Abstract
Non-myeloablative allogeneic transplant (NMAT) has a curative potential for patients who are not myeloablative allogeneic transplant (MAT) candidates. We report a phase II trial of a NMAT regimen with cyclophosphamide and fludarabine in 40 patients; 21 of whom had a prior MAT. Day +100 and 1-year transplant-related mortality (TRM) post-NMAT were 13% and 34%, respectively. Day +100 and 1-year Overall/Progression-Free Survival (OS/PFS) were 80%/65% and 43%/25%, respectively. OS was higher in patients with KPS≥90 and lower in recipient/donor CMV+/− vs. other combinations. FluCy has low TRM and is curative in about 20% of high-risk patients.
Keywords: Non-myeloablative, Allogeneic transplant, Fludarabine, Cyclophosphamide
Highlights
-
•
We report a phase II trial of a NMAT regimen with cyclophosphamide and fludarabine in 40 patients.
-
•
Transplant related mortality was low at Day+100 and 1-year.
-
•
Fludarabine and cyclophosphamide as NMAT is curative in about 1/5 high risk patients.
1. Introduction
NMAT has emerged over the last 2 decades as a promising alternative with reduced TRM for patients who would otherwise not be eligible for MAT. Aggressive disease poses a challenge, while patients in complete remission (CR) at the time of transplant can benefit from NMAT [1,2]. We summarize our prospective phase II clinical trial of fludarabine and cyclophosphamide for NMAT, with a focus on evaluating the efficacy of NMAT in patients with high-risk/aggressive disease.
2. Methods
This prospective IRB-approved phase II trial enrolled 40 patients from 4/2002 to 12/2008 with hematologic diseases. Eligibility included: age 4–75 years; HLA-suitable donor, KPS≥50, adequate organ function, no uncontrolled co-morbidity or infection, and ineligible for MAT.
2.1. Conditioning regimen and GvHD prophylaxis
Starting on day −5, fludarabine 25 mg/m2/d (actual body weight)×5 doses and cyclophosphamide 50 mg/kg/d IV×2 doses (lesser of ideal or adjusted body weight) were given. For graft-versus-host disease (GVHD) prophylaxis, patients received methotrexate 5 mg/m2 on day +1, +3, and +6, mycophenolate mofetil day −1 to +60, and tacrolimus starting day −1.
2.2. Cell dose and neutrophil recovery
Peripheral blood grafts had a minimum total cell dose of 2×106 CD34+cells/kg. Marrow grafts had a minimum of 1×108 nucleated cells/kg. Absolute neutrophil count (ANC)>500/μL for three consecutive days defined engraftment. Failure to engraft was no ANC recovery by day +45 post-NMAT. Platelet engraftment was defined as >20,000/mm3 after 7 days with no platelet transfusions.
2.3. Response evaluations
Disease response was measured on or before Day +100. The Center for International Blood and Marrow Transplant Registry (CIBMTR) criteria defined remission, relapse and progression. Disease status pre-NMAT was defined using CIBMTR definitions of low, intermediate and high risk [3].
2.4. Statistical analyses
Sample size and power calculations were based on the primary endpoint: Day +100 TRM, defined as death due to any cause except underlying disease. The null hypothesis was Day +100 TRM=25%. A two-stage study design was used with early stopping rules for patient safety. The decision rules yielded a type I error rate=0.0933 and power=0.8709. P<0.05 was considered statistically significant, with P≥0.05–0.09 considered a trend.
Secondary outcomes included OS, PFS and toxicity. Survival was updated through November 2012. The Kaplan–Meier method was used to construct survival curves, and the log rank test was used to determine statistical significance. OS was measured from day 0 (HCT infusion) to death due to any cause or last follow-up (censor). PFS was calculated from day 0 until date of last follow-up (censor), or documented relapse/progression/death (events). SPSS® version 21software was used for data analysis (IBM Corp., Armonk, NY).
