Abstract
Force and stress production within embryos and organisms are crucial physical processes that direct morphogenesis. In addition, there is mounting evidence that biomechanical cues created by these processes guide cell behaviors and cell fates. Here we review key roles for biomechanics during development to directly shape tissues, provide positional information for cell fate decisions, and enable robust programs of development. Several recently identified molecular mechanisms suggest how cells and tissues might coordinate their responses to biomechanical cues. Lastly, we outline long-term challenges in integrating biomechanics with genetic analysis of developing embryos.
Introduction
Behind the motions of cells and tissues in the early embryo lie forces and mechanics. Universal principles of mechanics reveal how forces shape the early embryo and drive tissues to move, strain, and deform (see Box 1 - Terminology of Mechanics - for a brief introduction to engineering terms and principles). The spatial and temporal regulation of gene expression and protein activity that guide cell physiology and behavior regulate the production of force and the mechanical response of embryonic cells and tissues to those forces. New findings suggest that mechanical cues may also directly alter gene expression and protein activity which in turn play a role in deciding cell fates and cell behaviors. Thus the developing form of the embryo and the phenotype of the organism are the direct consequence of these biomechanical processes and are constrained by the physical laws of mechanics.
Box 1. Engineering principles and terms.
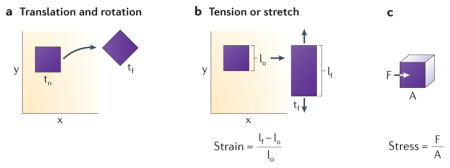
Translation and rotation
An object can move or translate by moving up, down, left, or right, and rotation can be described by the angle of change the object experiences as shown in the left hand panel below.
Deformation and Strain
Deformation of cells and tissues are changes in the shape of the cells and tissues over time or in response to an applied force, normally measured using live cell time lapse imaging. Engineers use the term strain, which is a measure of deformation normalized to the size of the structure, to quantify deformations. Also, from a measure of deformation over time a strain rate can be determined. The units are deformation are in units of length. Strain is generally dimensionless but sometimes noted as length/length (e.g. mm/mm) and the units of strain rate is per time.
Force and Stress
Force is any influence that causes an object to undergo a change such as translation, rotation, or deformation. Stress is a measure of force applied over a surface, either perpendicular to the surface, e.g. tension or compression, or within the plane of the surface, in shear. The units of force are mass times acceleration and the units of stress are force per unit area. The panel below illustrates its physical definition.
Fluids and solids
In addition to the ability to generate force, biological tissues all exhibit some resistance to mechanical force. If they flow in response to force they are considered a viscous fluid. If they deform in proportion to the applied force and recover their original shape when the force is removed they are considered an elastic solid. In contrast, a fluid will not recoil once the applied force, or load, is removed.
Viscoelastic
In practice, cells and tissues typically exhibit behaviors of both solids and fluids, deforming slowly under a load or adopting some new shape once the load is removed and are considered viscoelastic. Often, viscoelastic behaviors of a tissue are reported in terms of a combination of springs (elastic elements) and dashpots (viscous elements) but these are just convenient mathematical representations and do not necessarily mean the tissue consists of microscopic springs and fluids. The time-dependent behavior of a material to a force or stress applied between times “1” and “2” illustrate whether a material is considered elastic (material deforms immediately once force is applied or removed), viscous (material slowly deforms once force is applied and does not return to original shape once force is removed) or viscoelastic (material slowly deforms once force is applied but returns to the original shape once the force is removed).
Early studies of the physical and mechanical constraints on development 1–3 included the construction of physical analog models of morphogenesis to test hypotheses on the origin of forces and role of tissue architecture in guiding movements. For instance, assemblies of physical analogs consisting of elastic metal bands, bars and string allowed embryologists to simulate gastrulation in the amphibian Amphioxus maculatum and test their ideas about the cellular production of mechanical bending moments4. The goal of those early studies was to test the plausibility of the application of physical laws to morphogenesis.
Recent experimental biomechanical studies are exposing previously concealed forces and the roles of mechanics in cell and developmental biology. Additional experiments consider the capacity of cells to sense physical force and mechanical cues, similar to how they sense chemical gradients and guidance cues. From these initial studies, several broader roles for mechanics in development, such as force generation from actomyosin contractions to “pull” tissue edges, have been identified. To serve such functions, cells must first be able to evaluate or measure mechanical “signals” from their environment as well as their own internal mechanical state, secondly cells must be able to transduce those signals into changes in gene expression and cell behaviors, and thirdly cell must be able to generate and transmit mechanical forces to others.
However, one of the greatest challenges to understanding the role of mechanics in development is that the physics of mechanical structures cannot be “knocked out” in the same sense that individual genes can be mutated. Instead, mechanics must be investigated on the systems level, where the role of mechanics is studied through perturbation. The general experimental design of these studies is to introduce a molecular perturbation (such as by knock-down or acute acting inhibitor) or through mechanical perturbations (such as by laser cutting or other micromechanical manipulations) and identify the most proximal consequences of that perturbation on the cell or tissue scale. Molecular scale biochemistry and biophysics is the final arbiter of these perturbations but their action at the molecular scale is beyond the scope of this review (e.g. see 5 for detailed reviews of the biophysics of motor proteins and the cytoskeleton).
In this review we discuss recent studies in developmental biology, cell biology, and biophysics and how they are revitalizing the field of developmental biomechanics. These examples illustrate the classical, direct role of physical mechanics in shaping tissues as well as the potential role of mechanics in cell signaling and in patterning cell identity. We then turn to reviewing several examples of the sensory and signaling pathways that may play a role in these processes. Along the way we introduce engineering principles used to describe the important physical mechanics that shape tissues and conclude with a discussion of the challenges that remain in connecting molecular mechanisms to developmental mechanics.
The role of mechanics in sculpting tissues
Cell shape change: cause or effect?
Embryologists studying the role played by mechanics in shaping tissues, such as the vertebrate neural tube 6–11 and the echinoderm archenteron 12,13, recognized that the establishment of geometric patterns of cell shapes in tissues may either indicate programmed active cell shape changes, such as cells adopting wedge shapes in order to bend the tissue, or may reflect passive shape changes in the tissue in response to forces applied outside the field, for example, when a tissue is folded by the action of cells outside the folded region. In genetic terms, the passively shaped cell is responding to cell non-autonomous processes while the processes directly shaping the cell are cell-autonomous. To distinguish between these two cases, experimental embryologists microsurgically isolated specific layers, such as the vegetal plate of the starfish Dendraster excentricus blastula from surrounding ectoderm 14 to determine which tissue contained the “motor” driving tissue shape change (see Box 2 – for a brief introduction into tools and techniques). The modern genetic approach to the same question relies on localized expression of a mutant protein or mosaically knocking out a protein in a specific tissue.
Box 2. Tools used to measure and determine mechanical properties.
Cutting tissue to determine stresses
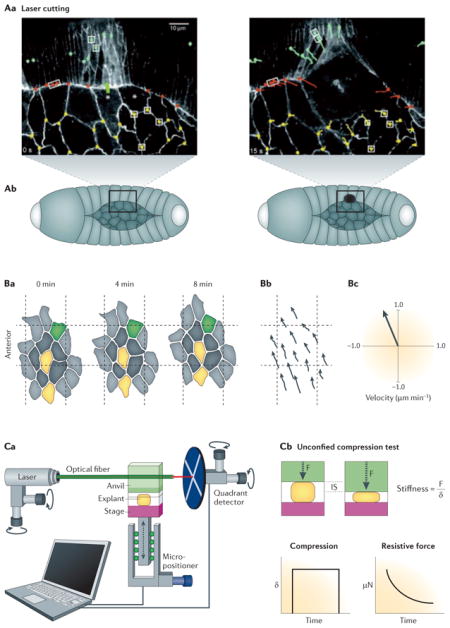
One cannot easily “see” stress in tissues, so to determine the mechanical stresses within a tissue early experimentalists turned to physically cutting the tissues or attaching tissues to deformable substrates. Laser cutting allows for a specific area of the tissue, such as a portion of the epithelial layer, to be targeted and ablated with a high powered laser After laser ablation, measurements are taken of the recoil of the surrounding cells and tissue within the first few seconds to infer the mechanical state of the tissue including the forces, such as tension, that were present in order to hold the tissue together before ablation 101–105. Another strategy involves fixing a tissue to a deformable substrate such as a silicone membrane that can be mechanically manipulated 75,106. This approach allows precise control over the magnitude and rate at which that local mechanical conditions are changed. Investigation of the stress at cellular and tissue scales, however, involves two different approaches: laser cutting, and microsurgery. Like laser cutting, microsurgery can be used to investigate local mechanics of tissue, but can also be used to isolate tissue specific mechanical processes without the influence of the tissue’s surroundings, which greatly simplifies the system.
