Key Points
The frequency of CD8+CD45RA+CCR7+ cells, a subset closest to T-memory stem cells, correlates with CAR–T-cell expansion in lymphoma patients.
IL-7 and IL-15 increase the frequency of CD8+CD45RA+CCR7+ cells during the ex vivo expansion of CAR+ T cells.
Abstract
Adoptive transfer of T lymphocytes expressing a CD19-specific chimeric antigen receptor (CAR.CD19) induces complete tumor regression in patients with lymphoid malignancies. Although in vivo persistence of CAR-T cells correlates with clinical responses, it remains unknown whether specific cell subsets within the CAR–T-cell product correlate with their subsequent in vivo expansion and persistence. We analyzed 14 patients with B-cell malignancies infused with autologous CAR.CD19-redirected T cells expanded ex vivo using IL-2, and found that their in vivo expansion only correlated with the frequency within the infused product of a CD8+CD45RA+CCR7+ subset, whose phenotype is closest to “T-memory stem cells.” Preclinical models showed that increasing the frequency of CD8+CD45RA+CCR7+ CAR-T cells in the infused line by culturing the cells with IL-7 and IL-15 produced greater antitumor activity of CAR-T cells mediated by increased resistance to cell death, following repetitive encounters with the antigen, while preserving their migration to secondary lymphoid organs. This trial was registered at www.clinicaltrials.gov as #NCT00586391 and #NCT00709033.
Introduction
Clinical experience with chimeric antigen receptor (CAR.CD19)-redirected T cells in patients with B-cell malignancies corroborates with previous studies with tumor infiltrating T lymphocytes in melanoma patients by showing a correlation between in vivo expansion and the persistence of adoptively transferred CAR-T cells and clinical outcome.1-6 It remains unclear, however, if specific T-cell subsets within the infused cellular products correlate with the capacity of CAR-T cells to expand and persist in vivo.
To generate CAR-T cells, T lymphocytes are generally activated from unselected peripheral blood mononuclear cells (PBMCs) through cross-linking antibodies (CD3 and/or CD28), transduced with γ retroviral or lentiviral vectors encoding the CAR, and then expanded ex vivo using the γc chain cytokine, IL-2.1,7,8 T-cell products obtained using these procedures are phenotypically heterogeneous, but predominantly composed of antigen-experienced T cells as they express the CD45RO isoform. Although the great majority of these cells are effector-memory cells, less than 10% co-express CD62L and CCR7,9-14 which are the canonical central-memory cells.2,8
Studies of adoptive T-cell transfer in mice and nonhuman primates suggest that although effector-memory T cells have robust cytolytic function, only central-memory T cells and other less differentiated T-cell subsets, such as naïve and the recently defined “T-memory stem cells,” are critical for in vivo expansion, survival, and long-term persistence. For instance, in nonhuman primates, only virus-specific cytotoxic T lymphocytes selectively expanded from CD62L+ cells as a surrogate marker of central-memory T cells, show robust and long-term persistence compared with T cells with identical antigen specificity but generated from the CD62L– fraction.15 In mouse xenograft models, human “T-memory stem cells” identified in the CD45RA+ T-cell compartment and expressing CD62L, CCR7, and high levels of CD95 show expansion, survival, and antitumor activity that are superior even to central-memory T cells.16,17
The translation of these fundamental discoveries into T-cell manufacturing protocols that will select or generate predominantly central-memory or “T-memory stem cells” is a matter of intense investigation. However, the clinical relevance of these specific subsets in the context of adoptive T-cell therapies in cancer patients remains to be validated. In this study, we demonstrate for the first time, in a clinical setting, that only the frequency of a subset of CD8+ T cells that phenotypically resemble T cells closely related to “T-memory stem cells” within the CAR–T-cell product correlates with in vivo expansion. We also found that substituting alternative γc chain cytokines for IL-2, namely IL-7 and IL-15, better preserves this T-cell subset ex vivo, suggesting that these cytokines will have a significant impact on the future clinical applications of CAR–T-cell therapies.
Material and methods
Patients enrolled in the clinical study
Fourteen patients with relapsed/refractory B-cell malignancies were infused with autologous T-cell products genetically manipulated to express a second generation (CD28 endodomain) CD19-specific CAR (CAR.CD19), according to protocols approved by regulatory agencies.8 Approval was obtained from the Baylor College of Medicine Institutional Review Board, and the study was conducted in accordance with the Declaration of Helsinki. T-cell lines were manufactured by stimulating unselected T cells with OKT3 and/or CD3/CD28 antibodies and IL-2.8 Persistence of CAR-T cells was evaluated in peripheral blood at different time points after infusion by a specific quantitative polymerase chain reaction (qPCR) assay.8
Cell lines and CAR-T cell generation
Raji (CD19+ lymphoma) was maintained in RPMI 1640 (Gibco-BRL) supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT) and 2 mM L-glutamine (Gibco-BRL, San Francisco, CA). Human PBMCs, obtained from healthy volunteer donors (Gulf Coast Regional Blood Center, Houston, TX), were stimulated by plate-bound OKT3 (1 ng/mL) and CD28 antibodies (1 ng/mL) (BD Biosciences, Mountain View, CA), and then cultured in media containing 45% Click’s media (Irvine Scientific, Santa Ana, CA), 45% RPMI 1640, 10% fetal bovine serum (Hyclone), 1% L-glutamine (Invitrogen, Carlsbad, CA), IL-2 (100 U/mL) (Teceleukin; Hoffmann-La Roche, Rockville, MD) or IL-7 (10 ng/mL) (PeproTech, Rocky Hill, NJ), and IL-15 (5 ng/mL) (Peprotech). Stimulated PBMCs were then transduced with a γ retroviral vector encoding the CAR.CD19.8 Transduced cells were fed with IL-2 (50 U/mL) or IL-7 (10 ng/mL) and IL-15 (5 ng/mL) twice a week for 12 to 14 days of culture before subsequent analysis.
Flow cytometry and antibodies
CAR–T-cell lines were stained with fluorescent-labeled antibodies against CD3, CD4, CD8, CD25, CD27, CD28, CD45RA, CD45RO, CD56, CD57, CD69, CD107a/b, and CD127 (BD, Franklin Lakes, NJ); CCR1-6, CXCR1, CXCR3, and CXCR4-6 (BioLegend, San Diego, CA); CCR7 (R&D Systems, Minneapolis, MN), and anti-IgG1-CH2-CH3 (Jackson ImmunoResearch, West Grove, PA). For intracellular staining, cells were fixed and permeabilized using Cytofix/Cytoperm solution, and stained with antibodies against IFN-γ, perforin, and granzyme-B in Perm/Wash buffer. Data were acquired using fluorescence-activated cell sorter (FACSCalibur; BD) and analyzed by FlowJo 9.3.2 software (Tree Star, Ashland, OR).
Cytokine production by CAR-T cells
CAR-T cells were cocultured with target tumor cells Raji (1:1 effector to target ratio [E:T]ratio) for 24 hours before the culture supernatant was collected. The presence of IL-2 and IFN-γ was quantified by enzyme-linked immunosorbent assay (R&D Systems).
Apoptosis analysis
CAR-T cells were cocultured with irradiated Raji tumor cells for 3 days, stained with CD4 and CD8 antibodies, then washed and stained with Annexin-V/7-AAD kit (BD) using the buffer provided.
Carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution assay
CAR-T cells were labeled with CFSE (Invitrogen) as previously described.18 CFSE-loaded CAR-T cells were then stimulated by irradiated Raji cells for 3 days without the addition of exogeneous cytokines, and then stained with CD4 and CD8 antibodies. The dilution of CFSE was determined by FACS. Proliferation and division index of CD4+ or CD8+ CAR-T cells were calculated by FlowJo 9.3.2 software.