3. Results
Patient characteristics are summarized in Table 1. Siblings were HLA-matched (N=14) or 1-Ag mismatched (N=1). Unrelated donors were HLA-matched (N=14) or 1-2 antigen HLA-mismatched (N=11). Median time to ANC recovery was 14 days (range 10–21); 3 died before neutrophil engraftment (Day +15, 27, 32) and there were no secondary graft failures. Median time to platelet recovery was 27 days (range 9–120); 7 patients did not nadir below 20,000/mm3.
Table 1.
Patient characteristics.
| Variable | Total N=40 |
|---|---|
| n (%) | |
| Gender | |
| Male | 24 (60) |
| Female | 16 (40) |
| Age (years) | |
| <40 | 7 (18) |
| 40–59 | 21 (53) |
| 60–75 | 12 (30) |
| Diagnosis | |
| NHL | 12 (30) |
| AML | 10 (25) |
| HL | 8 (20) |
| MDS | 5 (13) |
| MM | 2 (5) |
| ALL | 2 (5) |
| PLL | 1 (3) |
| Karnofsky Performance Score | |
| 90–100 | 13 (33) |
| 80 | 12 (30) |
| <80 | 15 (38) |
| Disease status at BMT | |
| Complete remission | 11 (28) |
| Primary induction failure/untreated | 12 (30) |
| Relapse | 17 (43) |
| Prior BMT | |
| No prior BMT | 19 (48) |
| Autologous | 19 (48) |
| Allogeneic | 2 (5) |
| Hematopoietic cell source | |
| Peripheral Blood | 37 (93) |
| Bone Marrow | 3 (8) |
| GvHD prophylaxis | |
| FKMMF+/−MTX | 38 (95) |
| FKMTX | 2 (5) |
| Donor type | |
| Unrelated | 25 (63) |
| Related | 15 (38) |
| Gender matching | |
| Matched | 23 (58) |
| Mismatched | 17 (43) |
| HLA match | |
| Matched | 28 (70) |
| Mismatched | 12 (30) |
| Cytomegalovirus serology recipient/donor | |
| CMV+/+ | 13 (33) |
| CMV+/− | 13 (33) |
| CMV−/− | 10 (25) |
| CMV−/+ | 4 (10) |
| ABO matching | |
| Matched | 20 (50) |
| Major Mismatch | 10 (25) |
| Minor Mismatch | 10 (25) |
| Ejection fraction pre-NMAT | |
| ≥60% | 23 (58) |
| <60% | 17 (43) |
| CIBMTR disease risk category | |
| High | 26 (65) |
| Intermediate | 8 (20) |
| Low | 6 (15) |
FK: tacrolimus; MTX: methotrexate; MMF: mycophenolate; CMV: cytomegalovirus.
HLA-match for sibling donors is 6/6 or 8/8, HLA-match for unrelated donors is 8/8 or 10/10.
HLA-mismatch for sibling donors is 7/8, HLA-mismatch for unrelated donors is 8/10, 9/10, or 11/12.
% May not add up to 100% due to rounding.
3.1. Treatment related mortality (TRM)
The observed cumulative incidence of TRM at Day +100 was statistically significantly lower than expected at 12.6% (95% CI 2–23%, P=0.03). The causes of Day +100 TRM were infection (n=4) and regimen-related toxicity (n=1).
3.2. Infections
Viral infections post-HCT were documented in 44% of patients (n=18). Fungal infections were documented in 17% of patients. Two patients died of bacterial sepsis before engraftment (Day +15, 27).
3.3. GvHD
Cumulative incidence of Grades II–IV acute GvHD by Day +100 was 35% (95% CI 20–51%). Cumulative incidence of extensive chronic GvHD at 1-year was 64% (95% CI 45–83%).
3.4. Overall Survival (OS)
The median follow-up was 5 years (range 2–8 years). Day +100 OS was 80% (95% CI 68–92%); 1 year OS was 43% (95% CI 27–58%); 3 year OS was 19% (CI 7–31%).
The univariate analysis of factors associated with OS is in Table 2. Three pre-NMAT variables, recipient/donor CMV serological status, KPS and disease status were significantly associated with OS (Table 2, Figs. 1a and b), whereas age and donor relation/HLA match were not (Table 2, Fig. 1c).
