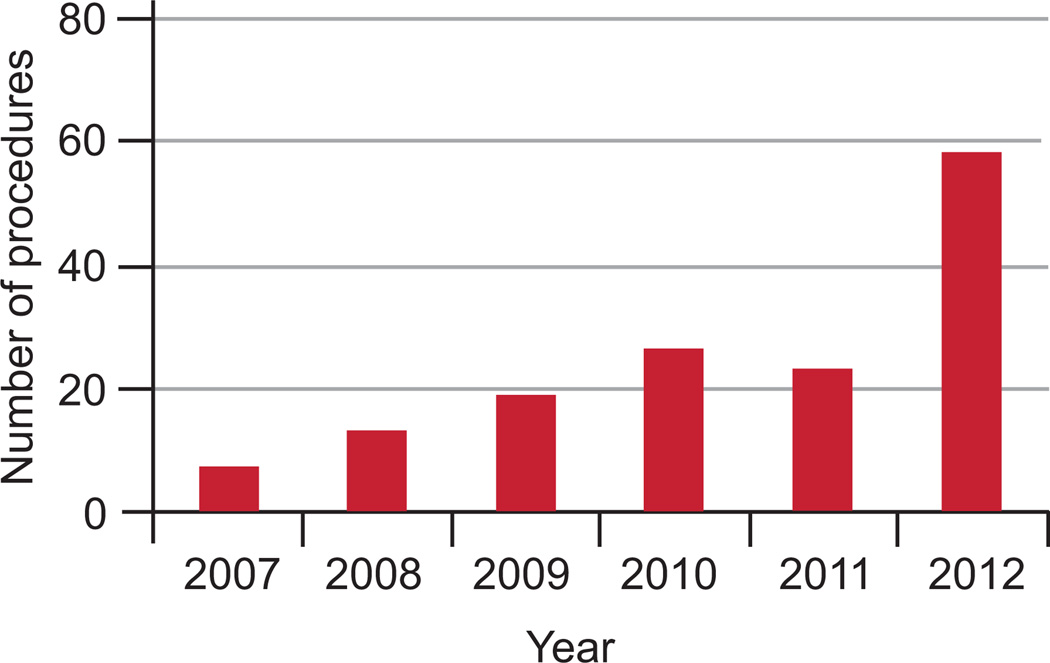
The number of mesh removal procedures performed at our institution over the 5-year period was reviewed, and the number of procedures performed per year continues to increase (Figure 1). During the study interval, 108 patients were identified that had a mesh removal procedure performed by the urogynecologists at our institution. All of the records were reviewed, and 18 patients were excluded due to excision of a midurethral sling only (n=10) or sacrocolpopexy mesh (n=8). Demographics of the remaining 90 patients are shown in Table 1, as well as surgical procedures performed before presentation to our office. Of the 90 patients, 39 (43%) had prior mesh removal procedures: most had one or two prior mesh removal procedures, but 3 patients had 3 or more removal procedures including one patient that had 6 prior mesh removal procedures.
Figure 1.
Mesh removal procedures performed in the urogynecology division at a tertiary referral center.
Table 1.
Demographic Characteristics and Previous Procedures
| Characteristic | N=90 |
|---|---|
| Age | 58±11 |
| Parity | 3 (0–10) |
| Body mass index | 29.5±11 kg/m2 |
| Current smokers | 19 (21) |
| Postmenopausal without daily hormone therapy | 58 (64) |
| Sexually active | 55 (61) |
| Surgeries before initial mesh placement | |
| Hysterectomy | 52 (58) |
| Anterior colporrhaphy/bladder suspension | 15 (17) |
| Posterior colporrhaphy | 9 (10) |
| Apical suspension/paravaginal defect repair | 3 (3) |
| Anti-incontinence procedure | 11 (12) |
| Prior mesh revision procedures | |
| One prior procedure | 28 (31) |
| Two prior procedures | 8 (9) |
| Three or more prior procedures | 3 (3) |
Data are mean ± standard deviation (age and body mass index), median with range (parity) or n (%).
