Abstract
A novel series of synthetic mimics of antimicrobial peptides (SMAMPs) containing triazole linkers were assembled using click chemistry. While only moderately active in buffer alone, an increase in antimicrobial activity against Staphylococcus aureus and Escherichia coli was observed when these SMAMPs were administered in the presence of mouse serum. One compound had minimum inhibitory concentrations (MICs) of 0.39 μg/mL and 6.25 μg/mL, respectively, and an HC50 of 693 μg/mL. These values compared favorably to peptide-based antimicrobials. A correlation between the net positive charge and SMAMP antimicrobial activity was observed. The triazole linker, an amide surrogate, was found to provide better antimicrobial activity against both S. aureus and E. coli when compared to other analogues.
Keywords: Synthetic mimics of antimicrobial peptides; click chemistry; antibiotics; Gram-positive and Gram-negative bacteria; 1,2,3-triazoles
Infections caused by bacteria are an increasing threat to human safety and well-being. Every year a large number of deaths are caused by bacterial infections.1 Traditional antibiotics used to treat these bacterial infections, such as β-lactams, are becoming ineffective as these bacteria develop resistance to them. While bacteria are developing resistance at an alarming rate, the development of new antibiotics has slowed considerably, with many pharmaceutical companies abandoning their antibiotic research, resulting in fewer and fewer antibiotics approved by the FDA (Food and Drug Administration).2 Antimicrobial peptides (AMPs) represent a class of antibiotics currently being investigated as an alternative to traditional antibiotics.3 They are peptides that show broad-spectrum antimicrobial activity against various pathogens. Unlike traditional antibiotics, AMPs do not target bacterial enzymes, which can mutate easily. Although the exact mechanisms of how AMPs operate are not completely clear, their ability to permeabilize the bacterial cell membrane4−7 has been identified along with several other targets.8,9
Since their initial discovery, hundreds of AMPs have been catalogued by scientists around the world, and web-based databases have been established to keep track of the vast number of AMPs.10,11 Despite this, there has been little success of developing AMPs into FDA-approved drugs. One of the very few examples is pexiganan, also known as MSI-78, which reached phase III clinical trials but was not approved by the FDA due to the fact that it was no more effective than existing treatments.12 A major difficulty in AMP drug development is production.13 AMPs are typically isolated from nature in minute amounts. Peptide synthesis methodologies are too expensive for manufacturing AMPs on the scale necessary for clinical testing. Using genetic engineering to produce AMPs in yeast or bacteria has proven to be ineffective due to the toxicity of AMPs toward their expression host.
These disadvantages of AMPs thus motivated the development of synthetic mimics of antimicrobial peptides (SMAMPs), synthetic molecules designed to capture the active structural features of AMPs, such as amphiphilicity. SMAMPs can exhibit improved antimicrobial activity and are easier to synthesize than AMPs due to their simplified structure. Examples of SMAMPs include peptoids,14 β-peptides,15−17 short linear18,19 and cyclic peptides,20 synthetic polymers,21−24 oligo-acyl lysines,25,26 ceragenins,27 and aromatic oligomers.28−33 In our research group, we previously synthesized SMAMPs possessing triaryl scaffolds linked with urea,29 amide,30 and acetylene34 moieties. Of the three, SMAMPs with amide linkers proved to be the most active, and one of these has completed a phase II clinical trial for pan-Staph infections in humans. 1,2,3-Triazoles are commonly used as bioisosteres of amides35,36 in medicinal chemistry, and replacing the amide functionality with a triazole can lead to improved activities.37−39 Therefore, it seemed reasonable to determine if SMAMPs containing triazole linker would be more active as well. Additionally, 1,2,3-triazoles are formed via click chemistry, which is highly reliable, regiospecific, and functional group tolerant. To the best of our knowledge, the use of click chemistry to create a SMAMP scaffold has not been demonstrated before. Hence, we set out to synthesize SMAMPs of the general structure 1 (Scheme 1) and characterize their antimicrobial properties.
Scheme 1. General Synthetic Design of SMAMPs Containing Triazoles Utilizing Click Chemistry.