Repeated stimulation and in vitro coculture experiments
CAR-T cells (0.5 × 106 cells) were stimulated by irradiated Raji cells (1:1 E:T) for 3 days, counted by Trypan Blue dye exclusion, and then re-stimulated for a total of 3 rounds without the addition of exogeneous cytokines. To assess antitumor activity in vitro, CAR-T cells were cocultured with “live” target tumor cells (1:2 E:T). After 3 days of coculture, cells were stained with CD3 and CD19 antibodies, and the percentage of tumor cells (CD19+CD3−) was measured by FACS.
In vitro migration assay
CCL21 (400 ng/mL) (R&D Systems) was added to the lower chamber of transwell plates (5 μM membrane) in serum-free media. CAR-T cells were cultured in serum-free media overnight and equal numbers of total T cells (105) were added to the upper chamber for 6 hours. The number of cells migrating toward the CCL21-compartment was quantified via FACS bead-counting.
In vivo migration assay
Eight-week-old nonobese diabetic/severe combined immunodeficiency/γc−/− (NSG) mice (The Jackson Laboratory, Bar Harbor, ME) were subcutaneously engrafted with Epstein-Barr virus (EBV) transformed human B lymphocyte cell lines (EBV-LCL). Two to 3 weeks after engraftment, 107 CAR-T cells were infused intravenously. Three days after T-cell infusion, mice were euthanized and peripheral blood, spleen, and tumor were collected, and CAR-T cells were enumerated in the blood and spleen using bead counting.
In vivo antitumor activity
Eight-week-old NSG mice were infused with 0.5 × 106 Raji cells intravenously. One week after infusion, mice were intravenously injected with 107 T cells transduced with the CAR and Firefly luciferase. Signal from infused T cells was monitored using the Xenogen-IVIS Imaging System (Caliper Life Sciences, Hopkinton, MA). Because the tumor burden in mice infused with tumor cells according to the schedule described above induces rapid hind-limb paralysis, to assess the antitumor effects, we reduced the tumor load and NSG mice were infused with 0.2 × 106 Raji tumor cells, intravenously. Two days later, mice were injected with 107 CAR-T cells. Mice developing hind-limb paralysis, which indicates tumor progression, were euthanized. Survival curves were generated by GraphPad Prism version 5.0 for Mac (GraphPad Software, San Diego, CA). Institutional Animal Care and Use Committee approval was obtained from the Baylor College of Medicine.
Results
CD8+CD45RA+CCR7+ subset correlates with in vivo expansion of CAR-T cells infused in patients with B-cell malignancies
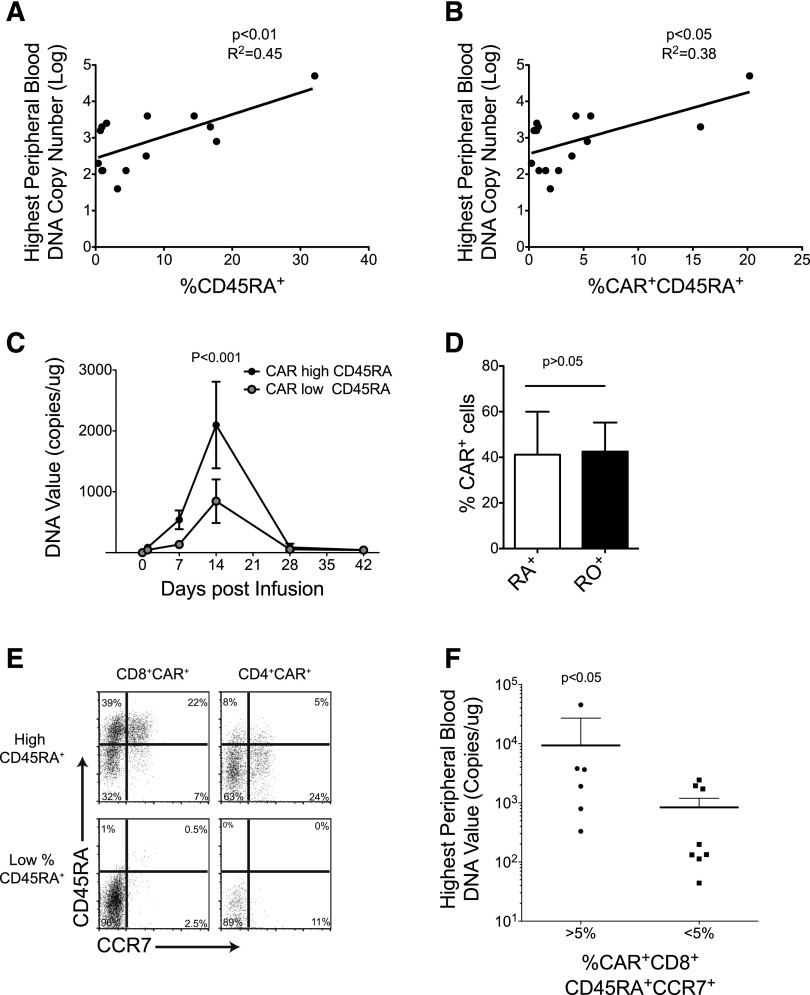
We analyzed 14 autologous CAR-T cell lines generated and infused in 14 patients with relapsed/refractory B-cell malignancies (C.A.R and B.S., manuscript in preparation).8 All T-cell lines were manufactured using recombinant IL-2 as a growth factor and were genetically modified with a γ retroviral vector encoding a CAR.CD19 that includes the CD28 co-stimulatory endodomain.8 The time required to generate sufficient T cells for infusion in our dose escalation study was 15 ± 6 days. T-cell infusions were well tolerated in all patients, and there were no symptoms or signs associated with systemic inflammatory response syndrome or cytokine storm that required a specific medication. We found no significant correlation between in vivo CAR–T-cell expansion/persistence and the following: CAR–T-cell dose; time for ex vivo culture; fold expansion ex vivo; cytotoxic activity ex vivo; proportion of CD4+ or CD8+ T-cell subsets, or CD45RO, CD62L, or CD28 expression in CAR-T cells (supplemental Table 1, available on the Blood Web site). By contrast, we found that the percentage of both total CD45RA+ and CAR+CD45RA+ T cells infused, correlated with the peak height of the qPCR CAR transgene signals in vivo (Figure 1A-C). A similar correlation was also found considering the absolute numbers of total CD45RA+, CAR+CD45RA+, and CD45RA+CCR7+ T cells infused (supplemental Figure 1), and the area under the curve during the first 6 weeks after CAR–T-cell infusion (supplemental Figure 1). This correlation with CD45RA+ cells was not determined by a preferential expression of the CAR in this subset because CAR detection by phenotypic analysis was equally distributed in all subsets analyzed, based on the expression of CD45RA, CD45RO, CCR7, CD27, or CD28 (Figure 1D and supplemental Figure 2). When we further characterized the CD45RA+ subset, we found that CAR+CD8+CD45RA+CCR7+ cells (Figure 1E) correlated with the in vivo expansion/persistence. Indeed, the infused lines containing >5% of CAR+CD8+CD45RA+CCR7+ cells produced significantly higher PCR signals in peripheral blood (Figure 1F), and had greater ex vivo expansion following T-cell–receptor (TCR) stimulation (supplemental Figure 1) than lines containing <5% of CAR+CD8+CD45RA+CCR7+ cells. These clinical data indicate that the frequency of CAR.CD19-expressing CD8+CD45RA+CCR7+ T cells in the infused product correlates with the subsequent expansion of CAR-T cells in the first 6 weeks after infusion, and reinforces the need to develop manufacturing strategies that consistently preserve this subset in the final product.
Figure 1.
