Abstract
Neuroimaging studies of post-traumatic stress disorder (PTSD)-related memory impairments have consistently implicated abnormal activities in the frontal and parietal lobes. However, most studies have used block designs and could not dissociate the multiple phases of working memory. In this study, the involvement of the prefrontal cortex in working memory phases was assessed among veterans with PTSD and age-/gender-matched healthy controls. Multichannel functional near infrared spectroscopy (fNIRS) was utilized to measure prefrontal cortex hemodynamic activations during memory of neutral (i.e., not trauma-related) forward and backward digit span tasks. An event-related experimental design was utilized to dissociate the different phases (i.e., encoding, maintenance and retrieval) of working memory. The healthy controls showed robust hemodynamic activations during the encoding and retrieval processes. In contrast, the veterans with PTSD were found to have activations during the encoding process, but followed by distinct deactivations during the retrieval process. The PTSD participants, but not the controls, appeared to suppress prefrontal activity during memory retrieval. This deactivation was more pronounced in the right dorsolateral prefrontal cortex during the retrieval phase. These deactivations in PTSD patients might implicate an active inhibition of dorsolateral prefrontal neural activity during retrieval of working memory.
Keywords: Post-traumatic stress disorder (PTSD), Functional near infrared spectroscopy (fNIRS), Working memory, Digit span, Hemispheric emotional valence (HEV), Topography
Highlights
-
•
Multiple phases of working memory in PTSD and healthy controls were assessed.
-
•
Brain atlas-based optical topography and multi-regression analysis were implemented.
-
•
Prefrontal deactivation in the retrieval phase of working memory was found in PTSD.
1. Introduction
Post-traumatic stress disorder (PTSD) is a neuropsychiatric disorder that can develop after exposure to any traumatic event. The estimated lifetime prevalence of PTSD is 7.8% among adult Americans (Kessler et al., 1995). Military combat is the most common trauma among men with PTSD. Combat-related PTSD is found in 9%–25% of war-zone veterans (Kessler et al., 1995; Dohrenwend et al., 2006; Erickson et al., 2001; Hoge et al., 2004) and is often persistent and comorbid with other mental disorders (Arbanas, 2010), even after the veterans return to their civilian lives.
Numerous neuropsychological studies have identified cognitive dysfunctions associated with PTSD, such as memory impairments, attention deficits, and learning disabilities (Bremner et al., 1993; Vasterling et al., 1998, 2002; Brandes et al., 2002; Koenen et al., 2001; Roca et al., 2006). In recent years, various neuroimaging techniques have been used to investigate neural mechanisms underlying stress-related and trauma-related memory impairments. Such imaging techniques include event-related potentials (ERPs) (Galletly et al., 2001; Weber et al., 2005; Veltmeyer et al., 2009), positron emission tomography (PET) (Shaw et al., 2002; Clark et al., 2003), functional magnetic resonance imaging (fMRI) (Elzinga et al., 2007; Moores et al., 2008; Shaw et al., 2009), and functional near infrared spectroscopy (fNIRS) (Matsuo et al., 2003). The most replicable findings from the neuroimaging studies suggest abnormal activities in the frontal and parietal cortices, two of the key cerebral regions consistently implicated in the neurobiology of PTSD (Bremner, 2002; Francati et al., 2007; Aupperle et al., 2012).
Working memory involves temporary brain storage and manipulation of information necessary for executing complex cognitive tasks. It is composed of multiple component processes or phases, such as encoding, maintenance, and retrieval of the given information (Baddeley, 1992, 1996). Neural correlates of these processes can be temporally dissociated and identified by using event-related experimental designs (Aguirre and D’Esposito, 1999). However, most studies of PTSD-related working memory using PET, fMRI, and fNIRS techniques have used block designs. Block designs do not allow reliable dissociation of overlapping brain responses during multi-phase processes of working memory. Subtracting brain responses during blocked trials with and without specific memory processes are often challenged by the untenable assumption of pure insertion (Zarahn et al., 1999; D’Esposito et al., 1999). While ERP provides a direct measure of event-related electrophysiological response of the brain, the source of a single evoked potential cannot be easily localized and isolated. To date, little is known on how temporal and spatial responses of the brain are altered by PTSD during the multi-phase processes of working memory.
Functional near infrared spectroscopy (fNIRS) is a noninvasive neuroimaging technology that measures the cerebral hemodynamics and oxygenation with near infrared light (670–900 nm) (Ferrari et al., 2012; Boas et al., 2004). The near infrared light can penetrate through the scalp and skull to reach a depth of several centimeters within the cortex (Dehghani et al., 2009; Cui et al., 2011; Tian and Liu, 2014), while being partially absorbed by the oxygenated hemoglobin (HbO2) and deoxygenated hemoglobin (Hb) in the cerebral blood flow. By measuring changes of near infrared light intensity from the baseline at two or more wavelengths, the changes of HbO2 and Hb concentrations in the cerebral vasculature can be quantified (Cope et al., 1988). Compared with other neuroimaging modalities, this technology has an excellent temporal resolution (in ms) and also has such advantages as being portable, relatively inexpensive, and feasible to be used in natural environments without too much body confinement (Hoshi et al., 2007). Recent studies have demonstrated that fNIRS could be utilized effectively and successfully to study prefrontal cortex activation associated with the working memory processes in human participants (Moro et al., 2013; Sato et al., 2013).
In this study, we used fNIRS to image the right and left prefrontal activations during working memory phases (encoding, maintenance, and retrieval) in veterans with PTSD and compared their prefrontal responses with those in age- and gender- matched healthy controls. We chose the forward and backward digit span tasks because they have been intensively used to assess working memory in neuropsychological research (Gerton et al., 2004). Both tasks require immediate, serial recall of a list of digits that were presented to the participants. In a digit forward task, participants repeat the digits in the same order as they are presented; in a digit backward task, participants repeat the digits in reverse order. A digit backward task is believed to share many of the cognitive components of a digit forward task except that the digit backward task requires an additional manipulation component. We used an event-related experimental design to image and dissociate the multi-phase processes of working memory during the digit span tasks. In this way, we were able to reveal both temporal and spatial patterns of prefrontal responses that could be specifically altered in patients with PTSD. The overall goal of this study was to demonstrate that fNIRS could be a portable and complementary neuroimaging tool to identify abnormal prefrontal activities in PTSD patients during working memory processes.
2. Material and methods
2.1. Participants
2.1.1. Veterans with PTSD
Combat-exposed male veterans who were preparing for or enrolled in higher education were recruited via a supported education intervention clinical trial conducted by the mental health translational research center of the School of Social Work at the University of Texas at Arlington. The veterans were referred for the fNIRS scan (to be described in the following Subsections 2.2 and 2.3) if they had medical records confirming a prior diagnosis with PTSD, and were experiencing clinically significant distress and functional impairment affecting their cognitive and related academic performance at the time of study. Confirmation of their PTSD diagnosis and comorbid conditions was conducted prior to the fNIRS scan by an experienced licensed clinician using the Structured Clinical Interview for DSM Disorders — research version (SCID-RV, http://www.scid4.org/info/refscid.html) (First et al., 2002) and scores on three self-report questionnaires: the PTSD checklist — military version (PCL–M) (Weathers et al., 1993; Wilkins et al., 2011), Mississippi scale for combat-related PTSD (Hyer et al., 1991; Keane et al., 1988), and the PTSD subscale of the Minnesota Multiphasic Personality Inventory (Keane et al., 1984). At least one of the three self-report scores was required to be above its respective clinical cutoff value (i.e., PCL-M > 50, Mississippi scale > 130, or PTSD subscale > 30) for the veterans to be enrolled, besides their prior diagnosis and current diagnostic evaluation confirmation of PTSD and comorbid conditions.