Measuring deformation and strain
Since mechanical properties are a measure of the ratio of deformation under externally or internally applied forces, quantifying the deformation and calculating the strain is the first step in estimating the stresses and forces present in the tissue. Live cell and tissue imaging plays a key role in these measurements. Confocal time-lapse microscopy allows quantitative analysis of cell movements and shape changes that can be related to deformation and engineering strain. Since the big question is how tissues and cells generate forces or what forces are acting upon cells and tissues during morphogenesis analysis must be framed by assumptions about the basic mechanical nature of the cells and surrounding tissues 107–109. Other crucial aspects of measuring and quantifying deformations involve deducing additional principles that guide moving tissues and developing new models and methods to test these new principles. For example, by measuring the gradient velocities of moving cells 110, or identifying geometries common to developing tissues and deducing the reason behind these emergent geometries 111.
Engineering-based tensile/compression tests
The physiologically relevant mechanical properties of the tissue or embryo can be determined using microindentation compression tests or microaspiration tensile tests (see Box Figure 2c). From these tests, the stiffness of the tissue can be calculated and can be used in conjunction with cutting experiments to complete the mechanical profile of morphogenic tissues. There are many different tools available for carrying out tensile or compression tests such as the nano Newton Force Measuring Device (nNFMD; 112,113), glass or metal needles114,115, parallel plate compression27,116,117, and microaspiration100. Furthermore, tools designed to measure mechanical properties of the whole embryo can be combined with tools that perturb mechanics such as electrical stimulation100, laser activation of proteins, or nano-perfusion to reveal ways in which electrophysiological and biochemical pathways might interface with mechanical pathways 118.
The interaction of forces during tissue movement
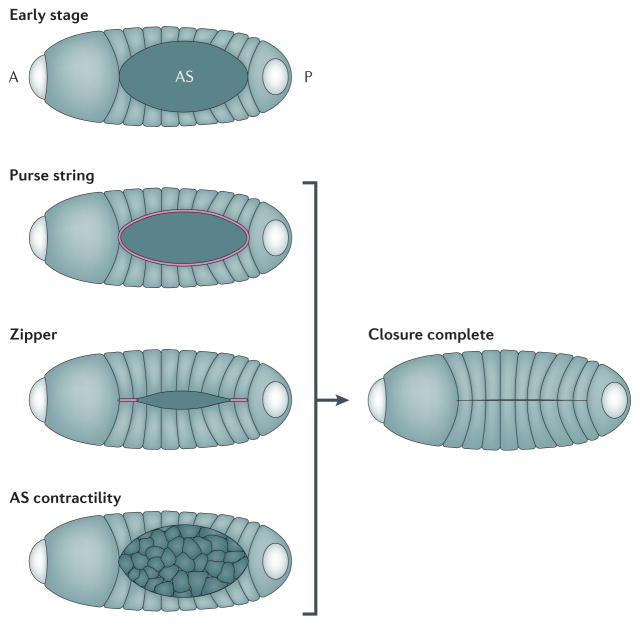
The transmission of force through a tissue integrates the activities of multiple cells and tissues enabling otherwise disconnected cellular processes to contribute to the same morphogenetic movement (see Box 1). An elegant series of papers on dorsal closure in Drosophila melanogaster demonstrates this principle 15–18. Using high resolution live time-lapse confocal imaging of embryos mutant in key morphogenetically active proteins, biophysical modeling, and laser microdissection, it was shown that dorsal closure relies on multiple discrete “motors” (see Box 2). Large scale tissue movements in the whole embryo are coordinated by long-range stresses generated by pulsatile actomyosin contractions within the amnioserosa, an epithelium that covers a transient hole in the early dorsal epidermis19–21, a contractile actin purse-string at the margin of the lateral epidermis 22–24, and fusion of epidermis at the anterior and posterior ends25, or canthii, of the amnioserosa (Figure 1). Large laser cuts across many cells were used to isolate and identify force generating tissues within target tissues (see Box 2). Local recoil from smaller laser-induced wounds on single cell-cell junctions were used to quantify the relative contribution of each motor to the global movements. Together with genetic manipulation, these biophysical studies demonstrate how multiple sources of force combine to shape a complex structure and how none of these individual motors acting in isolation can reliably seal the dorsal epidermis. Thus, to understand the role of mechanics in driving morphogenesis, it is critical to describe both the molecular mechanisms that generate force as well as those mechanisms that transmit and coordinate forces within complex tissues.
Figure 1. Forces contributing to tissue movements in development.
This cartoon shows the process of Drosophila dorsal closure and illustrates the individual mechanical components which help complete closure. In the early stage embryo, the amnioserosa (AS) cells are located in the “hole” which prospective epidermal cells will cover. The embryo is already polarized with anterior (A), where the head will form, and posterior (P) axes. Closure is driven to completion by three distinct cellular processes that contribute to global tissue movements: 1) The actomyosin purse string is shown in pink and is located at the edge of the epithelial cells and AS cells. 2) As epidermal cells meet at the anterior and posterior ends of the AS they fuse and create a zipper, shown in pink, that progressively seals the epidermal sheet over the AS. .3) Cells in the AS, shown in pink, undergo cycles of actomyosin contractions (AS contractility) to narrow their exposed apical faces and draw the epidermal margins of the AS together.
Mechanical equivalency of cellular processes
A range of different cellular and molecular mechanisms are capable of driving nearly identical morphogenetic movements. Two or more cellular or molecular mechanisms may be mechanically equivalent in that they contribute similar forces or maintain equivalent mechanical properties in the embryo. Similar patterns of force or stress may not necessarily be attributed to the same molecular mechanism. For example, a computer simulation used to study the invagination of a simple single-cell layered epithelial sheet in the sea urchin demonstrated how this movement could be driven by five distinctly different cellular mechanisms26 (see Box 3 for a brief introduction into the approach of applying theory and simulation). First, apical constriction within the vegetal plate can drive cytoplasm to move basally. Second, cells lateral to the vegetal plate tractoring towards the center of the plate can cause buckling. Third, contraction of a multicellular actomyosin ring or purse-string surrounding the vegetal plate can cause buckling. Fourth, apical basal contraction in the cytoskeleton shortens the height of vegetal plate cells and can generate a compressive force within the vegetal plate cells resulting in buckling. Fifth, differential swelling by localized secretion of a hydrophilic chondroitin sulfate proteoglycan can causing a swelling in the apical lamina which causes buckling of the vegetal plate.26 Subsequent experimental measurement of the mechanical properties of the embryo (see Box 2), separating the contributions of the extracellular matrix from the cytoskeleton, were then used to rule out the plausibility of theories requiring a compliant extracellular matrix (ECM) leaving those requiring a stiff ECM to be tested further 27.
Box 3. Computational approaches to modeling mechanics in development.
Theory and computer simulation are routinely used in mechanical engineering and developmental mechanics to test the plausibility of new hypotheses and to aid interpretation of complex mechanical experiments. Modeling allows one to assess the contribution of specific aspects of an experiment that are difficult to control experimentally, for instance the rates of actin polymerization. Further, because the models are explicitly defined, in silico experiments and the resulting kinematic can provide insights into which aspects of the biology are most important. These tools can suggest new research directions by identifying aspects of the biology that most affect the overall mechanics and behavior of the cells and tissue. For example, measuring the gradient velocities of moving cells 110, or identifying geometries common to developing tissues and deducing the reason behind these emergent geometries 111.