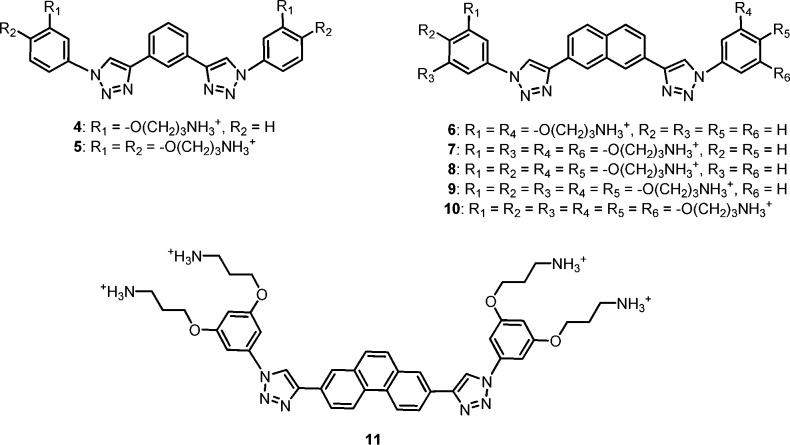
Previous studies showed that the central aromatic core and charge density were important parameters in optimizing SMAMP activity.33 As a result, three groups of SMAMPs were targeted in this endeavor. The first group (SMAMPs 4–5) consists of SMAMPs with a central phenyl ring flanked by two phenyl groups and a net positive charge of two or four (Figure 1). The second group (SMAMPs 6–10) consists of SMAMPs with a central naphthyl ring flanked by two phenyl groups and a net positive charge ranging from two to six. The third group consists of SMAMP 11, with a central phenanthryl ring flanked by two phenyl groups and a net positive charge of four.
Figure 1.
Three groups of SMAMPs investigated for their antimicrobial activity.
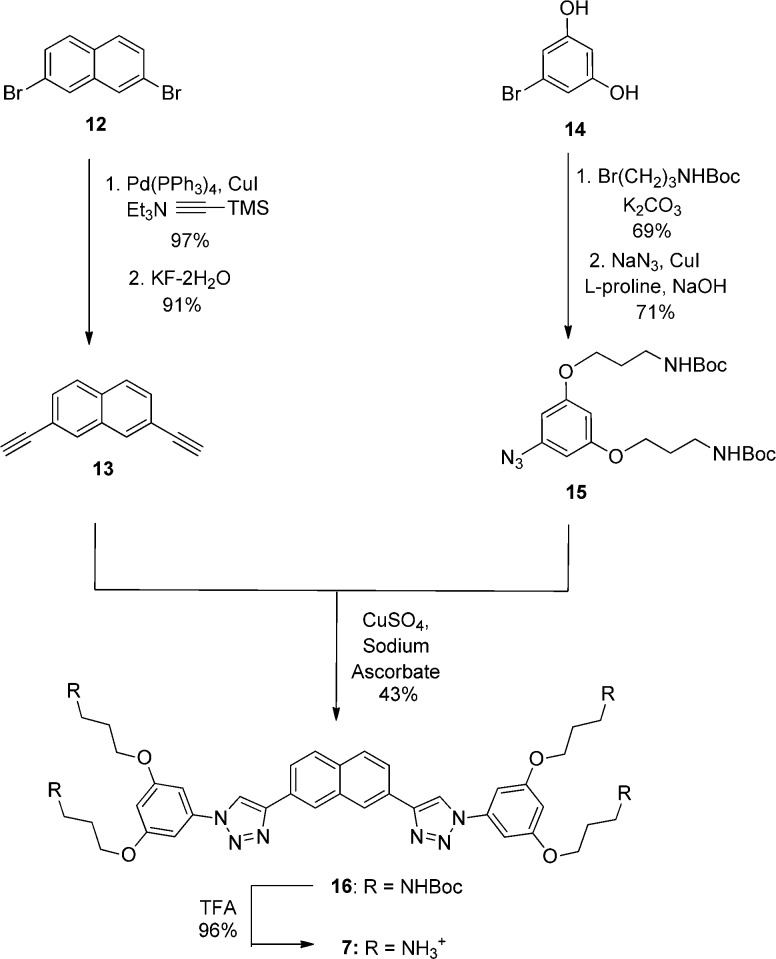
These SMAMPs were synthesized by reacting two equivalents of azide with one equivalent of bis-alkyne. A representative procedure for the synthesis of SMAMP 7 is depicted in Scheme 2. Starting with dibromide 12, a Sonogashira reaction40 with trimethylsilylacetylene gave the bis-trimethylsilylalkyne intermediate in excellent yields. Removal of the trimethylsilyl groups with KF furnished bis-alkyne 13. The azide fragment was prepared by the alkylation of diphenol 14 with Br(CH2)3NHBoc, followed by the conversion of the intermediate bromide to azide 15 via procedures developed by Zhu and Ma.41 Standard 1,3-dipolar cycloaddition conditions developed by Sharpless et al.,42 using CuSO4 as the copper source and sodium ascorbate as the reductant, gave 16 in 43% yield. The remainder of the mass balance consisted of the product in which bis-alkyne 13 reacted with only one equivalent of azide 15 and additional heating to 50 °C was required for 13 to react completely with two equivalents of 15. This difference in reactivity of the two alkyne moieties enabled the synthesis of asymmetrical SMAMPs such as 9. Finally, deprotection of 16 with trifluoroacetic acid (TFA) provided SMAMP 7 as its TFA salt. Further details for the synthesis of all SMAMPs can be found in the Supporting Information.
