Abstract
Activated protein C (APC) is a serine protease that regulates thrombin (IIa) production through inactivation of blood coagulation factors Va and VIIIa. APC also has non-hemostatic functions related to inflammation, proliferation, and apoptosis through various mechanisms. Using two breast cancer cell lines, MDA-MB-231 and MDA-MB-435, we investigated the role of APC in cell chemotaxis and invasion. Treatment of cells with increasing APC concentrations (1–50 μg/ml) increased invasion and chemotaxis in a concentration-dependent manner. Only the active form of APC increased invasion and chemotaxis of the MDA-MB-231 cells when compared to 3 inactive APC derivatives. Using a modified “checkerboard” analysis, APC was shown to only affect migration when plated with the cells; therefore, APC is not a chemoattractant. Blocking antibodies to endothelial protein C receptor (EPCR) and protease-activated receptor-1 (PAR-1) attenuated the effects of APC on chemotaxis in the MDA-MB-231 cells. Finally, treatment of the MDA-MB-231 cells with the proliferation inhibitor, Na butyrate, showed that APC did not increase migration by increasing cell number. Therefore, APC increases invasion and chemotaxis of cells by binding to the cell surface and activating specific signaling pathways through EPCR and PAR-1.
Keywords: Activated protein C, EPCR, PAR-1, Migration, Transwell invasion, chemotaxis assay, Na butyrate, Hirudin
Introduction
Activated protein C (APC) is a liver-derived serine protease [1], circulating in the blood as a zymogen [2,3]. When the blood vessel wall is injured, the coagulation pathway is initiated to produce thrombin (IIa). IIa will cleave fibrinogen to fibrin, which polymerizes to form a clot. Circulating zymogen protein C (PC) localizes to the endothelial cell surface at the site of injury by binding to Endothelial Protein C Receptor (EPCR) [4]. EPCR facilitates the interaction of PC with the receptor complex of IIa:thrombomodulin (TM) on the endothelial cell surface, properly aligning PC with IIa [5]. IIa cleaves PC to generate activated protein C (APC) [6]. Once it releases from EPCR, APC will inactivate coagulation factors Va and VIIIa [7,8], in the presence of a cofactor, protein S (PS) [9,10]. A reduction of factors Va and VIIIa around the area of the clot prevents any further generation of IIa and effectively helps regulate hemostasis [11,12]. Therefore, a key role of the protein C system is to control fibrin clot formation from expanding into the vessel without jeopardizing hemostasis and vessel repair.
Recent work has focused on other functions of the protein C system beyond hemostasis. Animal models using blocking antibodies to EPCR showed an important regulatory role of the protein C system with inflammation and coagulation related to Escherichia coli infection [13]. In the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis Study (PROWESS), patients diagnosed with sepsis and acute organ dysfunction were treated with recombinant human APC, which resulted in a mortality reduction of 19.4% [14–18]. It was initially believed that APC increased patient survival through its anticoagulant properties, reducing microvasculature thrombi formation and promoting blood flow. However, through in vitro and in vivo models, APC has been shown to not only regulate coagulation in the microvasculature but also affect inflammation, apoptosis, proliferation, and angiogenesis. APC inhibits apoptosis through upregulation of anti-apoptotic Bcl-2 [19–21], and downregulation of p53, Bax [20,21], and caspases 3 [20–22], 8, and 9 [21,22] all through interactions with EPCR and protease-activated receptors (PAR). Using a murine focal ischemic stroke model, human and mouse APC treatment restored blood flow, reduced infarct volume and edema, eliminated neutrophil infiltration, and reduced fibrin deposition [20–24], all mediated through EPCR [20], PAR-1 [20,21], and PAR-3 [21].
APC has also been shown to have a role in both cell migration and proliferation. APC/PC reduces the migration of immune cells towards chemoattractants through its binding to EPCR [17] and to epidermal growth factor receptor (EGFR) [25]. In a concentration-dependent manner, APC increases human umbilical vein endothelial cells (HUVEC) proliferation through the activation of the MAPK, PI3K, and eNOS pathways primarily via binding to EPCR rather than to PAR-1 [26]. In a mouse cornea angiogenesis assay, APC was shown to increase angiogenesis via the eNOS pathway [26]. APC increases proliferation and migration in keratinocytes by increasing the expression and activation of matrix metalloprotease-2 (MMP-2) [27]. Further, using a rat wound healing model, APC treatment reduced neutrophil infiltration and increased angiogenesis through MMP activation [28]. The initial studies looking at APC and cancer cell migration used ovarian cancer and choriocarcinoma cells in a transwell invasion assay [29]. These studies showed that APC formed a stable complex with PAI-1, which allowed for uPA to activate extracellular matrix proteases and increase invasion [29]. From these results, it is hypothesized that APC has a regulatory role in migration that is mediated through various pathways both intracellularly and extracellularly.
The first goal of this study was to characterize the effects of APC on cell invasion and chemotaxis using two breast cancer cell lines, MDA-MB-231 and MDA-MB-435, with transwell assays. The second goal was to study the interaction of APC with EPCR and PAR-1, receptors involved in mediating other non-hemostatic effects of APC on expression of apoptotic genes and cell proliferation [20,30,31]. The following results show that APC increases chemotaxis and invasion when incubated with the cancer cells, which requires active protease and is dependent on an interaction with both EPCR and PAR-1.
Materials and methods
Cell culture
MDA-MB-231 and MDA-MB-435, two breast cancer cell lines, were grown in Minimum Essential Media (Gibco) with 10% FBS (Gemini), 1% sodium pyruvate (Gibco), and 1% antibiotic/ antimycotic (Gibco). Both of these cell lines were obtained from the UNC Lineberger Comprehensive Cancer Center Tissue Culture Core Facility. Human Umbilical Vein Endothelial Cells (HUVEC) were grown in Endothelial Basal Media (EBM) with 2% FBS, 0.4% bovine brain extract with heparin, 0.1% hydrocortisone, 0.1% human epidermal growth factor, and 0.1% Gentamicin/Amphotericin B-1000 (Cambrex). HUVEC were obtained from Cambrex. All cell lines were maintained at 37 °C and 5% CO2.
Transwell invasion and chemotaxis assay
Invasion and chemotaxis [32,33] were assayed using a transwell system in which cells are plated onto a porous membrane insert (pore diameter of 8 μm; BD Biosciences) and migrate through the pores to the underside towards a chemotactic agent placed in the well below. The inserts were either uncoated, indicative of chemotaxis, or coated with 1.67 μg/μl of Matrigel (BD Biosciences) per insert, indicative of invasion. 50,000 cells were plated per insert in serum-free media (SFM) with 0.1% BSA, 1% sodium pyruvate, and 1% antibiotic/antimycotic. The chemotactic agent for the breast cancer cells in the well below was media containing 10% FBS, 1% sodium pyruvate, and 1% antibiotic/antimycotic.