Table 2 shows the vaginal compartment from which mesh was removed, the specific brand of mesh removed, and the concomitant procedures performed at the time of mesh removal. In addition to those listed, 3 patients had rectovaginal fistula repairs and 1 patient had a vaginoplasty with a full-thickness skin graft for vaginal stenosis. The median length of time between mesh placement and mesh removal was 24 months (range 5–96 months).
Table 2.
Procedures Performed at Time of Mesh Removal
| n (%) | |
|---|---|
| Compartment of mesh removal | |
| Removed anterior mesh only | 50 (56) |
| Removed posterior mesh only | 19 (21) |
| Removed anterior and posterior mesh | 21 (23) |
| Specific mesh removed* | |
| Perigee | 22 (24) |
| Apogee | 18 (20) |
| Anterior Prolift | 17 (19) |
| Anterior Avaulta | 15 (17) |
| Posterior Prolift | 11 (12) |
| Posterior Avaulta | 10 (11) |
| Elevate | 6 (7) |
| Gynemesh | 6 (7) |
| Anterior Pinnacle | 5 (6) |
| Uphold | 4 (4) |
| Avaulta | 3 (3) |
| Anterior unspecified biologic graft | 3 (3) |
| Posterior unspecified biologic graft | 3 (3) |
| Posterior Pinnacle | 2 (2) |
| Proxima | 1 (1) |
| Pelvisoft | 1 (1) |
| Unknown | 6 (7) |
| Concomitant procedures | |
| Concomitant hysterectomy | 5 (6) |
| Concomitant prolapse repair | 50 (56) |
| Concomitant anti-incontinence procedure | 9 (10) |
The risk of complication from specific mesh kits cannot be extrapolated from this data, as this is a case series.
Findings at the time of surgery revealed that in 63 of the 90 patients (70%), the mesh was not found to be lying flat or tension-free at the time of mesh removal. Descriptions of the mesh in the operative report from our mesh removal procedure include: bunched, rolled, tight-band, wadded, gathered, or taut. Many different meshes were removed as seen in Table 2.
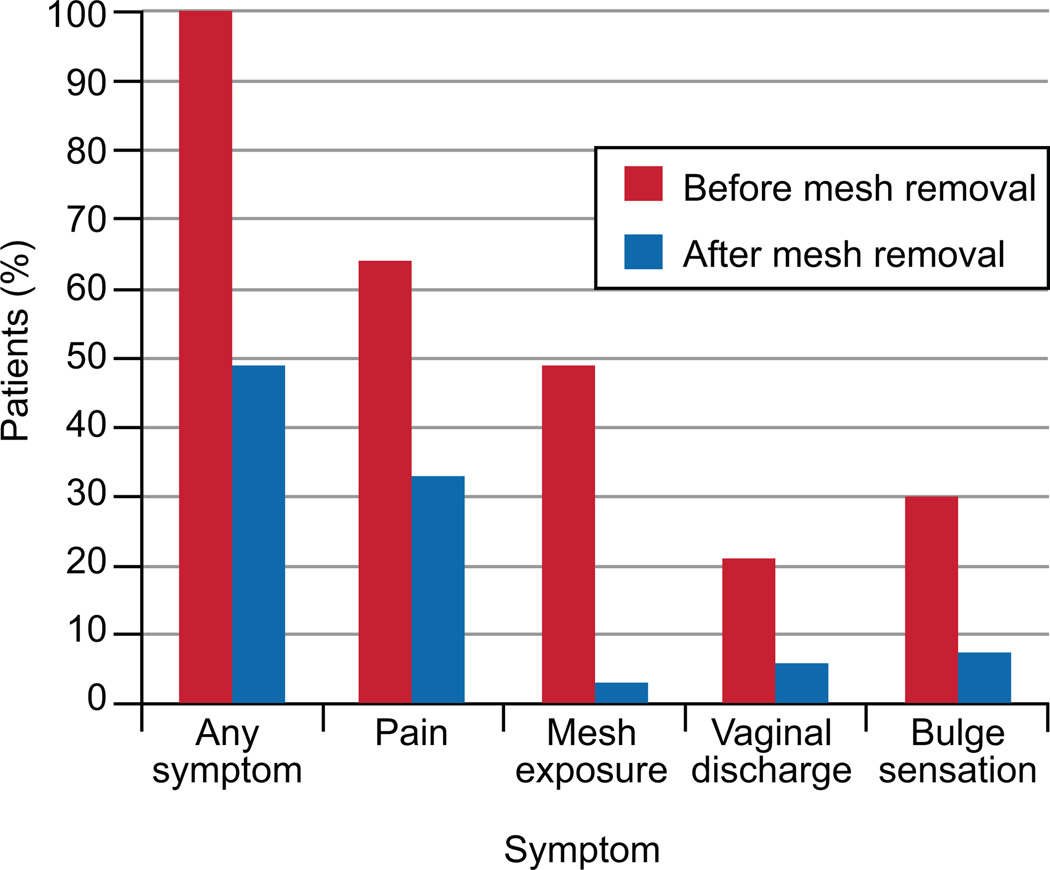
The most common presenting signs and symptoms were: pelvic or vaginal pain 64% (n=58), mesh exposure 62% (n=56), and a bulge sensation 30% (n=27) with most patients reporting more than one symptom (see Figure 2). Another common presenting symptom was dyspareunia, reported by 48% (n=43). Other presenting symptoms include recurrent infection 9% (n=8), stress urinary incontinence 28% (n=25), rectovaginal fistula 3% (n=3) and defecatory dysfunction 35% (n=32). Of the 56 patients that presented with a mesh exposure, 26 had vaginal bleeding or bothersome vaginal discharge, 20 had pain or dyspareunia, 4 were bothered by the exposure with no specific symptom mentioned, 1 had recurrent vaginal infections, 4 had recurrent prolapse with an asymptomatic exposure and 1 had stress urinary incontinence with an asymptomatic exposure.