Theoretical and computational tools can take many forms but the process of creating the models, however, is mostly uniform. For example, when observing the formation of the heart tube, one study began by characterizing the in vivo folding, and then creating a model that describes the necessary stresses the tube would be experienced in order to replicate the same folding. They further validate the theoretical model by creating computational simulations with finite element techniques which are able to match their model to the experimental results 115,119,120. We also point readers to more examples with sea urchin primary invagination 26,27, head fold formation in the avian embryo 104, coordination of cell behaviors during ascidian gastrulation 28, ventral furrow formation in Drosophila 121,122, and looping of the gut 123.
Computer simulation is a common strategy to deal with the “ill-posed” problem where there is incomplete understanding of the biology of a particular mechanical process. For example, in trying to understand which cellular behaviors (lamellipodial protrusion, cell shape change, or mitotic divisions) contribute to the mechanical morphogenetic event of convergent extension, one study created a cellular finite element model where the different cellular behaviors could be implemented in order to determine which emergent behavior of the model was most closely correlated to experimentally observed tissue shape and behavior 124 (see figure below). Computer simulations can be used to test the basic plausibility of a hypothesis. The best simulations and models are ones where the behavior is emergent and predictive, based on a few fundamental facts about the cell and tissue behavior, and the outcome that emerges closely correlates with both existing observations and those from new experiments.
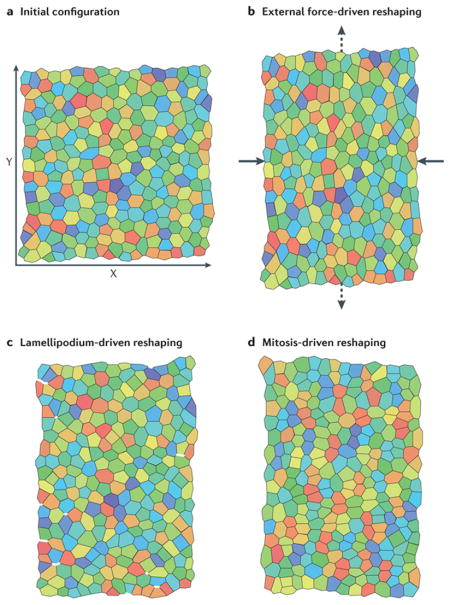
Another example can be found in the biomechanical analysis of gastrulation movements in ascidian embryos 28. This study tested the contributing role of different tissues and different cellular programs of mechanical contractility in driving the large epithelial in-folding of mesoderm progenitors. Detailed immunofluorescence studies of the location and activation of myosin regulatory light chain allowed the construction of a computer simulation of the process (see Box 3). These computer simulations provided testable predictions of the “trajectory” of both cell and tissue shape changes in the embryo as the program of force production was altered. The predicted shapes were then compared with those found within gastrulating embryos subjected to inhibitors of actomyosin contractility. Studies such as these involving whole embryos provide a basic framework for investigating the biomechanics of morphogenesis 29 and have been inspired by advances in biophysics and cell mechanics. These examples suggest that different cellular processes may be mechanically equivalent since they can drive the same movement.
Biomechanical cues and cell fate and behavior
Many of the early studies of cell mechanics were motivated by a desire to understand the cellular basis of morphogenesis (see the classic and still relevant monograph 30). Qualitative measurements of the forces generated by individual cells 31 have given way to precise measurements collected using traction force microscopy 32. The application of traction force microscopy, sophisticated micro-fabrication methods, and advanced imaging tools have revealed a central role of focal adhesions in both guiding cell movement 33 and in signaling 34. For instance, cultured cells can “sense” the mechanical properties of their micro-environment through focal adhesions and can use these cues to guide movements.
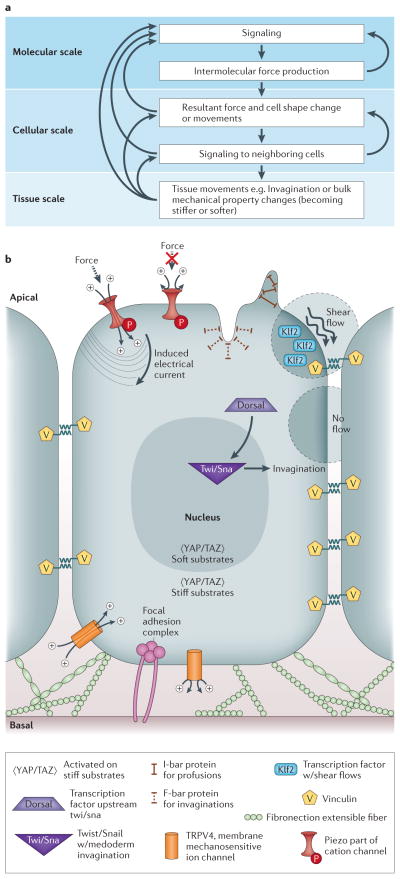
To investigate the role of force in directing stem cell fate, a key study cultured muscle-derived stem cells on stiffness-tunable elastic substrates in the physiological ranges of 100 Pascal to over 100 kPa 35. The authors were able to direct these cells into osteogenic fates with stiff substrates, neurogenic fates with soft substrates, and myogenic fates with intermediate substrates; substrate stiffnesses were tuned to mimic the stiffness of the endogenous microenvironment for each of these cell types. Thus, information from a cell’s mechanical microenvironment can complement information from growth factor signaling to pattern cell identities. In these studies cell fate decisions appear to take several days to progress through the stages of commitment, specification, and differentiation analogous to developmental programs. This leads to further questions concerning the specific signaling factors that mediate these choices, how cells integrate mechanical and chemical signaling, and whether cells sense bulk stiffness or fine scale features of ECM compliance 36. It is unclear whether the biological principles of cell mechanics in cultured cells, many of which have evolved to provide physiological adaptation or to allow cells to thrive on tissue culture plastic, behave similarly to cells during normal development. Another study has shown that substrate geometry can regulate human mesenchymal stem cell differentiation37. However, microfabricated geometry cues might not reflect endogenous geometry cues present during development. Numerous questions remain on the role of biomechanics during development and the nature of these potential cues in the embryo. Where and when these cues operate, whether there are hidden patterns of mechanical cues, and how the mechanical cues might be regulated by gene expression and protein activity remain elusive (Figure 2A).
Figure 2. Information flow and molecular origins of mechanics.
(A) This flow chart shows the intricacies involved with connecting molecular, cellular, and tissue scale behaviors and mechanisms. At the molecular scale, there is molecular signaling which causes intermolecular force production. This force production then feeds back into more molecular signaling. From the molecular scale, there are resultant forces and cell shape changes/movements on the cellular scale, which induce signaling into neighboring cells. The cellular scale can then feedback into molecular scale dynamics or result in tissue scale movements or bulk mechanical property changes. Isolating any portion of this intricate feedback loop is extremely difficult without considering all upstream and downstream effects. (B) Different molecules involved in sensing and signaling to force are shown. Vinculin is located inside the cell at sites of focal adhesions and is also a candidate for sensing forces during development. Fibronectin is an extensible ECM fiber that may sense tension within the tissue. TRPV4 is a membrane mechanosensitive ion channel, believed to open based on tension in the cell membrane. Piezo is a protein part of a cation channel which induces an electrical current based on force. I-bar protein is located at protrusions of the cell membrane and F-bar protein is located at invaginations of the cell membrane. KLF2 is a transcription factor which becomes transcribed when the cell experiences shear flow. YAP/TAZ is activated when the cell is on stiff substrates. Dorsal is a transcription factor upstream of Twist/Snail which is responsible for mesoderm invagination.