Scheme 2. Synthesis of SMAMP 7 via Click Chemistry.
SMAMPs 4–11 were tested against five different bacteria strains: Gram-positive Staphylococcus aureus (ATCC 27660) and Enterococcus faecalis (ATCC 29212), and Gram-negative Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 10145), and Klebsiella pneumoniae (ATCC 13883). Their antimicrobial activity was expressed in terms of minimum inhibitory concentration (MIC). These values were determined according to Hancock’s method for cationic antimicrobial peptides, which is a slight modification of the classical microbroth dilution method recommended by the Clinical and Laboratory Standards Institute (CLSI), and they were measured either in the absence or presence of 40% mouse serum.43,44 The upper limit of mouse serum was 40%, as the bacteria had difficulty growing when the percentage was increased further. The results are shown in Table 1. SMAMPs 4–11 possessed a variable spectrum of activity, generally showing lower MIC values toward S. aureus and E. coli than E. faecalis, P. aeruginosa, and K. pneumoniae. Interestingly, SMAMP 10 showed modest MIC values against P. aeruginosa, which is a difficult bacteria to kill.
Table 1. Antimicrobial Activities with and without Mouse Serum and Hemolysis of the SMAMPs.
| MIC (μg/mL) |
||||||||
|---|---|---|---|---|---|---|---|---|
| SMAMP | SAa | SA+40%MSb | ECc | EC+40%MSd | EFe | PAf | KPg | HC50 (μg/mL) |
| 4 | 50 | 12.5 | 25 | 25 | 25 | 25 | >50 | Ndh |
| 5 | 12.5 | 0.39 | >50 | 6.25 | 125.5 | 50 | >50 | >1000 |
| 6 | >50 | 12.5 | >50 | >50 | 50 | >50 | >50 | Ndh |
| 7 | 12.5 | 0.39 | >50 | 6.25 | 25 | >50 | 50 | 693 |
| 8 | 25 | 0.19 | >50 | 3.13 | 25 | 50 | 50 | 81 |
| 9 | 12.5 | 0.39 | 50 | 25 | 25 | 50 | >50 | 94 |
| 10 | 12.5 | 0.19 | 25 | 25 | 50 | 12.5 | >50 | 828 |
| 11 | 25 | 0.39 | >50 | 12.5 | 50 | 25 | >50 | 109 |
| pexiganani | 4 | >256 | 8 | 128 | 120 | |||
S. aureus (ATCC 27660).
S. aureus (ATCC 27660) in the presence of 40% mouse serum.
E. coli (ATCC 25922).
E. coli (ATCC 25922) in the presence of 40% mouse serum.
E. faecalis (ATCC 29212).
P. aeruginosa (ATCC 10145).
K. pneumoniae (ATCC 13883).
Not determined due to low antimicrobial activity.
MIC and HC50 of pexiganan were reported previously: Ge et al. Antimicrob. Agents Chemother.1999, 43, 782–788.
The hemolytic activity of SMAMPs 4–11 toward mammalian cells was also evaluated, where the ability to induce lysis in human erythrocytes was measured as an HC50 value, the lowest concentration that causes 50% lysis of the red blood cells. Surprisingly, SMAMPs 4–11 all displayed higher antimicrobial activity (lower MIC values) toward S. aureus in the presence of 40% mouse serum. This serum effect was less pronounced toward E. coli but was still observed for the majority of SMAMPs. This result was unexpected because it is well-known that antibiotics are generally less effective when administered in the presence of serum, as the binding of antibiotics to serum proteins usually deactivates the antibiotic.45 Svenson et al. had previously studied the binding of cationic peptides to albumin, and the results indicated that the binding of peptides to albumin lowers the effective concentration of peptides needed to combat bacteria.46 Hence, the MIC values of the peptides increased in the presence of albumin. There are only a limited number of examples47−49 in which an increase in antimicrobial activity was observed when an antibiotic was administered in the presence of serum. The exact reasons for this phenomena are not completely clear. It has been previously suggested that changes in the pH has lowered the MIC.50 In addition, the presence of other antibacterial peptides51 in serum or unidentified synergistic host factors52 could contribute to the observed lower MIC. Another explanation is that serum increases the solubility or internalization of the antibiotic.
