Abstract
An important role of the type 2 diabetes risk variant rs7903146 in TCF7L2 in metabolic actions of various tissues, in particular of the liver, has recently been demonstrated by functional animal studies. Accordingly, the TT diabetes risk allele may lead to currently unknown alterations in human. Our study revealed no differences in the kinetics of glucose, insulin, C-peptide and non-esterified fatty acids during an OGTT in homozygous participants from a German diabetes risk cohort (n = 1832) carrying either the rs7903146 CC (n = 15) or the TT (n = 15) genotype. However, beta-cell function was impaired for TT carriers. Covering more than 4000 metabolite ions the plasma metabolome did not reveal any differences between genotypes. Our study argues against a relevant impact of TCF7L2 rs7903146 on the systemic level in humans, but confirms the role in the pathogenesis of type 2 diabetes in humans as a mechanism impairing insulin secretion.
Genome-wide association studies have established the TCF7L2 variant rs7903146, which is located in a non coding region, as the strongest association with diabetes of all known single nucleotide polymorphisms (SNP) so far1. The risk allele is associated with lower insulin production and release2 and impaired GLP-1 induced insulin secretion3,4. Functional studies in cell culture and animal models as well as investigation of humans elucidated an important role of TCF7L2 in β-cell function5,6, as well as islet morphology7. The selective elimination of TCF7L2 function in the pancreas of mice is sufficient to replicate many of the key features observed in human diabetes-risk-allele carriers, as recently demonstrated by da Silva Xavier et al.8.
Beside pancreatic islets, TCF7L2 is highly expressed in a variety of glucose sensing and metabolizing tissues. Animal studies point to important roles in hepatic metabolic action such as the regulation of LDL, VLDL, triglycerides levels, gluconeogenesis and modulation of hepatic insulin sensitivity9. In adipose tissue, TCF7L2 is associated with adipogenesis10, in the intestine with the expression of the glucagon gene GCG11. Furthermore, in the brain it is involved in GCG expression and anorectic effects of GLP-111.
In the arena of metabolic traits TCF7L2 is a key and intriguing factor12. Genes bound by this transcription factor are strongly enriched in pathway categories related to metabolism. Very recently, the hepatic action of TCF7L2 came into focus. Unexpectedly, tissue specific manipulation of islet TCF7L2 expression in mice had no effect on glucose-stimulated insulin secretion, but hepatic overexpression led to enhanced hepatic glucose production and to glucose intolerance9. However, in humans the overall mechanisms and pathophysiological consequences by which TCF7L2 alters metabolic processes that are important in the pathogenesis of T2DM remain elusive.
In our study, metabolic alterations associated with the TCF7L2 variant rs7903146 were studied on the systemic level in humans by functional clinical, clinical chemical and non-targeted metabolomics investigations.
Results
Subject characteristics
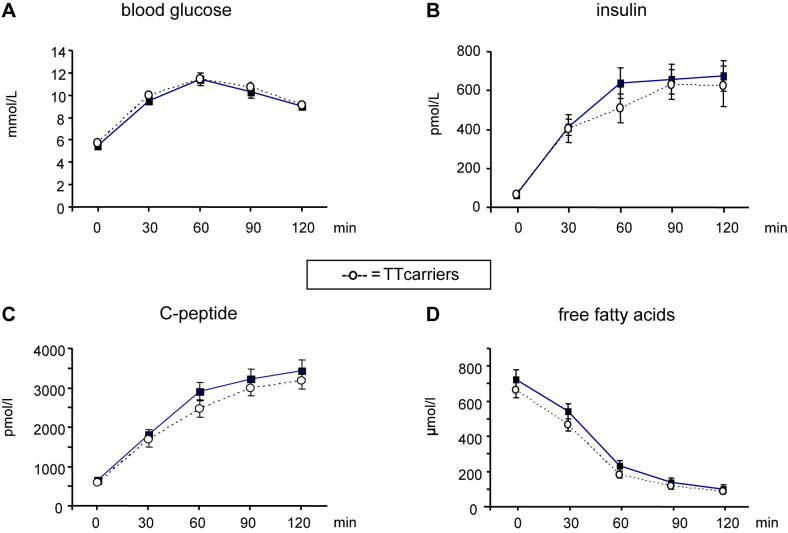
The clinical characteristics of the study group are given in table 1. Laboratory parameters of fasted plasma such as creatinine, γ-GT, ALT, AST, uric acid, urea, total protein and albumin showed no significant differences (data not shown). In the investigations of the OGTT time courses the two groups showed also no difference in glucose, insulin, C-peptide and free fatty acids (Fig. 1). However, the HOMA-β index was significantly lower in the TT genotype group (Table 1). We continued by non-targeted metabolomics investigation of potential metabolic effects of TCF7L2 reflected by unexpected plasma metabolites which are not covered by common clinical profiling.