A total of 17 veterans who met the eligibility criteria were successfully recruited and scanned by fNIRS. One veteran's results were dropped from the study database during data analysis because all of his trials of correct responses had motion artifacts. For the remaining 16 veterans, current comorbid conditions included attention deficit hyperactivity disorder (ADHD) (n = 6), major depressive disorder (n = 5), alcohol dependence (n = 4), musculoskeletal pain (n = 3), insomnia (n = 3), history of blast exposure (n = 3), anxiety disorder (n = 2), mild traumatic brain injury (mTBI) (n = 2), and learning disorder (n = 2).
2.1.2. Healthy controls
A total of 17 healthy male adults were also recruited from the local community of the University of Texas at Arlington and matched with the PTSD group on gender and age. Potential participants were screened initially with a questionnaire to exclude those who had history of any psychiatric illness, history of severe TBI, or intake of any medication at the time of study. Corresponding to the veteran who was excluded during data analysis, we also excluded the data from his healthy counterpart, yielding data from 16 healthy controls.
All of the 32 remaining participants in data analysis were right-handed, with age ranging from 21 to 56 years, mean ± SD age = 29.4 ± 9.6 years. The study protocols for both veterans with PTSD and matched healthy controls were approved by the University of Texas at Arlington Institutional Review Board. Written informed consent was obtained from every participant prior to the fNIRS scan.
2.2. Tasks and paradigm
All participants sat comfortably before a computer, and completed a session of digit forward task (8 trials) and a session of digit backward task (8 trials) sequentially while they were scanned by fNIRS. As modified from a previous study (Sun et al., 2005), each trial started from a string of six digits (randomly generated by the computer) that were presented in sequence on an LCD monitor, one digit per second. Then a blank screen was displayed for 10 s, during which the participants were instructed to look at the screen and covertly rehearse the given digits continuously. After this retention interval, a number pad was displayed on the monitor. The participants were instructed to recall the digits either in the same order (forward task) or reverse order (backward task) as they were presented, by clicking the on-screen number buttons. All participants were able to finish the forward or backward recall within 10 s. At last, an inter-trial interval of 10 s was given before the next trial. According to the study by Sun et al. (2005), a 10-s inter-trial interval would be sufficient for the hemodynamic signal to recover to the baseline level, while maintaining participants' patience on the task.
Before each formal measurement session was started, all participants were trained to practice a few trials. An experimenter observed the course of practice to confirm that the participants understood the protocol and were able to complete the task correctly.
It is known that for both digit forward and backward tasks, the task difficulty depended on the length of digit string (Gerton et al., 2004). We selected six-digit tasks because they are at an intermediate level and can be completed by all of the control and PTSD participants without extreme difficulty. In addition, the participants were always instructed to complete the digit forward task first. Because the digit forward task was easier than the digit backward task, this sequence allowed the participants to adapt to the following more difficult task ssession. The accuracy of each participant's performance in each type of task was measured by the percentage of trials of correct retrieval relative to the total number of trials (=8) in each session.
2.3. Functional near infrared spectroscopy
2.3.1. Instrument
A high-performance fNIRS system (Cephalogics LLC, Boston, MA) (Zeff et al., 2007) was used to acquire each participant's prefrontal hemodynamic activities during performance of the tasks. The system used light-emitting diodes (LEDs) at two wavelengths (750 and 850 nm) as light sources and avalanche photodiodes (APDs) as detectors. The data sampling rate was 10.8 Hz. The configuration of the fNIRS probe is shown in Fig. 1A. It was composed of 12 light sources and 16 detectors (6 light sources and 8 detectors on each hemisphere). The probe was placed bilaterally and symmetrically on the participant's forehead. The bottom line of 6 light sources in the probe was just above the eyebrows, and its midpoint was ˜3.5 cm in distance from the nasion (see Fig. 1A).
Fig. 1.
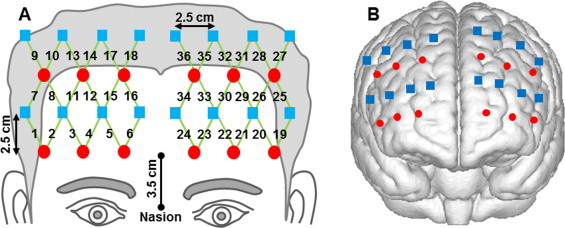
Configuration and cortical position of the fNIRS probe: (A) configuration of the fNIRS probe. Red circles represent light sources, blue squares represent detectors, and green lines represent the nearest source-detector pairs (channels) to measure the brain activities. (B) Co-registered positions of the optodes on a standard brain atlas. The probe partially covered the anterior, dorsolateral, and ventrolateral prefrontal regions on the right and left hemispheres. The anatomical position of each channel on the brain atlas is reported in detail in Table 1.
The fNIRS probe provided a total of 36 measurements (channels) when only the nearest source-detector pairs were considered (the nearest source-detector distance was 2.8 cm). Other larger source-detector distances were not included because their signals were too weak to be scientifically meaningful. The probe assembly was constructed with low-weight optical fibers (TechEn Inc., Boston, MA) and thin polyethylene film to ensure participants' comfort during the experiment.
2.3.2. Spatial registration
To estimate the cortical regions that the fNIRS probe covered, a spatial registration procedure (Singh et al., 2005) was performed among six randomly selected participants: Once the fNIRS probe was set in place, the positions of optodes (i.e., light sources and detectors) along with five cranial landmarks (the nasion, inion, left and right preauricular points, and vertex) were measured using a PATRIOT motion tracking system (Polhemus, Colchester, Vermont, USA). The cranial landmarks served as mediators to convert the real-world stereotaxic coordinates of the optodes to the Montreal Neurological Institute (MNI) coordinates used in a standard brain MRI atlas (Tian et al., 2012) based on the affine transformation. Fig. 1B shows the registered optode positions (averaged over six participants) on the standard brain atlas. The probe partially covered the anterior, dorsolateral and ventrolateral prefrontal regions, including Brodmann areas (BAs) 9, 10, 44, 45, and 46. A detailed report is given in Table 1. Because the fNIRS probe was carefully placed on each individual participant's forehead by referring to the nasion and eyebrows, the registered optode positions across individual participants were relatively consistent (positional variations were about 5 mm) in comparison with the separation of two neighboring measurement channels (about 12.5 mm).
Table 1.
Registered positions of fNIRS measurement channels on the standard brain MRI atlas. The channel numbers are defined in Fig. 1A.
| Hemisphere | Brodmann area | Channel |
|---|---|---|
| Right | BA 9 | 13, 14, 15, 16, 17, 18 |
| BA 10 | 4, 5, 6 | |
| BA 44 | 9, 10 | |
| BA 45 | 1, 7, 8 | |
| BA 46 | 2, 3, 11, 12 | |
| Left | BA 9 | 31, 32, 33, 34, 35, 36 |
| BA 10 | 22, 23, 24 | |
| BA 44 | 27, 28 | |
| BA 45 | 19, 25, 26 | |
| BA 46 | 20, 21, 29, 30 |
2.3.3. Data screening and processing
The fNIRS data from each measurement session was screened and processed using a publically available toolbox (Huppert et al., 2009). First, the raw data were visually inspected to exclude trials associated with significant data discontinuities (with a signal swing of 15% or larger from the baseline intensities), which were usually attributed to motion artifacts during the experiment. Then the qualified data in optical density were low-pass filtered at a cut-off frequency of 0.2 Hz to remove electronic noise and fast-oscillating cardiac waves, and high-pass filtered at a cut-off frequency of 0.01 Hz to remove possible slow baseline drift. Then, changes of hemoglobin concentrations relative to the baseline, ?[HbO2] and ?[Hb], were computed following the modified Beer–Lambert Law (Cope et al., 1988). At this step, we estimated the differential pathlength factor (DPF) to be 6.2 at 750 nm and 5.8 at 850 nm, according to published data for adult heads (Essenpreis et al., 1993). Event-related temporal profiles were averaged over the remaining trials in each session to obtain individual hemodynamic responses (in ?[HbO2] and ?[Hb]) that were evoked by the digit forward and backward tasks. At this step, only the trials of correct retrievals, in which the participants' competence of the memory processes could be confirmed, were averaged.