Co-expression of CD8, CD45RA, and CCR7 in CAR-T cells correlates with their in vivo expansion in lymphoma patients. (A-B) Illustrates the correlation between the percentage of CD45RA+ and CAR+CD45RA+ cells in the T-cell products and the peaks of the qPCR signals of CAR-T cells in the peripheral blood of infused patients. (C) Detection of CAR-T cells by qPCR in the peripheral blood of patients who received T-cell products with low or high CAR+CD45RA+ cells, respectively. (D) Equal CAR expression by phenotypic analysis in CD45RA+ and CD45RO+ cells. (E) Representative flow cytometry plots of CAR-T cells with low and high frequency of CD8+CD45RA+CCR7+ cells. (F) Peaks of the qPCR signals of CAR-T cells in the peripheral blood of patients infused with CAR-T cell lines containing >5% or <5% CAR+CD8+CD45+CCR7+ cells.
IL-7 and IL-15 are superior to IL-2 for preserving the CD8+CD45RA+CCR7+ population in ex vivo expanded CAR-T cells
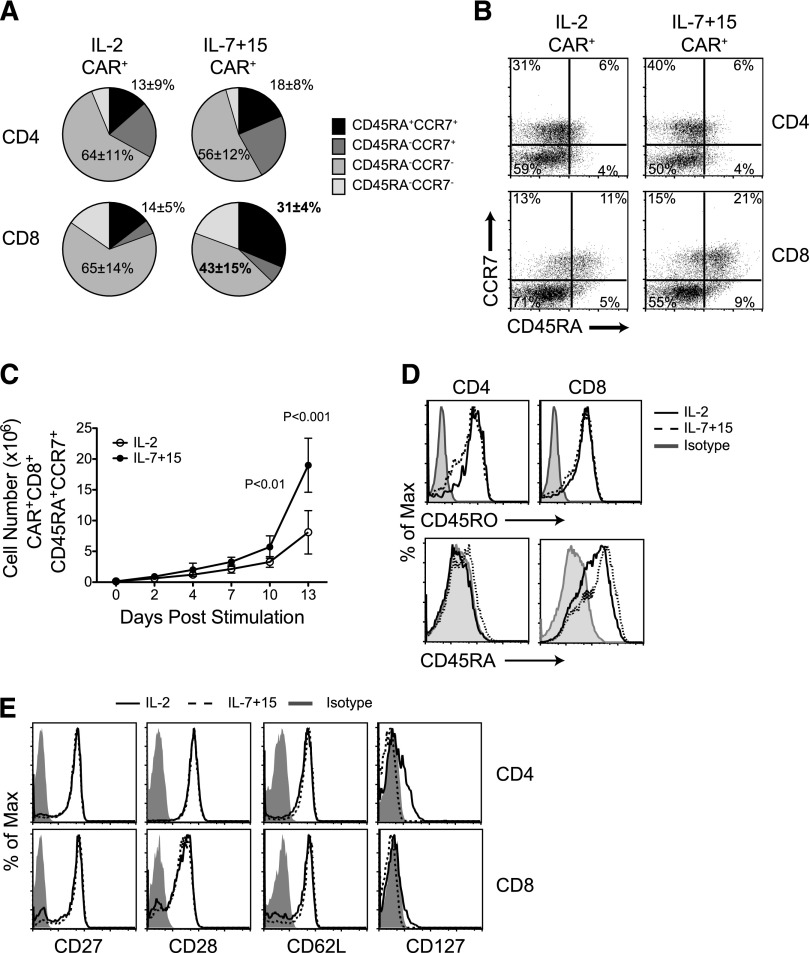
Having established that the frequency of CD8+CD45RA+CCR7+ CAR-T cells in the infused product correlates with subsequent in vivo engraftment, we investigated if alternative γ-chain cytokines to IL-2 better preserved this specific subset when used as growth factors to prepare the final T-cell products. Because of their known activity on the naïve and central-memory compartments,19 we selected IL-7 and IL-15 and compared their activity to IL-2 in our cultures. We found that IL-7 and IL-15 produced greater expansion of CAR.CD19-expressing CD8+ cells, retaining both CD45RA and CCR7 expression (31% ± 4%) compared with IL-2 (14% ± 5%; P < .01) (Figure 2A-C and supplemental Table 2). Of note, CD45RO expression was conserved in CAR-T cells cultured in either condition, while CD45RA expression was higher in IL-7 and IL-15 expanded CAR-T cells (Figure 2D), suggesting that these CD8+CD45RA+CCR7+ T cells have indeed entered the pool of antigen-experienced T cells, but still retain some naïve phenotypic markers. Other well-defined memory-associated surface markers such as CD27, CD28, CD62L, and CD127 were expressed equally by CAR-T cells, irrespective of whether the cells had been grown in IL-2 or in IL-7, and IL-15 (Figure 2E). These data further highlight that phenotypically only the frequency of cells co-expressing CD45RA and CCR7 discriminates between T cells exposed to IL-2 or IL-7, and IL-15.
Figure 2.
IL-7 and IL-15 better preserve CD8+ CD45RA+ CCR7+ cells in ex vivo expanded CAR-T cells than IL-2. (A) T-cell memory phenotypic analysis based on CD45RA and CCR7 expression by CAR-T cells exposed to either IL-2 or IL-7 and IL-15 (n = 4). (B) Representative flow cytometry analysis. (C) Numeric expansion of CAR+CD8+CD45RA+CCR7+ cells in culture conditions with either IL-2 or IL-7 and IL-15 (n = 3). (D) Expression of the CD45RO and CD45RA isoforms by CAR-T cells. Data shown are representative of 3 independent experiments. (E) Expression of CD27, CD28, CD62L, and CD127 in CAR-T cells. Data shown are representative of 3 independent experiments.
The preservation and expansion of CD8+CD45RA+CCR7+ CAR-T cells in the presence of IL-7 and IL-15 did not diminish their functional properties compared with CAR-T cells grown in IL-2. In particular, transduction efficiency, cell expansion (up to 2 weeks), and CD4:CD8 ratios were all equal (supplemental Figure 3). CAR-T cells also displayed similar intrinsic activation responses to TCR and CD28 stimulation, since the expression of activation markers including CD95, CD137, CD69, and CD25 were comparable (supplemental Figure 3). Lytic proteins (perforin and granzyme B) and the extent of degranulation upon CAR-specific engagement were also similar. Finally, CAR-T cells had similar Th1/Tc1 responses, as demonstrated by the production of IFN-γ and IL-2 following coculture with tumor target cells (supplemental Figure 3).