Cells were plated with increasing concentrations of recombinant human APC (also known as Xigris®; 1–50 μg/ml; Eli Lilly and Co.), human 5-dimethylaminonaphthalene-1-sulfonyl-glutamylglycylarginyl chloromethyl ketone (DEGR)-APC (10 μg/ml; Haematologic Technology), human PC (10 μg/ml; Haematologic Technology), S195A recombinant human APC (10 μg/ml; provided by Dr. Alireza R. Rezaie, St. Louis University, St. Louis, MO), 5 nM human α-IIa (Haematologic Technology), and 50 nM hirudin (Centerchem). Cells were pretreated at room temperature for 15 min with mouse serum IgG (4 or 30 μg/ml; Sigma), JNK 1494 anti-EPCR antibody (4 μg/ml; provided by Dr. Charles T. Esmon, OMRF, Oklahoma, OK), wede15 (20 μg/ml; Immunotech) and atap2 (10 μg/ml; Zymed) (wede15 and atap2 are anti-PAR-1 antibodies) prior to treatment with APC or α-IIa. Cells were also pretreated for 1 h at room temperature with 10 mM Na butyrate (Sigma) prior to treatment with APC. The MDA-MB-231 breast cancer cells were incubated at 37 °C, 5% CO2 for 12 h for the chemotaxis assay and 24 h for the invasion assay. The MDA-MB-435 breast cancer cells were incubated for 24 h for the chemotaxis assay and 48 h for the invasion assay.
The MDA-MB-231 cells were also used in modified “checkerboard” invasion and chemotaxis assays in order to determine if APC could also serve as a chemotactic agent. APC (10 μg/ml) in either SFM with 0.1% BSA or in media containing 10% FBS was plated with the cells onto Matrigel coated or uncoated inserts. These conditions were alternated with APC (10 μg/ml) in either SFM with 0.1% BSA or media containing 10% FBS placed in the well as the chemotactic agent. “Checkerboard” invasion and chemotaxis assays were incubated for 24 h.
After incubation, cells that did not migrate through to the underside of the membrane were removed with a cotton-tipped applicator. The cells that migrated through to the underside of the membrane were fixed in 100% methanol, washed in 1× PBS, and stained with a nuclear fluorescence dye, Hoechst (1:20000 in 1× PBS; Molecular Probes). Membranes were then cut out from the inserts and mounted on glass sides in a 50% glycerol solution. Using a fluorescent microscope, the total number of cells was counted in 4–400× fields, averaged, and compared to no treatment [32,33]. Each individual experiment was done in duplicate and data shown represent at least 3 experiments.
APC activity assay
APC activity was assessed by measuring the cleavage of an APC-specific chromogenic substrate. Excess conditioned media from setting up the transwell invasion and chemotaxis assays were centrifuged for 5 min at 500×g to remove cells. Fifty microliters of the conditioned media was added to the APC substrate, Pefachrome Pca (final concentration of 0.3 mM; Centerchem) in a 96-well plate coated with 0.2% BSA. Change in absorbance was read at 405 nm for 2 min. At the end of each transwell assay, 50 μl conditioned media from the insert was added to substrate and the change in absorbance was measured. Each condition was done in triplicate and averaged.
Generation of APC on a cell monolayer
Assays were based on experiments done previously [5,34–36], with modifications. Briefly, either HUVEC or MDA-MB-231 cells were grown to confluency in a 96-well plate. Cells were washed with 1× PBS and serum starved overnight. Cells were washed two times with Hanks' Balanced Salt Solution (HBSS) without phenol red. Either JNK 1494 anti-EPCR antibody (2–20 μg/ml) or mouse serum IgG (20 μg/ml) was added and incubated at room temperature for 15 min. Zymogen PC (100 nM) was added to the wells and incubated for an additional 15 min at room temperature. Finally, 2 nM α-IIa was added to each well, giving a final volume of 170 μl. The reaction was incubated for 5 min to 24 h. At each timepoint, 20 μl from each well was added to 5 nM hirudin (Haematologic Technologies), a specific IIa inhibitor, in a separate 96-well plate. Pefachrome Pca (0.15 mM) was added to each well and read at 405 nm for 30 min. Each condition was done in triplicate.
Western blots
Cells were grown to form a confluent monolayer and washed with 1× PBS. For 24 h, cells were treated with serum-free media and collected. Cell lysate or conditioned media protein concentrations were determined with a dye-binding assay (Biorad), using BSA as a standard. 25 μg of total protein for the cell lysates or 10 μg of total protein for the conditioned media was loaded per sample, run reduced on a 12% polyacrylamide gel, and transferred to PVDF (Millipore). Membranes were probed with a mouse monoclonal anti-EPCR antibody (1:1000; JNK 1494), mouse monoclonal anti-PAR-1 (1:1000; Immunotech), rabbit polyclonal anti-Erk2 (1:1000; Santa Cruz Biotechnology), and a rabbit polyclonal anti-PAI-1 antibody (1:1000; Molecular Innovations).
Immunofluorescence
Cells were grown on Lab-Tek II chamber slides (Nunc) until they were at least 80% confluent. The cells were fixed in 2% paraformaldehyde in 1× PBS for 30 min at room temperature, then washed 2 times in 1× PBS. Cells were next treated with 0.2 M glycine and incubated for 20 min at room temperature and then washed an additional 2 times in 1× PBS. Cells were blocked in 10% goat serum in 1× PBS, 1% BSA for 30 min at room temperature. This was followed by incubation with either 50 μg/ml anti-EPCR (JNK 1494) or 40 μg/ml PAR-1 (Zymed) in 1× PBS, 1% BSA for 1 h at room temperature. Negative controls were treated with buffer containing no primary antibody. Cells were then washed 5 times with 1× PBS, 1% BSA and treated with 1:20 secondary anti-mouse IgG F(ab′)2 fragment-R-phycoerythrin sheep antibody (Sigma) in 1× PBS, 1% BSA for 1 h at room temperature. Cells were finally washed 5 times with 1× PBS and stored covered at 4 °C in 1× PBS until photos were taken. Photographs were taken with an Olympus DP70 Microscope Digital Camera and DP70-BSW Software using an Olympus BX51WI fluorescent microscope with a TRITC filter. Photographs were taken at a 200× magnification with exposure time and sensitivity levels specific for each cell line and each antibody.
Statistical analysis
For each transwell experiment, conditions were done in duplicate and averaged. Experiments were repeated as indicated in the figure legends. Averages of each condition were compared to No Treatment, APC, or α-IIa. All experiments were averaged and the percentages of No Treatment were reported. Averages of the comparisons of various APC or α-IIa treatments to APC or α-IIa alone treatments were also done (not reported). Statistical analysis was performed using a one sample t-test with a normal distribution, a theoretical mean of 100, and significance of p<0.05 comparing back to No Treatment, APC, or α-IIa treatment.