In vivo studies in developing amphibian38–40 and Drosophila melanogaster 41–43 embryos suggest that differentiation can be triggered by high levels of mechanical strain. There are opportunities to investigate the role of mechanics in the model systems of D. melanogaster epithelial morphogenesis44 and the early stages of development in Xenopus laevis45. Since one of the primary roles of mechanical strain is to drive tissue movement it is often difficult to distinguish the direct or immediate roles of mechanical cues in differentiation from the classical secondary inductions as these movements bring new contacts between signaling and responding tissues. For instance, mechanical processes that drive extension of anterior mesoderm into the forming head result in novel signaling between these cells and overlying neural tissues46. The forces driving mesoderm into contact with new ectodermal cells enable a new round of nodal signaling that divides a single domain of gene expression driving eye formation into separate left and right domains. Force and mechanics are clearly necessary for this secondary induction but mechanical signaling events are not directly controlling gene expression within the prospective eye field. Several in vivo studies, where exogenously applied strains produce changes in gene expression have suggested that mechanical signals could create autoregulatory feedback loops. Such feedback would be triggered as an endogenous mechanical cue initiates a cell signaling pathway which then drives force production and a morphogenetic movement. Such a case may operate in Drosophila melanogaster ventral furrow formation or midgut invagination during gastrulation where high levels of exogenously applied mechanical strain can trigger Twist (twi) expression and activate myosin II contractility 42,47. Temporal control of myosin II contractility48 and the restriction of actomyosin to the apical caps of these epithelial cells drive folding and gastrulation49. Portions of these mechanosensing and mechanotransduction signaling pathways have been observed after application of external forces via stiff probes or electromagnetically-controlled ferrofluids result in expression of twi and activation of myosin II contractility. Mechanically-induced twi expression can drive ectopic invagination and redirect the cell fates of induced cells. Whether redirection of cell fate decisions is due to mechanical cues or the product of more conventional downstream signaling pathways such as secondary induction is an active area of investigation50.
Sensing mechanical signals
One of the difficulties in understanding the molecular biology of cell mechanics is that molecular factors that generate and transmit forces may also sense forces and mediate cellular responses. In this section we consider a set of candidate molecular mechanosensors (Figure 2B) capable of detecting mechanical conditions both outside and inside cells and transducing that information to intracellular signaling pathways that regulate cell fate and behaviors. Distinguishing between factors that transmit mechanical information and those factors involved in sensing and transducing mechanical information is difficult, for instance, classical knock-down or knock-out of factors responsible for maintaining a tissue’s mechanical integrity, may produce the same effect as removing a factor that senses biomechanical cues conveyed by a tissue. In the sections below we describe the role each candidate mechanosensor plays in cultured cells and, where possible, in developmental model systems.
Sensing mechanical signals from the cell’s microenvironment
Elements of the extracellular matrix (ECM) such as fibronectin (FN) and proteins within the focal adhesion complex such as vinculin have been implicated as key parts of mechanosensing signaling pathways but they also serve to transmit force and maintain tissue integrity. The ECM may play roles in providing mechanical stiffness, serving as a scaffold for migration or cell rearrangement, and providing polarity cues for tissue architecture. Fibronectin is an ECM fiber that is extremely extensible, experiences large strain, and contains cryptic cell-binding sites51 that open with increased molecular strain52. Furthermore, fibronectin fibril assembly requires the mechanical action of cells. FRET analysis has shown that fibril assembly occurs only after cryptic sites in the molecule, which are kept in a partially unfolded state, are opened at the cell surface 53, and refold when cell tension is reduced 54. This suggests that fibronectin may sense tension within the tissue. Antisense morpholino knockdown and genetic mutations reveal fibronectin is essential to both early development and later organogenesis. For instance, fibronectin is required for Xenopus gastrulation55,56 as well as zebrafish heart development 57, endothelial invasion or migration in the developing kidney 58, and establishment of left-right asymmetry 59. The requirement of fibronectin during development and its tension-dependent assembly into fibrils make it a key candidate in mechanosensing pathways.
Several candidates for mechanosensing during development can be found at sites of cell-cell adhesion such as adherens junctions or desmosomes. Plakoglobin60, beta-catenin42, alpha-catenin61,62, and vinculin63 have all been implicated as potential sites where physical cues are transduced into intracellular signaling pathways. Another candidate for mechanosensing in development is vinculin, a cytoskeletal protein found in focal adhesions and cell-cell adherens junctions63. Vinculin is also required for normal development. During mouse development, vinculin mutants show a lack of midline fusion of the neural tube, have delayed heart development accompanied by structural defects, most likely due to vinculin’s function in regulating cell adhesion and motility 64. Beyond the adhesion complex, these factors transmit and may sense tension between the ECM and intracellular actomyosin contractile structures. Vinculin also appears to play a role in sensing tension at cell-cell junctions. For instance, the mechanosensing role of vinculin within E-cadherin complexes has been observed with magneto twisting cytometry (MTC), a tool that allows application of force to a cell through adherent microbeads, applied to both Madine Darby Canine kidney (MDCK) and F9 cell cultures 65. Vinculin’s role in F9 cells has been further dissected by direct determination of the adhesion forces and creep modulus using magnetic tweezers another tool for applying force to cells. This finding suggests that vinculin contributes to the cell’s mechanical properties under large external forces through the regulation of contractile stresses in actomyosin 66. Using magnetic tweezers, total internal reflection fluorescence microscopy (TIRF) and atomic force microscopy (AFM) another study revealed that forces transmitted via talin (another scaffold protein linking ECM integrin receptors to the cytoskeleton) to vinculin altered cytoskeletal reorganization by exposing cryptic binding sites on vinculin 67. It is important to recognize that the principles of cell mechanics have largely explored cultured cells with only a few rigorous studies carried out in developing embryos or intact embryonic tissues.
Sensing mechanical signals from the plasma membrane and cell cortex
Mechanical cues may be sensed along the apical or basolateral faces of cells by stretch sensitive channels such as transient receptor potential cation channel, subfamily V, member 4 (TRPV4), BAR proteins, or Piezo proteins. Whereas the previous section discussed a cell’s ability to sense forces exerted on it, in this section we discuss the ability of a cell to sense intracellular mechanical conditions. These sensors may still reflect external cues but may also detect stresses and forces produced inside the cell. A role for stretch sensitive channels in transducing mechanical cues at the membrane can be seen in the ability of mammalian endothelial cells to reorient when exposed to fluid shear or to cyclic stretch, for instance, with the failure of cells to reorient to flow after TRPV4 channels are knocked down68. TRPV4 triggers reorientation by activating PI3K which then activates β1 integrins 69. In addition to TRPV4’s role in transducing fluid shear forces, its more general role in mechanotransduction has been studied in single muscle fibers 68. In zebrafish, TRPV4 within renal cilia are thought to sense mechanical stimuli and are also thermosensitive 70. In Drosophila, TRPV4 homologs play a role in photo-transduction mechanical sensing 71 and in C. elegans play a role in olfactory cue sensing mechanotranduction 72. Piezo proteins are another family of channel proteins thought to be responsible for converting mechanical forces into electrophysiological signals. Patch clamp studies of the D. melanogaster homolog piezo expressed in human embryonic kidney cells demonstrated that piezo channels can open in response to mechanical forces applied by a fine glass pipette73. In vivo Piezo mediates noxious response to high temperature and is essential for sensing noxious mechanical stimulus 74. In zebrafish, Piezo1 regulates cell extrusion in order to maintain homeostatic epithelial cell numbers in the growing tail fin 75 a function that may parallel its role in regulating cell density within confluent MDCK cultures. In mouse, Piezo1 is required for the initiation of mechanical activated currents in neuroblastoma cells 76. These examples have been identified in mechanosensing pathways in cultured cells and physiology studies, and likely play important roles in mechanical feedback signaling during development. How these endogenous biosensors trigger behavioral or cell shape changes in cells is largely unknown.
It has long been recognized that cell protrusive activity can be triggered by mechanical events at the cell membrane and cell cortex, for instance during contact inhibition of cell migration or tension-induced protrusion 60. Recent examples of responses to applied tension include the response of Xenopus embryonic mesendoderm cells60 and single neutrophils77. One candidate family of proteins that might mediate sensing of these types of mechanical events in the cortex are the BAR family of proteins. BAR proteins are membrane-deforming or membrane curvature-sensing proteins that connect F-actin structures to the plasma membrane 78 and have been suggested to sense mechanical cues. Due to their conformation F-Bar proteins accumulate within intracellular invaginations, or concave shaped membrane structures whereas I-Bar proteins accumulate within cellular protrusions, or convex shaped membrane structures. Deformed membranes also correlate with positive or negative membrane strains 79. Signaling to the actin cytoskeleton can occur via the I-bar domain since it can induce strong PI(4,5)P2 clustering 80. In zebrafish, loss of function studies also find defects in ciliary structures which were directly related to BAR protein function 81. These proteins have not yet been shown to have a role in early development or morphogenesis.