Table 1. Subject characteristics. The subjects were matched for sex, age and BMI. Significant difference: p < 0.05.
| TCF7L2 rs7903146 | |||
|---|---|---|---|
| CC (n = 15) | TT (n = 15) | ||
| Mean ± SD | Mean ± SD | p | |
| Female/Male | 8/7 | 7/8 | 1 |
| Age | 50.6 ± 6.3 | 50.2 ± 9.6 | 0.7 |
| BMI | 28.7 ± 2.3 | 28.6 ± 3.2 | 0.72 |
| Glucose 0 min | 5.5 ± 0.6 | 5.7 ± .8 | 0.43 |
| Glucose 120 min | 9.0 ± 1.1 | 9.1 ± .9 | 0.46 |
| Insulin 0 min | 63.8 ± 28.4 | 63. ± 32.8 | 0.71 |
| Insulin 120 min | 412.4 ± 162.3 | 403.3 ± 273.6 | 0.29 |
| C-peptide 0 min | 658.2 ± 199.4 | 597.9 ± 228.8 | 0.53 |
| C-peptide 120 min | 1813.7 ± 495.7 | 1677.5 ± 707.7 | 0.27 |
| HOMA-β | 102.2 ± 19.6 | 88.1 ± 20.7 | 0.04 |
| Insulin sensitivity index (Matsuda) | 9.4 ± 3.7 | 10.7 ± 6. | 0.79 |
Figure 1. Metabolite and hormone kinetics during an oral glucose tolerance test (OGTT) in plasma.
Homozygous T-allele transcription factor 7-like 2 (TCF7L2) rs7903146 carriers (n = 15) were compared with homozygous C-allele carriers (n = 15), both having isolated impaired glucose tolerance. A comparison of (A) glucose, (B) insulin, (C) C-peptide, and (D) non-esterified fatty acid kinetics during an OGTT was performed. Multivariate ANOVA after ln-transformation of the variables was applied and no significant difference was detected. Each value represents the mean ± SEM.
The metabolic fingerprints of CC and TT carriers
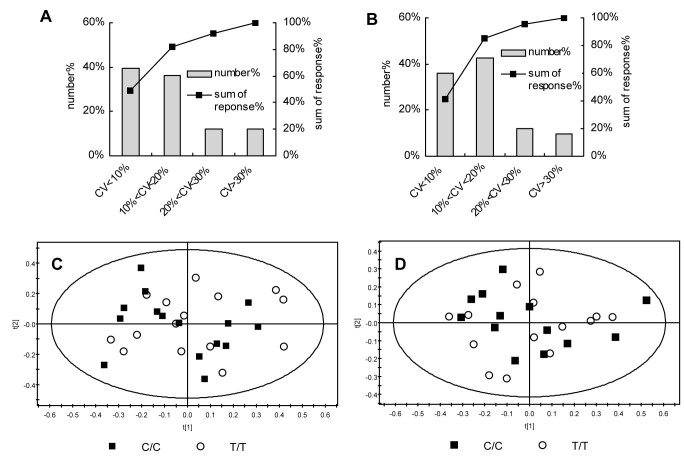
The non-targeted metabolomics investigation revealed in total 3728 and 1089 plasma metabolite ions in ESI(+) and ESI(−) analysis, respectively. After preprocessing of these data, applying 80% rule and QC evaluation, 3083 ions in ESI(+) and 962 ions in ESI(−) remained and were used for subsequent analysis. The control of the analytical stability and reproducibility indicated robust conditions as presented by QC evaluation (Figure 2A and Figure 2B). In ESI(+), about 88% ions showed RSD below 30%, and the summed response of these ions accounted for ~92% of total response; similarly, in ESI(−), about 90% of ions showed RSD below 30%, and the summed response account for more than 95%; both median values of RSD of all detected ions in ESI(+) and ESI(−) were below 12%.