2.3.4. General linear model (GLM) analysis
GLM analysis has been increasingly utilized to analyze fNIRS data over the last decade to identify cortical areas significantly stimulated by given tasks (Plichta et al., 2006; Ye et al., 2009; Tian and Liu, 2014; Hassanpour et al., 2014). In this work, we also employed GLM to analyze channel-wise, event-related hemodynamic responses in the prefrontal cortex stimulated by both digit forward and backward tasks. In particular, we used multiple regression analysis to account for multi-phase working memory performance. Similar to Sun et al. (2005), three memory phases were considered in each of the two tasks: stimulus (or encoding), delay (or active maintenance), and recall (or retrieval). Accordingly, three regressors (encoding, maintenance, and retrieval) were generated after convolving three corresponding boxcar functions with a hemodynamic response function (HRF) (Tian and Liu, 2014). Ideally, the HRF would be derived from the fNIRS signals via an event-related experimental paradigm. To the best of our knowledge, however, such an HRF is not available for memory-evoked responses in the prefrontal cortex. Therefore, we used a standard HRF derived from BOLD fMRI as a surrogate (Glover, 1999). By fitting the three regressors to the channel-wise, event-related hemodynamic responses, the amplitudes (expressed by ß-values) of prefrontal activations or deactivations in responses to each memory phase were estimated by GLM (Tian and Liu, 2014). A schematic diagram of GLM analysis with three regressors is shown in Fig. 2.
Fig. 2.
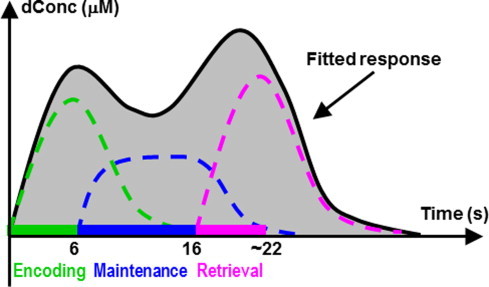
Schematic diagram of GLM analysis with three regressors that were generated to fit the hemodynamic responses evoked during the encoding, maintenance, and retrieval phases of working memory.
2.4. Statistical analysis
For behavioral measures, the Wilcoxon rank-sum test was used to compare performance accuracies between the control and PTSD groups as well as between the forward and backward tasks. The Wilcoxon rank-sum test is a nonparametric alternative to the t test and is often used when the data cannot be assumed to be normally distributed.
For hemodynamic measures, the channel-wise ß-values were examined with the interaction model of analysis of variance (ANOVA). Three factors were considered: group (control and PTSD), task (forward and backward), and phase (encoding, maintenance, and retrieval). If the main effect and/or interaction were found to be significant, separate t-tests were performed to identify the regions of interest (ROIs) that showed the significant difference. Both t-statistic values (expressed by t-values) and p-values were derived from the t-tests for each channel.
Lastly, correlations between the behavioral and hemodynamic measures were tested based on linear regression analyses. These analyses determined if the hemodynamic measures could be objective predictors of the participants' performance at the individual level. Here the hemodynamic changes were computed as the mean ß-values within the respective ROIs identified above.
2.5. Topography
Topographic images of prefrontal activation were generated using EasyTopo, an optical topography toolbox we developed recently (Tian et al., 2012). EasyTopo is based on a standard brain MRI atlas and implements 2D angular interpolation of the channel-wise data in a spherical coordinate system. In this study, the channel-wise ß-values derived from GLM analysis and t-values from subsequent statistical comparisons were interpolated to generate activation maps (ß-maps and t-maps) in each memory phase.
3. Results
3.1. Behavioral measures
Table 2 reports the performance accuracies of control and PTSD groups for the digit forward and backward tasks, respectively. The healthy controls had higher accuracies than the veterans with PTSD in both tasks. The group difference was highly significant in the forward task (p < 0.01) and significant in the backward task (p = 0.04 < 0.05). In terms of task differences, the healthy controls had reasonably higher accuracies in the forward task than those in the backward task (p < 0.01). In contrast, the veterans with PTSD had approximately equal accuracies in both tasks (p = 0.36 > 0.05).
Table 2.
Task-dependent performance accuracy of control and PTSD groups.
| Control group |
PTSD group |
|||
|---|---|---|---|---|
| Forward | Backward | Forward | Backward | |
| Accuracy (%) | 95.0 ± 7.9 | 78.3 ± 20.0 | 70.3 ± 25.4 | 62.5 ± 27.8 |
3.2. Hemodynamic responses
For hemodynamic responses, ANOVA analysis revealed that the group effect (control vs. PTSD) was statistically significant in 28 of the total 36 channels (p < 0.01). The interaction between group (control vs. PTSD) and task (forward vs. backward) was also significant in 12 of the total 36 channels (p < 0.05). Therefore, the group difference within each task and task difference within each group were identified in separate t-tests.
3.2.1. HbO2 changes induced by forward and backward tasks in control group
For the healthy controls, the digit forward task evoked robust HbO2 changes, while the Hb changes were relatively low and insignificant for all three phases, as shown in Fig. 3A. Specifically, the task-evoked HbO2 time course clearly showed two peaks corresponding to the encoding and retrieval phases (Fig. 3A), which are in accordance with a previous fMRI report using a similar stimulation protocol (Sun et al., 2005). Furthermore, the respective topographic images (first row in Fig. 4) showed that the regions of activation were primarily located in the dorsolateral prefrontal cortex (DLPFC, BAs 9 and 46), and the left hemisphere had greater activation than the right hemisphere.
Fig. 3.
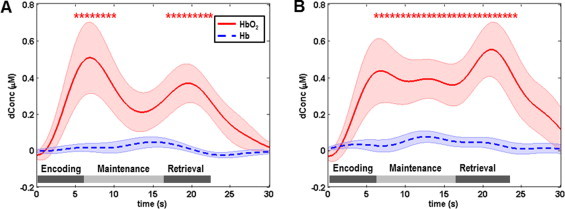
Mean task-evoked prefrontal hemodynamic responses (channel 30 in Fig. 1A) in the control group: (A) mean hemodynamic responses to the digit forward task, in which the mean HbO2 changes show two robust peaks in the encoding and retrieval phases; (B) mean hemodynamic responses to the digit backward task, in which the mean HbO2 changes show higher magnitudes in the maintenance and retrieval phases compared with those in the digit forward task. In both panels, the solid lines represent the mean time courses of HbO2, the dotted lines represent the mean time courses of Hb, the shaded regions represent the standard errors, the * symbols indicate the period of significant HbO2 changes (one-sample t-test, p < 0.01) from the baseline, and the gray bars in the bottom indicate the three phases of the task. It is noted that the actual retrieval time was slightly variable among participants. Therefore the retrieval phases labeled in both panels are schematic and approximate.
Fig. 4.
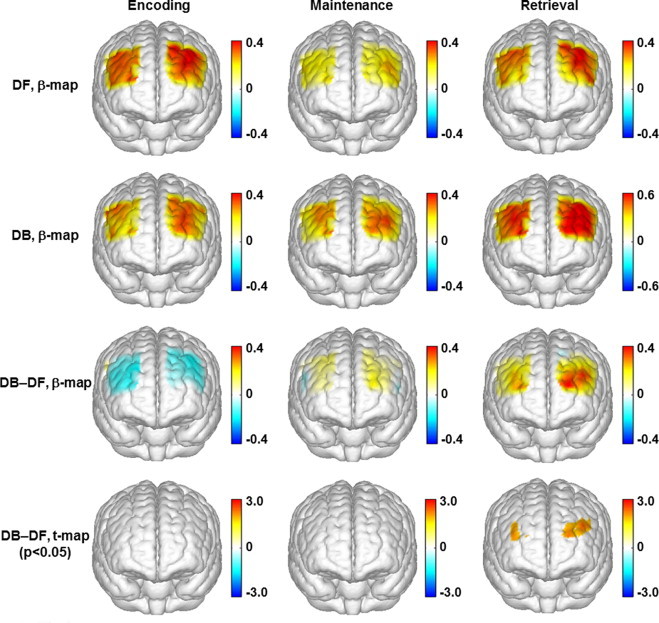
Topographic images of the task-evoked prefrontal activations (derived from ?[HbO2]) in the control group. First row: mean prefrontal activations (ß-maps, in microM) evoked by the digit forward (DF) task in the encoding, maintenance, and retrieval phases. Second row: mean prefrontal activations (ß-maps, in microM) evoked by the digit backward (DB) task in the encoding, maintenance, and retrieval phases. Third row: mean differences of activations (ß-maps, in microM) between the backward and forward tasks (DB-DF) in the encoding, maintenance, and retrieval phases. Fourth row: t-statistical maps (t-maps) which outline the regions of significant difference (paired t-test, p < 0.05) between the backward and forward tasks in the encoding, maintenance, and retrieval phases.