Results
APC increases breast and endothelial cell invasion and chemotaxis
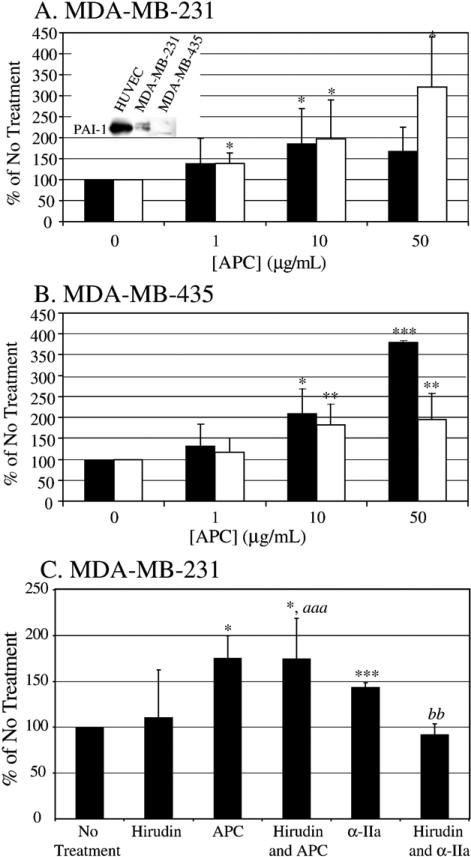
Incubation of the MDA-MB-231 cells with increasing concentrations of APC (1–50 μg/ml) increased both invasion and chemotaxis 150–300% compared to no treatment (Fig. 1A) in the transwell assays. Unexpectedly, the same effect was seen with the MDA-MB-435 cell line when treated with increasing concentrations of APC (Fig. 1B). APC increased the MDA-MB-435 cell invasion and chemotaxis 125–375% compared to no treatment. It was previously reported that APC increased ovarian cancer and choriocarcinoma cell invasion through Matrigel only when PAI-1 was present in the culture media [29]. This suggested that APC complexed to PAI-1, allowing uPA to increase activation of extracellular proteases. APC alone was believed to have no effect in the absence of PAI-1 [29]. From this study, we hypothesized that APC would only increase invasion and chemotaxis in the MDA-MB-231 cells because they express PAI-1 (inset in Fig. 1A), and have no effect on the MDA-MB-435 cells since they do not express PAI-1 (inset in Fig. 1A). Because APC treatment of both cell lines yielded the same result, it was concluded that APC was activating other mechanisms to increase cell invasion and chemotaxis in the transwell assay that are not dependent on the presence of endogenous PAI-1. Similar results were generated using HUVEC in the transwell assays, but with lower concentrations of APC, 0.1–10 μg/ml (data not included). These results indicate that the effects of APC on migration in the transwell assay are not isolated to cancer cells and, further, there is a common pathway among these three cell lines that is activated by APC. The remainder of the studies presented here will focus on the effects of APC on the MDAMB-231 cells as a model system for the effects of APC on cellular invasion and chemotaxis.
Fig. 1.
APC increases invasion and chemotaxis of breast cancer cells. Increasing concentrations of APC (0–50 μg/ml) are incubated with MDA-MB-231 cells (A) for 12-h transwell chemotaxis assay (white bars) and 24-h transwell invasion assay (black bars). Inset is a representative Western blot showing the presence of PAI-1 in the conditioned media of HUVEC and MDA-MB-231 cells but PAI-1 is absent in the MDA-MB-435 cells. Increasing concentrations of APC were also incubated with MDA-MB-435 cells (B) for 24-h transwell chemotaxis assay (white bars) and 48-h transwell invasion assay (black bars). Cells migrate towards media containing 10% FBS as the chemotactic agent. The graphs represent the average of 8 separate experiments; *p <0.05, **p <0.01, ***p <0.001 compared to No Treatment. (C) Hirudin (50 nM) is added with or without APC (10 μg/ml) to the MDA-MB-231 cells in a 12-h transwell chemotaxis assay. As control, 5 nM α-IIa is added with or without hirudin (50 nM) in a 12-h transwell chemotaxis assay to verify effectiveness of hirudin. Cells migrated towards media containing 10% FBS as the chemotactic agent. The graphs represent the average of 5 experiments; *p <0.05, **p <0.01, ***p <0.001 compared to No Treatment, aaa p <0.001 compared to Hirudin treatment, bb p <0.01 compared to α-IIa treatment.
We also verified that thrombin, which could potentially be present in the APC preparation, was not responsible for promoting the increase in migration seen with APC treatment. Cells were treated with hirudin, a specific thrombin inhibitor, and APC in the 12-h transwell chemotaxis assay. As seen in Fig. 1C, hirudin alone has no effect on cell migration when plated with the cells. APC significantly increases chemotaxis of the MDA-MB-231 cells by 175% in either the presence or absence of hirudin. As a control, cells were also treated with α-IIa in the presence or absence of hirudin. α-IIa alone increases chemo-taxis of the MDA-MB-231 cells by 144%. This effect is lost with α-IIa and hirudin. Therefore, the effect of APC on cellular migration is due to APC alone and not the presence of trace amounts of α-IIa.
Active protease is necessary to increase invasion and chemotaxis of the MDA-MB-231 cells
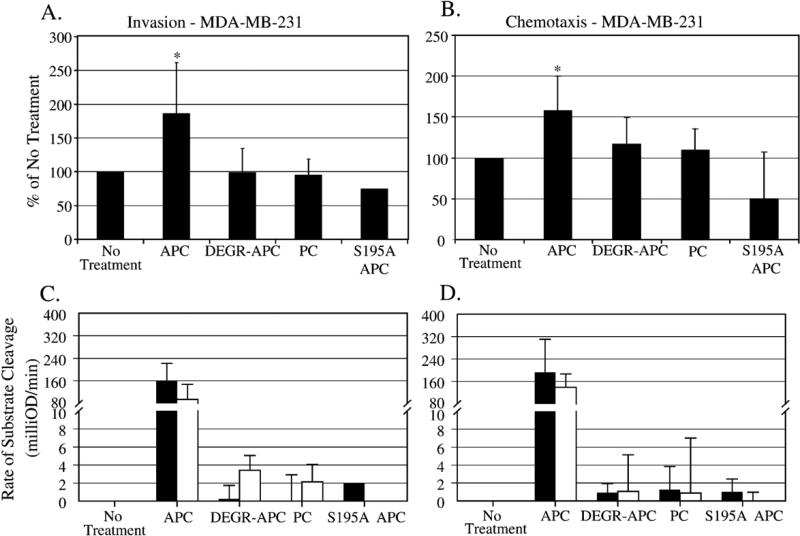
It is important to determine if active protease is necessary to increase cell migration in the transwell assays. The MDA-MB-231 cells were treated with APC, inactive forms of APC, or PC in a 12-h transwell chemotaxis assay and a 24-h transwell invasion assay. Active APC (10 μg/ml) was the only protease that significantly increased cell invasion by 190% (Fig. 2A). The addition of inactive forms of APC – DEGR-APC, active site mutant APC (S195A) and zymogen PC – all at the same concentration, had no effect on cell invasion. The same results were seen in the transwell chemotaxis assay with the MDAMB-231 cells (Fig. 2B). APC activity was verified by measuring the rate of cleavage of an APC-specific chromogenic substrate. Conditioned media were sampled at the beginning and end of the experiment to verify the activity of the active and the inactive forms of APC, as seen in Figs. 2C and D pre- and post-experiment. These results indicate that the active form of APC is necessary to increase invasion and chemotaxis in the MDA-MB-231 cells using the transwell system.
Fig. 2.
Active protease is necessary to increase invasion and chemotaxis in the MDA-MB-231 cells. 10 μg/ml APC, DEGR-APC, zymogen PC, and S195A APC were used in a 24-h transwell invasion assay (A) and 12-h transwell chemotaxis assay (B). Cells migrated towards media containing 10% FBS as the chemotactic agent. APC activity assays were done to verify the presence or absence of activity of each protease at the beginning (black bars) and at the end (white bars) of the transwell invasion (C) and chemotaxis (D) assays. The graphs represent the average of 4 separate experiments with the exception of S195A APC, which was done only 1–2 times due to the limited amount of protein available; *p <0.05 compared to No Treatment.