IL-7 and IL-15 do not promote preferential transduction of naïve T cells
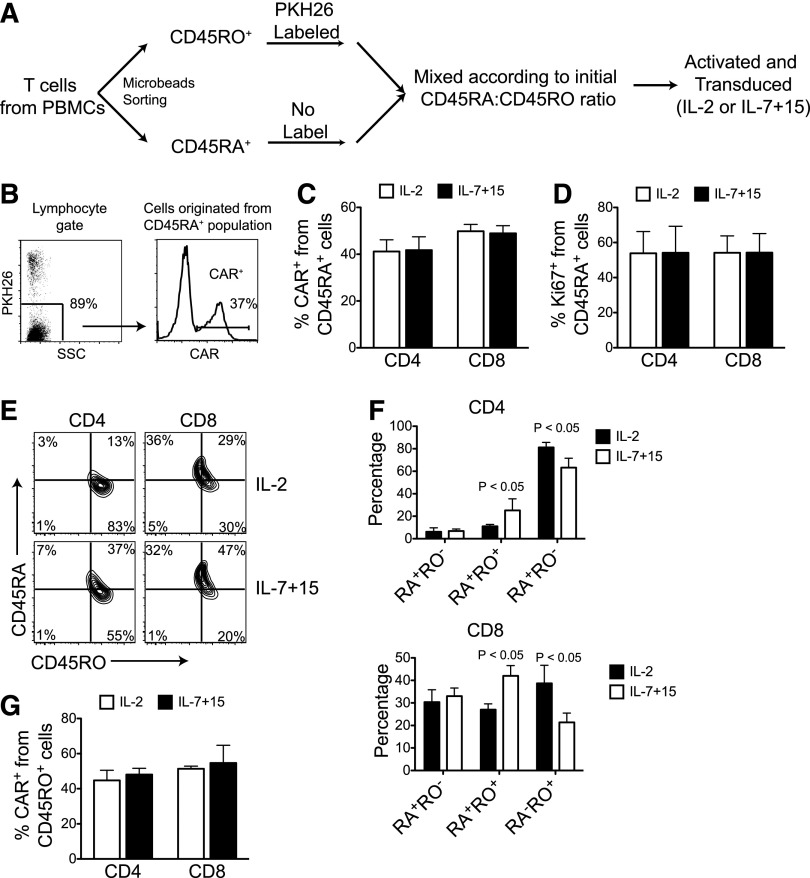
To determine whether the higher frequency of CD8+CD45RA+CCR7+ CAR-T cells promoted by IL-7 and IL-15 is due to an increased susceptibility of initial naïve subset to retroviral transduction, we isolated antigen-experienced cells (CD45RO+) from naïve cells (CD45RA+), and labeled the former population with the PKH26 dye. Labeled CD45RO+ cells were then mixed with unlabeled naïve cells to the same CD45RO:CD45RA ratio detected in the unsorted population (Figure 3A). Mixed cells were activated and transduced in the presence of either IL-2 or IL-7, and IL-15. As shown in Figure 3B-C, the transduction frequencies of PKH26– cells (derived from CD45RA+ cells) were similar both in IL-2 and in IL-7, and IL-15 culture conditions. Moreover, IL-7 and IL-15 did not enhance naive T-cell–cycling, as the percentage of Ki67+ cells was similar in both culture conditions at the time of retroviral transduction (Figure 3D). Notably, CD45RO expression was detected by day 3 after retroviral transduction on unlabeled CD45RA+ cells, and IL-7 and IL-15 significantly enriched cells co-expressing CD45RA and CD45RO (Figure 3E-F). Lastly, using reverse labeling (CD45RA+ cells labeled with PKH26 and unlabeled CD45RO+ cells), we found that transduction of CD45RO+ cells was also comparable in IL-2 and IL-7, and IL-15 culture conditions (Figure 3G). Hence, IL-7 and IL-15 cytokines appear to directly preserve the CD45RA+CCR7+ subset ex vivo rather than promoting more efficient retroviral transduction of naïve cells.
Figure 3.
IL-7 and IL-15 do not promote preferential transduction of naïve T cells. (A) Schematic representation of the labeling process to distinguish T cells originated from naïve (CD45RA+) and antigen-experienced (CD45RO+) T cells by labeling with PKH26 dye before activation and retroviral transduction with either IL-2 or IL-7 and IL-15. (B) Gating strategy and representative data showing CAR expression by T cells originated from naive T cells. (C) CAR expression in PKH26– cells (originally CD45RA+) after activation and transduction (n = 5). (D) Percentage of Ki67+ cells on PKH26– cells (originally CD45RA+) prior to retroviral transduction (n = 5). (E-F) Expression of CD45RA and CD45RO on PKH26– cells (originally CD45RA+) after activation and transduction. Representative dot plots (E) and summary of 3 experiments (F). (G) CD45RA+ cells were labeled with PKH26, and transduction on PKH26- cells (originally CD45RO+) was assessed (n = 3).
Compared with IL-2, IL-7 and IL-15 produce superior survival for CAR-T cells exposed to serial antigen stimulation
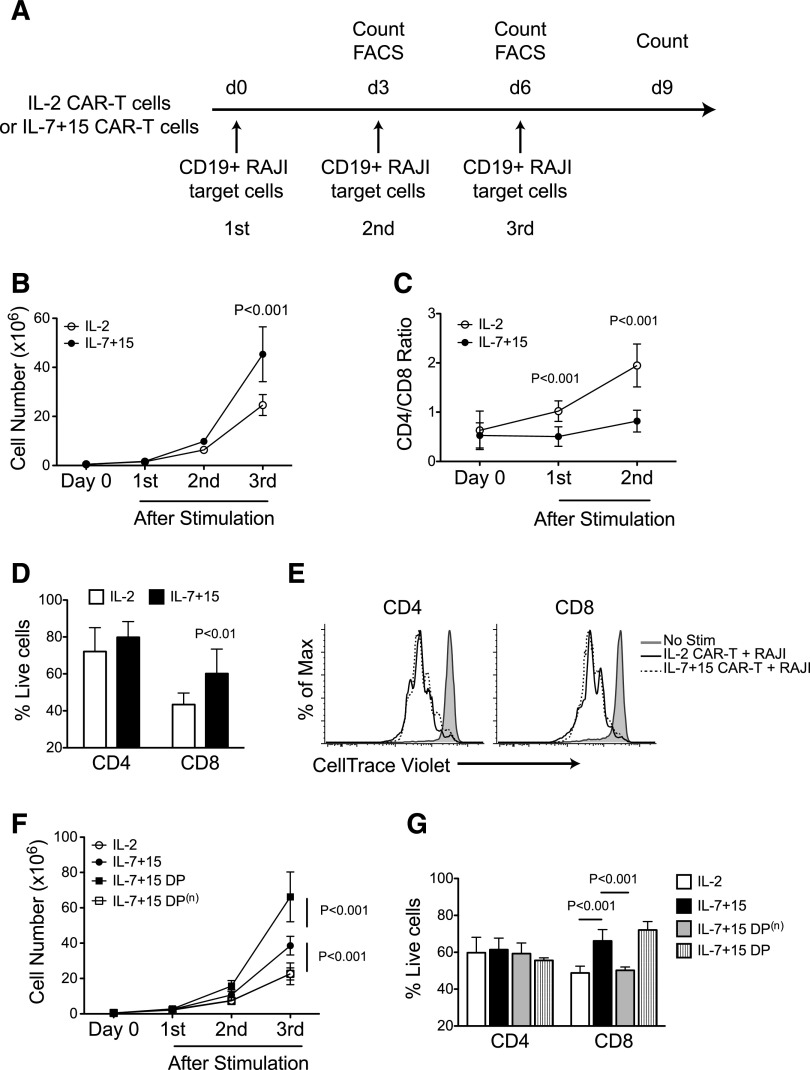
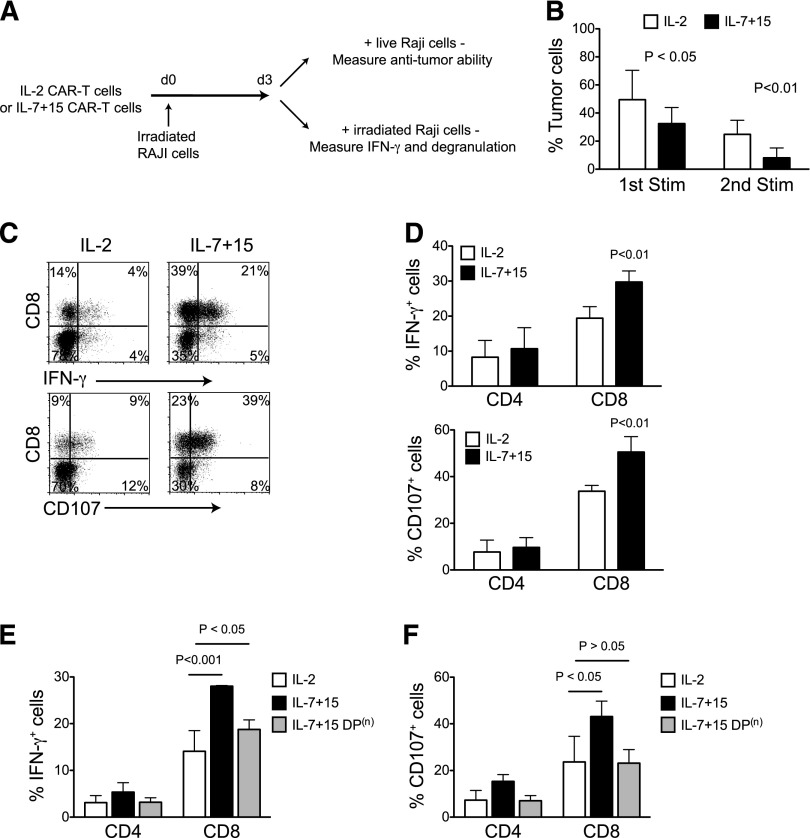
Having shown that IL-7 and IL-15 cytokines preserve CAR-T cells co-expressing the CD45RA and CCR7 markers, we determined if this subset had functional advantages. We assessed the proliferation and effector function of T cells after repeated stimulation through the CAR, to mimic the in vivo conditions of CAR-T cells with multiple sequential tumor encounters in the absence of exogenous cytokines (Figure 4A). In parallel to our observations in patients infused with CAR–T-cell products containing CD8+CD45RA+CCR7+ T cells, we found that CAR-T cells generated in IL-7 and IL-15 showed greater and more sustained expansion than those generated in IL-2 (45-fold vs 25-fold; P < .001) (Figure 4B). Consistent with IL-7 and IL-15 preservation of CD45RA+CCR7+ cells within the CD8+ cells, but not in the CD4+ subset, we observed that CD4+ T cells progressively dominated CD8+ T cells in the IL-2 expanded cells, whereas the CD4:CD8 ratio remained relatively stable in IL-7 and IL-15 expanded cells (Figure 4C). The preservation of the CD8+ subset in CAR-T cells generated in IL-7 and IL-15 compared with IL-2 was mostly attributed to their reduced activation-induced cell death (Figure 4D), as their proliferative potential was essentially unaffected (Figure 4E). When the CD45RA+CCR7+ double-positive (DP) subset was removed from CAR–T-cell products generated with IL-7 and IL-15, we found that these depleted cells lost their advantage and had similar responses after repetitive CAR stimulation as CAR-T cells exposed to IL-2, while sorted CD45RA+CCR7+ cells showed the greatest proliferation and viability after repetitive stimulation (Figure 4F-G). This indicates a strict relationship between the presence of IL-7 and IL-15 and the maintenance of the CD8+CD45RA+CCR7+ subset.