In the control group, the HbO2 changes evoked by the digit backward task (Fig. 3B) had a much higher peak (˜0.58 µM or microM) in the retrieval phase than those (˜0.4 µM or microM) evoked by the digit forward task (paired t-test, p < 0.05). The digit backward task also evoked a subtle peak of HbO2 changes in the maintenance phase. According to the topographic images (second row in Fig. 4), the main regions of activation evoked by the digit backward task were also located in the DLPFC. The predominant difference between the forward and backward tasks was seen in both the DLPFC (BAs 9 and 46) and the anterior prefrontal cortex (APFC, BA 10) in the retrieval phase (third and fourth rows in Fig. 4).
3.2.2. HbO2 changes induced by forward and backward tasks in the PTSD group
For the veterans with PTSD, all Hb changes evoked by the forward and backward tasks were low and insignificant (Fig. 5A and B). The HbO2 changes evoked by the two types of tasks were very similar to each other: a subtle shoulder in the encoding phase before reaching a predominant peak in the maintenance phase, followed by a distinct dip in the retrieval phase. In the forward task, the dip of HbO2 changes in the retrieval phase reached below the baseline. The topographic images (first and second rows in Fig. 6) of the PTSD group showed a focused response region in the left DLPFC during the encoding and maintenance phases, and a distinct deactivation towards the APFC (BA 10) in the retrieval phase especially when completing the forward task. Additionally, the HbO2 changes evoked by the forward and backward tasks were similar to each other, with only a narrow region in APFC (BA 10) showing a significant task difference in the retrieval phase (third and fourth rows in Fig. 6).
Fig. 5.
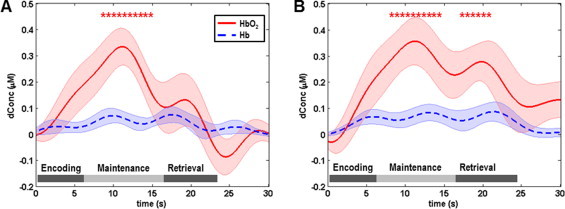
Mean task-evoked prefrontal hemodynamic responses (channel 30 in Fig. 1A) in the PTSD group: (A) mean hemodynamic responses to the digit forward task, in which the mean HbO2 changes show a predominant peak in the maintenance phase followed by a distinct dip in the retrieval phase; (B) mean hemodynamic responses to the digit backward task, in which the mean HbO2 changes are similar to those evoked by the digit forward task. In both panels, the solid lines represent the mean time courses of HbO2, the dotted lines represent the mean time courses of Hb, the shaded regions represent the standard errors, the * symbols indicate the period of significant HbO2 changes (p < 0.01) from the baseline, and the gray bars in the bottom indicate the three phases of the task. It is noted that the actual retrieval time was slightly variable among participants. Therefore the retrieval phases labeled in both panels are schematic and approximate.
Fig. 6.
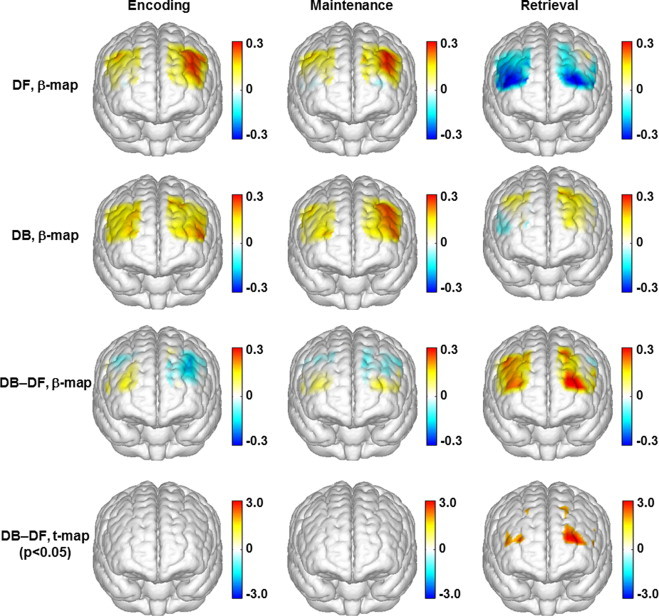
Topographic images of the task-evoked prefrontal activations (derived from ?[HbO2]) in the PTSD group. First row: mean prefrontal activations (ß-maps, in microM) evoked by the digit forward (DF) task in the encoding, maintenance, and retrieval phases. Second row: mean prefrontal activations (ß-maps, in microM) evoked by the digit backward (DB) task in the encoding, maintenance, and retrieval phases. Third row: mean differences of activations (ß-maps, in microM) between the backward and forward tasks (DB-DF) in the encoding, maintenance, and retrieval phases. Fourth row: t-statistical maps (t-maps) which outline the regions of significant difference (p < 0.05) between the backward and forward tasks in the encoding, maintenance, and retrieval phases.
3.2.3. Control group vs. PTSD group
For the digit forward task, since both the control and PTSD groups had activations during encoding and maintenance, between-group comparisons did not identify any channel with significance differences (p < 0.05) in these two phases. In the retrieval phase, because the control group had a distinct activation and the PTSD group showed a distinct deactivation, a large portion of the channels showed highly significant differences between the two participant groups (p < 0.01). According to the topographic images in Fig. 7, two ROIs with significant group differences were identified in the retrieval phase: 1) a broader region in the right prefrontal cortex, which involved mainly the right middle frontal gyrus, and 2) a narrower region in the left prefrontal cortex, which was mainly located within the left superior frontal gyrus.
Fig. 7.
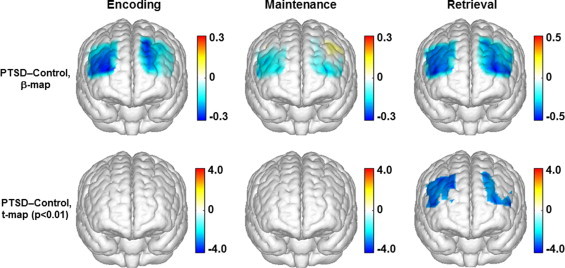
Topographic images (derived from ?[HbO2]) showing the differences between the control and PTSD groups in response to the digit forward task. Top row: mean differences of activations (ß-maps, in microM) between the control and PTSD groups in the encoding, maintenance, and retrieval phases of the digit forward task. Bottom row: t-statistical maps (t-maps) which outline the regions of significant group differences (p < 0.01) in the encoding, maintenance, and retrieval phases of the digit forward task.
For the digit backward task, there were also a large portion of the channels having highly significant between-group differences in the retrieval phase (p < 0.01). As shown in Fig. 8, two identified ROIs included a broader region in the right prefrontal cortex and a narrower region in the left prefrontal cortex, both of which were mainly located in the middle frontal gyrus.
Fig. 8.