Several trends were observed in the antimicrobial activity in the presence of mouse serum and hemolysis of SMAMPs 4–11. Regardless of the size of the central ring in SMAMPs 4–11, the antimicrobial activity increased dramatically when the net positive charge was increased from two (SMAMPs 4 and 6) to four (SMAMPs 5 and 7). Further increases in net positive charge to five or six did not seem to impact the antimicrobial activity significantly. The size of the aromatic core appeared to have a proportional effect on hemolytic activity but not on MIC values. When comparing SMAMPs 5 and 8, both with a net positive of four, there was approximately a 12-fold increase in hemolytic activity with the addition of one phenyl moiety in the aromatic core. The hemolytic activity also varied with the substitution pattern as the ortho-substituted SMAMP 8 had a much higher hemolytic activity than its meta-substituted counterpart, SMAMP 7. The effect of charge and hydrophobicity had been previously described by Strøm et al. in the context of short cationic peptides.53 These peptides required a minimal charge of +2 for antistaphylococcal activity, whereas our SMAMPs 4–11 required a minimal charge of +4. The presence of a larger hydrophobic group gave lower MIC values in Strøm’s peptides; however in this report increasing the size of the central aromatic ring did not have a significant effect on MIC values.
Out of all of these SMAMPs, 5, 7, and 10 were among the most potent and showed the highest selectivity even compared with pexiganan, the most thoroughly studied AMP to date. For example, SMAMP 5 is much more potent than pexiganin and is approximately 10 times less hemolytic giving selectivity values (HC50/MIC) of >2500 and >160 for S. aureus and E. coli, respectively. When 5, 7, and 10 were compared to various SMAMPs in the literature, it was found that 5, 7, and 10 possessed better antimicrobial activity against S. aureus than most peptoids, β-peptides, short linear and cyclic peptides, synthetic polymers, and oligo-acyl lysines, which typically possessed MIC values of 2–15 μg/mL.
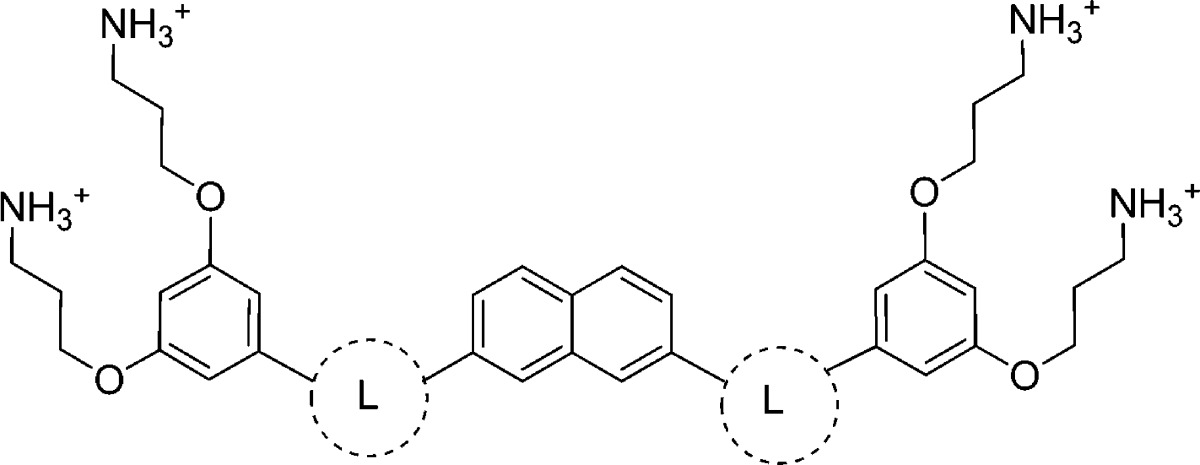
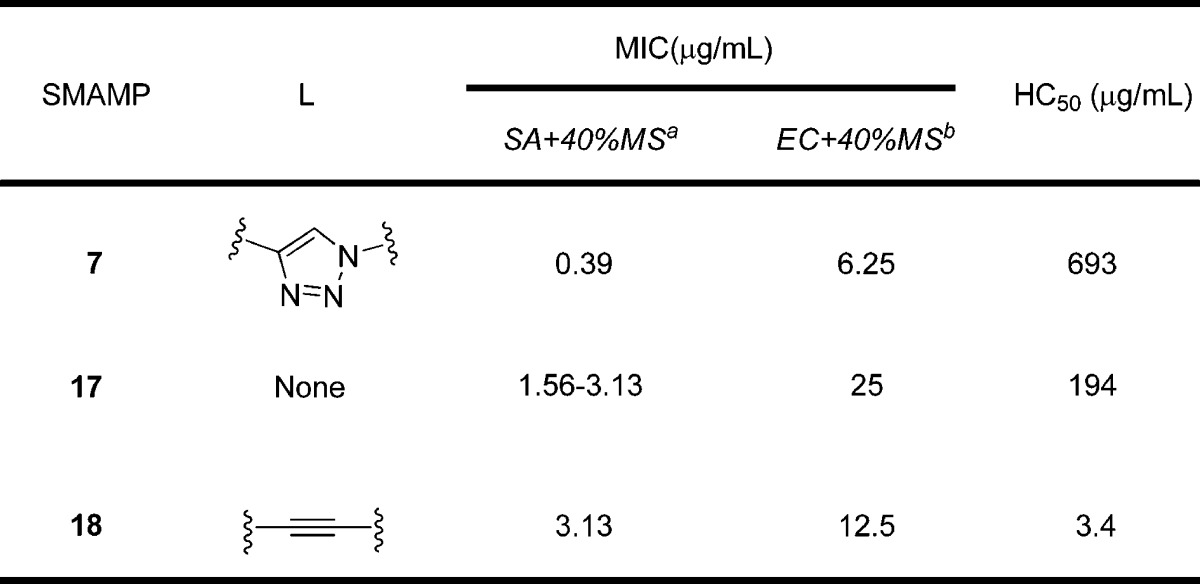