Figure 4.
CAR-T cells exposed to IL-7 and IL-15 display superior proliferative capacity after serial stimulations. (A) CAR-T cells expanded with IL-2 or IL-7 and IL-15 were stimulated 3 times with irradiated CD19+ target tumor cells (Raji) in the absence of exogenous cytokines. After each stimulation, cells were counted and phenotypic analysis was performed by flow cytometry. (B) Cell count of CAR-T cells after each antigen stimulation (n = 4). (C) Ratio of CD4+ and CD8+ T cells before and after 2 consecutive antigen stimulations (n = 5). (D) Apoptosis of CD4+ and CD8+ CAR-T cells after antigen stimulation assessed by Annexin-V and 7-AAD staining (n = 3). (E) CAR-T cells were labeled with CFSE before being stimulated by tumor cells. CFSE dilution was determined by flow cytometry on CD4+ and CD8+ T cells by day 3 of culture. Data shown are representative of 3 independent experiments. (F-G) CD45RA and CCR7 DP T cells were sorted by flow cytometry from IL-7 and IL-15 expanded CAR-T cells (IL-7+15 DP). Negative fraction was also collected as IL-7+15 DP(n). After sorting, IL-2 expanded (IL-2), IL-7 and IL-15 expanded (IL-7+15), IL-7/15 DP, IL-7, and IL-15 DP(n) CAR-T cells were stimulated as in (A). Cell counts (F) and apoptosis on CD4+ or CD8+ T cells (G) were measured by flow cytometry (n = 3).
The effector function, and thus the antitumor activity of CAR-T cells generated in IL-7 and IL-15 after repeated antigen-specific stimulation, was also stronger at high E:T ratios compared with IL-2 (Figure 5A-B) (residual tumor cells 8% vs 25%; P < .01 after the 2nd stimulation), and accompanied by significantly increased production of IFN-γ and more robust degranulation (Figure 5C-D). This effect can also be attributed to the CD45RA+CCR7+ subset enriched by IL-7 and IL-15 insofar as its removal abolished the enhanced IFN-γ production and degranulation (Figure 5E-F).
Figure 5.
CAR-T cells exposed to IL-7 and IL-15 display superior effector function after serial stimulations. (A) CAR-T cells expanded with IL-2 or IL-7 and IL-15 were first stimulated with irradiated CD19+ target tumor cells (Raji). Three days after stimulation, cells were collected and subjected to coculture and intracellular cytokine production assays. (B) CAR-T cells were cocultured with target tumor cells at 1:3 E:T ratio. Percentages of residual tumor cells in the culture were determined by flow cytometry by day 3 of culture (n = 4). (C-D) Intracellular IFN-γ staining and degranulation analysis of CAR-T cells after the 2nd consecutive antigen stimulation (n = 4). Minimal (<2%) expression of IFN-γ or CD107 was observed when cells were not stimulated by Raji. (E-F) Intracellular IFN-γ staining and degranulation analysis of CAR-T cells after the 2nd consecutive antigen stimulation after depletion of the DP subset (n = 3).
CCR7 expression by IL-7 and IL-15 expanded CAR-T cells facilitates efficient homing to secondary lymphoid organs
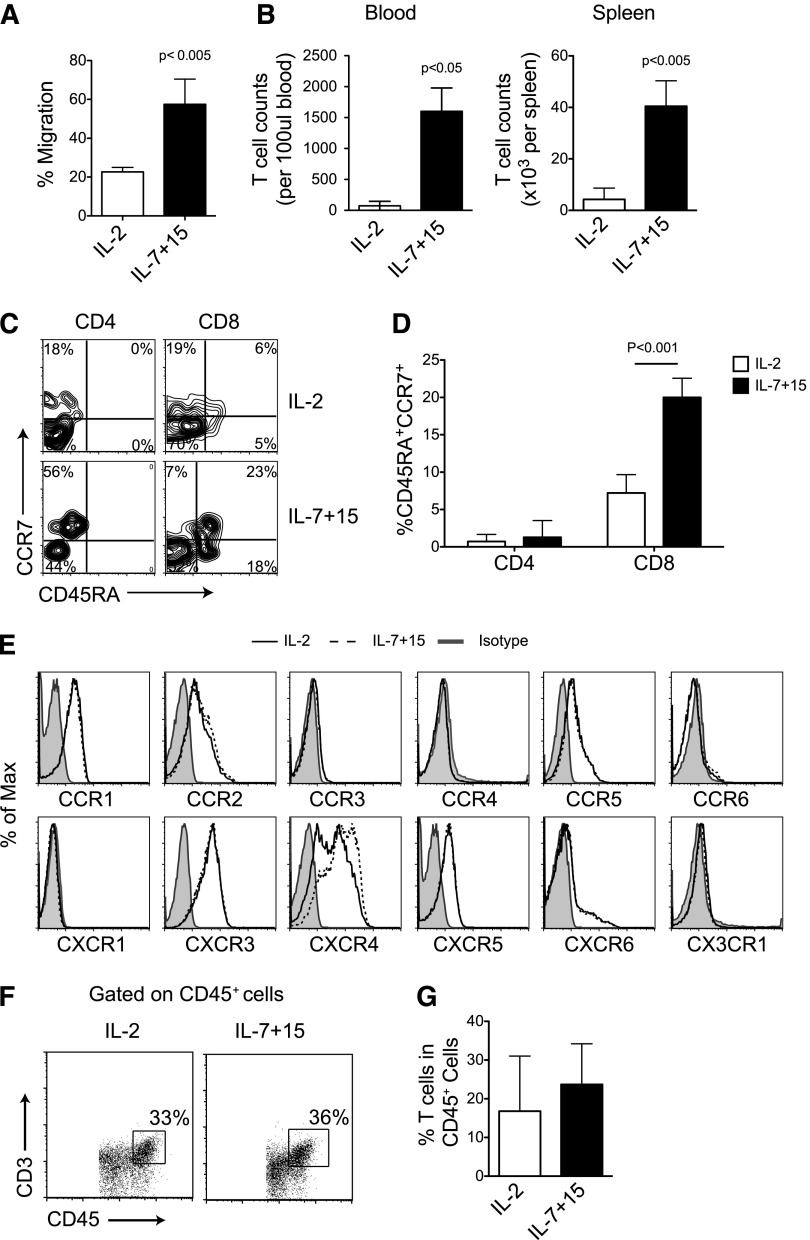
Because CCR7 is an essential chemokine receptor for T-cell homing to secondary lymphoid organs, we evaluated whether the differential expression of CCR7 in CAR-T cells driven by IL-7 and IL-15 modifies trafficking toward lymphoid-specific homing signals, including CCL21 and CCL19. As shown in Figure 6A, CAR-T cells exposed to IL-7 and IL-15, that overall express higher levels of CCR7, migrated more effectively along the CCL21 gradient in a conventional migration assay than CAR-T cells exposed to IL-2 (57% ± 13% vs 23% ± 3%; P < .005) that overall