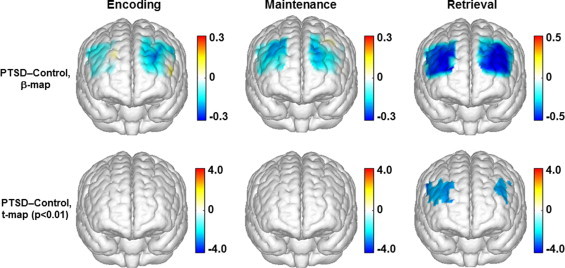
Topographic images (derived from ?[HbO2]) showing the differences between the control and PTSD groups in responses to the digit backward task. Top row: mean differences of activations (ß-maps, in microM) between the control and PTSD groups in the encoding, maintenance, and retrieval phases of the digit backward task. Bottom row: t-statistical maps (t-maps) which outline the regions of significant group differences (p < 0.01) in the encoding, maintenance, and retrieval phases of the digit backward task.
3.3. Correlations between behavioral and hemodynamic measures
For both the forward and backward tasks, linear regression analyses between the individual behavioral and hemodynamic measures were conducted only in the retrieval phase. This was because while the behavioral measures were significantly different between the two participant groups, the hemodynamic measures showed significant group differences only in the retrieval phase, as described above. For the forward task, there was a positive, significant correlation (R = 0.42, p = 0.02) between the individual performance accuracies and the within-ROIs hemodynamic responses in the retrial phase, as shown in Fig. 9A. For the backward task, the correlation between these two measures was low and insignificant (R = 0.002, p = 0.99), which is shown in Fig. 9B.
Fig. 9.
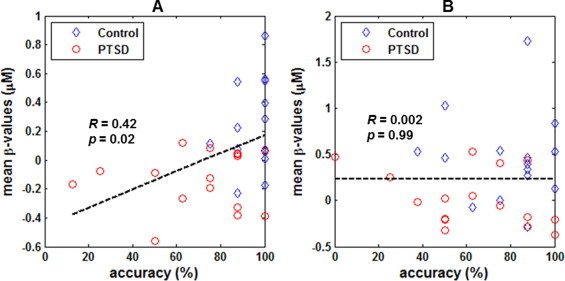
Correlations between the individual performance accuracies and hemodynamic responses to: (A) digit forward task, and (B) digit backward task. For each individual participant and task, the hemodynamic responses were computed as the mean ß-values (?[HbO2], in microM) in the retrieval phase within the identified ROIs which show significant group differences.
4. Discussion
The present study investigated the prefrontal hemodynamic responses to the forward and backward digit span tasks among veterans with PTSD and comorbid conditions, by using multichannel fNIRS. An event-related stimulation design was utilized to reveal the neural correlates of three working memory processes, namely the encoding, maintenance, and retrieval phases. By comparing with the results from the gender- and age-matched healthy controls, PTSD in veterans was found to be associated with distinct deactivations during the retrieval process. Significant prefrontal response differences between the two participant groups were seen predominantly in the right prefrontal cortex as well as a narrow region in the left prefrontal cortex. The results of the present study and the possible neural mechanisms underlying these results are discussed in more detail below.
4.1. Results from the healthy controls
In the control group, the digit forward task evoked robust hemodynamic activations during the encoding and retrieval phases, which is consistent with a previous fMRI report using a similar stimulation protocol (Sun et al., 2005). The digit backward task evoked a much greater activation in the retrieval phase, which is also consistent with the previous fMRI report (Sun et al., 2005). Compared with the forward retrieval, the backward retrieval requires the digits to be held in short-term memory and a mental manipulation to be performed on them, and therefore is believed to engage more executive processes. A greater hemodynamic activation in the backward retrieval is in agreement with a greater neural demand induced by this task.
In the present study, the digit backward task also evoked a subtle peak of HbO2 changes in the maintenance phase, which, however, was not seen in the fMRI study of Sun et al. (2005). This may be presumably because the present study had a longer duration of maintenance (10 s) than in the previous study (6 s). As a result, some participants in the present study might have started to reverse the digit string in the maintenance phase, which could evoke higher HbO2 changes.
The healthy controls also showed significant task differences in behavioral measures, as their accuracies in the backward task were significantly lower than those in the forward task. The reduced accuracies in the backward task are in agreement with the more complex processes involved in this task, as described above.
4.2. Results from the veterans with PTSD
For the veterans with PTSD, both the forward and backward tasks evoked hemodynamic activations during the encoding and maintenance phases, which were consistent with the healthy controls, followed by distinct deactivations in the retrieval phase. The hemodynamic responses to the two tasks were similar in terms of temporal evolutions and magnitudes. The veterans with PTSD also showed similar performance accuracies across the two tasks. It seems that there was a ceiling effect in the veterans with PTSD when completing the forward and backward tasks: while their performance was already poor in the forward task compared with their healthy counterparts, it did not become worse in the backward task. This ceiling effect could also explain the high similarity of the hemodynamic responses to the two tasks in veterans with PTSD.
4.3. Differences between the two participant groups
In the present study, statistical comparisons of task-evoked hemodynamic responses between the two participant groups revealed that veterans with PTSD had significantly abnormal prefrontal responses compared with healthy controls while processing the working memory tasks. A remarkable finding is that the healthy controls had nearly equal activations in the two hemispheres during all three phases (see Fig. 3), but veterans with PTSD showed dominant activations in the left DLPFC in the encoding and maintenance phases (see Fig. 5). These observations were consistent with a study by Schiffer et al. (1995) that demonstrated differences in activity between right and left hemispheres evoked by negative vs. neutral (i.e., not trauma-related) memories in patients with a history of trauma, but not in controls. According to their hemispheric emotional valence (HEV) model, the right frontal cortex is more associated with the processing of negative emotions, and the left frontal cortex is more associated with the processing of positive and neutral emotions. When recalling emotional memories, this right–left difference was significant in patients with a history of trauma, whereas control subjects had smaller, insignificant changes (Schiffer et al., 1995). The digit span tasks used in our study represented a neutral (i.e., not trauma-related) memory, and should therefore show more lateralized activations to the left prefrontal cortex of PTSD participants than in controls, as predicted by the HEV model. Although another study (Schiffer et al., 2007) pointed out that HEV-laterality is more individually determined, one could still expect a majority of PTSD patients to have predominantly left-sided prefrontal activations during a neutral working memory task, as we found in our study.
More importantly, veteran PTSD was found to be associated with distinct deactivations during the retrieval process, especially on the right prefrontal cortex (see Figs. 6 and 7). The identified ROIs that showed significant group differences included the right prefrontal cortex, localized at the right middle frontal gyrus, and a narrow region in the left prefrontal cortex, localized within the superior frontal gyrus in the forward task and within the middle frontal gyrus in the backward task. While the present study could not determine the exact neural mechanisms underlying this deactivation involved in memory retrieval, several possible interpretations are discussed below.
First, the veterans with PTSD had significantly poorer performance accuracies, indicating that they might have greater difficulty than the healthy controls in completing both the forward and backward tasks. However, the hemodynamic deactivations during retrieval in veterans with PTSD are unlikely a result of greater task difficulty. Previous studies (Hoshi et al., 2000; Gerton et al., 2004) on a group of healthy controls had demonstrated that a greater task difficulty (or with a longer length of digit string) would not cause deactivation during the retrieval process. To further confirm this conclusion, we also retested nine healthy participants in the control group with eight-digit forward tasks. When the task difficulty was increased from six digits to eight digits, these participants still showed clear activations (significant HbO2 increase) in the retrieval phase (Fig. 10).
Fig. 10.

Mean prefrontal hemodynamic responses (channel 30 in Fig. 1A) to the eight-digit forward task among nine retested healthy controls. In this graph the solid lines represent the mean time courses of HbO2, the dotted lines represent the mean time courses of Hb, the shaded regions represent the standard errors, the * symbols indicate the period of significant changes (p < 0.01) from the baseline, and the gray bars in the bottom approximately indicate the three phases of the task. The mean time course of HbO2 shows two robust peaks in the encoding and retrieval phases, similar to the responses evoked by the six-digit forward task (Fig. 3A). It demonstrates that for healthy controls, an increased task difficulty (from six digits to eight digits) still causes prefrontal activation rather than any deactivation.
