Abstract
Much research has been done on positive self-evaluation and its relationship to mental health. However, little is known about its neural underpinnings. Imaging studies have suggested that the brain’s default network is involved with self-related processing, and that one portion of the default network, medial prefrontal cortex (MPFC), is particularly involved with self-evaluation. Here we used transcranial magnetic stimulation (TMS) to causally demonstrate that this network, and particularly MPFC, is involved with self-evaluative processing. In a first experiment, 27 healthy volunteers judged whether adjectives, evenly-divided between desirable and undesirable traits, described themselves or their best friends, and a robust self-enhancement bias effect was found. In a second experiment, single-pulse TMS was applied targeting three locations (MPFC and left and right parietal cortex) in a different group of healthy volunteers while they performed the adjective task. In each trial, TMS was applied at one of five different times relative to onset of the adjective ranging from 0–480ms. TMS affected self-enhancement bias in a site- and latency-specific manner: at MPFC, the self-enhancement bias actually reversed at 160ms, with subjects favoring their best friend over themselves. TMS may thus be of use in investigating areas of mental illness in which self-evaluation is abnormal, potentially as a diagnostic tool. In addition, the present study, combined with our previous reports (Lou etal., 2004, 2010) causally demonstrate two kinds of self-related processing within the default network, one centered in parietal cortex and concerned with retrieval of self-related associations, and the other MPFC-centered, involved in self-evaluative processing.
Keywords: TMS, self, self-enhancement, medial prefrontal, parietal cortex, default network
Introduction
The “self” remains an ill-defined concept and yet it remains central to psychological theory and psychiatric practice. One neurobiological approach to understanding and defining the self concept is to examine the structures in the brain that participate in self-specific processing. One leading candidate for such a structure is the “default” network of the brain, a network which was initially recognized as a common pattern of deactivations occurring in imaging contrasts during PET or fMRI over a wide spectrum of tasks when images obtained during the task conditions were contrasted with resting or control conditions (Raichle, 1998). This same network has typically been activated in imaging studies in which tasks are used that contrast self-related stimuli with non-self-related stimuli, for example using self vs. other faces (Kircher et al., 2000, 2001; Platek et al., 2004), names (Perrin et al., 2005; Sugiura et al., 2006), information (Maguire and Mummery, 1999; Vinogradov et al., 2006; Nunez et al., 2004), traits, appearance, attitudes, or feelings (Craik et al., 1999; Gusnard et al., 2001; Kircher et al., 2000, 2002; Kelly et al., 2002; Johnson et al., 2002; Lou et al., 2004; Kjaer et al., 2002; Fossati et al., 2004; Schmitz et al., 2004; Ochsner et al., 2005). Given its activation when subjects are inwardly directed and also when processing self-related items, it has been suggested that the default network supports a tonically active system that continuously evaluates external and internal context and permits the experience of self-awareness and consciousness (Andreasen et al., 1995; Cavanna & Trimble, 2006; Damasio, 2010; Gusnard and Raichle, 2001).
These suggested roles for the default network remain speculative to the degree that they are based on brain imaging data, which can only be correlative. In fact, the same brain imaging studies have been used to make a case that the default network is not specifically activated by self-related content, but is involved in a more general process involved with reasoning and memory (Ruby and Legrand, 2008). On the other hand, transcranial magnetic stimulation (TMS), a noninvasive means of temporarily altering the function of precise brain regions, offers a way of testing whether direct causal relationships link brain with behavior. TMS has been used to examine processing of self/other relationships in the default network and has found evidence for its involvement in self-specific processing (Kwan et al., 2007, Lou et al., 2004, 2010; Uddin, 2006). In our own work, we used a task in which subjects rated adjectives as like or unlike themselves or their best friend. Almost all subjects demonstrated a self reference effect (SRE) in this task, a performance superiority for processing self- as opposed to other-related stimuli when speed and accuracy of responses are compared. Such SREs may indicate a functionally distinct cognitive system for self knowledge (e.g., Rogers et al., 1977). We used single-pulse TMS applied to default network nodes (midline prefrontal cortex in Lou et al., 2004 and 2010, precuneus in Lou et al. 2004, and left and right lateral parietal cortex in Lou et al., 2010) at various times ranging from 0 to 480 msec after the onset of the visually-presented adjective. The SRE was abolished (with disruption for self-related adjectives but no effect on performance in best-friend trials) with single TMS pulses to precuneus at 160 msec and to right parietal cortex at 480 msec. At the left parietal site, TMS nullified the SRE at all latencies tested, and may have reversed the SRE at 160 ms, such that performance in the best friend condition was better than in the self condition. These results support the idea that the posterior portion of the default network, centered on left parietal cortex, is involved with self-specific processing, more specifically with the categorization and recall of self-related traits.
While midline prefrontal cortex (MPFC) was tested in both studies (Lou et al., 2004, 2010), a TMS effect on self-related processing was not found. This was somewhat surprising, given the ubiquity of activation results in this region in self/other contrasts in imaging experiments, and specifically, MPFC involvement during evaluation of self-relevant verbal stimuli (Craik et al., 1999; Fossati et al., 2003, 2004; Heatherton et al., 2006; Johnson et al., 2002). The lack of a MPFC self/other TMS effect in the Lou et al. studies may have been due to the exact MPFC site chosen, the time of TMS application, or the relative unpleasantness or distractability of stimulation to frontal scalp. Another possibility is that the MPFC does not contribute substantially to the SRE in the adjective task, leaving the source of the SRE to posterior regions. Instead, its contribution to self-specific processing might be more evaluative in nature. There is considerable evidence that MPFC may be involved in networks associated with self-monitoring and self-evaluation (Gusnard et al., 2001; Johnson et al., 2005; Ochsner et al., 2004, 2005). Gusnard et al. (2001) in particular demonstrated MPFC activation in self-referential judgments evaluating pleasantness vs. unpleasantness of affectively normed pictures.
In this regard, a self-related TMS effect has been produced using the same adjective task as Lou et al., but employing a different behavioral measure (Kwan et al., 2007). They took advantage of the fact that the adjectives in the task included equal amounts of desirable and undesirable traits. They found subjects were typically biased towards agreeing with more desirable traits and not agreeing with undesirable traits when describing themselves as opposed to their best friend, a self-enhancement effect. In applying TMS, a single pulse to MPFC at 500 ms SOA, but not to SMA or precuneus, eliminated this bias. This outcome suggested that the MPFC portion of the default network does indeed process self-specific information, albeit specialized for different functions than parietal cortex.