As an effort to showcase the importance of the 1,2,3-triazole linker used in this study, the activity of SMAMP 7 was compared to analogues previously synthesized in our laboratory (Table 2, SMAMPs 17 and 18). When the 1,2,3-triazole linker was removed from the scaffold (SMAMP 17(33)), there was a 4–8-fold decrease in antimicrobial activity toward S. aureus and a 4-fold decrease toward E. coli in the presence of mouse serum. Additionally, the hemolytic activity was approximately four times higher. When the 1,2,3-triazole linker was replaced with an acetylene moiety (SMAMP 18), again a significant decrease in antimicrobial activity toward S. aureus in the presence of mouse serum was observed as well as a lower HC50 value. Overall, the presence of the 1,2,3-triazole linker enabled SMAMP 7 to outperform analogues with other linkers in terms of both antimicrobial and hemolytic activities.
Table 2. Effect of the Linker Moiety on the Antimicrobial and Hemolytic Activities of SMAMPs.
S. aureus (ATCC 27660) in the presence of 40% mouse serum.
E. coli (ATCC 25922) in the presence of 40% mouse serum.
In conclusion, the first example of utilizing click chemistry to generate a SMAMP scaffold was reported. The size of the aromatic core and the number and substitution pattern of the ammonium groups were varied; the effects of these variables on SMAMP antimicrobial and hemolytic activities were measured. All SMAMPs had higher antimicrobial activities when administered in the presence of mouse serum and exhibited higher antimicrobial activity toward S. aureus over E. coli. SMAMPs 5, 7, and 10 possessed comparable antimicrobial activities toward S. aureus as the most potent SMAMPs reported in the literature while maintaining lower hemolytic activity. The importance of the linker was also investigated by comparing 7 to 17–18, and a pronounced difference in antimicrobial and hemolytic activities was observed, thus validating the use of the 1,2,3-triazole moiety. As a result of this study, we have identified three SMAMPs, 5, 7, and 10, that outperformed pexiganan, and they are currently being investigated for use as new antibiotics.
Acknowledgments
This work was supported by the NIH (AI-074866 and U01 AI-082192). Mr. Michael Lis and Ms. Katie Gibney are acknowledged for helpful and challenging discussions. Mass spectral data were obtained at the University of Massachusetts Mass Spectrometry Facility which is supported, in part, by the National Science Foundation.