express lower levels of CCR7 (Figure 2B). Because human CCR7 is also responsive to murine-derived CCL19 and CCL21 chemokines,20 we assessed the in vivo migration of CAR-T cells to secondary lymphoid organs. We engrafted NSG mice with subcutaneous EBV-LCLs, which served as a competitive migratory signal for lymphoid homing to CCL19/21 chemokines.21 We found that CAR-T cells exposed to IL-7 and IL-15 were present at a higher frequency in the spleen by day 3 after systemic infusion compared with CAR-T cells exposed to IL-2 (4 ± 0.5 cells × 104 vs 4 ± 1 cell × 103 per spleen; P < .005) and in the peripheral blood (1269 ± 370 cells vs 73 ± 70 cells per 100 μl blood; P < .05) (Figure 6B). Notably, when analyzing the T cells detected in mouse peripheral blood, the expression of CCR7 and CD45RA by CAR-T cells exposed to IL-7 and IL-15 was significantly higher than IL-2–cultured CAR-T cells (Figure 6C-D), suggesting that the ex vivo exposure to these cytokines imprints T cells to retain the naïve phenotypic markers even when the cytokines are no longer available in vivo. Finally, IL-7 and IL-15 did not affect the expression of chemokine receptors sensing inflammatory signals, such as RANTES, CXCL9, and CXCL10 (Figure 6E), and thus did not impair their migration to the tumor site (Figure 6F-G).
Figure 6.
High CCR7 expression of CAR-T cells exposed to IL-7 and IL-15 facilitates homing to secondary lymphoid organs. (A) Equal numbers of CAR-T cells expanded either with IL-2 or IL-7 and IL-15 were subjected to migration assay toward CCL21 (n = 4). (B) NSG mice were subcutaneously implanted with EBV-LCL and then infused with CAR-T cells. CD45+CD3+ cells were numerated by flow cytometry in blood and spleen 3 days after T-cell infusion (n = 5). (C-D) Expression of CD45RA and CCR7 in CAR-T cells in vivo. Data summarize 3 mice per group from 2 independent experiments. (E) Expression of other chemokine receptors by CAR-T cells. Data shown are representative of 3 independent experiments. (F-G) Detection of CAR-T cells at the tumor sites in vivo. Data are obtained from 3 mice per group from 2 independent experiments.
CAR-T cells exposed to IL-7 and IL-15 have improved in vivo persistence and antitumor activity
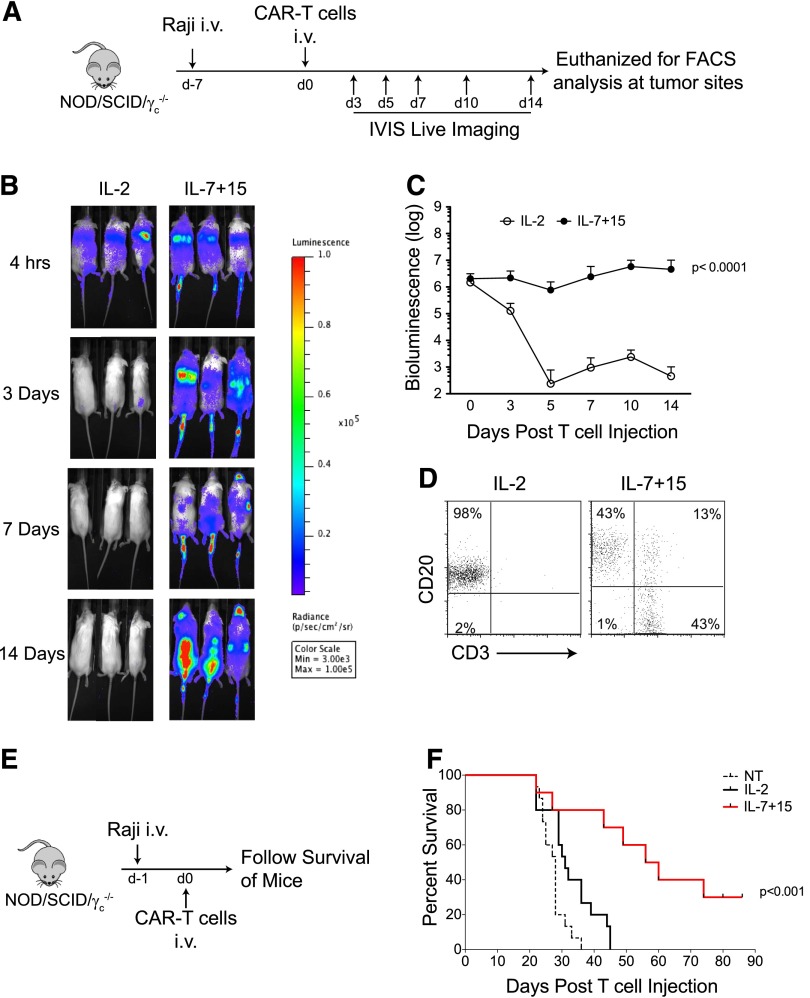
Our in vitro data showing that CAR-T cells exposed to IL-7 and IL-15 have an increased subset of CD8+ T cells retaining CD45RA and CCR7 expression, superior lymphoid homing capacity and prolonged functionality after repeated antigen stimulations, would suggest superior persistence and antitumor activity in vivo. In NSG mice engrafted with a high tumor burden of Raji cells and infused with CAR-T cells labeled with FFLuc (Figure 7A), we consistently observed that CAR-T cells exposed to IL-2 had a short half-life and disappeared by day 3 post-infusion (Figure 7B-C). In sharp contrast, IL-7 and IL-15 expanded CAR-T cells were detectable for more than 14 days after infusion and accumulated in the spinal cord where tumor cells were primarily localized (Figure 7B-C). Bioluminescence imaging data were also supported by FACS analysis, showing infiltration of T cells in the spinal cord by day 14 (Figure 7D). When we followed mice for antitumor effects of CAR-T cells, we found that IL-2 expanded CAR-T cells only modestly delayed the onset of hind-limb paralysis compared with control nontransduced T cells, whereas mice infused with IL-7 and IL-15 expanded CAR-T cells had significant delays in disease/paralysis progression (P < .001) (Figure 7E-F).
Figure 7.