APC Increases chemotaxis of the MDA-MB-231 cells, but it is not a chemotactic agent
α-IIa, another serine protease, has been shown to be both a chemotactic agent for the MDA-MB-231 cells [37] and an enhancer of the cell's response to chemotactic agents [38]. Using a modified “checkerboard” analysis, we next determined if APC could affect cell migration in the same manner as α-IIa. APC (10 μg/ml) was plated in the insert with the cells either in 10% FBS containing media or in SFM with 0.1% BSA. These conditions were varied with the conditions in the well below, where APC (10 μg/ml) was added with either 10% FBS containing media or SFM with 0.1% BSA. All assays were run for 24 h in order to give APC sufficient time as a putative chemotactic agent. In the transwell chemotaxis assay, APC is not a chemotactic agent since there was no difference in the number of cells that migrated towards SFM, 0.1% BSA or 10% FBS containing media with or without APC (Table 1). When the cells were plated in 10% FBS containing media with APC, there was no difference in the number of cells that migrated either to SFM, 0.1% BSA with or without APC or 10% FBS containing media with or without APC. When cells were plated in SFM, 0.1% BSA with APC and migrated towards SFM, 0.1% BSA with or without APC, there was, again, no difference in the number of migrating cells between the two conditions. In fact, there were very few cells that did migrate. The only difference seen with the modified “checkerboard” transwell chemotaxis assay was the increase in the number of cells that migrated when 10% FBS containing media was the chemotactic agent and APC was plated with the cells in SFM, 0.1% BSA. Similar results were seen in a modified “checkerboard” transwell invasion assay (data not shown). These results suggest that APC increases cell migration when plated with the MDA-MB-231 cells, enhancing the response of the cells to the chemotactic agent.
Table 1.
Modified checkerboard analysis of APC on chemotaxis of the MDA-MB-231 cellsa
| APC concentration (μg/mL) below membrane | APC concentration (μg/mL) above membrane |
|||
|---|---|---|---|---|
| 0 + SFM | 10 + SFM | 0+10% FBS | 10 + 10% FBS | |
| 0 + SFM | 5.0±6.5 | 2.4±2.3 | 113.4±28.4 | 64.3±33.9 |
| 10 + SFM | 1.6±1.7 | 1.9±3.1 | 82.0±29.1 | 104.2±27.0 |
| 0 + 10% FBS | 498.8± 112.9 | 590.7 ±148.5 | 156.1 ±46.2 | 188.1 ±68.7 |
| 10 + 10% FBS | 471.3± 120.9 | 341.7 ±84.2 | 148.1 ±42.6 | 144.1 ±44.1 |
Two different concentrations of APC (0 and 10 μg/mL) were added above or below the uncoated insert membrane in either SFM-0.1% BSA or media containing 10% FBS. After a 24h incubation, the number of cells that underwent chemotaxis were determined as described in the Material and methods. Data represents the mean number of cells that migrated ± standard deviation of 4 separate experiments, each done in duplicate.
APC binding to EPCR is necessary to increase chemotaxis of the MDA-MB-231 cells
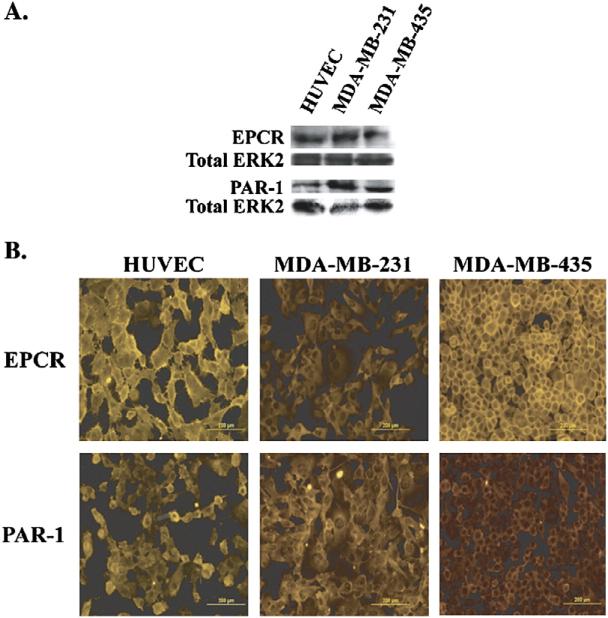
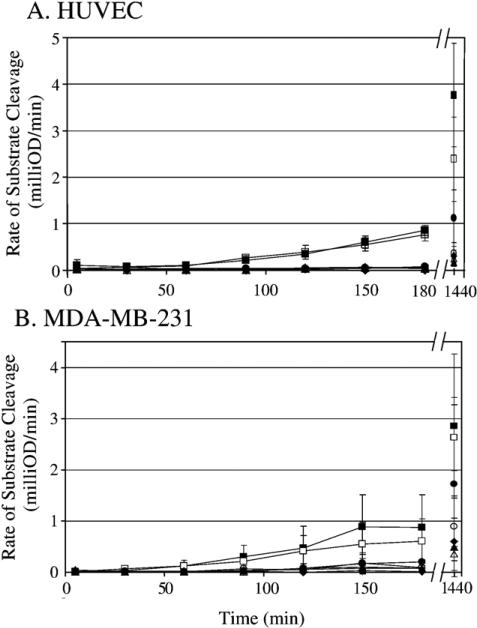
To study the role of EPCR in the pro-migratory effects of APC, we showed the presence of EPCR using both Western blots of cell lysates (Fig. 3A) and immunofluorescence staining of cell monolayers (Fig. 3B) of HUVEC, as the positive control, and both breast cancer cell lines. A blocking antibody (JNK 1494) to the PC/APC binding site on EPCR [5,34–36] was used in a 12-h transwell chemotaxis assay with the MDA-MB-231 cells. Initially, it was necessary to determine the optimal concentra tion of EPCR blocking antibody to use with the MDA-MB-231 cells. HUVEC, as a control, and MDA-MB-231 cells were treated with increasing concentrations of the EPCR blocking antibody to block the generation of APC on the cell surface [5,34–36]. Figs. 4A and B show the generation of APC on both HUVEC and MDAMB-231 cell surfaces increases over time in the absence and presence of control mouse IgG. The generation of APC is reduced in the presence of the anti-EPCR antibody, even at a concentration of 2 μg/ml. Reduction of APC generation was seen out to 24 h at concentrations as low as 4 μg/ml.
Fig. 3.
PAI-1, EPCR, and PAR-1 expression in HUVEC, MDA-MB-231, and MDA-MB-435 cell lines. Representative Western blots (A) showing the presence of EPCR and PAR-1 in cell lysates of HUVEC, MDA-MB-231, and MDA-MB-435 cells. Total Erk2 is used as a loading control. Immunofluorescence staining (B), as described in the Materials and methods, was done on the HUVEC, MDA-MB-231, and MDA-MB-435 cell lines to show the expression pattern of EPCR and PAR-1 on the cell surface.
Fig. 4.
Generation of APC on either HUVEC (A) or MDA-MB-231 (B) cell monolayer is blocked with anti-EPCR antibody. In a 96-well plate, serum starved confluent monolayers were treated with either buffer, 20 μg/ml mouse serum IgG, or (0–20 μg/ml) anti-EPCR (JNK 1494) antibody for 15 min. Cells were then treated with zymogen PC (100 nM) for an additional 15 min. α-IIa (2 nM) was added and samples were taken from each well at various timepoints from 5 min to 24 h. Samples were added to hirudin (5 nM) and APC substrate (0.15 mM). The change in absorbance was read at 405 nm. The graphs represent the average of 3 separate experiments for HUVEC (A) and 4 separate experiments for MDA-MB-231 (B) done in triplicate.  α-IIa only;
α-IIa only;  α-IIa and PC;
α-IIa and PC;  20 μg/ml mouse serum IgG, α-IIa, PC;
20 μg/ml mouse serum IgG, α-IIa, PC;  2μg/ml anti-EPCR IgG, α-IIa, PC;
2μg/ml anti-EPCR IgG, α-IIa, PC;  4 μg/ml anti-EPCR IgG, α-IIa, PC;
4 μg/ml anti-EPCR IgG, α-IIa, PC;  10 μg/ml anti-EPCR IgG, α-IIa, PC;
10 μg/ml anti-EPCR IgG, α-IIa, PC;  20μg/ml anti-EPCR IgG, α-IIa, PC.