Second, as reviewed in the Introduction, a few neuroimaging studies of working memory have consistently found that hypoactivation in the prefrontal cortex is associated with PTSD (Matsuo et al., 2003; Clark et al., 2003; Moores et al., 2008). These studies used block designs to measure the accumulated neural correlates of the working memory tasks. In the present study, if we assume that the accumulated neural correlates in a block design could be estimated as a summation of event-related changes across all of the transit memory processes (encoding, maintenance, and retrieval), the hemodynamic activations during encoding and maintenance would be reduced by the deactivation during retrieval in veterans with PTSD, that should lead to results consistent with the previous neuroimaging studies. Thus, some of the findings in the previous studies can be applied to interpret the results in this study. The study by Matsuo et al. (2003) using the same fNIRS technology found a strong correlation between the prefrontal memory-related hypoactivation with the lower scores on attention and concentration among PTSD patients (victims of the Tokyo Subway Sarin Attack). They concluded that the prefrontal hypoactivation might be secondary to a reduced attention and concentration capacity in PTSD patients; namely, the prefrontal cortex was not efficiently concentrated or engaged in the given memory task. The results in the present study revealed, however, that the prefrontal ‘hypoactivation’ could be a composite outcome of activations during the encoding and maintenance phases followed by a deactivation during the retrieval phase. The deactivation cannot be explained simply as an insufficient engagement of the prefrontal cortex, because an insufficient brain activity or engagement theoretically should cause hemodynamic activation with reduced magnitude, rather than hemodynamic deactivation.
Finally, we speculate that the distinct deactivation revealed by the present study may represent an active inhibition of prefrontal activities in the retrieval phase (Tomasi et al., 2006). The PTSD participants, but not the controls, appeared to suppress prefrontal activity during memory retrieval. This deactivation was more pronounced in the right DLPFC. This finding is consistent with a previous fMRI study of PTSD patients performing a memory task of 12-word-pair associates (Geuze et al., 2008). Similar to our digit span working memory tasks, the reported associative memory task consisted of neutral (not trauma related) material, and PTSD subjects also showed under-activation of the right prefrontal cortex during retrieval. Furthermore, both structural and functional neuroimaging studies support that impairment of the right DLPFC is involved in PTSD, and that normalization of the right DLPFC is linked to recovery from trauma (Lyoo et al., 2011). Repetitive transcranial magnetic stimulation (rTMS) of the left or right DLPFC is effective at reducing symptoms in PTSD (up to 3 months follow-up), but increasing cortical excitability in the right DLPFC induces a larger beneficial effect compared to the left (Boggio et al., 2010). Together with our findings, these studies suggest that normalization of DLPFC activity, particularly on the right hemisphere, might be a direction for developing treatments of PTSD.
4.4. Correlations between the behavioral and hemodynamic measures
For the forward task, a positive, significant correlation between the individual performance accuracies and hemodynamic responses in the retrieval phase was found for both groups. However, for the backward task, the correlation between these two measures was low. Such a low correlation in the backward task might be attributed to the potential ceiling effect in the PTSD group. As discussed above, although the backward task is apparently more difficult than the forward task, the veterans with PTSD showed small differences in both the behavioral and hemodynamic measures between the two tasks. In a scenario in which veterans with PTSD reach a response ceiling in the backward task, a low correlation between the behavioral and hemodynamic measures in this task is expected. A useful piece of information obtained from the behavioral–hemodynamic correlations is that, for future neuroimaging studies, the digit forward task seems a better task to use for objective assessment of the memory-related dysfunctions among PTSD patients. This task adapts well to participants with both normal and declined neurocognitive abilities. The task-evoked hemodynamic responses predict the participants' performance in a broad range. In contrast, the digit backward task seems too difficult for patients with impaired memory functions.
4.5. Limitations of the present study
We acknowledge several technical limitations in the present study. First, the fNIRS probe in the present study covered only a limited region of the prefrontal cortex. The involvement of other cortical regions during the digit span tasks was not investigated. The present study can be improved by using a multi-patch probe to cover more cortical regions that deal with memory and attention. Second, in the GLM analysis, we used a standard HRF derived from BOLD fMRI (Glover, 1999) as a surrogate to model the signals measured by fNIRS. This approach is reasonable because both fMRI and fNIRS detect similar hemodynamic changes in the brain. However, a previous study using a finger-tapping task did show that temporal profiles in HbO2 changes deviated from those of BOLD signals (Huppert et al., 2006). It means that an HRF derived from BOLD signals is not the best to model the HbO2 changes quantified by fNIRS. Lastly, the veterans with PTSD in the study had comorbid conditions, which may create hemodynamic signals that confound those resulting only from PTSD conditions.
5. Conclusions and future work
In the present study, multichannel fNIRS was utilized to assess the involvement of the prefrontal cortex in working memory processes among veterans with PTSD and age-/gender-matched healthy controls. The veterans with PTSD, but not the healthy controls, were found to have distinct deactivations during the retrieval phase of working memory, which might implicate an active inhibition of prefrontal neural activity. The deactivation in the retrieval phase was more pronounced in the right dorsolateral prefrontal cortex than the left side among the veterans with PTSD. The present study demonstrated that fNIRS could be a portable and complementary neuroimaging tool to study the cognitive dysfunctions associated with PTSD. We expect that a larger coverage of the cortex with more optodes and measurement channels will permit us to simultaneously reveal essential cortical regions involved in cognitive processes. In the meantime, future studies should seek a better HRF derived from fNIRS signals directly, while much effort on exclusion of subjects with comorbid conditions from the study is needed in order to accurately investigate prefrontal responses to memory phases in subjects with PTSD only.
Acknowledgments
This work was supported in part by the Hogg Foundation for Mental Health, UT Arlington Research Enhancement fund, the Dallas Foundation, and private donors in support of the Student Veteran Project (SVP). The authors would like to thank all of the SVP clinicians, case managers, and veteran peer facilitators involved in this program for their assistance in recruiting and providing services to the veteran participants with PTSD. The authors would also like to thank Bejoy Mathai, M.S., Neha Khandelwal, M.S., and Nikita S. Bapat, M.S., for their assistance in the collection of fNIRS data. Dr. North discloses employment by VA North Texas Health Care System, Dallas, Texas, USA. The points of view in this document are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs or the United States Government.