Table 2.
Univariate analysis of Progression-Free and Overall Survival.
| Factor | Progression-Free Survival |
Overall Survival |
||||||
|---|---|---|---|---|---|---|---|---|
| Day 100 | 1-Year | 3-Year | P | Day 100 | 1-Year | 3-Year | P | |
| Age (years) | NS | NS | ||||||
| <40 | 57% (20–94) | 29% (0–62) | 29% (0–62) | 71% (38–100) | 57% (20–94) | 29% (0–62) | ||
| 40–59 | 67% (47–87) | 24% (6–42) | 6% (0–17) | 81% (64–98) | 43% (22–64) | 17% (0–34) | ||
| ≥60 | 67% (40–93) | 25% (0–50) | 17% (0–38) | 83% (62–100) | 33% (7–60) | 17% (0–38) | ||
| CIBMTR disease risk | NS | NS | ||||||
| Low | 71% (38–100) | 29% (0–62) | 14% (0–40) | 100% | 29% (0–62) | 14% (0–40) | ||
| Intermediate | 89% (68–100) | 33% (3–64) | 22% (0–49) | 89% (68–100) | 67% (36–97) | 33% (3–64) | ||
| High | 50% (30–70) | 21% (5–37) | 10% (0–24) | 71% (53–89) | 38% (18–57) | 15% (0–30) | ||
| Disease status pre-NMAT | 0.075 | 0.025 | ||||||
| CR≥1 | 82% (59–100) | 36% (8–65) | 18% (0–41) | 100% | 46% (16–75) | 18% (0–41) | ||
| Relapse≥1 | 65% (42–87) | 29% (8–51) | 22% (1–43) | 77% (56–97) | 65% (42–87) | 34% (11–57) | ||
| Never in CR | 50% (22–78) | 8% (0–24) | 0% | 67% (40–93) | 8% (0–24) | 0% | ||
| KPS | 0.097 | 0.014 | ||||||
| 90–100 | 92% (78–100) | 39% (12–65) | 23% (0–46) | 100% | 54% (27–81) | 39% (13–65) | ||
| 80 | 58% (31–86) | 25% (0–50) | 17% (0–38) | 83% (62–100) | 58% (30–86) | 17% (0–38) | ||
| <80 | 40% (15–65) | 13% (0–31) | 0% | 60% (35–85) | 20% (0–40) | 13% (0–30) | ||
| Donor relation/HLA match | NS | NS | ||||||
| Matched related | 64% (39–89) | 43% (17–69) | 27% (3–51) | 79% (58–100) | 50% (24–76) | 36% (11–61) | ||
| Matched unrelated | 79% (57–100) | 21% (0–43) | 7% (0–21) | 86% (68–100) | 50% (24–76) | 7% (0–21) | ||
| HLA-mismatched | 42% (14–70) | 8% (0–24) | 8% (0–24) | 75% (50–100) | 25% (1–50) | 17% (0–38) | ||
| CMV R+/D− | 0.004 | <0.001 | ||||||
| R+/D− | 31% (6–56) | 8% (0–22) | 0% | 69% (44–94) | 8% (0–22) | 0% | ||
| Other | 82% (67–96) | 33% (16–51) | 21% (5–37) | 85% (72–98) | 59% (40–78) | 29% (12–46) | ||
KPS: Karnofsky Performance Score; CMV R+ indicates recipient is serologically IgG positive pre NMAT, CMV D− indicates donor is serologically IgG negative prior to stem cell collection, NS: not statistically significant P>0.1.
Fig. 1.
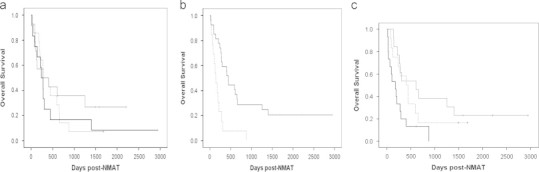
(a) P=0.014; thin solid line: KPS 90–100, dashed line: KPS 80, thick solid line=KPS<80. (b) P<0.001; dashed line: CMV R+/D−, solid line: all other combinations. (c) P>0.5, thin solid line: HLA-matched related donor, dashed line: HLA-matched unrelated donor, thick solid line: HLA-mismatched donor.