Figure 2. Analytical robustness and reproducibility of non-targeted metabolomics evaluated using QC samples and metabolite fingerprints of homozygous T-allele TCF7L2 rs7903146 carriers (n = 15) and homozygous C-allele carriers (n = 15) by principle component analysis (PCA).
(A) and (B) shows coefficient of variation (CV) distribution in QC samples in ESI(+) and ESI(-) mode, respectively. Number%, percentage of ions with CV within a specific range as indicated in X axis. Sum of response%, percentage of sum of response of ions below a criterion. (C) shows the PCA overview of the metabolite fingerprints detected in the positive ionization mode ESI (+). Each individual fingerprint is composed out of 3083 metabolite ions. (D) shows the PCA overview of the metabolite fingerprints detected in the negative ionization mode ESI (−). Each individual fingerprint is composed out of 962 metabolite ions.
First, aiming to get an overview of the metabolite patterns of the two groups an unsupervised multivariate data analysis was performed. Unexpectedly, the principle component analysis (PCA) model, given in Fig. 2, showed no differences in the plasma metabolome between TT and CC carriers, neither in ESI(+) (Fig 2C, R2X = 0.597, Q2 = 0.372), nor in ESI(−) mode (Fig 2D, R2X = 0.478, Q2 = 0.283). Next, supervised models, including partial least squares-discriminant analysis (PLSDA) and orthogonal signal correction (OSC)-PLSDA, were applied. However, a separation of the two groups could only be achieved in over-fitted supervised models (data not shown). Finally, significant changes between individual metabolite ions were investigated applying Wilcoxon, Mann-Whitney test coupled with FDR correction (FDR < 0.2). But again, no differences between TT and CC carriers were detected. Finally 183 metabolites, covering various metabolite categories and metabolic pathways (Table S1(a) and Table S1(b), for ESI(+) and ESI(−), respectively), were identified according to a currently built up in-house metabolite library which is based on authentic standards, exact mass, MS/MS fragmentation pattern and retention time.
Discussion
The genetic susceptibility for T2DM is conferred by the simultaneous occurrence of common genetic variations in many genes13. Hence, facing the more than 70 diabetes risk genes and an unknown number of additional genetic risk variants, a human study on the metabolic effects and consequences of one specific gene variant is challenging. In our study we aimed to narrow down on specific metabolic effects of TCF7L2 as effectively as possible. The two middle aged groups of 15 CC vs 15 TT carriers showed very similar characteristics (table 1). In line with current knowledge on TCF7L2 function, the HOMA-β index showed reduced insulin secretion for carriers of the risk allele. The studied kinetics during an oGTT were not different, as well as none of the analyzed laboratory parameters. Therefore we concluded that, at best, only minor potential effects of other diabetes risk genes could occur in our study.
Next we questioned which unexpected alterations in the human metabolite pattern may be detected applying non-targeted metabolomics, since it has recently been shown in cell culture and animal models that TCF7L2 is associated with miscellaneous metabolic actions in various tissues and organs8,9,10,11. We compared metabolite profiles in plasma of homozygous risk vs. non-risk allele carriers covering more than 4000 metabolite ions. But unexpectedly we did not detect any differences, even when we applied the supervised PLS-DA and OSC-PLSDA, which, in contrast to PCA, are suitable to detect even modest differences in metabolic fingerprints between two groups. The promising perspectives of metabolomic profiling in diabetes research have recently been reviewed14 and already differences in the prediabetic phase can be detected, as recently shown by Mai et al.15. Very recently, 80 phospholipids and a few other metabolites have been analyzed in plasma of a group of mixed CT/TT TCF7L2 risk gene vs a group of homozygous CC carriers by a targeted approach16. But only a non-physiological metabolic provocation (intravenous GTT) led to the group-specific detection of slightly significant differences in some phospholipids levels16. In contrast to that, metabolic profiling of monogenetic diabetic diseases (MODY1, MODY2, MODY3) revealed a clear separation of cases and controls by multivariate data analysis and several gene associated alterations of metabolite levels in plasma and urine were detected17,18.