CAR-T cells exposed to IL-7 and IL-15 have improved in vivo persistence and antitumor activity. (A) Schematic representation of the experiments in NSG mice to compare persistence of CAR-T cells expanded with either IL-2 or IL-7 and IL-15. (B-C) Time-course of T-cell luminescence in mice engrafted with Raji tumor cells. Data summarize 10 mice per group from 2 independent experiments. (D) Detection of CAR-T cells in the spinal cord 2 weeks after CAR-T cell adoptive transfer. Data shown are representative of 5 mice per group from 2 independent experiments. (E) Schematic representation of the experiments in NSG mice to measure the antitumor effects of CAR-T cells expanded with either IL-2 or IL-7 and IL-15. (F) Survival curve of mice after infusion of control and CAR-T cells. Data illustrate the summary of 3 independent experiments for a total of 15 mice per group.
Discussion
We show for the first time in a clinical study of adoptively transferred CAR-modified T cells that the frequency of a T-cell subset within the transferred lymphocytes phenotypically closest to “T-memory stem cells” positively correlates with their subsequent in vivo expansion during the first 6 weeks after infusion in patients with B-cell malignancies. In addition, we report that this subset can be significantly increased in proportion and in absolute numbers in the final product by substituting IL-7 and IL-15 combination for IL-2 as growth factor support during ex vivo generation of the CAR-T cells.
The use of unselected T lymphocytes collected from the peripheral blood remains the most convenient and simple means of generating CAR-redirected T cells for clinical use. Manufacturing these cells requires ex vivo TCR stimulation and the addition of exogenous cytokines, such as IL-2, to induce the proliferation and expansion of circulating memory T cells and naïve T cells, which then transition to antigen-experienced T cells and express the CD45RO isoform.8,22 Within the heterogeneous T-cell subsets composing ex vivo expanded clinical grade CAR–T-cell lines, we found that neither CD62L, a frequently used surrogate marker of central-memory cells, nor CD28, previously associated with the in vivo persistence of tumor infiltrating T lymphocytes,23-25 correlated with the expansion or persistence of infused CAR-T cells in lymphoma patients.
Importantly, we identified in the infused CAR-T cell lines, a subset of CD8+ T cells that while co-expressing the CD45RO isoform, retained expression of the naïve CD45RA isoform and CCR7, and is the only subset that correlates with in vivo expansion of CAR-T cells in lymphoma patients. The expression of CD45RO within a CD8+CD45RA+CCR7+ T-cell compartment places this subset in a differentiation stage that is closely related to the previously described “T-memory stem cells” that are phenotypically similar but lack CD45RO expression.16,26,27 CD95, another maker associated with “T-memory stem cells,” was not included in the phenotypic panel we routinely use to characterize clinical grade CAR-T cell lines infused into the patients, but we confirmed its co-expression in two clinical cell lines from which residual material was available. Moreover, our data from CAR-T cell lines subsequently generated from healthy donors, show that the subset of CAR-redirected CD8+CD45RA+CCR7+ also co-express CD95, CD25, CD28, and CD62L, further indicating that this antigen-experienced population co-expressing the CD45RO isoform is indeed closely related to “T-memory stem cells” as it retains both phenotypic and functional elements of “naïvity” or “stem-ness.”
Patients enrolled in our clinical study presented with relapsed/refractory lymphoma, frequently recurred even after autologous stem-cell transplantation.8 In addition, these patients did not receive specific lymphodepletion before infusion of CAR-T cells, thus, we did not observe sustained clinical responses in our cohort of patients. We cannot, therefore, determine a correlation between the numbers of CD8+CD45RA+CCR7+-infused CAR-T cells and clinical responses. Nevertheless, increasing the numbers of cells in the CD8+CD45RA+CCR7+ subset in CAR–T-cell products is a high priority to ensure robust expansion and persistence of human CAR-T cells.
In transgenic mouse models or severe combined immunodeficiency models of alloreactivity, it has been previously suggested that IL-7 and IL-15 favors the generation of central-memory and “T-memory stem cells.”26,28-30 We found that IL-7 and IL-15 can also significantly enrich for the CD8+CD4RA+CCR7+ subset in CAR–T-cell lines generated following routine clinical grade manufacturing procedures, doubling their amount compared with cultures using IL-2, and improving antitumor effects in a preclinical model. Of note, this benefit is achieved without any specific T-cell manipulation before initiating the manufacturing of the CAR-T cells, such as the selection of naïve precursors.26
The combination of IL-7 and IL-15 used to expand unselected T lymphocytes has beneficial effects on many of the T-cell subsets composing clinical grade CAR–T-cell products, so that the enrichment of the CD8+CD45RA+CCR7+ population may not be the only mechanism by which improved expansion and persistence of infused cells is obtained. For example, IL-15 can reverse anergy,31 promote survival by Bcl-2 upregulation,32-34 and confer resistance to the inhibitory effects of regulatory T cells of effector-memory T cells.18 Furthermore, IL-7 drives the homeostatic expansion of naïve T cells and contributes in maintaining the pool of central-memory T cells.35,36 The molecular signature we performed on CAR-T cells expanded either in IL-2 or IL-7 and IL-15 by day 14 of culture, also shows differences in their gene expression pattern, further underlining that IL-7 and IL-15 promote molecular modifications likely deriving from different T-cell subsets (supplemental Figure 4). However, our data clearly indicate that the increased frequency of the CAR-redirected CD8+CD4RA+CCR7+ cells induced by IL-7 and IL-15, compared with IL-2, plays an independent and critical role. Indeed, irrespective of their phenotypic characteristics and cytokine exposure, CAR.CD19-T cells have robust cytotoxic activity against tumor cells, but it is only the CD8+CD45RA+CCR7+ subset that numerically expands upon repetitive CAR stimulation, suggesting that only these cells are preserved after encountering multiple tumor cells in vivo. Removal of CD45RA+CCR7+ cells from the CAR–T-cell product exposed to IL-7 and IL-15 abrogates this advantage, even if all other T-cell subsets remain intact. Thus, the effects of IL-7 and IL-15 on the CD8+CD45RA+CCR7+ subset are direct and specific rather than the results of a more generalized perturbation on all T-cell subpopulations simultaneously exposed to these cytokines.
For long-term persistence, some adoptively transferred T cells must also enter secondary lymphoid organs and constantly replenish the pool of circulating T cells that then migrate to tumor sites.13,28 The CD8+CD45RA+CCR7+ subset contains CAR-T cells that preserve the lymphoid-homing receptor CCR7, and better migrate to lymphoid organs. Our experiments demonstrate that IL-7 and IL-15 preserve more cells expressing functional levels of CCR7 than IL-2, thereby allowing superior migration to murine spleen in our model. Overall, the greater expansion capacity, effector function, and migration to secondary lymphoid organs translate into longer in vivo persistence and greater antitumor activity of CAR-T cells grown in IL-7 and IL-15. It is, however, important to point out that our observation was derived from clinical experience with a CAR.CD19 that contains the CD28 co-stimulatory endodomain. Whether the CD8+CD45RA+CCR7+ subset is also relevant in CAR-T cells with different antigen specificity or alternative co-stimulatory domains such as 4-1BB remains to be elucidated. Finally, insofar as the subset of CAR+CD4+CD45RA+CCR7+ was not particularly abundant in T cells expanded either with IL-2 or with IL-7/IL-15, we did not specifically study if this subset also plays a relevant role.
Although IL-7 and IL-15 doubles the numbers of CD8+CD45RA+CCR7+ cells in CAR–T-cell products compared with IL-2, there may be approaches that can produce an even greater selective expansion. Manipulation of the Wnt/β-catenin pathway represents one possibility,16,17,22 although we and others have found that β-catenin accumulation often leads to a reduced T-cell expansion and differentiation arrest.22,37 These effects would preclude obtaining sufficient T cells for adoptive therapies. However, it is possible that a “therapeutic window” exists in which IL-7 and IL-15 can be combined with the Wnt/β-catenin pathway to balance adequate T-cell expansion with the preservation of CD8+CD45RA+CCR7+ cells.