3.5. Progression free survival (PFS)
By Day +100, 21 (54%) patients achieved a complete remission (half of these later relapsed), 5 (12%) had a partial response (2 died of disease, 3 received other therapy and are all alive >4 years post-NMAT), 2 (5%) had stable disease (both later progressed and died 9 months and 3.8 years post-NMAT), 8 (20%) had progressed (all of whom died 3–38 days later), and 4 (10%) were not evaluable due to early TRM. Day +100 PFS was 64% (CI 49–78%); 1-year PFS was 27% (CI 13–40%); 3-year PFS was 17% (CI 5–28%). The univariate analysis of factors associated with PFS is summarized in Table 2, demonstrating only recipient/donor CMV serological status as significantly associated with PFS.
4. Discussion
We observed a significantly lower than expected Day +100 TRM. Other NMAT studies [4,5] have shown similar findings, although 60% of our trial population had high risk disease per the CIBMTR classification. In this and other studies, late TRM and disease progression remains problematic and offsets the reduction in early TRM [6].
Outcomes of other NMAT studies may have been related to type of disease [7] and disease burden [8] at the time of transplant, however, our results in high-risk, advanced disease patients are comparable to McClune et al. [9]. We demonstrate that durable disease control can be achieved in 15% of patients who are not in remission pre-NMAT, with a 3-year OS of 34% in patients with relapsed disease vs. 0% in primary induction failure patients. One retrospective analysis of MDS and AML patients found that non-relapse mortality, 3-year OS and PFS did not vary between MAT and NMAT [10]. Two studies have documented a stronger Graft-versus-Leukemia (GvL) effect for those in remission vs. active disease pre-NMAT [10,11]. A randomized multi-center trial is currently accruing patients to compare MAT vs. NMAT regimens in AML and MDS patients [12].
In our study, lower KPS was a significant risk factor for shorter OS that is comparable to 2 other studies [13,14]. This factor could be studied further as a prospective, prognostic indicator of survival along with the HCT co-morbidity index [15]. A major cause of morbidity and mortality following NMAT is infection, in both our study and others [16,17]. Despite a decrease in early bacterial infection due to a shorter time to neutrophil recovery and less mucosal damage, there is an increased risk for later viral and fungal infections [17,18]. Our study confirms another [16] that CMV serologic status is associated with OS and needs to be considered in the donor selection process.
The development of NMAT has changed the application of allogeneic transplantation. The curative effect of NMAT is dependent on donor cell-mediated immunotherapy [19–21]. The reduction of early TRM must be balanced with PFS and late TRM. FluCy has a low rate of early TRM and is curative in approximately 20% of advanced disease patients. Identification of pre-NMAT factors which predict for long-term survival may allow more appropriate patient selection for FluCy versus alternative NMAT regimens and newer reduced toxicity regimens.
Acknowledgments
This work is supported by the Graduate Scholarship in Cancer Nursing Practice from the American Cancer Society (Grant no. GSCNP-10-218-01-SCN).
References
- 1.Shimoni A., Hardan I., Shem-Tov N. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20:322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 2.Arnold R., Massenkeil G., Bornhäuser M. Nonmyeloablative stem cell transplantation in adults with high-risk ALL may be effective in early but not in advanced disease. Leukemia. 2002;16:2423–2428. doi: 10.1038/sj.leu.2402712. [DOI] [PubMed] [Google Scholar]
- 3.〈http://www.asbmt.org/displaycommon.cfm?an=1&subarticlenbr=35〉 View “Disease Classifications”. [accessed 14.05.13].