Presupposing that TCF7L2 has an impact on human hepatic metabolism, as suggested from animal models9, our non-targeted metabolomics investigation of individual metabolic fingerprints composed of >4000 metabolite ions should have led to the detection of alterations in the metabolite pattern in plasma of homozygous risk variant carriers, since the liver is a major source of circulating metabolites. Of note, there is remarkably little consistency in functional studies regarding the directional relationship between glucose intolerance and TCF7L2 expression6,19. Some rodent studies reported that reduced TCF7L2 expression lead to glucose intolerance8,20 while others detected the opposite21. The same is true for studies in human islets, i.e. either TCF7L2 suppression5,8,20 or overexpression3 is reported to impair glucose stimulated insulin secretion. A potential explanation for the contradicting findings could be that it is quite challenging to mimic the situation in human body by experimental settings, since the rs7903146 SNP is located in a non coding region acting by altering gene expression. Also reports of functional studies in humans comparing insulin sensitivities between carriers of the risk SNP and controls are not consistent. Florez et al. detected impaired insulin secretion accompanied by higher insulin sensitivity in carriers of the T allele22. However, applying the gold standard to measure insulin sensitivity in humans, we recently could not confirm an association of the risk allele with insulin sensitivity, neither with the euglycemic-hyperinsulinemic nor with the hyperglycemic-hyperinsulinemic clamp23.
The findings of our study strongly argue against a relevant systemic impact of the variant rs7903146 in TCF7L2 on metabolism, at least in middle aged humans. Our data may support the concept that each single diabetes risk SNP has only a modest effect on metabolism, and a deleterious combination of genetic diabetes risk variants, in addition to interactions with other factors (environment, lifestyle)24,25 are needed to result in a dysregulation of whole body metabolism and finally diabetes.
It should be noted that we investigated subjects at risk to develop T2DM with a mean age of 50 years. Therefore, we can not exclude that the TT SNP exerts its deleterious effects leading to T2DM and alterations of the metabolome in a later phase of life when the glucose level is increasing based on age-dependent increase in obesity and insulin resistance. Furthermore, it has been shown that the genetic effect of TCF7L2 interacts with glycemia26. Therefore, we controlled for glycemia by selecting only participants with iIGT. Moreover, the stringent subject matching resulted in a relatively small number of subjects. However, despite the small group size, the ultra high resolution of our approach would have identified existing strong perturbations of circulating metabolite pattern caused by the diabetes risk genotype. This meticulous metabolomic profiling, the careful selection of participants suggests that by comparing the two extreme genotypes we should have been able to detect genotype-associated metabolomic differences. Notwithstanding the above, our findings can not absolutely rule out any association on the metabolite level although more than 4000 metabolite ions were detected by the applied methodology.
To the best of our knowledge this study is the first investigation of a diabetes risk SNP in a well-matched group of homozygous carriers by non-targeted metabolic profiling and careful clinical and clinical chemical investigations. Based on our results, the contribution of TCF7L2 rs7903146 to the pathogenesis of T2DM in humans can still be seen as a mechanism impairing insulin secretion. However, the impact of the polymorphism on the dysregulation of whole body metabolism, at least in middle aged individuals, seems to be minor or solely relevant for a particular subphenotype when specific interactions with other factors like a deleterious combination of genetic diabetes risk variants are present.