From mechanical cues to gene expression
Once a mechanical cue has been converted into a conventional intracellular signal, cell fate choices are made through the activation of mechanically-responsive transcription factors such as Twist41,42, Kruppel-like282–85, and the newly identified YAP/TAZ complex86–88. The downstream targets of Kruppel-like2 and YAP/TAZ are being elucidated but how targets of the transcription factor Twist (Twi)have been subject to detailed analysis 89.
Twi initiates a broad range of developmental modules including differentiation, cell cycle, cell migration, and morphogenesis89. The following examples highlight the prospective role of twi in the mechanics of mesoderm invagination, apical constriction, and in anterior endoderm compression by germ-band extension in Drosophila42. Conventional signaling pathways active during mesoderm patterning turn on overlapping expression of transcription factors twi and Snail (sna). It has been hypothesized that sna expression over a broad region activates myosin II and drives pulsatile contractions in the mesoderm48. This first wave of contractility activates the pathway downstream of the gene folded gastrulation (fog). Cells with an activated fog signaling pathway and expressing sna induced genes then initiate twi expression and a second contraction wave begins and stabilizes actomyosin contractility, driving cell shape changes and ventral furrow formation 47,49. Twi mutants revealed these two phases of furrowing in which the forces produced by the first wave of actomyosin contractions, activate signaling pathways to produce the next wave of stable contractions that drive the furrow to deepen. In twi mutants, the second constriction wave is defective, whereas in sna mutants with fog still expressed, both the first and second waves are defective. This suggests sna directs expression of factors that control the first contraction wave while twi targets, including fog are necessary for the second wave. In sna homozygous mutants, applying a local deformation with a micromanipulated needle succeeds in inducing furrowing in nearly 70% of the embryos 47. In sna twi double mutants, mechanical indentation does not rescue the defect but activation of fog signaling in mesoderm suggesting that twi directs expression of mechanical force sensors required for the mechanical rescue of sna mutants (see Figure 3)47. Further studies of apical constriction in ventral furrow formation have found that activated myosin II no longer accumulates at the apical cortex in twi and sna mutants while myosin remains in cell-cell junctions suggesting that mechanical cues may serve to reorganize the apical cortex, reinforcing it as ventral furrow formation progresses 48.
Figure 3. Mechanical feedback during morphogenesis.
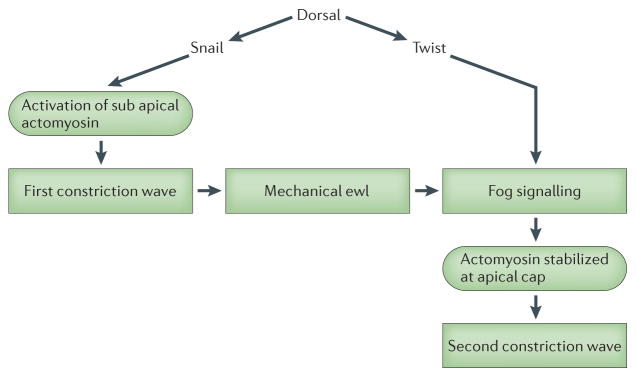
A pathway depicting genetic control of ventral furrow and anterior midgut invagination and a role in that network for mechanical cues. Snail expression is responsible for the mechanical cues produced by the first phase of myosin II contraction. Strain within the epithelium may redirect actomyosin to the apical cap and activate fog signaling which act during the second, Twist-dependent phase to stabilize actomyosin contractions and drive epithelial folding. Furrowing and invagination in Twist mutants can be rescued by exogenously applied strain either by laser ablation or by physical indentation. In the case of invagination-rescued anterior midgut differentiation, larval development was also reported to be rescued.
A similar role for twi in a mechanotransduction pathway has been proposed during invagination of the anterior midgut primordia in D. melanogaster. Magnetic tweezers were used in conjunction with laser ablation to rescue the differentiation of the stomodeal primordium. Twi expression in stomodeal primordia could be blocked by ablation of nearby cells and restored after magnetically controlled compressive forces were applied to the primordium 41. Mechanical perturbations of ventral furrow formation and anterior midgut invagination in Drosophila have been conducted on the tissue scale where forces may be transmitted and integrated before being sensed.
Another transcription factor, Kruppel-like factor 2 (klf2) is expressed in response to shear strains resulting from fluid flow. For example, artery, vein and capillary formation depends on klf2 signaling in response to blood flow. Klf2 is a member of the Kruppel-like factor family of zinc finger-containing transcription factors with homology to Drosophila segmentation gene product Kruppel 90. In vitro studies have been used to refine the nature of the mechanical cues that trigger klf2 expression. Cultured human umbilical vein endothelial cells (HUVECs) subjected to pulsatile shear flow, have increased klf2 expression and decreased klf2 expression after exposure to reciprocating oscillatory shear flow85. In vivo, klf2 expression correlates with disturbed patterns of local fluid flow. Klf2 expression in chick embryonic vasculature is found in regions of high shear stress 84. Similar studies in mouse also suggests klf2 expression correlates with the rise in fluid shear forces during development 83 Studies of zebrafish silent heart mutants where the heart does not beat reveals a highly altered vascular expression of klf2a, demonstrating a direct dependence on blood flow in vivo 82.
The role of mechanics in driving gene expression has been quantified more thoroughly in vitro with the application of defined shear forces85. These studies demonstrate cell fate and behavioral decisions can be responsive to mechanical cues but the molecular mechanisms for sensing biomechanical conditions and triggering signaling pathways have been difficult to identify. A direct connection between mechanical stimulation and transcriptional response has been made in the case of the transcription factor Yes-associated protein (YAP) and its transcriptional coactivator (TAZ). YAP and TAZ are significantly up regulated in mammary epithelial cells (MECs) cultured on high stiffness substrates91. The majority of work with YAP has been in cell culture, however, knockout and knock-down studies have implicated this transcription factor in vascularization and anterior-posterior axis elongation88. YAP knock-down studies in both fish and frog implicate a role in axis formation as well as epiboly during gastrulation 87. The strongest cases for a correlation between mechanics and YAP/TAZ activation are found when cells are cultured in well-defined mechanical conditions91. In order to determine if YAP/TAZ transcription activation reports ECM stiffness, transcriptional activity was measured in various cell types (MEC, MDA-MB-231, and HeLa) cultured on stiff and soft FN-coated hydrogels91. Stiff substrates had YAP/TAZ transcriptional activity comparable to cells grown on plastic, whereas on soft substrates YAP/TAZ activity was reduced to levels comparable YAP/TAZ knock-downs91. Furthermore, on soft substrates YAP/TAZ is found in the cytoplasm and moves into the nucleus on hard substrates. Micropatterned FN-substrates could also drive YAP/TAZ from the cytoplasm into the nucleus as cells adopt a spread out morphology. To determine if YAP/TAZ was reacting to the cell spreading or the ECM contacts, both the stiffness of the substrate and FN-density were controlled by culturing cells on micropillars, elastomeric molded microposts whose height corresponds to the rigidity of individual pillars 91. On shorter, or more stiff micropillars, YAP/TAZ remained in the nucleus even though the cell’s spreading area was the same. YAP/TAZ activation is also regulated by intracellular actomyosin contractility since inhibition of Rho or reduced F-actin assembly also inhibits YAP/TAZ transcriptional activity. Similar responses of YAP/TAZ to reduced Rho kinase (ROCK) and non-muscle myosin activity suggests cytoskeletal tension drives YAP/TAZ nuclear location. YAP/TAZ may play a key role in stem cell differentiation since YAP/TAZ depletion prevents stem cells from responding to changes in ECM stiffness 86. In D. melangogaster, increasing the activity of the homolog to YAP/TAZ, Yorkie, increases F-actin assembly.67
Mechanics, feedback, and robust programs of development
The ability of the embryo to develop amidst genetic, maternal and environmental variation has puzzled developmental biologists for decades. Considerable experimental evidence suggests that cells and embryos must be able to modulate cell signaling networks, gene expression patterns, and cell behaviors to accommodate variation (e.g. 92). Still, even with these robust programs there remains considerable phenotypic variation 93. Several factors may provide feedback between the mechanical environment and signaling networks as part of these programs. On the molecular scale, the motor complex of myosin II can adjust force production based on the mechanical loading 5. On the cell-scale, the shape of a cell can dictate its protrusive activity 94 as well as its commitment to specific cell lineages 37. Lastly, on the tissue scale the classical processes of secondary induction may serve to signal the success of a particular tissue movement or elicit further morphogenetic movements. The molecular and cellular scale processes that provide feedback from the mechanical processes of morphogenesis are poorly understood and their resolution will provide deeper insights into many larger questions in evolution as well as the nature of disease liability and birth defects 42,95–97.