20μg/ml anti-EPCR IgG, α-IIa, PC.
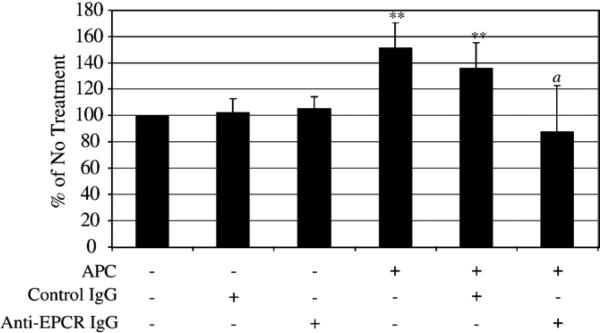
In the transwell chemotaxis assay, MDA-MB-231 cells were pre-incubated with 4 μg/ml control mouse IgG or anti-EPCR antibody for 15 min at room temperature prior to APC treatment. In Fig. 5, there was an 150 and 130% increase in chemotaxis when the cells were incubated with 10 μg/ml APC or APC and control IgG, respectively, compared to no treatment. Neither control IgG alone nor anti-EPCR antibody alone had an effect on chemotaxis. However, anti-EPCR antibody (4 μg/ml) attenuated the effects of APC, reducing chemotaxis back to baseline. These results indicate that an interaction between APC and EPCR is necessary to increase chemotaxis in the MDA-MB-231 cells.
Fig. 5.
APC binds to EPCR to increase chemotaxis in the MDA-MB-231 cells. Cells were pretreated with either anti-EPCR IgG (JNK1494; 4 μg/ml) or mouse serum IgG (4 μg/ml) for 15 min prior to the addition of APC (10 μg/ml) in a transwell chemotaxis assay incubated for 12 h. Cells migrated towards media containing 10% FBS as the chemotactic agent. The graphs represent an average of 6 separate experiments; **p <0.01 compared to No Treatment; a p <0.01 compared to APC treatment or APC and mouse serum IgG treatment.
APC interaction with PAR-1 is also necessary to increase chemotaxis of the MDA-MB-231 cells
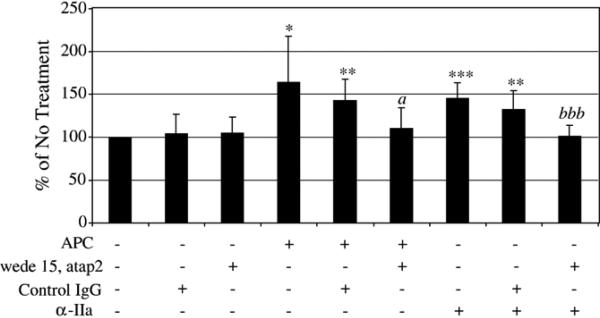
PAR-1 has been shown to be involved in mediating the nonhemostatic effects of APC [20,21,26,31]. With Western blots of cell lysates (Fig. 3A) and immunofluorescence staining of cell monolayers (Fig. 3B) of HUVEC, as a positive control, and both cancer cell lines, we verified the presence and expression of PAR-1 on the cell surface. To study the role of PAR-1 in the pro-migratory effects of APC with the MDA-MB-231 cells, two PAR-1 blocking antibodies were used in a 12-h transwell chemotaxis assay. The cells were pre-incubated with either PAR-1 blocking antibodies (wede15 and atap2) or control mouse IgG for 15 min at room temperature prior to APC treatment. In Fig. 6, 10 μg/ml APC (±control IgG) increased chemotaxis approximately 150%. In the presence of PAR-1 blocking antibodies, the effects of APC were attenuated. To verify that the PAR-1 blocking antibodies were properly blocking the binding of the protease to the receptor, cells were also treated with 5 nM α-IIa, a known ligand of the PAR-1 receptor that will affect migration of the MDA-MB-231 through its activation [37–39]. α-IIa alone or α-IIa with control IgG increased chemotaxis approximately 150%, and this increase was attenuated in the presence of the PAR-1 blocking antibodies. These results suggest that binding of APC to EPCR and PAR-1 is necessary to increase chemotaxis in the MDA-MB-231 cells.
Fig. 6.
Interaction with PAR-1 is necessary for APC to increase chemotaxis in the MDA-MB-231 cells. Cells were pretreated with either PAR-1 blocking IgG (10 μg/ml atap2 and 20 μg/ml wede15) or 30 μg/ml mouse serum IgG for 15 min prior to addition of APC (10 μg/ml) or α-IIa (5 nM) in a transwell chemotaxis assay incubated for 12 h. Cells migrated towards media containing 10% FBS as the chemotactic agent. The graphs represent the average of 8 separate experiments for studies done with APC and 7 separate experiments done with α-IIa; *p <0.05, **p <0.01, ***p <0.001 compared to No Treatment; a p <0.05 compared to APC treatment or APC and mouse serum IgG treatment; bbb p <0.05 compared to α-IIa treatment or α-IIa and mouse serum IgG treatment.
APC does not increases chemotaxis of the MDA-MB-231 cells by increasing cell number
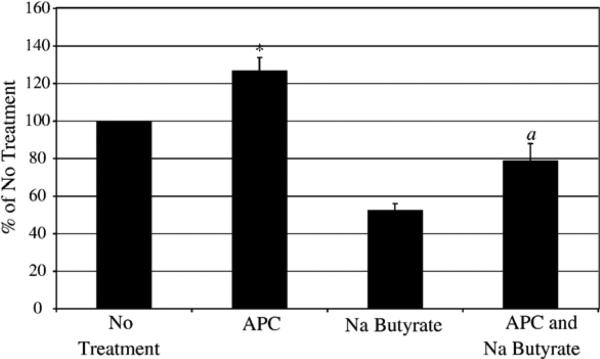
Na butyrate was used as an inhibitor of proliferation to determine if APC increases the number of cells that migrate in the transwell assays by increasing proliferation. Treatment of the MDA-MB-231 cells with 10 mM Na butyrate in 12 h reduces cell proliferation but does not induce apoptosis (data not shown). In a 12-h chemotaxis assay (Fig. 7), Na butyrate reduces chemotaxis compared to no treatment as expected due to differentiation and inhibition of undefined pathways in breast cancer cells. When APC is added with Na butyrate, APC increases cell chemotaxis over Na butyrate treatment alone (approximately 150% increase over Na butyrate treatment alone), which is the approximately the same ratio as APC treatment alone compared to no treatment (approximately 130% increase over No Treatment). Therefore, these results show APC increases chemotaxis by affecting other pathways involved in migration and not by increasing proliferation of the cells.
Fig. 7.
APC does not increases migration in the MDA-MB-231 cells by increasing cell number. Cells were pretreated for 1 h with 10 mM Na butyrate prior to addition of APC in the transwell chemotaxis assay incubated for 12 h. Cells migrated towards media containing 10% FBS as the chemotactic agent. The graph represents an average of 3 separate experiments; *p <0.05 compared to No Treatment; a p <0.05 compared to Na butyrate treatment.