To investigate this possibility, we have re-analyzed the data of Lou et al. (2010), by initially segregating all trials according to whether the adjectives exemplified desirable or undesirable traits, and tested whether a self-enhancement TMS effect was present in our own data. We did this with three goals in mind. First, given that we applied TMS to MPFC at approximately the same time (480 ms after adjective onset) in one of our conditions, we could replicate the effect seen in Kwan et al. (2007). Second, we could also considerably extend the Kwan et al. finding within the default network both dynamically and topographically, as we examined a range of time points from 0–480 ms after adjective onset, as well as two more regions within the network besides MPFC (left and right lateral parietal cortex). Third, we would be able for the first time to use TMS to demonstrate the simultaneous presence of two self-related processes in the same network (here, the default network), one giving rise to an SRE, the other to a self-enhancement effect. Most important in this regard is that by demonstrating the activity of two qualitatively different sorts of self-specific processing, evidence would be provided that the default network is involved with a system centered around self-specific processing.
Methods
Subjects
Forty-five healthy subjects were recruited and provided written informed consent for the study, which was approved by the NY State Psychiatric Institute IRB. Twenty-seven (sixteen male) with mean age of 26.9 +/− 1.5 years participated in an initial behavioral experiment, performing an adjective task with no TMS. Eighteen subjects (eight male) with mean age of 28.6 +/− 2.7 years volunteered for the TMS experiment, performing the adjective task while receiving TMS. Data from the eighteen subjects in this second group were presented in Lou et al. (2010). Subjects were required to have normal or corrected-to-normal vision. All subjects were screened for psychiatric disorders, substance abuse/dependence, a history of neurological disease, pregnancy, or seizure risk factors.
Adjective Task
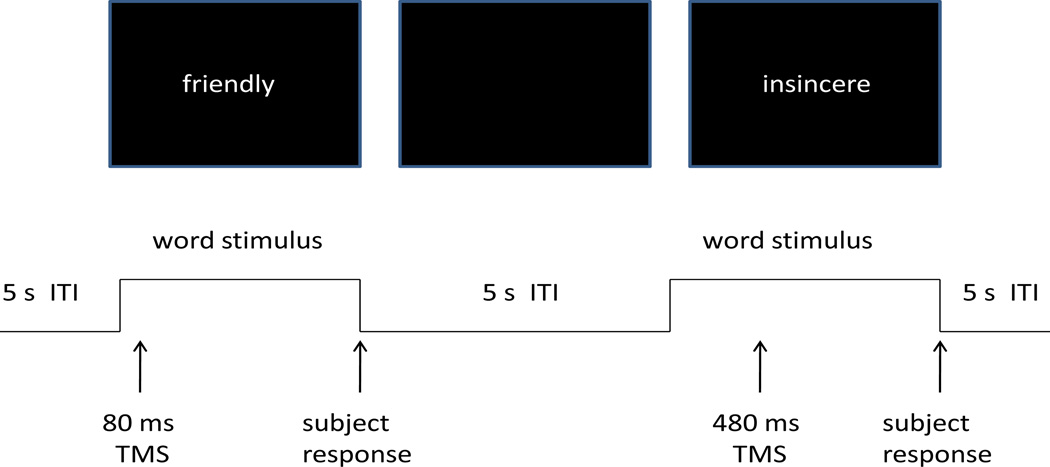
A set of 555 adjectives describing personality characteristics and which were rated according to likableness were obtained from Anderson (1968). From this set, six 90-word lists were randomly chosen (without replacement) for each subject to be used in the experimental session. In each set of 90 words, half of the words were chosen from those in Anderson (1968) that were rated as desirable, and the other half undesirable. In each trial an adjective appeared on a computer monitor and remained on the screen for a maximum of four seconds, and disappeared when the subject responded (Figure 1). As a list of adjectives was presented, subjects were asked to rate the applicability of each adjective to one's self or, in separate blocks, his/her best friend. Then each word was presented again with the requirement for the subject to indicate with a yes/no button press as quickly as possible whether or not the adjective had been judged to describe him/her self (or the best friend). The order of self and best friend conditions was counterbalanced between subjects.
Figure 1.
Schematic depiction of the adjective task.
In a first experiment, twenty-seven subjects performed this task for both self and best friend conditions in a single session without TMS. This was done in order to establish the size and predominance of the self-enhancement effect as it occurred in the adjective task in healthy subjects. In a second experiment, a new group of subjects performed the task while TMS was applied to different cortical regions, in order to assess the influence of TMS on the established self-enhancement effect.
TMS Application
In the second experiment, 18 participants received single pulse TMS, applied using a figure 8 coil (9 cm diameter) powered by a Magstim 200 stimulator (Magstim Co., Whitland, South West Wales, UK). TMS stimulus intensity was set at 150% of resting motor threshold of the left hemisphere, with motor threshold determined after Rothwell et al. (1999). Three cortical sites were selected for stimulation: left and right lateral parietal cortex (angular gyrus) and midline prefrontal cortex (Figure 2) based on previous imaging work (Lou et al., 2004). Order of stimulation sites in the session was counterbalanced between subjects. The sites were identified using high-resolution structural MRI scans obtained for each subject (except in 5 subjects without scans where the International 10/20 system was used). The coil was positioned and accuracy of placement continuously monitored during task performance using Brainsight, a computerized frameless stereotaxic system (Rogue Research, Montreal, Canada).
Figure 2.
Schematic diagram showing locations and TMS coil orientations for the three target sites (from left to right): medial prefrontal, right parietal and left parietal cortex sites.
For each trial of a list’s second presentation, a single pulse of TMS was delivered with a stimulus onset asynchrony (SOA) between the onset of an adjective and the TMS pulse of 0, 80, 160, 240, or 480 milliseconds. Choice of SOA was randomized for each trial, with the constraint that eighteen trials of each SOA occurred during each 90 trial block and no more than four trials in a row had the same SOA. A TMS block was performed for Self and Best Friend conditions, counterbalanced across subjects, at each of the three stimulation sites. Each session lasted approximately 2.5 hours.
Analysis
An index of self enhancement (SEI) was generated after Kwan et al. (2007). In the case of desirable adjectives, each “yes” response was assigned a value of 1, and each “no” response a 0. For undesirable adjectives, each “yes” response received a −1, and each “no” response a 0. In summing these scores, a total greater than zero indicated a tendency to respond positively and less than zero a tendency to respond negatively (within a total range of −45 to +45). Comparing self and best friend conditions can be achieved via self – best friend subtraction, where a positive value indicates a self-enhancement tendency. This comparison results in the SEI.