Methods
Subjects
The subjects of this study were selected from the TUEF (Tuebingen Family) study, originally designed to metabolically characterize a population at increased risk of T2Dm27. Subjects with elevated CRP (>0.5 mg/l), medication use, and elevated type 1 diabetes associated antibodies (anti-GAD2) were excluded, yielding a cohort of 1832 individuals. Of these participants, 183 had isolated impaired glucose tolerance (iIGT) defined as fasting plasma glucose ≤5.6 mmol/l, 120-min post-challenge glucose ≥7.8 mmol/l, but <11.1 mmol/l. Among the participants with iIGT, 75 were homozygous non-risk allele (CC) carriers and 23 carried the TT genotype. Very carefully matching for sex distributions, mean age and mean BMI 15 participants per genotype group remained (Table 1).
The rationale for choosing subjects with iIGT is the following: Despite TCF7L2 being the strongest gene associated with type 2 diabetes, its effect size, expressed as the odds ratio to increase diabetes risk per allele, is rather modest (OR ~ 1.35). However, there is evidence that the effect size of TCF7L2 is modulated by other factors such as glycemia (reviewed by: Wagner et al28). These data show that in a hyperglycemic setting, the effect allele of rs7903146 exerts a stronger impediment on insulin secretion. The most striking interaction of TCF7L2's effect on insulin secretion with glycemia has been shown for the post-challenge 120 minute glucose value. Therefore, we selected a population with elevated post-challenge glucose levels to augment the genetic effect of TCF7L2 on insulin secretion.
The protocol of the study was approved by the Institutional Review Board of the University of Tuebingen, Schleichstr. 8, 72076 Tuebingen (board chairman: Prof. Dr. D. Luft) according to the Declaration of Helsinki, and all subjects gave written informed consent before the study commenced. The investigation was conducted in accordance with the ethical principles of Good Clinical Practice.
Oral glucose tolerance test (OGTT), clinical chemical analyses, genotyping and calculations
All individuals underwent a 75 g OGTT at 8:00 am, after 12 hours overnight fast to study kinetics of non-esterified fatty acids (NEFA), glucose, insulin, C-peptide and pro-insulin. Blood glucose was determined using a bedside glucose analyzer (YSI, Yellow Springs, CO). Hormones were determined on an ADVIA Centaur XP, except pro-insulin (IBL international, Hamburg), blood cell count on an ADVIA 2120 and all other routine parameters on an ADVIA 1800 clinical chemistry system (Siemens Healthcare systems, Erlangen, Germany). The SNP rs7903146 was genotyped using the MassARRAY platform (Sequenom, USA). Insulin sensitivity was calculated from glucose and insulin values using the formula proposed by Matsuda and DeFronzo29. Insulin secretion was calculated from glucose and C-peptide values as HOMA-β using a publicly available calculator (http://www.dtu.ox.ac.uk/homacalculator/).
Non-targeted metabolomics profiling
Sample preparation for the metabolomics analysis was performed using a 200 μl aliquot of EDTA-plasma, which was deproteinized with two volumes of acetonitrile (final concentration 66%), followed by vortexing for 2 min and centrifugation (15,700 × g, 20 min, 4°C). The supernatant was dried in a vacuum centrifuge and stored at −80°C. For analysis, samples were reconstituted in 200 μl acetonitrile/water (4:1, v/v). An AccelaTM ultra high performance liquid chromatography (UHPLC) system (Thermo Fisher, San Jose, CA, USA) coupled online via an electrospray ionization source (ESI) with a LTQ-Orbitrap XL mass spectrometer (Thermo Fisher, San Jose, CA, USA) was employed for non-targeted metabolomics profiling. Plasma metabolite profiles were separated by a C8 AQUITYTM column (2.1 mm × 100 mm × 1.7 μm, Waters, Milford, MA, USA) according to previous method with slight modifications30. Briefly, in positive mode analysis, the elution started with 95% mobile phase A (water, containing 1% formic acid) and 5% mobile phase B (acetonitrile) and maintained for 0.5 min, then it linearly increased to 100% B in next 23.5 min, and was kept constant for 4 min, followed by returning to 5% B in next 0.1 min and equilibrating for 4 min before next injection. Flow rate was set as 0.35 mL/min and the column temperature was 35°C. In negative mode analysis, water and 95% MeOH/5% H2O, both containing 5 mM NH4HCO3, were used as elute A and B, respectively. The elution started with 20% B for 0.5 min, then linearly increased to 100% B in next 21.5 min and maintained for 5 min, followed by returning to 20% B in next 0.1 min and equilibrating for 5 min before next injection. Flow rate was set 0.35 mL/min and the column temperature was 50°C. Capillary temperature was 325°C and high resolution full scan started from m/z 100 to 1000. Source voltage was set 4.5 kV with sheath gas flow 40 arb and auxiliary gas flow 5 arb in positive mode. In negative mode, source voltage was set 3.5 kV with sheath gas flow 35 arb and auxiliary gas flow 5 arb. The data was acquired in profile mode. Samples were analyzed in random sequence with quality control (QC) samples31 analyzed every fifth injection.