- 4.Brown J.R., Haesook T., Kim S.L. Predictors of improved progression-free survival after non-myeloablative allogeneic stem cell transplantation for advanced chronic lymphocytic leukemia. Biol Blood Marrow Transplant. 2006;12:1056–1064. doi: 10.1016/j.bbmt.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Tauro S., Craddock C., Peggs K. Allogeneic stem cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2005;23:9387–9393. doi: 10.1200/JCO.2005.02.0057. [DOI] [PubMed] [Google Scholar]
- 6.Luger S.M., Ringdén O., Zhang M.J. Similar outcomes using myeloablative vs. reduced-intensity allogeneic transplant preparative regimens for AML and MDS. Bone Marrow Transplant. 2012;47:203–211. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michallet M., Bilger K., Garban F. Allogeneic hematopoietic stem-cell transplantation after nonmyeloablative preparative regimens: impact of pretransplantation and posttransplantation factors on outcome. J Clin Oncol. 2001;19:3340–3349. doi: 10.1200/JCO.2001.19.14.3340. [DOI] [PubMed] [Google Scholar]
- 8.Kebriaei P., Klein J., Stock W. Impact of disease burden at time of allogeneic stem cell transplantation in adults with acute myeloid leukemia and myelodysplastic syndromes. Bone Marrow Transplant. 2005;35:965–970. doi: 10.1038/sj.bmt.1704938. [DOI] [PubMed] [Google Scholar]
- 9.McClune D., Weisdorf D., Pedersen T.L. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott B.L., Sandmaier B.M., Storer B., Maris M.B., Sorror M.L., Maloney D.G. Myeloablative vs. nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20:128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 11.Gyurkocza B., Storb R., Storer B.E. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28:2859–2867. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.〈https://web.emmes.com/study/bmt2/protocol/0901_protocol/0901_protocol.html〉 A randomized multi-center phase III study of allogeneic stem cell transplantation comparing regimen intensity in patients with myelodysplastic syndrome or acute myeloid leukemia. [accessed 15.05.13].
- 13.Deschler B., Binek K., Ihorst G. Prognostic factor and quality of life analysis in 160 patients aged >60 years with hematologic neoplasias treated with allogeneic hematopoietic cell transplantation. Blood. 2010;16:967–975. doi: 10.1016/j.bbmt.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Marks D.I., Wang T., Pérez W.S. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with philadelphia chromosome- negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116:366–374. doi: 10.1182/blood-2010-01-264077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorror M.L., Sandmaier B.M., Storer B.E. Comorbidity and disease status-based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25:4246–4254. doi: 10.1200/JCO.2006.09.7865. [DOI] [PubMed] [Google Scholar]
- 16.Boeckh M., Nichols W.G., Papanicolaou G., Rubin R., Wingard J.R., Zaia J. Cytomegalovirus in hematopoietic stem cell transplant recipients: current status, known challenges, and future strategies. Biol Blood Marrow Transplant. 2003;9:543–558. doi: 10.1016/s1083-8791(03)00287-8. [DOI] [PubMed] [Google Scholar]
- 17.Junghanss C., Marr K.A. Infectious risks and outcomes after stem cell transplantation: are nonmyeloablative transplants changing the picture? Curr Opin Infect Dis. 2002;15:347–353. doi: 10.1097/00001432-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Boeckh M., Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103:2003–2008. doi: 10.1182/blood-2003-10-3616. [DOI] [PubMed] [Google Scholar]
- 19.Sorror M.L., Maris M.B., Sandmaier B.M., Storer B.E., Stuart M.J., Hegenbart U. Hematopoietic cell transplantation after nonmyeloablative conditioning for advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:3819–3829. doi: 10.1200/JCO.2005.04.569. [DOI] [PubMed] [Google Scholar]
- 20.Slavin S., Nagler A., Naparsteck E., Kapelushnik Y., Aker M., Cividalli G. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;913:756–763. [PubMed] [Google Scholar]
- 21.Slavin S. Reduced-intensity conditioning or nonmyeloablative stem cell transplantation: introduction, rationale, and historic background. Semin Oncol. 2004;31:1–3. doi: 10.1053/j.seminoncol.2003.10.015. [DOI] [PubMed] [Google Scholar]