Supporting Information Available
Procedure for the synthesis of 7, characterization data for 4–11, and procedures for determining antimicrobial and hemolytic activity. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
T-h.F. and Y.L. contributed equally.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Capita R.; Alonso-Calleja C. Antibiotic-Resistant Bacteria: A Challenge for the Food Industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [DOI] [PubMed] [Google Scholar]
- Wright G. D. Antibiotics: A New Hope. Chem. Biol. 2012, 19, 3–10. [DOI] [PubMed] [Google Scholar]
- Hadley E. B.; Hancock R. E. Strategies for the Discovery and Advancement of Novel Cationic Antimicrobial Peptides. Curr. Top. Med. Chem. 2010, 10, 1872–1881. [DOI] [PubMed] [Google Scholar]
- Baumann G.; Mueller P. A molecular model of membrane excitability. J. Supramol. Struct. 1974, 2, 538–557. [DOI] [PubMed] [Google Scholar]
- Pouny Y.; Rapaport D.; Mor A.; Nicolas P.; Shai Y. Interaction of antimicrobial dermaseptin and its fluorescently labeled analogs with phospholipid membranes. Biochemistry 1992, 31, 12416–12423. [DOI] [PubMed] [Google Scholar]
- Ludtke S. J.; He K.; Heller W. T.; Harroun T. A.; Yang L.; Huang H. W. Membrane Pores Induced by Magainin. Biochemistry 1996, 35, 13723–13728. [DOI] [PubMed] [Google Scholar]
- Leontiadou H.; Mark A. E.; Marrink S. J. Antimicrobial Peptides in Action. J. Am. Chem. Soc. 2006, 128, 12156–12161. [DOI] [PubMed] [Google Scholar]
- Li Y.; Xiang Q.; Zhang Q.; Huang Y.; Su Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 2012, 37, 207–215. [DOI] [PubMed] [Google Scholar]
- Zhu W.; Zhang Y.; Sinko W.; Hensler M. E.; Olson J.; Molohon K. J.; Lindert S.; Cao R.; Li K.; Wang K.; Wang Y.; Liu Y.-L.; Sankovsky A.; de Oliveira C. A. F.; Mitchell D. A.; Nizet V.; McCammon J. A.; Oldfield E. Antibacterial drug leads targeting isoprenoid biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 2013, 110, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMSDb database. http://www.bbcm.univ.trieste.it/∼tossi/pag1.htm (accessed February 1, 2013).
- Brahmachary M.; Krishnan S. P. T.; Koh J. L. Y.; Khan A. M.; Seah S. H.; Tan T. W.; Brusic V.; Bajic V. B. ANTIMIC: a database of antimicrobial sequences. Nucleic Acids Res. 2004, 32, D586–D589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden N. K.; Brogden K. A. Will new generations of modified antimicrobial peptides improve their potential as pharmaceuticals?. Int. J. Antimicrob. Agents 2011, 38, 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupuleti M.; Schmidtchen A.; Malmsten M. Antimicrobial peptides: key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [DOI] [PubMed] [Google Scholar]
- Chongsiriwatana N. P.; Patch J. A.; Czyzewski A. M.; Dohm M. T.; Ivankin A.; Gidalevitz D.; Zuckermann R. N.; Barron A. E. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 2794–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.; DeGrado W. F. De Novo Design, Synthesis, and Characterization of Antimicrobial Peptides. J. Am. Chem. Soc. 2001, 123, 7553–7559. [DOI] [PubMed] [Google Scholar]
- Porter E. A.; Weisblum B.; Gellman S. H. Mimicry of Host-Defense Peptides by Unnatural Oligomers: Antimicrobial β-Peptides. J. Am. Chem. Soc. 2002, 124, 7324–7330. [DOI] [PubMed] [Google Scholar]
- Porter E. A.; Wang X.; Lee H.-S.; Weisblum B.; Gellman S. H. Antibiotics: Non-haemolytic [beta]-amino-acid oligomers. Nature 2000, 404, 565–565. [DOI] [PubMed] [Google Scholar]
- Haug B. E.; Stensen W.; Kalaaji M.; Rekdal Y.; Svendsen J. S. Synthetic Antimicrobial Peptidomimetics with Therapeutic Potential. J. Med. Chem. 2008, 51, 4306–4314. [DOI] [PubMed] [Google Scholar]