Future Challenges
Advances in developmental mechanics are revealing the hidden contribution of biomechanics to patterning, morphogenesis, and organogenesis and extending our understanding of the origin of structural birth defects. These advances are being accomplished by unifying the efforts of geneticists, cell biologists, physicists and engineers. One of the critical challenges to progress is that one cannot simply “knock-out” a mechanical process in the same way as one knocks-out a gene. Furthermore, there are no one-to-one correspondence principles between gene functions and the mechanical events they affect. It is clear that mechanical processes have polygenic origin and that dozens if not hundreds of genes are responsible for physical processes operating on a range of scales from molecular to cellular to the tissue-level. There are many ways for mechanical processes to influence development, from direct and immediate impact on gene expression, to their role in guiding cell behaviors, to the long term consequences of tissue movements. The challenge of distinguishing between these roles for mechanics, e.g. distinguishing between cause and effect, are well understood by geneticists. To overcome these challenges developmental biologists are turning to systems biology with principles borrowed from control theory and computer simulation.
A systems or theoretical approach to studying morphogenesis involves breaking the biochemical and biological processes of force generation, transmission, and sensing into multiple separate processes operating on different scales and then recombining those processes in a mathematical or computational framework. Processes that are difficult to isolate experimentally in vivo are easily controlled in a computer model. For instance, motor proteins like myosin II play a role in force generation, transmission, and coordination within the tissue. Furthermore, single molecule studies have shown myosin can directly “sense” levels of applied force and adjust their output to compensate so it might seem likely to expect this occurs universally during morphogenesis. Alternatively, another report suggests cells directly sense force in vivo and trigger the recruitment of myosin II to the site where applied forces were sensed98. This is an attractive hypothesis but it is unclear what stresses are present in vivo and whether the magnitude or range of those forces are physiologically relevant at the scale of the cell or to physical demands of morphogenesis. Efforts to understand the role of myosin II feedback in vivo either in a cell or tissue setting will require sophisticated experimental designs that parallel earlier in vitro single molecule studies that controlled the loads and processivity of myosin II99, laser ablation and photo activation studies, methods for applying controlled forces, as well as loss of function mutants.
Future progress in developmental biomechanics will require new experimental techniques and theory to control mechanical loads on tissues 100, to assess changes in gene expression, protein activity and signaling, and evaluate their effects on the rates of morphogenesis and phenotypic variation. Experiments that rigorously evaluate the role of mechanics in directing cell fate or in providing feedback to improve the robustness of developmental programs are difficult to design. The long time-lag between a mechanical stimulus and cell differentiation and the difficulty ruling out a role for secondary induction are just two challenges. Falsification of hypotheses involving both mechanical and chemical signaling pathways requires testing tissue or cellular responses to well-defined mechanical stimuli. Advances in cell mechanics, tunable elastic substrates, and microfabrication provide increasingly sophisticated tools for controlling the mechanical microenvironment of cells and tissues. Advances in cell biology and imaging are providing tools for interrogating cell signaling pathways needed to report the status of mechanically stimulated cells. Together with theoretical and systems biology these experimental biomechanics approaches are leading to more complete, integrated views of developmental mechanics that will provide lasting insights into development and the self-organizing processes that assemble organs and lay the foundation for physiological function.
Online ‘at-a-glance’ summary.
Spatial, temporal, and mechanical cues control gene expression and protein activity that regulate force production and mechanical tissue responses in embryos.
Experiments to understand the role of mechanics during morphogenesis describe molecular and genetic manipulations, as well as spatial and temporal mechanisms that transmit and coordinate forces in the tissue.
Recent in vivo and in vitro studies identify the role of biomechanical cues in guiding cell fate and behavior.
Cellular mechanosensing is broken down into mechanical signals from the cell’s microenvironment and from the plasma membrane and cell cortex.
Highlight recent studies that identify the link between mechanical cues to gene expression.
Identify the role mechanics plays in a complex, and poorly understood feedback loop which underlies the robust programs of development.
Glossary
- Amnioserosa
layer of epithelial cells that covers dorsal regions of the early Drosophila embryo
- Anterior/Posterior
axis of the embryo defined by the tissues fated to form the head (anterior) and tail (posterior)
- Apical/Basal
axis perpendicular to the plane of the epithelium. Apical surfaces face the ‘outside’ or lumen. Basal surfaces lie opposite the apical
- Blastula
The early stage of a developing embryo after rapid cell divisions have created a sphere, sometimes hollow, of many cells
- Cell Cortex
the surface of the cell just inside the plasma membrane where cytoskeletal proteins maintain cell shape
- Dendraster excentricus
sand dollar species of echinoderm
- Desmosome
a spot-like junctional complex for cell-cell adhesion distinct from adherens junctions found connecting epithelial cells to neighbors at their apical ends
- Dorsal Closure
step in Drosophila development where the epidermis close over the exposed amnioserosa
- Drosophila melanogaster
fruit fly
- Ectoderm
outer most germ layer of the embryo. Cells from this layer differentiate into skin and neural tissues
- Epidermis
outermost epithelial layer of an embryo
- Focal adhesion complex
a dynamic, protein complex which connects the cell’s cytoskeleton to the extracellular matrix
- Gastrulation
stage of embryonic development when large cell rearrangements occur and the three germ layers, endoderm, mesoderm, and ectoderm of the embryo are established
- Invagination
the ‘in-folding’ of an epithelium
- Stomodeal primordium
tissues fated to give rise to the Drosophila foregut
- Traction force microscopy
a method used to determine how the force a cell or tissue exerts on a substrate to which it as adhered
- Xenopus laevis
African claw-toed frog
- Vegetal plate
The columnar epithelium at the vegetal pole of an echinoderm embryo. The thickened vegetal plate forms a pocket and tube by invagination during gastrulation to form the archenteron or primitive gut of the embryo
Biographies
Callie J Miller - Callie has a background in mathematics (BS from James Madison University and MA from the University of Pittsburgh) and is a PhD graduate student in the bioengineering department at the Univeristy of Pittsburgh. Her research is focused on understanding the biomechanics of actomyosin contractions.
Lance A Davidson - Lance has a background in biophysics and developmental biology (BS in Physics from University of Illinois; PhD in Biophysics from UC Berkeley) and works at the interfaces between engineering, physics, and biology. His research seeks to integrate the biomechanics of morphogenesis across a number of size scales from subcellular generation of forces to the macroscopic tissue properties that guide development of the developing embryo. The group he leads at the University of Pittsburgh seeks to understand, in mechanical terms, how single cell mechanical processes are coordinated to generate force, and how these forces are converted into tissue-scale movements.