References
- Aguirre G., D’Esposito M. Experimental Design for Brain fMRI. Functional MRI. Springer-Verlag; Berlin: 1999. pp. 369–380. [Google Scholar]
- Arbanas G. Patients with combat-related and war-related posttraumatic stress disorder 10 years after diagnosis. Croatian Medical Journal. 2010;51:209–214. doi: 10.3325/cmj.2010.51.209. 20564763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle R.L., Melrose A.J., Stein M.B., Paulus M.P. Executive function and PTSD: disengaging from trauma. Neuropharmacology. 2012;62:686–694. doi: 10.1016/j.neuropharm.2011.02.008. 21349277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;225:566–569. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13468–13472. doi: 10.1073/pnas.93.24.13468. 8942958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandes D., Ben-Schachar G., Gilboa A., Bonne O., Freedman S., Shalev A.Y. PTSD symptoms and cognitive performance in recent trauma survivors. Psychiatry Research. 2002;110:231–238. doi: 10.1016/s0165-1781(02)00125-7. 12127473 [DOI] [PubMed] [Google Scholar]
- Bremner J.D., Scott T.M., Delaney R.C., Southwick S.M., Mason J.W., Johnson D.R., Innis R.B., McCarthy G., Charney D.S. Deficits in short-term memory in posttraumatic stress disorder. American Journal of Psychiatry. 1993;150:1015–1019. doi: 10.1176/ajp.150.7.1015. 8317569 [DOI] [PubMed] [Google Scholar]
- Bremner J.D. Neuroimaging studies in post-traumatic stress disorder. Current Psychiatry Reports. 2002;4:254–263. doi: 10.1007/s11920-996-0044-9. 12126593 [DOI] [PubMed] [Google Scholar]
- Boas D.A., Dale A.M., Franceschini M.A. Diffuse optical imaging of brain activation: approaches to optimizing image sensitivity, resolution, and accuracy. NeuroImage. 2004;23(Suppl. 1):S275–S288. doi: 10.1016/j.neuroimage.2004.07.011. 15501097 [DOI] [PubMed] [Google Scholar]
- Boggio P.S., Rocha M., Oliveira M.O., Fecteau S., Cohen R.B., Campanhã C., Ferreira-Santos E., Meleiro A., Corchs F., Zaghi S., Pascual-Leone A., Fregni F. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. Journal of Clinical Psychiatry. 2010;71:992–999. doi: 10.4088/JCP.08m04638blu. 20051219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C.R., McFarlane A.C., Morris P., Weber D.L., Sonkkilla C., Shaw M., Marcina J., Tochon-Danguy H.J., Egan G.F. Cerebral function in posttraumatic stress disorder during verbal working memory updating: a positron emission tomography study. Biological Psychiatry. 2003;53:474–481. doi: 10.1016/s0006-3223(02)01505-6. 12644352 [DOI] [PubMed] [Google Scholar]
- Cope M., Delpy D.T., Reynolds E.O., Wray S., Wyatt J., van der Zee P. Methods of quantitating cerebral near infrared spectroscopy data. Advances in Experimental Medicine and Biology. 1988;222:183–189. doi: 10.1007/978-1-4615-9510-6_21. 3129910 [DOI] [PubMed] [Google Scholar]
- Cui X., Bray S., Bryant D.M., Glover G.H., Reiss A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage. 2011;54:2808–2821. doi: 10.1016/j.neuroimage.2010.10.069. 21047559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghani H., White B.R., Zeff B.W., Tizzard A., Culver J.P. Depth sensitivity and image reconstruction analysis of dense imaging arrays for mapping brain function with diffuse optical tomography. Applied Optics. 2009;48:D137–D143. doi: 10.1364/ao.48.00d137. 19340101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M., Zarahn E., Aguirre G.K. Event-related functional MRI: implications for cognitive psychology. Psychological Bulletin. 1999;125:155–164. doi: 10.1037/0033-2909.125.1.155. 9990848 [DOI] [PubMed] [Google Scholar]
- Dohrenwend B.P., Turner J.B., Turse N.A., Adams B.G., Koenen K.C., Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science (New York, N.Y.) 2006;313:979–982. doi: 10.1126/science.1128944. 16917066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga B.M., Ardon A.M., Heijnis M.K., De Ruiter M.B., Van Dyck R., Veltman D.J. Neural correlates of enhanced working-memory performance in dissociative disorder: a functional MRI study. Psychological Medicine. 2007;37:235–245. doi: 10.1017/S0033291706008932. 17018171 [DOI] [PubMed] [Google Scholar]
- Erickson D.J., Wolfe J., King D.W., King L.A., Sharkansky E.J. Posttraumatic stress disorder and depression symptomatology in a sample of gulf War veterans: a prospective analysis. Journal of Consulting and Clinical Psychology. 2001;69:41–49. doi: 10.1037//0022-006x.69.1.41. 11302276 [DOI] [PubMed] [Google Scholar]
- Essenpreis M., Elwell C.E., Cope M., van der Zee P., Arridge S.R., Delpy D.T. Spectral dependence of temporal point spread functions in human tissues. Applied Optics. 1993;32:418–425. doi: 10.1364/AO.32.000418. 20802707 [DOI] [PubMed] [Google Scholar]
- Ferrari M., Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. NeuroImage. 2012;63:921–935. doi: 10.1016/j.neuroimage.2012.03.049. 22510258 [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP), Biometrics Research. New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Francati V., Vermetten E., Bremner J.D. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depression and Anxiety. 2007;24:202–218. doi: 10.1002/da.20208. 16960853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletly C., Clark C.R., McFarlane A.C., Weber D.L. Working memory in posttraumatic stress disorder — an event-related potential study. Journal of Traumatic Stress. 2001;14:295–309. doi: 10.1023/A:1011112917797. 11469158 [DOI] [PubMed] [Google Scholar]
- Gerton B.K., Brown T.T., Meyer-Lindenberg A., Kohn P., Holt J.L., Olsen R.K., Berman K.F. Shared and distinct neurophysiological components of the digits forward and backward tasks as revealed by functional neuroimaging. Neuropsychologia. 2004;42:1781–1787. doi: 10.1016/j.neuropsychologia.2004.04.023. 15351627 [DOI] [PubMed] [Google Scholar]
- Geuze E., Vermetten E., Ruf M., de Kloet C.S., Westenberg H.G. Neural correlates of associative learning and memory in veterans with posttraumatic stress disorder. Journal of Psychiatric Research. 2008;42:659–669. doi: 10.1016/j.jpsychires.2007.06.007. 17698081 [DOI] [PubMed] [Google Scholar]
- Glover G.H. Deconvolution of impulse response in event-related BOLD fMRI. NeuroImage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. 10191170 [DOI] [PubMed] [Google Scholar]
- Hassanpour M.S., White B.R., Eggebrecht A.T., Ferradal S.L., Snyder A.Z., Culver J.P. Statistical analysis of high density diffuse optical tomography. NeuroImage. 2014;85:104–116. doi: 10.1016/j.neuroimage.2013.05.105. 23732886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge C.W., Castro C.A., Messer S.C., McGurk D., Cotting D.I., Koffman R.L. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. New England Journal of Medicine. 2004;351:13–22. doi: 10.1056/NEJMoa040603. 15229303 [DOI] [PubMed] [Google Scholar]
- Hoshi Y., Oda I., Wada Y., Ito Y., Yamashita Y., Oda M., Ohta K., Yamada Y., Tamura M. Visuospatial imagery is a fruitful strategy for the digit span backward task: a study with near-infrared optical tomography. Cognitive Brain Research. 2000;9:339–342. doi: 10.1016/s0926-6410(00)00006-9. [DOI] [PubMed] [Google Scholar]
- Hoshi Y. Functional near-infrared spectroscopy: current status and future prospects. Journal of Biomedical Optics. 2007;12:062106. doi: 10.1117/1.2804911. 18163809 [DOI] [PubMed] [Google Scholar]
- Huppert T.J., Hoge R.D., Diamond S.G., Franceschini M.A., Boas D.A. A temporal comparison of BOLD, ASL, and NIRS hemodynamic responses to motor stimuli in adult humans. Neuroimage. 2006;29:368–382. doi: 10.1016/j.neuroimage.2005.08.065. 16303317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert T.J., Diamond S.G., Franceschini M.A., Boas D.A. HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain. Applied Optics. 2009;48:D280–D298. doi: 10.1364/ao.48.00d280. 19340120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyer L., Davis H., Boudewyns P., Woods M.G. A short form of the Mississippi Scale for Combat-Related PTSD. Journal of Clinical Psychology. 1991;47:510–518. doi: 10.1002/1097-4679(199107)47:4<510::aid-jclp2270470407>3.0.co;2-f. 1939695 [DOI] [PubMed] [Google Scholar]