The goal of the first experiment was to establish that a self-enhancement effect was reliably present in subjects performing the adjective task, and that the SEI captured this effect. A three-way repeated measures ANOVA comparing number of answers was performed with factors of pleasant vs. unpleasant adjectives, agree vs. disagree response, and self vs. best friend condition. Here we looked for interactions of self/best friend with the other two factors, followed by a t-test of the SEI as greater than zero to indicate the presence of the self-enhancement bias.
It was important to establish that the SEI captured the enhancement effect in the first experiment, because otherwise the ANOVA needed would be 5-way, with the addition of five SOAs and three stimulation sites, resulting in too many degrees of freedom for the number of subjects. The SEI incorporates the effects of pleasant/unpleasant and agree/disagree in an index that directly combines these factors to indicate a bias towards self or best friend. Using the SEI also allows a comparison with Kwan et al. (2007), where results were reported only using that index.
Having established a reliable effect and the reliability of the SEI in capturing the effect in the first experiment, the goal of the second experiment was to observe the effect of TMS on the SEI in a site and time specific fashion. A repeated measures ANOVA comparing SEIs across Site (MPFC, right parietal, left parietal) and SOA (0, 80, 160, 240, 480 ms) was thus performed. Eighteen subjects participated in the TMS experiment. However, three subjects felt discomfort during prefrontal TMS and dropped out, with the result that the ANOVA was run on the data from fifteen subjects in the TMS experiment. ANOVAs using factors with three or more levels (here, Site and SOA) were tested for sphericity. In the case of departures from sphericity (if the Greenhouse-Geisser epsilon was < 0.70), a repeated measures MANOVA was used in place of the ANOVA. Otherwise, the probability value was Greenhouse-Geisser corrected. Post hoc comparisons were t-tests comparing each SEI against a value of zero to indicate the significant presence of a bias. Results were Bonferroni-corrected for 5 SOAs and 3 sites.
Reaction time data in both experiments was also analyzed, using repeated measures ANOVAs comparing RTs across pleasant vs. unpleasant adjectives and self vs. best friend. The factor of agree/disagree was not included in RT analyses because there were typically too few responses in the pleasant/disagree and unpleasant/agree categories to generate good individual estimates of RT.
Results
First Experiment: Task performance without TMS
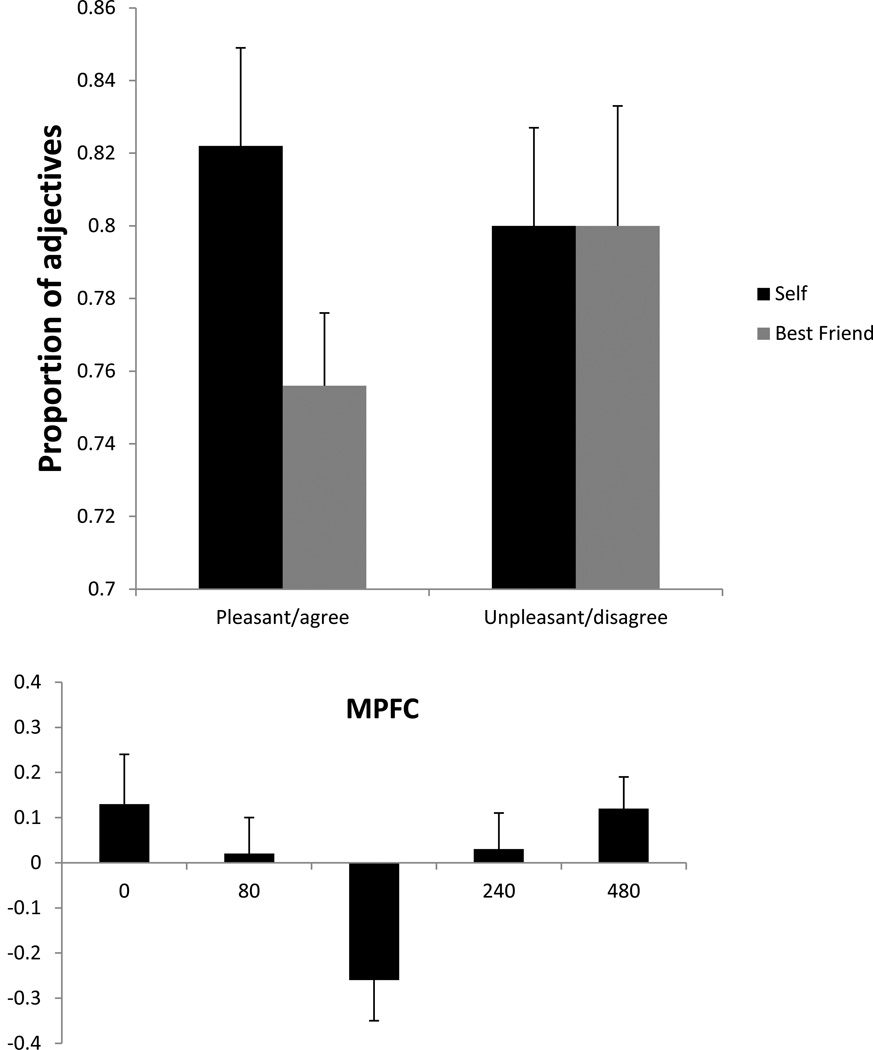
The three-way repeated measures ANOVA comparing number of answers across pleasant vs. unpleasant adjectives, agree vs. disagree response, and self vs. best friend condition resulted in two significant interactions: pleasant/unpleasant * agree/disagree (F1,26 = 193.1, p < 0.001) and self/best friend * pleasant/unpleasant * agree/disagree (F1,26 = 4.3, p < 0.05). The first significant result indicated the expected finding that agreement with pleasant and unpleasant traits is asymmetric, i.e., that in general subjects agree with pleasant traits and disagree with unpleasant ones. The second significant result was evidence for a self-enhancement effect. The mean proportion of answers for pleasant adjectives agreed with and unpleasant ones disagreed with (compared to the total number of pleasant and unpleasant adjectives, respectively) in self and best friend conditions for the 27 subjects who performed the task without TMS is shown in Figure 3. As can be observed in this figure, subjects were more likely to attribute desirable traits to themselves than to their best friends. On the other hand, they were equally likely to reject an undesirable trait, whether about themselves or their friend. When their responses were entered into the SEI equation, two-thirds of the subjects had a positive index, indicating a self-enhancement bias, while three had a index of 0.0, indicating no bias, and six had a negative index, with a bias towards enhancement of their friend’s traits. The group mean (and standard error) SEI was 5.5% ± 2.7, which was significantly greater than zero (t26 = 2.0, p < 0.03), demonstrating an overall self-enhancement in the task.
Figure 3.