In conclusion, the frequency of a CD8+CD45RA+CCR7+ T-cell subset that is phenotypically closest to “T-memory stem cells” correlates with overall in vivo expansion of CAR-T cells in lymphoma patients. The combination of IL-7 and IL-15 plays a crucial role in increasing the number of CD8+CD45RA+CCR7+ within the CAR–T-cell products, enhancing their persistence and antitumor activity in preclinical models. It may be beneficial to substitute this combination of cytokines in the manufacturing protocols, and thereby increase the therapeutic benefits of CAR-T cells.
Acknowledgments
The authors thank Malcolm Brenner (Center for Cell and Gene Therapy, Baylor College of Medicine, Houston, TX) for the critical revision of the manuscript and Yiqun Zhang for his technical assistance.
This work was supported in part by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (RO1 NHLBI 1145564), the National Cancer Institute (RO1 CA 138188), the Specialized Programs of Research (SPOR) Excellence in Lymphoma (P50 CA 126752), and the Shared Resources of the National Cancer Institute for Bioinformatics and Cell and Vector Production at the Dan L. Duncan Cancer Center (P30 CA125123).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.X., B.S., and G.D. designed experiments, analyzed data, and wrote the manuscript; Y.X. and M.Z. performed the experiments; C.A.R (PI of clinical studies), enrolled patients, and monitored clinical responses; A.D. performed the phenotypic analysis of clinical grade CAR-T cell lines; E.L. and O.D. performed qPCR on patient samples; H.L. and C.J.C. performed the statistical analysis; A.P.G. and H.E.H. ensured compliance with regulatory requirements for the clinical trial; C.M.R. contributed to the experimental design; and H.E.H. and C.M.R. contributed to the preparation of the manuscript.
Conflict-of-interest disclosure: The Center for Cell and Gene Therapy (Houston, TX) has a collaborative research agreement with Celgene and Bluebird Bio.
Correspondence: Gianpietro Dotti, Center for Cell and Gene Therapy, Baylor College of Medicine, 6621 Fannin St., MC 3-3320, Houston, TX 77030; e-mail: gdotti@bcm.edu
References
- 1.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scholler J, Brady TL, Binder-Scholl G, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4(132):132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3(9):666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savoldo B, Dotti G. Chimeric antigen receptors (CARs) from bench-to-bedside. Immunol Lett. 2013;155(1-2):40–42. doi: 10.1016/j.imlet.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang S, Gattinoni L, Liu F, et al. In vitro generated anti-tumor T lymphocytes exhibit distinct subsets mimicking in vivo antigen-experienced cells [published correction appears in Cancer Immunol Immunother. 2011;60(5):751]. Cancer Immunol Immunother. 2011;60(5):739–749. doi: 10.1007/s00262-011-0977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hombach AA, Chmielewski M, Rappl G, Abken H. Adoptive immunotherapy with redirected T cells produces CCR7- cells that are trapped in the periphery and benefit from combined CD28-OX40 costimulation. Hum Gene Ther. 2013;24(3):259–269. doi: 10.1089/hum.2012.247. [DOI] [PubMed] [Google Scholar]
- 11.Neeson P, Shin A, Tainton KM, et al. Ex vivo culture of chimeric antigen receptor T cells generates functional CD8+ T cells with effector and central memory-like phenotype. Gene Ther. 2010;17(9):1105–1116. doi: 10.1038/gt.2010.59. [DOI] [PubMed] [Google Scholar]
- 12.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 14.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 15.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gattinoni L, Zhong XS, Palmer DC, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perna SK, De Angelis B, Pagliara D, et al. Interleukin 15 provides relief to CTLs from regulatory T cell-mediated inhibition: implications for adoptive T cell-based therapies for lymphoma. Clin Cancer Res. 2013;19(1):106–117. doi: 10.1158/1078-0432.CCR-12-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 20.Jenh CH, Cox MA, Kaminski H, et al. Cutting edge: species specificity of the CC chemokine 6Ckine signaling through the CXC chemokine receptor CXCR3: human 6Ckine is not a ligand for the human or mouse CXCR3 receptors. J Immunol. 1999;162(7):3765–3769. [PubMed] [Google Scholar]
- 21.Miyauchi K, Urano E, Yoshiyama H, Komano J. Cytokine signatures of transformed B cells with distinct Epstein-Barr virus latencies as a potential diagnostic tool for B cell lymphoma. Cancer Sci. 2011;102(6):1236–1241. doi: 10.1111/j.1349-7006.2011.01924.x. [DOI] [PubMed] [Google Scholar]
- 22.Muralidharan S, Hanley PJ, Liu E, et al. Activation of Wnt signaling arrests effector differentiation in human peripheral and cord blood-derived T lymphocytes. J Immunol. 2011;187(10):5221–5232. doi: 10.4049/jimmunol.1101585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175(10):7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117(6):1888–1898. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Naranjo A, Brown CE, et al. Phenotypic and functional attributes of lentivirus-modified CD19-specific human CD8+ central memory T cells manufactured at clinical scale. J Immunother. 2012;35(9):689–701. doi: 10.1097/CJI.0b013e318270dec7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cieri N, Camisa B, Cocchiarella F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121(4):573–584. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 27.Gattinoni L, Restifo NP. Moving T memory stem cells to the clinic. Blood. 2013;121(4):567–568. doi: 10.1182/blood-2012-11-468660. [DOI] [PubMed] [Google Scholar]
- 28.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102(27):9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S, Ji Y, Gattinoni L, et al. Modulating the differentiation status of ex vivo-cultured anti-tumor T cells using cytokine cocktails. Cancer Immunol Immunother. 2013;62(4):727–736. doi: 10.1007/s00262-012-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarling S, Berenzon D, Dalai S, Liepinsh D, Steers N, Krzych U. The survival of memory CD8 T cells that is mediated by IL-15 correlates with sustained protection against malaria. J Immunol. 2013;190(10):5128–5141. doi: 10.4049/jimmunol.1203396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu J, Giuntoli RL, II, Omiya R, Kobayashi H, Kennedy R, Celis E. Interleukin 15 promotes antigen-independent in vitro expansion and long-term survival of antitumor cytotoxic T lymphocytes. Clin Cancer Res. 2002;8(12):3877–3884. [PubMed] [Google Scholar]
- 32.Bianchi T, Gasser S, Trumpp A, MacDonald HR. c-Myc acts downstream of IL-15 in the regulation of memory CD8 T-cell homeostasis. Blood. 2006;107(10):3992–3999. doi: 10.1182/blood-2005-09-3851. [DOI] [PubMed] [Google Scholar]
- 33.Wu TS, Lee JM, Lai YG, et al. Reduced expression of Bcl-2 in CD8+ T cells deficient in the IL-15 receptor alpha-chain. J Immunol. 2002;168(2):705–712. doi: 10.4049/jimmunol.168.2.705. [DOI] [PubMed] [Google Scholar]
- 34.Yajima T, Yoshihara K, Nakazato K, et al. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol. 2006;176(1):507–515. doi: 10.4049/jimmunol.176.1.507. [DOI] [PubMed] [Google Scholar]
- 35.Tan JT, Dudl E, LeRoy E, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98(15):8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boyman O, Létourneau S, Krieg C, Sprent J. Homeostatic proliferation and survival of naïve and memory T cells. Eur J Immunol. 2009;39(8):2088–2094. doi: 10.1002/eji.200939444. [DOI] [PubMed] [Google Scholar]
- 37.Driessens G, Zheng Y, Locke F, Cannon JL, Gounari F, Gajewski TF. Beta-catenin inhibits T cell activation by selective interference with linker for activation of T cells-phospholipase C-γ1 phosphorylation. J Immunol. 2011;186(2):784–790. doi: 10.4049/jimmunol.1001562. [DOI] [PMC free article] [PubMed] [Google Scholar]