Footnotes
References
- 1.His W. On the principles of animal morphology. Proceedings of Royal Society of Edinburgh. 1888;15:287–298. [Google Scholar]
- 2.Rhumbler L. Zur mechanik des gastrulations vorganges insbesondere der invagination. Archiv Fur Entwicklungs mechanic. 1902;14:401–476. [Google Scholar]
- 3.Morgan TH. Experimental Embryology. Columbia University Press; 1927. [Google Scholar]
- 4.Lewis WH. Mechanics of Invagination. Anatomical Record. 1947;97:139–56. doi: 10.1002/ar.1090970203. [DOI] [PubMed] [Google Scholar]
- 5.Howard J. Mechanics of Motor Proteins and the Cytoskeleton. Sinauer Associates; Sunderland, MA: 2001. p. 367. [Google Scholar]
- 6.Selman GG. Studies on the forces producing neural closure in amphibia. Proceedings of the Royal Physical Society of Edinburgh. 1955;24:24–27. [Google Scholar]
- 7.Selman GG. The forces producing neural closure in amphibia. Journal of Embryology and Experimental Morphology. 1958;6:448–465. [PubMed] [Google Scholar]
- 8.Smith JL, Schoenwolf GC. Further evidence of extrinsic forces in bending of the neural plate. J Comp Neurol. 1991;307:225–36. doi: 10.1002/cne.903070206. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez IS, Schoenwolf GC. Expansion of surface epithelium provides the major extrinsic force for bending of the neural plate. J Exp Zool. 1992;261:340–8. doi: 10.1002/jez.1402610313. [DOI] [PubMed] [Google Scholar]
- 10.Smith JL, Schoenwolf GC, Quan J. Quantitative analyses of neuroepithelial cell shapes during bending of the mouse neural plate. J Comp Neurol. 1994;342:144–51. doi: 10.1002/cne.903420113. [DOI] [PubMed] [Google Scholar]
- 11.Smith JL, Schoenwolf GC. Neurulation: coming to closure. Trends Neurosci. 1997;20:510–7. doi: 10.1016/s0166-2236(97)01121-1. [DOI] [PubMed] [Google Scholar]
- 12.Ettensohn CA. Mechanisms of epithelial invagination. Quarterly Review of Biology. 1985;60:289–307. doi: 10.1086/414426. [DOI] [PubMed] [Google Scholar]
- 13.Gustafson T, Wolpert L. The cellular basis of morphogenesis and sea urchin development. Int Rev Cytol. 1963;15:139–214. doi: 10.1016/s0074-7696(08)61117-1. [DOI] [PubMed] [Google Scholar]
- 14.Moore AR, Burt AS. On the locus and nature of the forces causing gastrulation in the embryos of Dendraster excentricus. Journal of Experimental Zoology. 1939;82:159–71. [Google Scholar]
- 15.Hutson MS, et al. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300:145–9. doi: 10.1126/science.1079552. Described the mechanical steps required for Drosophila dorsal closure with laser ablation to describe the degree of mechanical contributions and qualitative models to show the physiological forces. [DOI] [PubMed] [Google Scholar]
- 16.Peralta XG, et al. Upregulation of forces and morphogenic asymmetries in dorsal closure during Drosophila development. Biophys J. 2007;92:2583–96. doi: 10.1529/biophysj.106.094110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyama Y, Peralta XG, Wells AR, Kiehart DP, Edwards GS. Apoptotic force and tissue dynamics during Drosophila embryogenesis. Science. 2008;321:1683–6. doi: 10.1126/science.1157052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–90. doi: 10.1083/jcb.149.2.471. Examined forces responsible for dorsal closure in Drosophila using three techniques: time-lapse confocal microscopy of actomyosin dynamics, laser cutting, and repeated ablation of cells in each of the specific tissue regions to examine the roles tissue types play in dorsal closure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solon J, Kaya-Copur A, Colombelli J, Brunner D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell. 2009;137:1331–42. doi: 10.1016/j.cell.2009.03.050. [DOI] [PubMed] [Google Scholar]
- 20.Gorfinkiel N, Blanchard GB, Adams RJ, Martinez Arias A. Mechanical control of global cell behaviour during dorsal closure in Drosophila. Development. 2009;136:1889–98. doi: 10.1242/dev.030866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David DJ, Tishkina A, Harris TJ. The PAR complex regulates pulsed actomyosin contractions during amnioserosa apical constriction in Drosophila. Development. 2010;137:1645–55. doi: 10.1242/dev.044107. [DOI] [PubMed] [Google Scholar]
- 22.Young PE, Richman AM, Ketchum AS, Kiehart DP. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes and Development. 1993;7(1):29–41. doi: 10.1101/gad.7.1.29. [DOI] [PubMed] [Google Scholar]
- 23.Edwards KA, Demsky M, Montague RA, Weymouth N, Kiehart DP. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev Biol. 1997;191:103–17. doi: 10.1006/dbio.1997.8707. [DOI] [PubMed] [Google Scholar]
- 24.Jacinto A, et al. Dynamic analysis of actin cable function during Drosophila dorsal closure. Curr Biol. 2002;12:1245–50. doi: 10.1016/s0960-9822(02)00955-7. [DOI] [PubMed] [Google Scholar]
- 25.Millard TH, Martin P. Dynamic analysis of filopodial interactions during the zippering phase of Drosophila dorsal closure. Development. 2008;135:621–6. doi: 10.1242/dev.014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson LA, Koehl MA, Keller R, Oster GF. How do sea urchins invaginate? Using biomechanics to distinguish between mechanisms of primary invagination. Development. 1995;121:2005–18. doi: 10.1242/dev.121.7.2005. Used finite element modeling to test hypothesized mechanisms for sea urchin primary invagination and applied the model results to develop experiments which would ascertain the in vivo mechanism. [DOI] [PubMed] [Google Scholar]
- 27.Davidson LA, Oster GF, Keller RE, Koehl MA. Measurements of mechanical properties of the blastula wall reveal which hypothesized mechanisms of primary invagination are physically plausible in the sea urchin Strongylocentrotus purpuratus. Dev Biol. 1999;209:221–38. doi: 10.1006/dbio.1999.9249. [DOI] [PubMed] [Google Scholar]
- 28.Sherrard K, Robin F, Lemaire P, Munro E. Sequential Activation of Apical and Basolateral Contractility Drives Ascidian Endoderm Invagination. Curr Biol. 2010;20:1499–1510. doi: 10.1016/j.cub.2010.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koehl MAR. Biomechanical approaches to morphogenesis. Seminars in Developmental Biology. 1990;1:367–78. [Google Scholar]
- 30.Trinkaus JP. Cells into Organs: the forces that shape the embryo. Vol. 560. Prentice-Hall Inc; Englewood Cliffs: 1984. [Google Scholar]
- 31.Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208(4440):177–9. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 32.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–16. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci U S A. 2001;98:11295–300. doi: 10.1073/pnas.201201198. To examine the role of focal adhesion kinase in mechanosensing, the researchers applied forces by pulling or pushing the substrate near the cell with a microneedle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–8. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 35.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. Tuned the elasticity of in vitro gels to physiological elasticity and observed corresponding differentiation of human mesenchymal stem cells. [DOI] [PubMed] [Google Scholar]
- 36.Trappmann B, et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642–9. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 37.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–95. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 38.Brodland GW, et al. Furrowing surface contraction wave coincident with primary neural induction in amphibian embryos. Journal of Morphology. 1994;219(2):131–42. doi: 10.1002/jmor.1052190203. [DOI] [PubMed] [Google Scholar]
- 39.Beloussov LV, Lakirev AV, Naumidi II, Novoselov VV. Effects of relaxation of mechanical tensions upon the early morphogenesis of Xenopus laevis embryos. International Journal of Developmental Biology. 1990;34(4):409–19. [PubMed] [Google Scholar]
- 40.Beloussov LV, Luchinskaya NN, Ermakov AS, Glagoleva NS. Gastrulation in amphibian embryos, regarded as a succession of biomechanical feedback events. Int J Dev Biol. 2006;50:113–22. doi: 10.1387/ijdb.052057lb. [DOI] [PubMed] [Google Scholar]
- 41.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15:470–7. doi: 10.1016/j.devcel.2008.07.009. Used laser ablation and magnetic tweezers to deform Drosophila embryos and draw conclusions about mechanically induced factors. [DOI] [PubMed] [Google Scholar]
- 42.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–77. doi: 10.1016/s0960-9822(03)00576-1. Applied compression to deform Drosophila embryos and observed transcription patterns of multiple genes after compression. [DOI] [PubMed] [Google Scholar]
- 43.Kumar A, Shivashankar G. Mechanical force alters morphogenetic movements and segmental gene expression patterns during Drosophila embryogenesis. PLoS One. 2012;7:e33089. doi: 10.1371/journal.pone.0033089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lecuit T, Lenne PF, Munro E. Force generation, transmission, and integration during cell and tissue morphogenesis. Annu Rev Cell Dev Biol. 2011;27:157–84. doi: 10.1146/annurev-cellbio-100109-104027. [DOI] [PubMed] [Google Scholar]
- 45.Keller R, Davidson LA, Shook DR. How we are shaped: The biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- 46.Sampath K, et al. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature. 1998;395:185–9. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- 47.Pouille PA, Ahmadi P, Brunet AC, Farge E. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci Signal. 2009;2:ra16. doi: 10.1126/scisignal.2000098. [DOI] [PubMed] [Google Scholar]