Mean proportion (and SE) of total pleasant adjectives agreed with and unpleasant ones disagreed with in self and best friend conditions for the 27 subjects who performed the task without TMS.
Subjects also were faster in responding to pleasant traits, especially when they were answering about themselves. Group mean (and SD) RT for the pleasant/self condition was 786 ± 148 ms, for pleasant/best friend, 844 ± 151 ms, for unpleasant/self, 835 ± 142, and for unpleasant/best friend, 872 ± 132 ms. A repeated measures ANOVA comparing RTs across pleasant vs. unpleasant adjectives and self vs. best friend condition showed main effects of pleasant/unpleasant and self/best friend, with no interaction. RTs were significantly faster for desirable adjectives (F1,26 = 13.1, p < 0.002) and for the self condition (F1,26 = 8.8, p < 0.01).
Second Experiment: Task performance with single-pulse TMS
An initial repeated measures ANOVA comparing self and best friend responses before they were joined into a single SEI indicated that overall, the self-enhancement effect was present in the new group of subjects, with a main effect of self/best friend (F1,14 = 9.41, p<.01).
The repeated measures ANOVA comparing SEIs across Site (MPFC, right parietal, left parietal) and SOA (0, 80, 160, 240, 480 ms) resulted in a strong departure from sphericity for SOA, and a repeated measures MANOVA was substituted. The MANOVA showed that TMS affected the self-enhancement effect in both a location- and time-specific manner. There were significant main effects of Site (F2,13 = 4.5, p < 0.035) and SOA (F4,11 = 5.5, p < 0.015). In addition, the Site * SOA interaction exhibited a statistical trend (F8,7 = 3.5, p < 0.058).
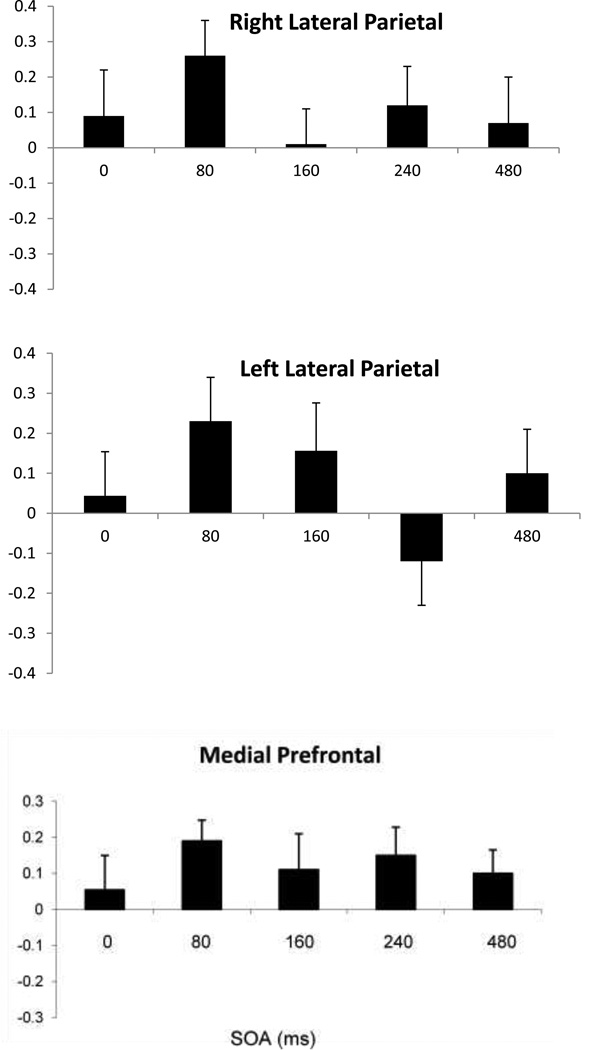
The SOA main effect provides evidence for a TMS effect, as it would be difficult to interpret it as anything other than an effect of TMS on processing in underlying cortex. While superficial effects of TMS can cause an SOA effect in performance measures such as accuracy or RT, it is unclear how they might cause time-sensitive changes in a measure of differences in trait judgments between Self and Best Friend. Likewise, while a superficial effect of TMS such as unpleasantness at one site can be distracting and can cause a site effect on performance measures such as RT or accuracy, it is difficult to imagine how that could cause a reversal in trait judgments between Self and Best Friend. The main effect of Site is due to the mean SEI going to 0% at MPFC, but averaging 8% and 11% at left and right parietal sites respectively, with a significant difference between MPFC and right parietal (t14 = 2.86, p<.02, Bonferroni-corrected for Site). The main effect of SOA is due to the SEI becoming negative (favoring Best Friend) at 160 ms, while remaining positive (favoring Self) at the other SOAs, a pattern seen most distinctly at MPFC (see Figure 4a). Given the main effects of SOA and Site, their likely interpretation as being due to the effects of TMS on neural processing, as well as the trend in the Site x SOA interaction, separate ANOVAs with SOA as a factor were performed at each site. A main effect of SOA was found only for the frontal location (F4,11 = 2.9, p < 0.05).
Figure 4.
Mean group SEI index (and SE) at the prefrontal, right and left lateral parietal sites at the five SOAs. Vertical axis is proportion of total adjectives.
The SEIs for each SOA at each of the three scalp sites are shown in Figure 4, expressed as proportion of total adjectives. At MPFC, the SEI across SOA forms a U-shaped function, with the self-enhancement effect diminished at 80 and 240 ms, and reversed at 160 ms. A one-tailed t test between SEI and zero at this latency indicated an enhancement toward Best Friend (t14 = 3.24, p=.045, Bonferroni-corrected for SOA and Site). Also at this latency, 11 of 15 subjects answered more positively to best friend than self, with the others showing no difference. At the right parietal site, the SEI at 160 ms vanished, while at the left parietal site, a negative SEI occurred at 240 ms, although this was not significant. In summary, while the group in Experiment 2 showed an overall self-enhancement effect, TMS modified this effect in a site- and time-specific manner, and in particular, TMS to MPFC at 160 ms reversed the self-enhancement and demonstrated enhancement of responses to Best Friend instead.
In the RT data, there was a main effect of pleasant/unpleasant (F1,14 = 27.8, p < 0.0001), with responses to undesirable traits (mean/SD 918 ± 252 ms) slower overall than to desirable ones (844 ± 218 ms), as was seen above in the subjects that performed the task without TMS. There was also a main effect of SOA (F4,11 = 5.81, p < 0.01), which was reported previously for this data (Lou et al., 2010).