Statistical analyses
Anthropometric data and clinical chemical parameters of the two groups were compared with the Wilcoxon-test for continuous variables. Fisher's exact test was used to compare sex distributions. Glucose, insulin and C-peptide, proinsulin and NEFA kinetics during the OGTT were compared with multivariate ANOVA after ln-transformation of the variables. Statistical tests were peformed with JMP 10.0 (SAS Inc).
For metabolomics, first the time alignment and framing of acquired LC-MS raw data were performed using the software SIEVE (V1.2, Thermo Fisher, USA). The 80% rule was applied to exclude missing values, i.e., variables were removed if their nonzero values are presented in less than 80% in both studied groups. Subsequently variables with a coefficient of variation >30% in QC samples were excluded after normalization to the sum of peak areas, according to the procedure described by Bijlsma et al.32. Multivariate analysis was conducted with SIMCA-P (Umetrics, Sweden). Principle component analysis (PCA), partial least squares-discriminant analysis (PLSDA) and orthogonal signal correction (OSC)-PLSDA were applied using pareto scaling. Wilcoxon, Mann-Whitney test and positive false discover rate (FDR) correction (according to Benjamini-Hochberg) was performed using MultiexperimentViewer. FDR < 0.2 was set as significance criterion.
Author Contributions
J.L., E.K., F.M. researched data; R.W., R.L. researched data, wrote the manuscript; H.-U.H., A.F., O.K., G.X. contributed to discussion, reviewed/edited the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
We gratefully acknowledge the technical assistance of Heike Runge, Department of Internal Medicine 4 (University Hospital Tuebingen). This work was supported in part by grants of the German Federal Ministry of Education and Research BMBF) to the German Centre for Diabetes Research (DZD e.V.; Grant 01GI0925 to HUH); the Kompetenznetz Diabetes mellitus (Competence Network for Diabetes mellitus) funded by the Federal Ministry of Education and Research (FkZ 01 GI 1104A to EK, OK, HUH and RL); the 973 Project of the State Ministry of Science and Technology of China (No. 2012CB517506 to GX), the Sino-German Center for Research Promotion (GZ 753 by DFG and NSFC to GX and RL and LE 1391/1-1 by DFG to RL); the key foundation and creative research group project (No. 21175132 and No. 21021004 by NSFC to GX).