Figure 2.
Symptoms before and after mesh removal.
Operative reports for the initial mesh placement surgery were reviewed before mesh removal and the expected location of the mesh noted. During the mesh removal procedure, mesh was encountered unexpectedly in a second area of the vagina in 5% (n=4); for example an operative report only described mesh in the anterior vaginal wall and examination under anesthesia revealed mesh in the posterior vaginal wall as well. Mesh was encountered in the bladder in 1% (n=1), and in the bowel in 2% (n=2). The presenting symptoms most bothersome to the patient with mesh in the bladder were pain, urinary incontinence and recurrent urinary tract infection. One patient found to have mesh involving the bowel complained of recurrent prolapse and pain and the other patient complained of pain and dyspareunia. Seven patients required reoperation after mesh removal at our institution. One patient was found to have mesh in the bladder that required subsequent cystoscopic laser removal. Two patients had an autologous fascia pubovaginal sling placed after the mesh removal procedure: one had recurrent urinary incontinence after a portion of a synthetic midurethral sling was removed and the other had a planned staged procedure for removal of her mesh and then treatment of her incontinence. Four patients (5%) required an additional vaginal mesh removal procedure.
Follow up data was available for 84 of the 90 patients. The median follow up length with interquartile range was 4 (2, 11.5) months. However, 29 of the 84 subjects had 2 month follow up, 29 had up to 6 months follow up, 8 had up to 1 year follow up and 18 had follow up beyond 1 year. After vaginal mesh removal, 51% (n=43) had resolution of all presenting symptoms. Of the patients that presented with mesh exposure, 95% were treated successfully and did not require any further treatment. Persistent symptoms were reported by 51% of those that presented with pain (Figure 2). The proportion of patients with each symptom before and after mesh removal were compared and there was a significant decrease in the proportion with each symptom after mesh removal, p<0.01. Of the 43 patients that reported dyspareunia, 30% reported persistent dyspareunia at most recent follow up.