A comparison of RT showed no significant differences between the first and second experiments. A mixed model ANOVA with between groups factor of experiment (1, 2) and repeated measures factors of pleasant/unpleasant and Self/Best Friend yielded the same significant main effects as in the first experiment alone, with RTs significantly faster for desirable adjectives (F1,40 = 40.2, p < 0.0001) and for the self condition (F1,40 = 7.8, p < 0.01), but with no significant main effect of or interactions with Experiment.
Discussion
A robust self-enhancement effect was found in a group of subjects performing the adjective task without TMS, linked to a positive self-enhancement index (SEI), and again in a second group with TMS, replicating previous studies (Kwan et al., 2007; Barrios et al., 2008). With single pulses of TMS, the SEI was diminished in a site-specific and time-specific manner, creating a dynamic topography across anterior and posterior portions of the default network. Specifically, TMS to MPFC generated a U-shaped function of SEI diminishment, with a negative peak with TMS at 160 ms after adjective onset, at which point subjects actually were biased towards enhancement of their best friends over themselves. This topography of effects is different than the one found previously with the same subjects using a different measure emphasizing speed and consistency of performance rather than judgment of desirability (Lou et al., 2010), providing evidence for two separate aspects of self-specific processing within the default network, one more frontally-centered, and the other centered in left posterior cortex.
It should be noted that without a no-TMS condition in Experiment 2, the direction and magnitude of the TMS effects cannot be interpreted with certainty. The data for the second experiment were a reanalysis of data from a TMS experiment (Lou et al., 2010) in which time and site controls were sufficient to demonstrate TMS effects, and a no TMS condition was not performed. While another no-TMS replication in the second experiment would of course have been desirable, the SOA technique used here appears to have successfully separated the normal SEI effect and TMS-disrupted SEI. The usefulness of the SOA technique is based on the assumption that in a given cortical region associated with a given task, there are critical time periods when a single TMS pulse will be sufficient to disrupt ongoing processing enough to create a behavioral manifestation of that disruption, while a pulse at a non-critical time will have little or no effect, and behavior will be more or less normal (e.g., subjects in the present case would show the typical SEI effect). The SOA method has been used in this way to study cortical processing dynamics with great success almost from the time TMS was invented (e.g., Amassian et al., 1989). The SOA assumption appeared to hold true in the present case as well. First, we convinced ourselves that the SEI effect was reliable. It was first shown using the adjective task in Kwan et al. (2007) with a smaller group (N=12) than used here in either experiment. We replicated the effect without TMS in the first experiment. In the second experiment it was then checked that the self-enhancement effect was present overall: this would be the case if the SOA Assumption held, and TMS had an effect only in a minority of the total time. This test was passed as well: as reported, an ANOVA comparing Self and Best Friend responses before they were joined into a single SEI had a strong main effect distinguishing Self and Best Friend. Moreover, inspection of Figure 4 indicates that at a time when TMS would not be expected to disturb ongoing processing (i.e., SOA = 0, before any visual information has left the retina), the SEI averages a positive 9% across the three sites, a degree of self-enhancement consistent with non-TMS results. The sensitive window for the task appears to center at a latency of 160 ms, and the SEI had gone back to a positive 10% across sites at 480 ms. Thus, while the relationship of the SEI and TMS effects cannot be known with certainty, the results suggest that the SOA Assumption was correct, and that a self-enhancement effect was generated, except at a critical time when TMS could disrupt it.
The result of the present study is in accord with Kwan et al. (2007), in which the SEI was eliminated with single pulse TMS to the same region of MPFC, and extends that study’s findings by demonstrating similar effects in two posterior cortical regions. However, the reduction in SEI was seen in Kwan et al. with stimulation at 500 ms after adjective onset, while none was apparent in the present study at a similar stimulation time (480 ms). There were considerable differences between how Kwan et al. and the present study applied TMS which may explain this discrepancy. First, the intensity of the TMS was much stronger in this study (150% MT) than in Kwan et al. (90% MT). The stronger stimulation certainly had both a more disruptive effect on the cortex immediately below the coil and also affected a more extensive region, both across the cortical surface as well as in depth. Either or both of these effects may have resulted in a different temporal pattern of disruption of ongoing self-specific processing, and the differing results suggest that parametric measurements across TMS intensity and SOA in future experiments might lead to richer understanding of the dynamic processing involved. A second important difference was that in Kwan et al. subjects were given TMS on first exposure to the adjectives, while in the present study subjects responded to the same lists of adjectives twice in succession, with TMS pulses only given during the second list. While this was done to reduce RT variability in the TMS trials, this added experience of the same stimuli may also have altered the timing of processing so that the sensitive latency was somewhat earlier.
One objection to the interpretation that TMS was modulating a self-specific processing system in the present study and in our previous reports (Lou et al., 2004, 2010) might be that people in general have thought much more about their own personality, and have observed more often their own names, faces, possessions, characteristics, etc., and so even in a general, non-self-specific processing system, representations related to the self might thus be stronger, leading to superior performance when dealing with self-related items. This argument has been made in reviews of self-enhancement effects in behavioral (Symons and Johnson, 1997) and physiological data (Gillihan and Farah, 2005). However, the TMS evidence from our previous reports and the present one argue against this interpretation, and instead provide causal evidence for an interpretation of default network activity involving a self-specific processing system. In our previous studies (Lou et al., 2004, 2010), TMS modulated the SRE found in the adjective task in two ways. First, TMS to midline and right parietal sites nullified the SRE by lowering performance in the Self condition without affecting performance in the Best Friend condition. Representations of best friends in a general memory store should be strong, although perhaps not as strong as those for one’s self: disruptions caused by TMS that lowered Self performance should also have disrupted Best Friend performance as well, although probably to a lesser degree. Instead, only disruptions in the Self conditions were observed, suggesting a system independently processing self information. Second, TMS to left parietal cortex resulted in a reversal of the SRE, such that both performance in the Self condition diminished to the level seen in the Best Friend condition, while the reverse was true in the Best Friend condition. This suggested that TMS to left parietal cortex not only disrupted processing that lead to an SRE, but that it nullified the output of this region that might normally compete with output from other areas processing Best Friend information, such as the immediately adjacent parieto-temporal cortex (Seghier et al., 2010), resulting in a paradoxical TMS facilitation effect (Walsh et al., 1999; Hayward et al., 2004). Again, this result suggests a self-specific processing system. The results of the present report, using the same data that supported self-specific processing resulting in an SRE, reinforce this possibility. Here we have shown that TMS modulated a self-enhancement bias. SREs, the performance facilitations for self-related items, might be explained by stronger self representations within a more general processing system (although our findings suggest otherwise). However, biases are not performance facilitations: they are an index of qualitative choices made, in this case directly about the self (or best friend). As such, they are not subject to the same sort of objections concerning relative matters of degree a continuous performance variable like RT is subject to. TMS affected this bias, and thus a judgment process, in left and right parietal cortex already implicated in the same data set in self-specific processing. The most profound modulations of the judgment process behind the self-enhancement bias occurred in MPFC, a region intimately connected with these parietal regions as part of the default mode network.