Discussion
Historically, studies on the protein C system have focused on the role of APC as an anticoagulant. However, there have been recent studies, such as the PROWESS trial [14], on the anti-inflammatory and anti-apoptotic role of APC. Many recent reports have looked into these non-hemostatic roles of APC in vivo and in vitro. In this study, we showed that APC affects breast cancer cell migration in the transwell invasion and chemotaxis assay. APC, in a concentration-dependent manner, increases invasion and chemotaxis in both the MDA-MB-231 and MDA-MB-435 breast cancer cell lines. Previously, it had been published that APC increased cancer cell invasion through Matrigel-coated membranes only in the presence of PAI-1 [29]. It was hypothesized that APC complexed with PAI-1 [29]. This stable complex removed active PAI-1 from the cell environment and allowed uPA to activate plasminogen to plasmin. Plasmin can go on to activate MMP-9 [40] and MMP-2 [41]. uPA can also activate MMP-2 and regulate MMP-9 expression [42–44]. Since the MDA-MB-435 cell line does not express PAI-1 but still was affected by APC treatment in the transwell assay, we concluded that another mechanism is activated by APC to increase cell migration.
As previously reported, IIa is approximately 104-fold more potent that APC at activating PAR-1 [45]. Cells were treated with hirudin in the absence and presence of APC to assess if trace amounts of IIa were influencing the APC-induced transwell migration assays. We showed that APC alone was able to increase chemotaxis of the MDA-MB-231 cells and this was not due to IIa. The amount of APC needed to promote cellular migration is greater than physiological protein C blood plasma levels (~4 μg/ml). In the PROWESS trial, sepsis patients that were treated with APC have a steady-state level of 45 ng/ ml [45]. However, our overall goal was to characterize the mechanism of APC to promote cell migration and we used similar APC concentrations as in previous in vitro studies (0.5– 50 μg/ml) [20,26,29]. Interestingly, levels of APC used in HUVEC experiments are closer to physiological concentrations of APC (0.1–10 μg/ml) (data not shown).
As part of the anti-inflammatory action of APC, it had been previously reported that either APC or zymogen PC, through EPCR and EGFR, inhibits lymphocyte migration [17,25]. Using zymogen PC, chemically inactivated DEGR-APC, and an active site mutant of APC (S195A) with the MDA-MB-231 cancer cells, one finding of our study showed that the active protease was needed to increase cell migration. Therefore, unlike the inhibitory role of APC with lymphocytes, the pro-migratory role of APC in the MDA-MB-231 cells requires the active site of the protease, most likely to bind and activate receptors, such as PAR-1 [20,21,26,30,31], and to activate extracellular matrix proteases, such as MMP-2 and MMP-9 [27,46,47]. It is possible that when bound to EPCR, APC may undergo modifications to its macromolecular substrate recognition. Thus, APC bound to EPCR could promote migration and invasion through both activation of signaling pathways and activation of extracellular proteases.
Another finding in our study was that APC itself is not a chemotactic factor for the MDA-MB-231 cells, but it promoted chemotaxis and invasion through direct interactions with the cells. By contrast, an analogous serine protease, IIa, can either promote migration when treated with the cell or when used as a chemotactic factor. Using the transwell invasion and chemo-taxis assay with the MDA-MB-231 cells and altering the treatment of APC from the insert to the well, we found that APC must interact with the cells directly to allow for the increase in migration. Our results parallel what is known about uPA as a mediator of cell migration. This serine protease is not considered a chemotactic factor [48,49]. When bound to uPAR, uPA is able to promote both cell migration and invasion. Therefore, APC does not act as a chemotactic agent, but functions similarly to uPA and not IIa.
Past studies have indicated that APC is either activating proteases that degrade the extracellular matrix [27,28] and/or activating receptor(s) that initiate signaling pathways to increase invasion and chemotaxis. Other studies have shown that the ability of APC to interact with EPCR and PAR-1 is a critical aspect of the mechanism responsible for altering inflammation, proliferation, and apoptosis [20–27]. We found that the relationship of APC, EPCR, and PAR-1 is similar to that shown in anti-apoptotic [20,31] and anti-inflammatory [20] studies, where binding and activating of both receptors by APC are important to initiate the effects of APC on cell migration. Using blocking antibodies to both of these receptors, we showed that APC must bind to EPCR and PAR-1 in order to increase chemotaxis in the MDA-MB-231 cells. Blocking one or the other receptor completely attenuated the effects of APC on the MDA-MB-231 cells. We hypothesize that APC binds to EPCR, which localizes the protease and aids in the interaction of APC with PAR-1. Upon activation of PAR-1, the heterodimer of EPCR and PAR-1 initiates signaling pathways that increase MDA-MB-231 chemotaxis. It is important to note that this heterodimer pair is what allows for the unique effect of APC on the cell and distinguishes it from the effects of IIa on the cell. IIa signaling through PAR-1 is the opposite of APC in many aspects, such as IIa promotes both apoptosis and inflammation.
There are other studies that describe slight variances for the requirements of APC, EPCR, and PAR-1 to modify cell responses, suggesting either some diversity in the response or some cellular adaptation to APC as a modulator of cell function. In one setting it was shown that APC binding to EPCR is necessary for increases in HUVEC proliferation while PAR-1 activation by APC is only important for initial signaling events and not in maintaining the effect [26]. In a different setting, APC binding to EPCR and EGFR was critical for lymphocyte migration, not any PARs [17,25]. It is still possible that other receptors, including EGFR [25], have a role in the effects of APC on MDA-MB-231 cell migration. Additional studies on the interactions of APC and the cell surface are needed to further our understanding of how APC affects various cellular processes, including migration.
Na butyrate has been used as an inhibitor of proliferation in the MDA-MB-231 cells [50,51]. Na butyrate inhibits histone deacetylase, increases p21 expression, and decreases cyclin D gene expression [50] resulting in an arrest of the cell in G2 [51]. Na butyrate also induces cell differentiation [50], as shown with an increase in lipid accumulation, and a reduction in migration across multiple extracellular matrices [52]. We found that unlike the effects of APC on HUVEC proliferation, in breast cancer cells, binding to EPCR and activation of PAR-1 by APC increase migration but not proliferation. APC is able to increase MDA-MB-231 cell migration over Na butyrate treatment alone by the same ratio as APC treatment compared to control. Further, the pathways activated by Na butyrate [52,53] are not the same pathways utilized by APC to increase migration in these cells. Other pathways, such as MAPK and PI3K pathways [26], that have been implicated in the mechanism by which APC increase HUVEC proliferation may have a role in increasing migration in the MDA-MB-231 cells. Within the context of the MDA-MB-231 cells as a model system of cell migration, our results imply that in the presence of APC a pro-migratory process is initiated that is not dependent on cell proliferation.
Acknowledgments
We are grateful to Dr. Charles T. Esmon for providing the anti-EPCR antibody (JNK 1494) and to Dr. Alireza R. Rezaie for providing the active site mutant APC (S195A APC). We also gratefully acknowledge Lilly Research Laboratories for supplying Xigris® (wild-type recombinant human APC) under study number F1K-US-V016 (to F.C. Church). Current stipend support for L.M. Beaulieu is through F31 NS054590-01A1 (NRSA-NIH), and previously from the Integrative Vascular Biology Program (T32 HL69768, NIH) and from the Susan G. Komen Breast Cancer Foundation (BCTR0503475). Work in the authors' laboratory is supported in part by Research Grant HL-32656 from the National Institutes of Health and BCTR0503475 and BCTR45206 from the Susan G. Komen Breast Cancer Foundation (F.C. Church).