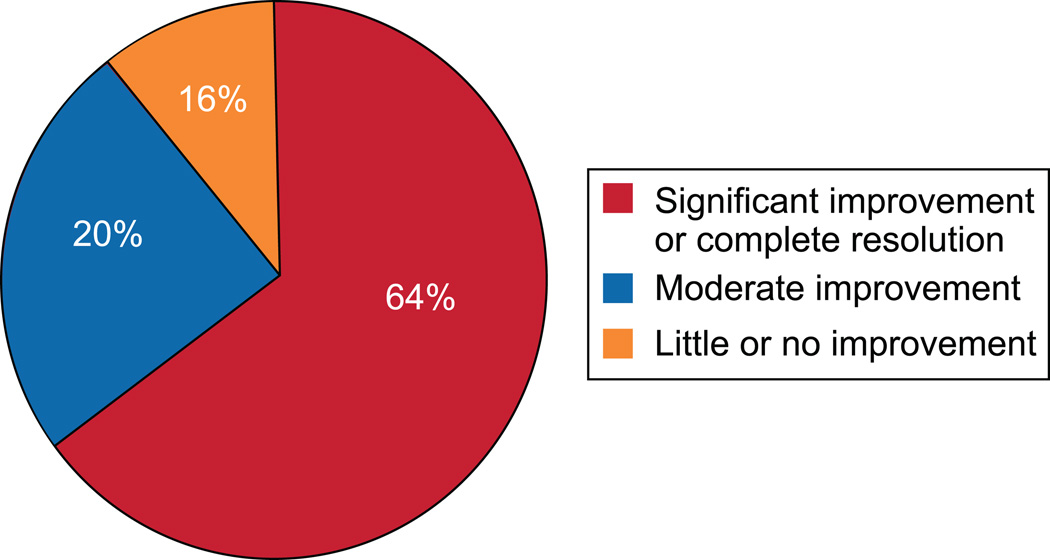
Improvement in pain symptoms was analyzed due to the relatively high persistence of symptoms after surgery (see Figure 3). There were 16 women (25%) who carried a preoperative diagnosis of a chronic pain condition and all presented with pain. Of these 16 women, 6 had little or no improvement in pain compared to 5 of the 39 women who presented with pain and did not have a chronic pain condition (37% vs. 13%, p=0.06). Subjects that had a removal of all vaginally-accessible mesh were not more likely to have a significant improvement in pain compared with those that had a partial mesh removal (58.1% vs. 70.1%, p=0.4). The number of prior mesh removal procedures was also not associated with pain resolution. There was one patient that had de novo pain after mesh removal. She had an autologous fascia pubovaginal sling placed at the time of mesh removal and had persistent pain at the Pfannensteil incision site at last follow up.
Figure 3.
Change in pain after mesh removal.
Discussion
In this study, complete resolution of all symptoms including vaginal discharge, bleeding, mesh exposure, and pain was achieved for about 50% of patients on whom follow-up was available. The symptom most difficult to relieve with surgical management was pain. Significant improvement or complete resolution of pain was achieved in 64% of patients while 36% of patients had moderate or no improvement in pain after mesh removal.
The persistence of pain after mesh removal is consistent with several other series in the literature2,6–10. The reported rate at which pain is relieved with surgical management in these prior reports ranges from 50–78% but little is known about risk factors for persistent pain. In our series, we found that patients with a chronic pain disorder were almost three times more likely to have continued pain after mesh removal than those without a chronic pain disorder (37% versus 13%, p=0.06). We hypothesize that patients with preexisting pain syndromes have underlying pathophysiology predisposing them to persistent pain, even after mesh has been removed. In general, our approach is to manage expectations by explaining that mesh removal may be one component of a broader treatment plan, which may also include other modalities such as neuropathic pain medications, trigger point injections and physical therapy.