Self-enhancement and MPFC
In our previous report (Lou et al., 2010), we found no TMS effects on the SRE in MPFC. At that time, we discussed a number of possible reasons for this: that the timing of the pulses was not correct to disrupt MPFC activity, that the unpleasantness and/or distractibility of TMS pulses to that part of the head may have interfered with subjects’ ability to perform the task, or that the type of adjective task-related processing occurring in MPFC may have been different than the parietal processing that was shown to be sensitive to TMS. The results of the present re-analysis of the same data indicate that the third possibility was likely the correct one, since MPFC was sensitive to TMS disruption of judgmental biases in the adjective task. This suggests that MPFC was involved with processing of an evaluative nature which was sensitive to TMS disruption as reflected in changes in the SEI, while parietal cortex was involved with episodic retrieval processes in which TMS could disrupt SRE performance effects.
A strong empirical case has been made that self-enhancement is a robust phenomenon occurring in most people, in which they view themselves in unrealistically positive terms, believe they have greater control over events than they actually do, and hold unjustifiably optimistic views of their future (Taylor and Brown, 1988, 1994). These illusions were held to contribute to positive mental health, and it has been suggested that these biases are lost in depression (Alloy and Abramson, 1988; Beck, 1967). While both the present study and Kwan et al. (2007) provide causal evidence for MPFC involvement in self-specific processing involving self-enhancement in MPFC, little is known regarding the neurophysiological mechanisms underlying self-enhancement. No imaging studies of self-enhancement have been reported. Some imaging studies have employed task manipulations using positive and negative trait adjectives as stimuli to observe self-related processing (Fosatti et al., 2004; Gusnard et al., 2001), but self-enhancement effects were not explicitly studied. On the other hand, a great deal of evidence has been amassed for MPFC participation in self processing in general. In particular, there have been a number of imaging studies that used trait adjectives like the present study to provide evidence for this role (Craik et al., 1999; Fosatti et al., 2003. 2004; Heatherton 2006; Kelly et al., 2002; Lou et al., 2004; Ochsner et al., 2005; Schmitz et al., 2004). One distinction that has been observed in imaging (and also in line with differing anatomical connections) has been between dorsal and ventral MPFC (D’Argenbeau et al., 2007; Mitchell et al., 2006; Ochsner et al., 2005; Schmitz and Johnson, 2007). It has been suggested that MPFC is sensitive overall to self-relevant task features, with ventral MPFC supporting the representation of affective and motivational states connected to the detection of and orientation to self-relevant content, while similar information is handled in dorsal MPFC at a more symbolic level allowing introspective judgments and evaluation of internal states (D’Esposito et al., 2000; Miller and Cohen, 2001; Ochsner et al., 2005; Schmitz and Johnson, 2007). TMS would be expected to directly influence the shallower dorsal MPFC rather than the deeper lying ventral MPFC, suggesting that self-enhancement is integral to the self-evaluative judgments occurring there.
Simultaneous self-specific processes operating within the default network
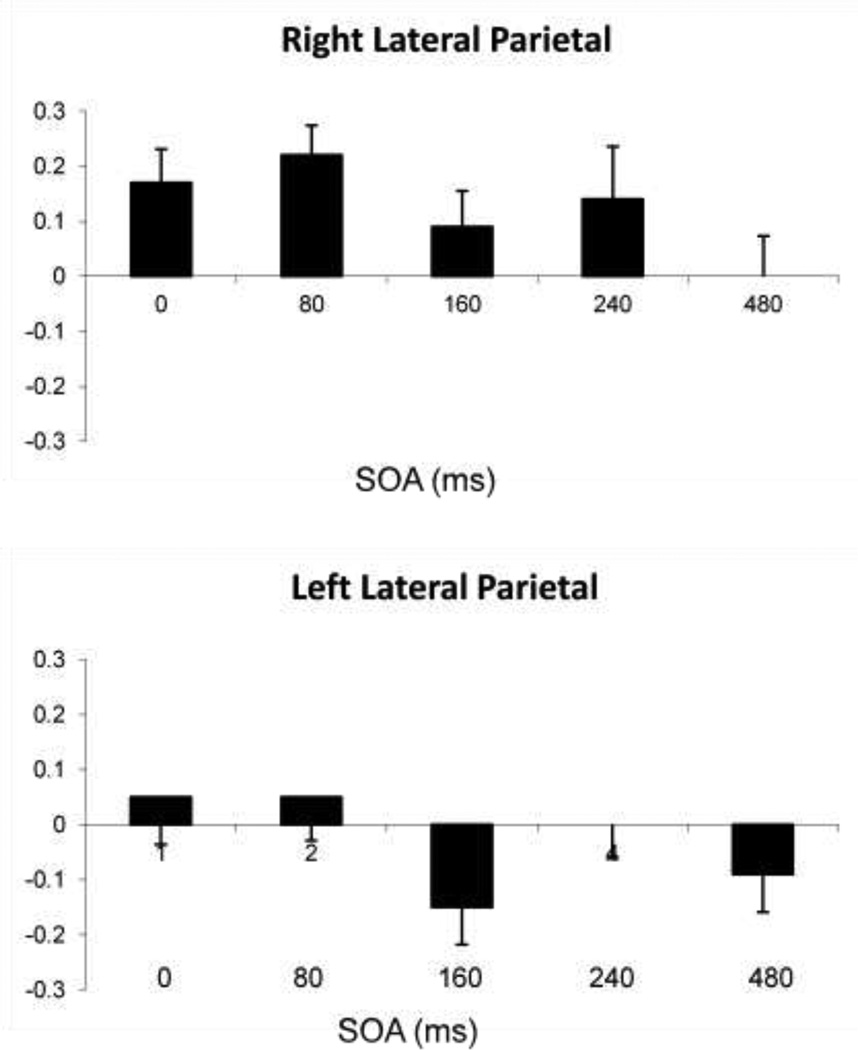
The data from Lou et al. (2010), which were reanalyzed here to demonstrate self-enhancement, exhibited in that study a robust SRE on adjective task performance. SREs were seen as subjects performing the task consistently performed faster and more accurately when responding to traits describing themselves than those describing their best friend. It is typical to find such an SRE when processing stimuli related to the self compared to another (see Gillahan and Farah, 2005 for a review). Of interest, the dynamic topographical patterns of TMS effects on self-enhancement processing as indexed by the SEI (Figure 4) and on processing underlying the SRE (Figure 5) were quite different. The SEI was most profoundly affected at MPFC, where a U-shaped function across SOAs was evident, centered on a reversal of the index at 160 ms, while there was no effect of TMS on the SRE there at all. Instead, the SRE was most strongly affected by TMS over left lateral parietal cortex, eliminating it entirely between 160–480 ms SOA, while the SRE showed an effect of TMS only at 240 ms. With TMS to right lateral parietal cortex, the SRE was nullified at 160 ms, echoing the strongest prefrontal effect, while the SRE was affected only late, at 480 ms. Thus, while the use of TMS provided evidence for self-specific processing at all sites tested in the default network, its use also differentiated two subprocesses, one centered on MPFC and indexed by the SEI, and another, indexed by the SRE and centered in left lateral and medial parietal cortex (Lou et al., 2004). As discussed above, the prefrontal process may be involved with introspective evaluation and judgment of trait adjectives in a self-specific context. On the other hand, the posterior process may be more involved with the retrieval and placement of episodic memories within that self-oriented context to enable such introspective evaluation (Lou et al., 2010). In that study TMS results differentiated a more general memory system that retrieved episodic and semantic information involving the trait adjectives from a self-specific process that takes that information as input. Recent convergent evidence supporting this separation of general memory and self-specific processes was an fMRI study of 94 healthy subjects of activity in left parietal cortex (Seghier et al., 2010), cortex where the strongest TMS effects on the SRE occurred in Lou et al. In Seghier et al., areas within left lateral parietal cortex processing semantic information retrieval were spatially segregated from default network regions (presumably involved with self-specific processing) lying in the same region.