- Makovitzki A.; Avrahami D.; Shai Y. Ultrashort antibacterial and antifungal lipopeptides. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 15997–16002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartois V.; Sanchez-Quesada J.; Cabezas E.; Chi E.; Dubbelde C.; Dunn C.; Granja J.; Gritzen C.; Weinberger D.; Ghadiri M. R.; Parr T. R. Systemic Antibacterial Activity of Novel Synthetic Cyclic Peptides. Antimicrob. Agents Chemother. 2005, 49, 3302–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew G. N.; Liu D.; Chen B.; Doerksen R. J.; Kaplan J.; Carroll P. J.; Klein M. L.; DeGrado W. F. De novo design of biomimetic antimicrobial polymers. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 5110–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowery B. P.; Lee S. E.; Kissounko D. A.; Epand R. F.; Epand R. M.; Weisblum B.; Stahl S. S.; Gellman S. H. Mimicry of Antimicrobial Host-Defense Peptides by Random Copolymers. J. Am. Chem. Soc. 2007, 129, 15474–15476. [DOI] [PubMed] [Google Scholar]
- Arnt L.; Nüsslein K.; Tew G. N. Nonhemolytic abiogenic polymers as antimicrobial peptide mimics. J. Polym. Sci., Part A: Polym. Chem 2004, 42, 3860–3864. [Google Scholar]
- Lienkamp K.; Madkour A. E.; Musante A.; Nelson C. F.; Nüsslein K.; Tew G. N. Antimicrobial Polymers Prepared by ROMP with Unprecedented Selectivity: A Molecular Construction Kit Approach. J. Am. Chem. Soc. 2008, 130, 9836–9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzishevsky I. S.; Kovachi T.; Porat Y.; Ziserman L.; Zaknoon F.; Danino D.; Mor A. Structure-activity relationships of antibacterial acyl-lysine oligomers. Chem. Biol. 2008, 15, 354–362. [DOI] [PubMed] [Google Scholar]
- Zaknoon F.; Sarig H.; Rotem S.; Livne L.; Ivankin A.; Gidalevitz D.; Mor A. Antibacterial Properties and Mode of Action of a Short Acyl-Lysyl Oligomer. Antimicrob. Agents Chemother. 2009, 53, 3422–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X.-Z.; Feng Y.; Pollard J.; Chin J. N.; Rybak M. J.; Bucki R.; Epand R. F.; Epand R. M.; Savage P. B. Ceragenins: Cholic Acid-Based Mimics of Antimicrobial Peptides. Acc. Chem. Res. 2008, 41, 1233–1240. [DOI] [PubMed] [Google Scholar]
- Liu D.; Choi S.; Chen B.; Doerksen R. J.; Clements D. J.; Winkler J. D.; Klein M. L.; DeGrado W. F. Nontoxic Membrane-Active Antimicrobial Arylamide Oligomers. Angew. Chem., Int. Ed. 2004, 43, 1158–1162. [DOI] [PubMed] [Google Scholar]
- Tang H.; Doerksen R. J.; Tew G. N. Synthesis of urea oligomers and their antibacterial activity. Chem. Commun. 2005, 1537–1539. [DOI] [PubMed] [Google Scholar]
- Tang H.; Doerksen R. J.; Jones T. V.; Klein M. L.; Tew G. N. Biomimetic Facially Amphiphilic Antibacterial Oligomers with Conformationally Stiff Backbones. Chem. Biol. 2006, 13, 427–435. [DOI] [PubMed] [Google Scholar]
- Choi S.; Isaacs A.; Clements D.; Liu D.; Kim H.; Scott R. W.; Winkler J. D.; DeGrado W. F. De novo design and in vivo activity of conformationally restrained antimicrobial arylamide foldamers. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 6968–6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker H. D.; Sgolastra F.; Clements D.; Scott R. W.; Tew G. N. Synthetic Mimics of Antimicrobial Peptides from Triaryl Scaffolds. J. Med. Chem. 2011, 54, 2241–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaker H. D.; Som A.; Ayaz F.; Lui D.; Pan W.; Scott R. W.; Anguita J.; Tew G. N. Synthetic Mimics of Antimicrobial Peptides with Immunomodulatory Responses. J. Am. Chem. Soc. 2012, 134, 11088–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnt L.; Tew G. N. New Poly(phenyleneethynylene)s with Cationic, Facially Amphiphilic Structures. J. Am. Chem. Soc. 2002, 124, 7664–7665. [DOI] [PubMed] [Google Scholar]
- Kolb H. C.; Sharpless K. B. The growing impact of click chemistry on drug discovery. Drug Discovery Today 2003, 8, 1128–1137. [DOI] [PubMed] [Google Scholar]
- Tron G. C.; Pirali T.; Billington R. A.; Canonico P. L.; Sorba G.; Genazzani A. A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [DOI] [PubMed] [Google Scholar]