References
- Helgason A. et al. Refining the impact of TCF7L2 gene variants on type 2 diabetes and adaptive evolution. Nat. Genet. 39, 218–225 (2007). [DOI] [PubMed] [Google Scholar]
- Renstrom E. Impact of transcription factor 7-like 2 (TCF7L2) on pancreatic islet function and morphology in mice and men. Diabetologia 55, 2559–2561 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssenko V. et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J. Clin. Invest 117, 2155–2163 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjesing A. P. et al. Carriers of the TCF7L2 rs7903146 TT genotype have elevated levels of plasma glucose, serum proinsulin and plasma gastric inhibitory polypeptide (GIP) during a meal test. Diabetologia 54, 103–110 (2011). [DOI] [PubMed] [Google Scholar]
- Shu L. et al. Transcription factor 7-like 2 regulates beta-cell survival and function in human pancreatic islets. Diabetes 57, 645–653 (2008). [DOI] [PubMed] [Google Scholar]
- McCarthy M. I., Rorsman P. & Gloyn A. L. TCF7L2 and Diabetes: A Tale of Two Tissues, and of Two Species. Cell Metab 17, 157–159 (2013). [DOI] [PubMed] [Google Scholar]
- Le Bacquer O. et al. TCF7L2 rs7903146 impairs islet function and morphology in non-diabetic individuals. Diabetologia 55, 2677–2681 (2012). [DOI] [PubMed] [Google Scholar]
- da Silva Xavier G. et al. Abnormal glucose tolerance and insulin secretion in pancreas-specific Tcf7l2-null mice. Diabetologia 55, 2667–2676 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boj S. F. et al. Diabetes risk gene and Wnt effector Tcf7l2/TCF4 controls hepatic response to perinatal and adult metabolic demand. Cell 151, 1595–1607 (2012). [DOI] [PubMed] [Google Scholar]
- Ross S. E. et al. Inhibition of adipogenesis by Wnt signaling. Science 289, 950–953 (2000). [DOI] [PubMed] [Google Scholar]
- Shao W. et al. The Wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis. Diabetes 62, 789–800 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S. F. Understanding the elusive mechanism of action of TCF7L2 in metabolism. Diabetes 61, 2657–2658 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer S. A., Machicao F., Fritsche A., Haring H. U. & Kantartzis K. New type 2 diabetes risk genes provide new insights in insulin secretion mechanisms. Diabetes Res. Clin. Pract. 93 Suppl 1 S9–24 (2011). [DOI] [PubMed] [Google Scholar]
- Bain J. R. et al. Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes 58, 2429–2443 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai M. et al. Serum levels of acylcarnitines are altered in prediabetic conditions. PLoS. ONE. 8, e82459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then C. et al. Plasma metabolomics reveal alterations of sphingo- and glycerophospholipid levels in non-diabetic carriers of the Transcription Factor 7-Like 2 polymorphism rs7903146. PLoS. ONE. 8, e78430 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spegel P. et al. Metabolite profiling reveals normal metabolic control in carriers of mutations in the glucokinase gene (MODY2). Diabetes 62, 653–661 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloyn A. L. et al. Metabolic profiling in Maturity-onset diabetes of the young (MODY) and young onset type 2 diabetes fails to detect robust urinary biomarkers. PLoS. ONE. 7, e40962 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega M. A. TCF7L2 and glucose metabolism: time to look beyond the pancreas. Diabetes 62, 706–708 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Xavier G. et al. TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes 58, 894–905 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic D. et al. Alterations in TCF7L2 expression define its role as a key regulator of glucose metabolism. Genome Res. 21, 1417–1425 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez J. C. et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N. Engl. J. Med. 355, 241–250 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer S. A. et al. Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia 50, 2443–2450 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan C. J., Damm P. & Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 378, 169–181 (2011). [DOI] [PubMed] [Google Scholar]
- Linder K. et al. Allele summation of diabetes risk genes predicts impaired glucose tolerance in female and obese individuals. PLoS. ONE. 7, e38224 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heni M. et al. Glycemia determines the effect of type 2 diabetes risk genes on insulin secretion. Diabetes 59, 3247–3252 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamer C. et al. Reduced skeletal muscle oxygen uptake and reduced beta-cell function: two early abnormalities in normal glucose-tolerant offspring of patients with type 2 diabetes. Diabetes Care 26, 2126–2132 (2003). [DOI] [PubMed] [Google Scholar]
- Wagner R. et al. Untangling the interplay of genetic and metabolic influences on beta-cell function: Examples of potential therapeutic implications involving TCF7L2 and FFAR1. Mol. Metab 3, 261–267 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M. & DeFronzo R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999). [DOI] [PubMed] [Google Scholar]
- Huang Q. et al. Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics. Cancer Res. 73, 4992–5002 (2013). [DOI] [PubMed] [Google Scholar]
- Gika H. G., Theodoridis G. A., Wingate J. E. & Wilson I. D. Within-day reproducibility of an HPLC-MS-based method for metabonomic analysis: application to human urine. J. Proteome. Res. 6, 3291–3303 (2007). [DOI] [PubMed] [Google Scholar]
- Bijlsma S. et al. Large-scale human metabolomics studies: a strategy for data (pre-) processing and validation. Anal. Chem. 78, 567–574 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information