Figure 5.
Mean change (and SE) in performance efficiency scores between Self and Best Friend conditions. A positive value indicates an SRE. Scores are shown for each SOA, for TMS to A) midline prefrontal cortex, B) left lateral parietal cortex, and C) right lateral parietal cortex. (Adapted from Luber et al., 2010).
While it has been argued here that the regions of the default network appear to be involved in self-specific processing, it might also be argued that the same prefrontal and parietal areas have been shown to be involved with many diverse and general processes such as attention, cognitive control, and evaluation and judgment rather than being specific to processing self-related content. This latter line of thought goes back to some of the earliest PET imaging studies whose results led to a theory of general attention centered on midline PFC and parietal cortex (Posner and Petersen, 1995). On the other hand, there has been a great amount of convergent evidence in brain imaging studies that the default network is involved in self-specific processing. The regions associated with this network- midline parietal and MPFC and left and right angular gyri- consistently emerge in self/other image contrasts within a context of a diverse array of tasks involving a wide range of perceptual and cognitive processes, whether the tasks are centered on perceptual discriminations of self vs. other faces (Kircher et al., 2000, 2001; Platek et al., 2004), episodic or semantic recall of personal vs. impersonal information (Maguire and Mummery, 1999; Vinogradov et al., 2006; Nunez et al., 2004), or conceptual evaluation of one’s own vs. another's personality traits, appearance, attitudes, or feelings (Craik et al., 1999; Gusnard et al., 2001; Kircher et al., 2000, 2002; Kelly et al., 2002; Johnson et al., 2002; Kjaer et al., 2002; Fossati et al., 2003; Schmitz et al., 2004; Ochsner et al., 2005). For example, one fMRI study investigated the brain substrate underlying deception (Nunez et al., 2004). Subjects were to lie or tell the truth about a series of statements, some involving themselves and others concerning more general semantic information. In contrasting lie vs. truth trials, they found a number of brain regions activated such as lateral and ventral PFC. But when they contrasted personal vs. impersonal information, the regions associated with the default network emerged.
There is no conflict between the areas comprising the default network being part of general executive, affective, motor or perceptual systems and also processing self-specific information. In the present study multiple processes of evaluation and episodic recall were shown to be simultaneously active in the default network. Each process could be part of larger systems involved with the recall and evaluation of concepts. What the TMS evidence suggests is that when parts of these systems are in the default network, the content worked on may be self-specific, or rather that all content processed in that network is seen through a self-specific lens. This may be due to the anatomical connectivity of medial parietal and MPFC directly with the cingulate gyrus, the cingulum tract, and the superior fronto-occipito fasciculus (van den Heuvel et al. 2009; Mufson and Mesulam 1984; Beer et al. 2002), and with the medial dorsal, ventrolateral, pulvinar and ventral posterior lateral nuclei of the thalamus, as shown through analyses of functional connectivity with the default network portion of postero-medial cortex, using slow oscillations in BOLD imaging data recorded in the resting state (Cauda et al., 2010). This forms a loop of reciprocal cortico-cortical and cortico-thalamic connections (Tononi and Edelman 2000) which may form a neural substrate for self-awareness, and perhaps act as a substrate for or with one that underlies the “working self”, a postulated system of control processes centered around the goals of the individual (Conway and Pleydell-Pearce, 2000).
Conclusion
Overall, the present study and Lou et al. (2010) together provide evidence that spatially and temporally targeted TMS combined with appropriate performance measures can be used to distinguish anterior and posterior subsystems within the default network involved in retrieval, contextualization, and evaluation of self-specific information. In addition, the present study, together with Kwan et al. (2007) and Barrios et al. (2008) provide convergent evidence that MPFC is involved with self-evaluative processing and self-enhancement, and suggest that TMS might be used as a probe to examine these phenomena and possibly aid in diagnosis where deficits occur in self-awareness.
Acknowledgments
Dr. Lisanby has received research support, for topics not presented here, from Magstim Company, Neuronetics, Cyberonics, and ANS. Columbia University has applied for a patent for novel TMS technology developed in Dr. Lisanby’s Lab, for work unrelated to the topic presented here. This research was in part funded by the Lundbeck Foundation and the Mind Brain Program of the Danish Ministry of Science.