- Keane T.M., Malloy P.F., Fairbank J.A. Empirical development of an MMPI subscale for the assessment of combat-related post-traumatic stress disorder. Journal of Consulting and Clinical Psychology. 1984;52:888–891. doi: 10.1037//0022-006x.52.5.888. [DOI] [PubMed] [Google Scholar]
- Keane T.M., Caddell J.M., Taylor K.L. Mississippi Scale for Combat-Related Posttraumatic Stress Disorder: three studies in reliability and validity. Journal of Consulting and Clinical Psychology. 1988;56:85–90. doi: 10.1037//0022-006x.56.1.85. 3346454 [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. 7492257 [DOI] [PubMed] [Google Scholar]
- Koenen K.C., Driver K.L., Oscar-Berman M., Wolfe J., Folsom S., Huang M.T., Schlesinger L. Measures of prefrontal system dysfunction in posttraumatic stress disorder. Brain and Cognition. 2001;45:64–78. doi: 10.1006/brcg.2000.1256. 11161363 [DOI] [PubMed] [Google Scholar]
- Lyoo I.K., Kim J.E., Yoon S.J., Hwang J., Bae S., Kim D.J. The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma. Longitudinal brain imaging study among survivors of the South Korean subway disaster. Archives of General Psychiatry. 2011;68:701–713. doi: 10.1001/archgenpsychiatry.2011.70. 21727254 [DOI] [PubMed] [Google Scholar]
- Matsuo K., Taneichi K., Matsumoto A., Ohtani T., Yamasue H., Sakano Y., Sasaki T., Sadamatsu M., Kasai K., Iwanami A., Asukai N., Kato N., Kato T. Hypoactivation of the prefrontal cortex during verbal fluency test in PTSD: a near-infrared spectroscopy study. Psychiatry Research. 2003;124:1–10. doi: 10.1016/s0925-4927(03)00093-3. 14511791 [DOI] [PubMed] [Google Scholar]
- Moores K.A., Clark C.R., McFarlane A.C., Brown G.C., Puce A., Taylor D.J. Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Research. 2008;163:156–170. doi: 10.1016/j.pscychresns.2007.08.011. 18455372 [DOI] [PubMed] [Google Scholar]
- Moro S.B., Cutini S., Ursini M.L., Ferrari M., Quaresima V. Prefrontal cortex activation during story encoding/retrieval: a multi-channel functional near-infrared spectroscopy study. Frontiers in Human Neuroscience. 2013;7:925. doi: 10.3389/fnhum.2013.00925. 24427131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta M.M., Herrmann M.J., Baehne C.G., Ehlis A.C., Richter M.M., Pauli P., Fallgatter A.J. Event-related functional near-infrared spectroscopy (fNIRS): are the measurements reliable? NeuroImage. 2006;31:116–124. doi: 10.1016/j.neuroimage.2005.12.008. 16446104 [DOI] [PubMed] [Google Scholar]
- Roca V., Hart J., Kimbrell T., Freeman T. Cognitive function and dissociative disorder status among veteran subjects with chronic posttraumatic stress disorder: a preliminary study. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18:226–230. doi: 10.1176/jnp.2006.18.2.226. 16720800 [DOI] [PubMed] [Google Scholar]
- Sato H., Yahata N., Funane T., Takizawa R., Katura T., Atsumori H., Nishimura Y., Kinoshita A., Kiguchi M., Koizumi H., Fukuda M., Kasai K. A NIRS–fMRI investigation of prefrontal cortex activity during a working memory task. Neuroimage. 2013;83:158–173. doi: 10.1016/j.neuroimage.2013.06.043. 23792984 [DOI] [PubMed] [Google Scholar]
- Schiffer F., Teicher M.H., Papanicolaou A.C. Evoked potential evidence for right brain activity during the recall of traumatic memories. Journal of Neuropsychiatry and Clinical Neurosciences. 1995;7:169–175. doi: 10.1176/jnp.7.2.169. 7626959 [DOI] [PubMed] [Google Scholar]
- Schiffer F., Teicher M.H., Anderson C., Tomoda A., Polcari A., Navalta C.P., Anderson S.L. Determination of hemispheric emotional valence in individual subjects: a new approach with research and therapeutic implications. Behavioral and Brain Functions: BBF. 2007;3:13. doi: 10.1186/1744-9081-3-13. 17341309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M.E., Strother S.C., McFarlane A.C., Morris P., Anderson J., Clark C.R., Egan G.F. Abnormal functional connectivity in posttraumatic stress disorder. NeuroImage. 2002;15:661–674. doi: 10.1006/nimg.2001.1024. 11848709 [DOI] [PubMed] [Google Scholar]
- Shaw M.E., Moores K.A., Clark R.C., McFarlane A.C., Strother S.C., Bryant R.A., Brown G.C., Taylor J.D. Functional connectivity reveals inefficient working memory systems in post-traumatic stress disorder. Psychiatry Research. 2009;172:235–241. doi: 10.1016/j.pscychresns.2008.07.014. 19398308 [DOI] [PubMed] [Google Scholar]
- Singh A.K., Okamoto M., Dan H., Jurcak V., Dan I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. NeuroImage. 2005;27:842–851. doi: 10.1016/j.neuroimage.2005.05.019. 15979346 [DOI] [PubMed] [Google Scholar]
- Sun X., Zhang X., Chen X., Zhang P., Bao M., Zhang D., Chen J., He S., Hu X. Age-dependent brain activation during forward and backward digit recall revealed by fMRI. NeuroImage. 2005;26:36–47. doi: 10.1016/j.neuroimage.2005.01.022. 15862203 [DOI] [PubMed] [Google Scholar]
- Tian F., Kozel A., Yennu A., Croarkin P.E., McClintock S.M., Mapes K.S., Husain M.M., Liu H. Test–retest assessment of cortical activation induced by repetitive transcranial magnetic stimulation with brain atlas-guided optical topography. Journal of Biomedical Optics. 2012;17:116020. doi: 10.1117/1.JBO.17.11.116020. 23139044 [DOI] [PubMed] [Google Scholar]
- Tian F., Liu H. Depth-compensated diffuse optical tomography enhanced by general linear model analysis and an anatomical atlas of human head. NeuroImage. 2014;85:166–180. doi: 10.1016/j.neuroimage.2013.07.016. 23859922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Ernst T., Caparelli E.C., Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Human Brain Mapping. 2006;27:694–705. doi: 10.1002/hbm.20211. 16404736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling J.J., Brailey K., Constans J.I., Sutker P.B. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. 9460740 [DOI] [PubMed] [Google Scholar]
- Vasterling J.J., Duke L.M., Brailey K., Constans J.I., Allain A.N., Jr, Sutker P.B. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. 11853357 [DOI] [PubMed] [Google Scholar]
- Veltmeyer M.D., Clark C.R., McFarlane A.C., Moores K.A., Bryant R.A., Gordon E. Working memory function in post-traumatic stress disorder: an event-related potential study. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2009;120:1096–1106. doi: 10.1016/j.clinph.2009.03.024. 19442579 [DOI] [PubMed] [Google Scholar]
- Weathers F., Litz B., Herman D., Huska J., Keane T. The PTSD checklist: reliability, validity, & diagnostic utility. Annual Meeting of the Internation Society of Traumatic Stress Studies, San Antonio, TX. 1993 [Google Scholar]
- Weber D.L., Clark C.R., McFarlane A.C., Moores K.A., Morris P., Egan G.F. Abnormal frontal and parietal activity during working memory updating in post-traumatic stress disorder. Psychiatry Research. 2005;140:27–44. doi: 10.1016/j.pscychresns.2005.07.003. 16202566 [DOI] [PubMed] [Google Scholar]
- Wilkins K.C., Lang A.J., Norman S.B. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depression and Anxiety. 2011;28:596–606. doi: 10.1002/da.20837. 21681864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J.C., Tak S., Jang K.E., Jung J., Jang J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. NeuroImage. 2009;44:428–447. doi: 10.1016/j.neuroimage.2008.08.036. 18848897 [DOI] [PubMed] [Google Scholar]
- Zarahn E., Aguirre G.K., D’Esposito M. Temporal isolation of the neural correlates of spatial mnemonic processing with fMRI. Brain Research. Cognitive Brain Research. 1999;7:255–268. doi: 10.1016/s0926-6410(98)00029-9. 9838152 [DOI] [PubMed] [Google Scholar]
- Zeff B.W., White B.R., Dehghani H., Schlaggar B.L., Culver J.P. Retinotopic mapping of adult human visual cortex with high-density diffuse optical tomography. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12169–12174. doi: 10.1073/pnas.0611266104. 17616584 [DOI] [PMC free article] [PubMed] [Google Scholar]