REFERENCES
- 1.Foster D, Davie EW. Characterization of a cDNA coding for human protein C. Proc. Natl. Acad. Sci. U. S. A. 1984;81:4766–4770. doi: 10.1073/pnas.81.15.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esmon CT, Stenflo J, Suttie JW. A new vitamin K-dependent protein. A phospholipid-binding zymogen of a serine esterase. J. Biol. Chem. 1976;251:3052–3056. [PubMed] [Google Scholar]
- 3.Kisiel W, Ericsson LH, Davie EW. Proteolytic activation of protein C from bovine plasma. Biochemistry. 1976;15:4893–4900. doi: 10.1021/bi00667a022. [DOI] [PubMed] [Google Scholar]
- 4.Fukudome K, Kurosawa S, Stearns-Kurosawa DJ, He X, Rezaie AR, Esmon CT. The endothelial cell protein C receptor. Cell surface expression and direct ligand binding by the soluble receptor. J. Biol. Chem. 1996;271:17491–17498. doi: 10.1074/jbc.271.29.17491. [DOI] [PubMed] [Google Scholar]
- 5.Stearns-Kurosawa DJ, Kurosawa S, Mollica JS, Ferrell GL, Esmon CT. The endothelial cell protein C receptor augments protein C activation by the thrombin-thrombomodulin complex. Proc. Natl. Acad. Sci. U. S. A. 1996;93:10212–10216. doi: 10.1073/pnas.93.19.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kisiel W, Canfield WM, Ericsson LH, Davie EW. Anticoagulant properties of bovine plasma protein C following activation by thrombin. Biochemistry. 1977;16:5824–5831. doi: 10.1021/bi00645a029. [DOI] [PubMed] [Google Scholar]
- 7.Walker FJ, Sexton PW, Esmon CT. The inhibition of blood coagulation by activated protein C through the selective inactivation of activated Factor V. Biochim. Biophys. Acta. 1979;571:333–342. doi: 10.1016/0005-2744(79)90103-7. [DOI] [PubMed] [Google Scholar]
- 8.Marlar RA, Kleiss AJ, Griffin JH. Human protein C: inactivation of factors V and VIII in plasma by the activated molecule. Ann. N. Y. Acad. Sci. 1981;370:303–310. doi: 10.1111/j.1749-6632.1981.tb29743.x. [DOI] [PubMed] [Google Scholar]
- 9.Walker FJ. Regulation of activated protein C by a new protein. A possible function for bovine protein S. J. Biol. Chem. 1980;255:5521–5524. [PubMed] [Google Scholar]
- 10.Walker FJ. Regulation of activated protein C by protein S. The role of phospholipid in factor Va inactivation. J. Biol. Chem. 1981;256:11128–11131. [PubMed] [Google Scholar]
- 11.Hoffman M, Monroe DM., III A cell-based model of hemostasis. Thromb. Haemost. 2001;85:958–965. [PubMed] [Google Scholar]
- 12.Esmon CT. The protein C pathway. Chest. 2003;124:26S–32S. doi: 10.1378/chest.124.3_suppl.26s. [DOI] [PubMed] [Google Scholar]
- 13.Taylor FB, Jr., Stearns-Kurosawa DJ, Kurosawa S, Ferrell G, Chang ACK, Laszik Z, Kosanke S, Peer G, Esmon CT. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood. 2000;95:1680–1686. [PubMed] [Google Scholar]
- 14.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ., Jr. Efficacy and safety of recombinant human activated protein C for severe sepsis. N. Engl. J. Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 15.Esmon CT. Protein C pathway in sepsis. Ann. Med. 2002;34:598–605. doi: 10.1080/078538902321117823. [DOI] [PubMed] [Google Scholar]
- 16.Yuda H, Adachi Y, Taguchi O, Gabazza EC, Hataji O, Fujimoto H, Tamaki S, Nishikubo K, Fukudome K, D'Alessandro-Gabazza CN, Maruyama J, Izumizaki M, Iwase M, Homma I, Inoue R, Kamada H, Hayashi T, Kasper M, Lambrecht BN, Barnes PJ, Suzuki K. Activated protein C inhibits bronchial hyperresponsiveness and Th2 cytokine expression in mice. Blood. 2004;103:2196–2204. doi: 10.1182/blood-2003-06-1980. [DOI] [PubMed] [Google Scholar]
- 17.Feistritzer C, Sturn DH, Kaneider NC, Djanani A, Wiedermann CJ. Endothelial protein C receptor-dependent inhibition of human eosinophil chemotaxis by protein C. J. Allergy Clin. Immunol. 2003;112:375–381. doi: 10.1067/mai.2003.1609. [DOI] [PubMed] [Google Scholar]
- 18.Isobe H, Okajima K, Uchiba M, Mizutani A, Harada N, Nagasaki A, Okabe K. Activated protein C prevents endotoxin-induced hypotension in rats by inhibiting excessive production of nitric oxide. Circulation. 2001;104:1171–1175. doi: 10.1161/hc3501.093799. [DOI] [PubMed] [Google Scholar]
- 19.Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J. Biol. Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 20.Cheng T, Liu D, Griffin JH, Fernandez JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 21.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 22.Liu D, Cheng T, Guo H, Fernandez JA, Griffin JH, Song X, Zlokovic BV. Tissue plasminogen activator neurovascular toxicity is controlled by activated protein C. Nat. Med. 2004;10:1379–1383. doi: 10.1038/nm1122. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez JA, Xu X, Liu D, Zlokovic BV, Griffin JH. Recombinant murine-activated protein C is neuroprotective in a murine ischemic stroke model. Blood Cells Mol. Dis. 2003;30:271–276. doi: 10.1016/s1079-9796(03)00034-2. [DOI] [PubMed] [Google Scholar]
- 24.Shibata M, Kumar SR, Amar A, Fernandez JA, Hofman F, Griffin JH, Zlokovic BV. Anti-inflammatory, antithrombotic, and neuroprotective effects of activated protein C in a murine model of focal ischemic stroke. Circulation. 2001;103:1799–17805. doi: 10.1161/01.cir.103.13.1799. [DOI] [PubMed] [Google Scholar]
- 25.Feistritzer C, Mosheimer BA, Sturn DH, Riewald M, Patsch JR, Wiedermann CJ. Endothelial protein C receptor-dependent inhibition of migration of human lymphocytes by protein C involves epidermal growth factor receptor. J. Immunol. 2006;176:1019–1025. doi: 10.4049/jimmunol.176.2.1019. [DOI] [PubMed] [Google Scholar]
- 26.Uchiba M, Okajima K, Oike Y, Ito Y, Fukudome K, Isobe H, Suda T. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ. Res. 2004;95:34–41. doi: 10.1161/01.RES.0000133680.87668.FA. [DOI] [PubMed] [Google Scholar]
- 27.Xue M, Thompson P, Kelso I, Jackson C. Activated protein C stimulates proliferation, migration and wound closure, inhibits apoptosis and upregulates MMP-2 activity in cultured human keratinocytes. Exp. Cell. Res. 2004;299:119–127. doi: 10.1016/j.yexcr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Jackson CJ, Xue M, Thompson P, Davey RA, Whitmont K, Smith S, Buisson-Legendre N, Sztynda T, Furphy LJ, Cooper A, Sambrook P, March L. Activated protein C prevents inflammation yet stimulates angiogenesis to promote cutaneous wound healing. Wound Repair Regen. 2005;13:284–294. doi: 10.1111/j.1067-1927.2005.00130311.x. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi H, Moniwa N, Gotoh J, Sugimura M, Terao T. Role of activated protein C in facilitating basement membrane invasion by tumor cells. Cancer Res. 1994;54:261–267. [PubMed] [Google Scholar]