Some patients that present with pain have a finding on exam that may explain their symptoms, such as mesh that is bunched or folded, or have an obvious mesh contracture. These women often have a single point or focal area that is painful on examination. Pain relief after mesh removal has been reported in up to 100% of women with obvious mesh contracture9. However in other patients, we found no anatomic abnormality on exam; the vagina was supple and the mesh seemed to be lying flat and tension-free, and yet they have had considerable pain since the mesh was placed. It is now our practice to perform removal of all vaginally-accessible mesh on those without focal tenderness or clear bunching or folding of mesh, and to perform a partial mesh removal procedure on those with focal tenderness or other finding, such as one tender arm of mesh, etc. However, there are instances where removal of all vaginally-accessible mesh was the goal, but a partial removal was done due to the risk of increased bleeding or damage to the bladder or bowel during the dissection. At times, one must consider the risk of an intraoperative complication during aggressive removal of all vaginally-accessible mesh, as it is not known if this yields better outcomes than partial removal.
Firoozi, et al., reported on the operative management of complications from vaginal mesh placed for prolapse in a series of 23 patients11. In that series, 11 (48%) of patients presented with pain or dyspareunia and all but one patient had resolution of the pain or dyspareunia at last follow up. The mesh removal technique for patients with pain as described in that study is similar to our practice. The median length of time between mesh placement and mesh removal in our study was 24 months. It was not reported in the Firoozi study, so it is possible that those patients had a shorter latency to mesh removal which may impact the risk of persistent pain. It may also be related to the smaller size of that study, or the specific mesh kits that were removed. The mesh removed in that study was from one of four types of mesh kits (Prolift, Apogee, Perigee or Avaulta). In contrast, there were 10 different mesh kits in our series. However, it is important to note that any statement about the risk of persistent pain amongst different mesh kits should not be extrapolated from a case series such as this, as it is impossible to know the number of patients who received each kit and did not have problems. The role of our study is to provide information on symptom resolution in those women who require surgery for these problems.
Though 30% of our patients had persistent dyspareunia after mesh removal, many patients had not yet had intercourse at the time of last follow up. Furthermore, it is not known if this and other symptoms are likely to resolve with time.
This study describes the degree to which symptoms resolve in the short term in one of the largest series of prolapse mesh removal cases reported. There are, however, several limitations to consider in interpreting the results of our study. Due to its retrospective design, intraoperative data and outcomes were not collected in a standardized fashion and a validated pain assessment was not performed. It is a custom, however, at our institution to have a very detailed operative findings section of the operative note including, for example, details of the location of mesh exposure, presence or absence of banding and its location and a detailed description of any prolapse present. In addition, a focus of our postoperative visits is to assess success or failure of symptom resolution. The IUGA/ICS graft complication classification system was published during the time when this cohort of patients had surgery and collecting information required by this classification system was not a part of our practice12. Furthermore, as a tertiary referral center, it is difficult to obtain some components of the IUGA/ICS classification system, for example T (time from mesh placement to initial clinical diagnosis of a complication). Many patients had a concomitant prolapse repair at the time of mesh removal and it is unclear how much this contributes to their symptom burden postoperatively. A quality of life assessment was not performed and the metric used for pain improvement was based at times on subjective phrases in the medical record. Because it is a case series, we cannot comment on the frequency or risk factors of complications of individual vaginal mesh kits. Similarly, because we do not place transvaginal mesh for prolapse, we cannot make any comparative statements about our cohort of patients and those that have transvaginal mesh placed and do not require additional treatment.
There are myriad clinical problems due to transvaginal mesh that clinicians must manage with little data or experience to guide them. The persistence of pain in 30% of patients suggests that treatment of persistent pain may be among the most difficult. In our experience, issues that may have an impact on outcomes of mesh removal include partial versus complete removal and patient willingness and ability (financial, logistical) to pursue physical therapy, trigger point medications and to tolerate neuropathic pain medications postoperatively. However, we were unable to analyze these variables due to the size of the study. This analysis suggests that patients with a history of chronic pain diagnoses may not be ideal candidates for the use of synthetic materials/implants and are at a higher risk for persistent pain after mesh removal.