References
- Alloy LB, Abramson LY. Depressive realism: Four theoretical perspectives. In: Alloy LB, editor. Cognitive processes in depression. New York: Guilford Press; 1988. pp. 223–265. [Google Scholar]
- Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell A, Eberle L. Suppression of visual perception by magnetic coil stimulation of human occipital cortex. Electroenceph Clin Neurophysiol. 1989;74:458–462. doi: 10.1016/0168-5597(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Anderson N. Likeableness ratings of 555 personality-trait adjectives. J Personality Soc Psychol. 1968;9(3):272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD. Remembering the past: Two facets of episodic memory explored with positron emission tomography. Amer J Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Barrios V, Kwan VSY, Ganis G, Gorman J, Romanowski J, Keenan JP. Elucidating the neural correlates of egoistic and moralistic self-enhancement. Consciousness and Cogn. 2008;17:451–456. doi: 10.1016/j.concog.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: Clinical, experimental, and theoretical aspects. New York: Harper and Row; 1967. [Google Scholar]
- Beer J, Blakemore C, Previc FH, Liotti M. Areas of the human brain activated by ambient visual motion, indicating three kinds of self movement. Exp Brain Res. 2002;143:78–88. doi: 10.1007/s00221-001-0947-y. [DOI] [PubMed] [Google Scholar]
- Cauda F, Geminiani G, D’Agata F, Sacco K, Duca S, Bagshaw AP, Cavanna AE. Functional connectivity of the posteromedial cortex. PLoS One. 2010;5:e13107. doi: 10.1371/journal.pone.0013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovich M, Stuss DT, Winocur G, Tulving E, Kapur S. In search of the self: A positron emission tomography study. Psychol Sci. 1999;10:26–34. [Google Scholar]
- Damasio A. Self Comes to Mind: Constructing the Conscious Brain. New York: Pantheon; 2010. [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp. Brain Res. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, et al. In search of the emotional self: An FMRI study using positive and negative emotional words. Amer J Psychiatry. 2003;160(11):1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Lepage M, Graham SJ, Grady C, Keightley ML, et al. Distributed self in episodic memory: Neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage. 2004;22(4):1596–1604. doi: 10.1016/j.neuroimage.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychol Bull. 2005;131:76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Soc Cogn Affective Neurosci. 2006;1(1):18. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G, Goodwin GM, Harmer CJ. The role of the anterior cingulate cortex in the counting Stroop task. Exp Brain Res. 2004;154:355–358. doi: 10.1007/s00221-003-1665-4. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Johnson S, Schmitz T, Kawahara-Baccus T, Rowley H, Alexander A, Lee J, et al. The cerebral response during subjective choice with and without self-reference. J Cogn Neurosci. 2005;17(12):1897–1906. doi: 10.1162/089892905775008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, et al. Towards a functional neuroanatomy of self processing: Effects of faces and words. Cogn Brain Res. 2000;10:133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Senior C, Phillips ML, Rabe-Hesketh S, Benson PJ, Bullmore ET, et al. Recognizing one's own face. Cognition. 2001;78:B1–B15. doi: 10.1016/s0010-0277(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage. 2002;17:1080–1086. [PubMed] [Google Scholar]
- Kwan VS, Barrios V, Ganis G, Gorman J, Lange C, Kumar M, Shepard A, Keenan JP. Assessing the neural correlates of self-enhancement bias: a transcranial magnetic stimulation study. Exp Brain Res. 2007;182:379–385. doi: 10.1007/s00221-007-0992-2. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan J, Nowak M, Kjaer T, Sackeim H, Lisanby SH. Parietal cortex and representation of the mental self. Proc Natl Acad Sci USA. 2004;101(17):6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Luber B, Stanford A, Lisanby SH. Self-specific processing in the default network: a single-pulse TMS study. Exp Brain Res. 2010;207:27–38. doi: 10.1007/s00221-010-2425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9:54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol. 1984;227:109–120. doi: 10.1002/cne.902270112. [DOI] [PubMed] [Google Scholar]
- Nuňez JM, Casey BJ, Egner T, Harre T, Hirsch J. Intentional false responding shares neural substrates with response conflict and cognitive control. NeuroImage. 2005;25:267–277. doi: 10.1016/j.neuroimage.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16(10):1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Perrin F, Maquet P, Peigneux P, et al. Neural mechanisms involved in the detection of our first name: a combined ERP and PET study. Neuropsychologia. 2005;43:12–19. doi: 10.1016/j.neuropsychologia.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Platek SM, Keenan JP, Gallup GG, Mohamed FB. Where am I? The neurological correlates of self and other. Cogn Brain Res. 2004;19:114–122. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu. Rev Neurosci. 1995;78:507–545. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Behind the scenes of functional brain imaging: A historical and physiological perspective. Proc Natl Acad Sci USA. 1998;95(3):765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. J Pers Soc Psychol. 1977;35:677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The international federation of clinical neurophysiology. Electroenceph Clin Neurophysiol Suppl. 1999;52:97–103. [PubMed] [Google Scholar]
- Ruby P, Legrand D. Neuro imaging the self? In: Haggard P, Rosetti Y, Kawato M, editors. Sensorimotor Foundations of Higher Cognition. Attention and Performance XXII. London, UK: Oxford University Press; 2008. [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. NeuroImage. 2004;22:1168–1177. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: A brief review and framework of neural systems underlying appraisal. Neurosci Biobehav Rev. 2007;31(4):585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Fagan E, Price CJ. Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. J. Neurosci. 2010;30(50):16809–16817. doi: 10.1523/JNEUROSCI.3377-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, Watanabe J, et al. Cortical mechanisms of person representation: recognition of famous and personally familiar names. NeuroImage. 2006;31:853–860. doi: 10.1016/j.neuroimage.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Symons CS, Johnson BT. The self-reference effect in memory: A meta-analysis. Psychol Bull. 1997;121:371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Brown JD. Illusion and well-being: A social psychological perspective on mental health. Psychol Bull. 1988;103(2):193–210. [PubMed] [Google Scholar]
- Taylor SE, Brown JD. Positive illusions and well-being revisited: Separating fact from fiction. Psychol Bull. 1994;116(1):21–27. doi: 10.1037/0033-2909.116.1.21. [DOI] [PubMed] [Google Scholar]
- Tononi G, Edelman GM. Schizophrenia and the mechanism of conscious integration. Brain Res Rev. 2000;31:391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Molnar-Szakacs I, Zaidel E, Iacoboni M. rTMS to the right parietal lobule disrupts self-other discrimination. Soc Cogn Affective Neurosci. 2006;1:65–71. doi: 10.1093/scan/nsl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RCW, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Human Brain Mapping. 2009 doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Luks TL, Simpson GV, Schulman BJ, Glenn S, Wong AE. Brain activation patterns during memory of cognitive agency. NeuroImage. 2006;31:896–905. doi: 10.1016/j.neuroimage.2005.12.058. [DOI] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Ashbridge E, Cowey A. The role of the parietal cortex in visual attention- hemispheric asymmetries and the effects of learning: a magnetic stimulation study. Neuropsychologia. 1999;37:245–251. doi: 10.1016/s0028-3932(98)00099-2. [DOI] [PubMed] [Google Scholar]