- 30.Riewald M, Petrovan RJ, Donner A, Ruf W. Activated protein C signals through the thrombin receptor PAR1 in endothelial cells. J. Endotoxin. Res. 2003;9:317–321. doi: 10.1179/096805103225002584. [DOI] [PubMed] [Google Scholar]
- 31.Mosnier LO, Griffin JH. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem. J. 2003;373:65–70. doi: 10.1042/BJ20030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitley BR, Palmieri D, Twerdi CD, Church FC. Expression of active plasminogen activator inhibitor-1 reduces cell migration and invasion in breast and gynecological cancer cells. Exp. Cell. Res. 2004;296:151–162. doi: 10.1016/j.yexcr.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Palmieri D, Lee JW, Juliano RL, Church FC. Plasminogen activator inhibitor-1 and -3 increase cell adhesion and motility of MDA-MB-435 breast cancer cells. J. Biol. Chem. 2002;277:40950–40957. doi: 10.1074/jbc.M202333200. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Esmon NL, Esmon CT. Reconstitution of the human endothelial cell protein C receptor with thrombomodulin in phosphatidylcholine vesicles enhances protein C activation. J. Biol. Chem. 1999;274:6704–6710. doi: 10.1074/jbc.274.10.6704. [DOI] [PubMed] [Google Scholar]
- 35.Liaw PC, Mather T, Oganesyan N, Ferrell GL, Esmon CT. Identification of the protein C/activated protein C binding sites on the endothelial cell protein C receptor. Implications for a novel mode of ligand recognition by a major histocompatibility complex class 1-type receptor. J. Biol. Chem. 2001;276:8364–8370. doi: 10.1074/jbc.M010572200. [DOI] [PubMed] [Google Scholar]
- 36.Kurosawa S, Stearns-Kurosawa DJ, Hidari N, Esmon CT. Identification of functional endothelial protein C receptor in human plasma. J. Clin. Invest. 1997;100:411–418. doi: 10.1172/JCI119548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamath L, Meydani A, Foss F, Kuliopulos A. Signaling from protease-activated receptor-1 inhibits migration and invasion of breast cancer cells. Cancer Res. 2001;61:5933–5940. [PubMed] [Google Scholar]
- 38.Booden MA, Eckert LB, Der CJ, Trejo J. Persistent signaling by dysregulated thrombin receptor trafficking promotes breast carcinoma cell invasion. Mol. Cell. Biol. 2004;24:1990–1999. doi: 10.1128/MCB.24.5.1990-1999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henrikson KP, Salazar SL, Fenton JW, II, Pentecost BT. Role of thrombin receptor in breast cancer invasiveness. Br. J. Cancer. 1999;79:401–406. doi: 10.1038/sj.bjc.6690063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makowski GS, Ramsby ML. Binding of latent matrix metalloproteinase 9 to fibrin: activation via a plasmin-dependent pathway. Inflammation. 1998;22:287–305. doi: 10.1023/a:1022300216202. [DOI] [PubMed] [Google Scholar]
- 41.Murphy G, Atkinson S, Ward R, Gavrilovic J, Reynolds JJ. The role of plasminogen activators in the regulation of connective tissue metalloproteinases. Ann. N. Y. Acad. Sci. 1992;667:1–12. doi: 10.1111/j.1749-6632.1992.tb51590.x. [DOI] [PubMed] [Google Scholar]
- 42.Rao NK, Shi GP, Chapman HA. Urokinase receptor is a multifunctional protein: influence of receptor occupancy on macrophage gene expression. J. Clin. Invest. 1995;96:465–474. doi: 10.1172/JCI118057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lakka SS, Gondi CS, Yanamandra N, Dinh DH, Olivero WC, Gujrati M, Rao JS. Synergistic down-regulation of urokinase plasminogen activator receptor and matrix metalloproteinase-9 in SNB19 glioblastoma cells efficiently inhibits glioma cell invasion, angiogenesis, and tumor growth. Cancer Res. 2003;63:2454–2461. [PubMed] [Google Scholar]
- 44.Keski-Oja J, Lohi J, Tuuttila A, Tryggvason K, Vartio T. Proteolytic processing of the 72,000-Da type IV collagenase by urokinase plasminogen activator. Exp. Cell. Res. 1992;202:471–476. doi: 10.1016/0014-4827(92)90101-d. [DOI] [PubMed] [Google Scholar]
- 45.Ludeman MJ, Kataoka H, Srinivasan Y, Esmon NL, Esmon CT, Coughlin SR. PAR1 cleavage and signaling in response to activated protein C and thrombin. J. Biol. Chem. 2005;280:13122–13128. doi: 10.1074/jbc.M410381200. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen M, Arkell J, Jackson CJ. Activated protein C directly activates human endothelial gelatinase A. J. Biol. Chem. 2000;275:9095–9098. doi: 10.1074/jbc.275.13.9095. [DOI] [PubMed] [Google Scholar]
- 47.Pekovich SR, Bock PE, Hoover RL. Thrombin-thrombomodulin activation of protein C facilitates the activation of progelatinase A. FEBS Lett. 2001;494:129–132. doi: 10.1016/s0014-5793(01)02296-7. [DOI] [PubMed] [Google Scholar]
- 48.de Paulis A, Montuori N, Prevete N, Fiorentino I, Rossi FW, Visconte V, Rossi G, Marone G, Ragno P. Urokinase induces basophil chemotaxis through a urokinase receptor epitope that is an endogenous ligand for formyl peptide receptor-like 1 and -like 2. J. Immunol. 2004;173:5739–5748. doi: 10.4049/jimmunol.173.9.5739. [DOI] [PubMed] [Google Scholar]
- 49.Okada SS, Grobmyer SR, Barnathan ES. Contrasting effects of plasminogen activators, urokinase receptor, and LDL receptor-related protein on smooth muscle cell migration and invasion. Arterioscler. Thromb. Vasc. Biol. 1996;16:1269–1276. doi: 10.1161/01.atv.16.10.1269. [DOI] [PubMed] [Google Scholar]
- 50.Davis T, Kennedy C, Chiew YE, Clarke CL, deFazio A. Histone deacetylase inhibitors decrease proliferation and modulate cell cycle gene expression in normal mammary epithelial cells. Clin. Cancer Res. 2000;6:4334–4342. [PubMed] [Google Scholar]
- 51.Steinert M, Wobus M, Boltze C, Schutz A, Wahlbuhl M, Hamann J, Aust G. Expression and regulation of CD97 in colorectal carcinoma cell lines and tumor tissues. Am. J. Pathol. 2002;161:1657–1667. doi: 10.1016/S0002-9440(10)64443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basson MD, Turowski GA, Rashid Z, Hong F, Madri JA. Regulation of human colonic cell line proliferation and phenotype by sodium butyrate. Dig. Dis. Sci. 1996;41:1989–1993. doi: 10.1007/BF02093601. [DOI] [PubMed] [Google Scholar]
- 53.Ogawa H, Rafiee P, Fisher PJ, Johnson NA, Otterson MF, Binion DG. Sodium butyrate inhibits angiogenesis of human intestinal microvascular endothelial cells through COX-2 inhibition. FEBS Lett. 2003;554:88–94. doi: 10.1016/s0014-5793(03)01110-4. [DOI] [PubMed] [Google Scholar]