In summary, there are many symptoms well-treated with mesh excision. Vaginal bleeding and discharge, erosion and urinary symptoms are relieved in the overwhelming majority. This analysis has demonstrated to us that we need to be careful to establish realistic patient expectations with respect to immediate and longer term pain relief. It is our hope that with additional care, including the aforementioned treatments, our patients will experience at least adequate or complete relief of pain.
Footnotes
Financial Disclosure: Dr. Delancey is a consultant for American Medical Systems. The other authors did not report any potential conflicts of interest.
References
- 1.Rogowski A, Bienkowski P, Tosiak A, Jerzak M, Mierzejewski P, Baranowski W. Mesh retraction correlates with vaginal pain and overactive bladder symptoms after anterior vaginal mesh repair. Int Urogynecol J. 2013 doi: 10.1007/s00192-013-2131-x. [DOI] [PubMed] [Google Scholar]
- 2.Skala CE, Renezeder K, Albrich S, et al. Mesh complications following prolapse surgery: management and outcome. Eur J Obstet Gynecol Reprod Biol. 2011;159:453–456. doi: 10.1016/j.ejogrb.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Baessler K, Hewson AD, Tunn R, Schuessler B, Maher CF. Severe mesh complications following intravaginal slingplasty. Obstet Gynecol. 2005;106:713–716. doi: 10.1097/01.AOG.0000177970.52037.0a. [DOI] [PubMed] [Google Scholar]
- 4.Huebner M, Hsu Y, Fenner DE. The use of graft materials in vaginal pelvic floor surgery. Int J Gynaecol Obstet. 2006;92:279–288. doi: 10.1016/j.ijgo.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 5.FDA Public Health Notification: Serious Complications Associated with Transvaginal Placement of Surgical Mesh in Repair of Pelvic Organ Prolapse and Stress Urinary Incontinence. [Accessed August 3, 2012];2011 doi: 10.1016/j.eururo.2009.01.055. at http://www.fda.gov/medicaldevices/safety/alertsandnotices/publichealthnotifications/ucm061976.htm.) [DOI] [PubMed]
- 6.Marcus-Braun N, Bourret A, von Theobald P. Persistent pelvic pain following transvaginal mesh surgery: a cause for mesh removal. Eur J Obstet Gynecol Reprod Biol. 2012;162:224–228. doi: 10.1016/j.ejogrb.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Margulies RU, Lewicky-Gaupp C, Fenner DE, McGuire EJ, Clemens JQ, Delancey JO. Complications requiring reoperation following vaginal mesh kit procedures for prolapse. Am J Obstet Gynecol. 2008;199:678 e1–678 e4. doi: 10.1016/j.ajog.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 8.Ridgeway B, Walters MD, Paraiso MF, et al. Early experience with mesh excision for adverse outcomes after transvaginal mesh placement using prolapse kits. Am J Obstet Gynecol. 2008;199:703 e1–703 e7. doi: 10.1016/j.ajog.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 9.Feiner B, Maher C. Vaginal mesh contraction: definition, clinical presentation, and management. Obstet Gynecol. 2010;115:325–330. doi: 10.1097/AOG.0b013e3181cbca4d. [DOI] [PubMed] [Google Scholar]
- 10.Hurtado EA, Appell RA. Management of complications arising from transvaginal mesh kit procedures: a tertiary referral center's experience. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:11–17. doi: 10.1007/s00192-008-0721-9. [DOI] [PubMed] [Google Scholar]
- 11.Firoozi F, Ingber MS, Moore CK, Vasavada SP, Rackley RR, Goldman HB. Purely transvaginal/perineal management of complications from commercial prolapse kits using a new prostheses/grafts complication classification system. J Urol. 2012;187:1674–1679. doi: 10.1016/j.juro.2011.12.066. [DOI] [PubMed] [Google Scholar]
- 12.Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA) / International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) & grafts in female pelvic floor surgery. Int Urogynecol J. 2011;22:3–15. doi: 10.1007/s00192-010-1324-9. [DOI] [PubMed] [Google Scholar]