- Pirali T.; Pagliai F.; Mercurio C.; Boggio R.; Canonico P. L.; Sorba G.; Tron G. C.; Genazzani A. A. Triazole-Modified Histone Deacetylase Inhibitors As a Rapid Route to Drug Discovery. J. Combin. Chem. 2008, 10, 624–627. [DOI] [PubMed] [Google Scholar]
- Ballard T. E.; Richards J. J.; Wolfe A. L.; Melander C. Synthesis and Antibiofilm Activity of a Second-Generation Reverse-Amide Oroidin Library: A Structure–Activity Relationship Study. Chem.—Eur. J. 2008, 14, 10745–10761. [DOI] [PubMed] [Google Scholar]
- Davis M. R.; Singh E. K.; Wahyudi H.; Alexander L. D.; Kunicki J. B.; Nazarova L. A.; Fairweather K. A.; Giltrap A. M.; Jolliffe K. A.; McAlpine S. R. Synthesis of sansalvamide A peptidomimetics: triazole, oxazole, thiazole, and pseudoproline containing compounds. Tetrahedron 2012, 68, 1029–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi E.-i.; Anastasia L. Palladium-Catalyzed Alkynylation. Chem. Rev. 2003, 103, 1979–2018. [DOI] [PubMed] [Google Scholar]
- Zhu W.; Ma D. Synthesis of aryl azides and vinyl azides via proline-promoted CuI-catalyzed coupling reactions. Chem. Commun. 2004, 888–889. [DOI] [PubMed] [Google Scholar]
- Rostovtsev V. V.; Green L. G.; Fokin V. V.; Sharpless K. B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem., Int. Ed. 2002, 41, 2596–2599. [DOI] [PubMed] [Google Scholar]
- Steinberg D. A.; Hurst M. A.; Fujii C. A.; Kung A. H.; Ho J. F.; Cheng F. C.; Loury D. J.; Fiddes J. C. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 1997, 41, 1738–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H.; Hancock R. E. W. Synergistic Interactions between Mammalian Antimicrobial Defense Peptides. Antimicrob. Agents Chemother. 2001, 45, 1558–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. A.; Welling P. G. Protein-Binding of Antimicrobials - Clinical Pharmacokinetic and Therapeutic Implications. Clin. Pharmacokinet. 1977, 2, 252–268. [DOI] [PubMed] [Google Scholar]
- Svenson J.; Brandsdal B.-O.; Stensen W.; Svendsen J. S. Albumin Binding of Short Cationic Antimicrobial Micropeptides and Its Influence on the in Vitro Bactericidal Effect. J. Med. Chem. 2007, 50, 3334–3339. [DOI] [PubMed] [Google Scholar]
- Perl T. M.; Pfaller M. A.; Houston A.; Wenzel R. P. Effect of serum on the in vitro activities of 11 broad-spectrum antibiotics. Antimicrob. Agents Chemother. 1990, 34, 2234–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruul H.; McDonald P. J. Potentiation of antibacterial activity of azithromycin and other macrolides by normal human serum. Antimicrob. Agents Chemother. 1992, 36, 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha R.; Rybak M. J. Influence of protein binding under controlled conditions on the bactericidal activity of daptomycin in an in vitro pharmacodynamic model. J. Antimicrob. Chemother. 2004, 54, 259–262. [DOI] [PubMed] [Google Scholar]
- Eagle H.; Levy M.; Fleischman R. The effect of pH of the medium on the antibacterial action of penicillin, streptomycin, chloramphenicol, terramycin, and bacitracin. Antibiot. Chemother. 1952, 2, 563–575. [PubMed] [Google Scholar]
- Carroll S. F.; Martinez R. J. Antibacterial peptide from normal rabbit serum. 1. Isolation from whole serum, activity, and microbicidal spectrum. Biochemistry 1981, 20, 5973–5981. [DOI] [PubMed] [Google Scholar]
- Leggett J. E.; Craig W. A. Enhancing effect of serum ultrafiltrate on the activity of cephalosporins against gram-negative bacteria. Antimicrob. Agents Chemother. 1989, 33, 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøm M. B.; Haug B. E.; Skar M. L.; Stensen W.; Stiberg T.; Svendsen J. S. The Pharmacophore of Short Cationic Antibacterial Peptides. J. Med. Chem. 2003, 46, 1567–1570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.