- 48.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–9. doi: 10.1038/nature07522. Apical constriction of ventral furrow cells in Drosophila is caused by pulsed actomyosin contractions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mason FM, Tworoger M, Martin AC. Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nat Cell Biol. 2013 doi: 10.1038/ncb2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Somogyi K, Rorth P. Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. Dev Cell. 2004;7:85–93. doi: 10.1016/j.devcel.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 51.Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–98. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krammer A, Lu H, Isralewitz B, Schulten K, Vogel V. Forced unfolding of the fibronectin type III module reveals a tensile molecular recognition switch. Proc Natl Acad Sci U S A. 1999;96:1351–6. doi: 10.1073/pnas.96.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klotzsch E, et al. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proc Natl Acad Sci U S A. 2009;106:18267–72. doi: 10.1073/pnas.0907518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci U S A. 2002;99:5139–43. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marsden M, DeSimone DW. Regulation of cell polarity, radial intercalation and epiboly in Xenopus: novel roles for integrin and fibronectin. Development. 2001;128:3635–3647. doi: 10.1242/dev.128.18.3635. [DOI] [PubMed] [Google Scholar]
- 56.Davidson LA, Marsden M, Keller R, Desimone DW. Integrin alpha5beta1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr Biol. 2006;16:833–44. doi: 10.1016/j.cub.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 57.Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–82. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- 58.Chiu CH, Chou CW, Takada S, Liu YW. Development and fibronectin signaling requirements of the zebrafish interrenal vessel. PLoS One. 2012;7:e43040. doi: 10.1371/journal.pone.0043040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pulina MV, et al. Essential roles of fibronectin in the development of the left-right embryonic body plan. Dev Biol. 2011;354:208–20. doi: 10.1016/j.ydbio.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber GF, Bjerke MA, Desimone DW. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell. 2012;22:104–15. doi: 10.1016/j.devcel.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raich WB, Agbunag C, Hardin J. Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr Biol. 1999;9:1139–46. doi: 10.1016/S0960-9822(00)80015-9. [DOI] [PubMed] [Google Scholar]
- 62.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–42. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 63.Kong F, et al. Cyclic mechanical reinforcement of integrin-ligand interactions. Mol Cell. 2013;49:1060–8. doi: 10.1016/j.molcel.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu W, Baribault H, Adamson ED. Vinculin knockout results in heart and brain defects during embryonic development. Development. 1998;125:327–37. doi: 10.1242/dev.125.2.327. [DOI] [PubMed] [Google Scholar]
- 65.le Duc Q, et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–15. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mierke CT, et al. Mechano-coupling and regulation of contractility by the vinculin tail domain. Biophys J. 2008;94:661–70. doi: 10.1529/biophysj.107.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.del Rio A, et al. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–41. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho TC, Horn NA, Huynh T, Kelava L, Lansman JB. Evidence TRPV4 contributes to mechanosensitive ion channels in mouse skeletal muscle fibers. Channels (Austin) 2012;6:246–54. doi: 10.4161/chan.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thodeti CK, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104:1123–30. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kottgen M, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–47. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–7. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 72.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coste B, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–81. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim SE, Coste B, Chadha A, Cook B, Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–12. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eisenhoffer GT, et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature. 2012;484:546–9. doi: 10.1038/nature10999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coste B, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Houk AR, et al. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148:175–88. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Itoh T, et al. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell. 2005;9:791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Suetsugu S. The proposed functions of membrane curvatures mediated by the BAR domain superfamily proteins. J Biochem. 2010;148:1–12. doi: 10.1093/jb/mvq049. [DOI] [PubMed] [Google Scholar]
- 80.Saarikangas J, et al. Molecular mechanisms of membrane deformation by I-BAR domain proteins. Curr Biol. 2009;19:95–107. doi: 10.1016/j.cub.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 81.Schuler S, et al. Ciliated sensory hair cell formation and function require the F-BAR protein syndapin I and the WH2 domain-based actin nucleator Cobl. J Cell Sci. 2012 doi: 10.1242/jcs.111674. [DOI] [PubMed] [Google Scholar]
- 82.Parmar KM, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee JS, et al. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell. 2006;11:845–57. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 84.Groenendijk BC, Hierck BP, Gittenberger-De Groot AC, Poelmann RE. Development-related changes in the expression of shear stress responsive genes KLF-2, ET-1, and NOS-3 in the developing cardiovascular system of chicken embryos. Dev Dyn. 2004;230:57–68. doi: 10.1002/dvdy.20029. [DOI] [PubMed] [Google Scholar]
- 85.Wang N, et al. Shear stress regulation of Kruppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun. 2006;341:1244–51. doi: 10.1016/j.bbrc.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 86.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 87.Gee ST, Milgram SL, Kramer KL, Conlon FL, Moody SA. Yes-associated protein 65 (YAP) expands neural progenitors and regulates Pax3 expression in the neural plate border zone. PLoS One. 2011;6:e20309. doi: 10.1371/journal.pone.0020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morin-Kensicki EM, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sandmann T, et al. A core transcriptional network for early mesoderm development in Drosophila melanogaster. Genes Dev. 2007;21:436–49. doi: 10.1101/gad.1509007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pearson R, Fleetwood J, Eaton S, Crossley M, Bao S. Krüppel-like transcription factors: a functional family. The international journal of biochemistry & cell biology. 2008;40:1996–2001. doi: 10.1016/j.biocel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 91.Fu J, et al. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7:733–6. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Manu, et al. Canalization of gene expression in the Drosophila blastoderm by gap gene cross regulation. PLoS Biol. 2009;7:e1000049. doi: 10.1371/journal.pbio.1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tyszka JM, Ewald AJ, Wallingford JB, Fraser SE. New tools for visualization and analysis of morphogenesis in spherical embryos. Dev Dyn. 2005;234:974–83. doi: 10.1002/dvdy.20561. [DOI] [PubMed] [Google Scholar]
- 94.Jiang X, Bruzewicz DA, Wong AP, Piel M, Whitesides GM. Directing cell migration with asymmetric micropatterns. Proc Natl Acad Sci U S A. 2005;102:975–8. doi: 10.1073/pnas.0408954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.von Dassow M, Davidson LA. Physics and the canalization of morphogenesis: a grand challenge in organismal biology. Phys Biol. 2011;8:045002. doi: 10.1088/1478-3975/8/4/045002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Farge E. Mechanotransduction in development. Curr Top Dev Biol. 2011;95:243–65. doi: 10.1016/B978-0-12-385065-2.00008-6. [DOI] [PubMed] [Google Scholar]
- 97.Shook DR, Keller R. Morphogenic machines evolve more rapidly than the signals that pattern them: lessons from amphibians. J Exp Zool B Mol Dev Evol. 2008;310:111–35. doi: 10.1002/jez.b.21204. [DOI] [PubMed] [Google Scholar]
- 98.Fernandez-Gonzalez R, de Simoes SM, Roper JC, Eaton S, Zallen JA. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell. 2009;17:736–43. doi: 10.1016/j.devcel.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sweeney HL, Houdusse A. Structural and functional insights into the Myosin motor mechanism. Annu Rev Biophys. 2010;39:539–57. doi: 10.1146/annurev.biophys.050708.133751. [DOI] [PubMed] [Google Scholar]
- 100.von Dassow M, Strother JA, Davidson LA. Surprisingly simple mechanical behavior from a complex embryonic tissue. PLoS One. 2010;5:e15359. doi: 10.1371/journal.pone.0015359. Used simple in vivo mechanical experiments and theoretical model to describe the viscoelastic properties of a vertebrate embryo and rule out the presence of mechanical feedback in Xenopus laevis embryonic tissues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma X, Lynch HE, Scully PC, Hutson MS. Probing embryonic tissue mechanics with laser hole drilling. Phys Biol. 2009;6:036004. doi: 10.1088/1478-3975/6/3/036004. [DOI] [PubMed] [Google Scholar]
- 102.Rodriguez-Diaz A, et al. Actomyosin purse strings: renewable resources that make morphogenesis robust and resilient. HFSP J. 2008;2:220–37. doi: 10.2976/1.2955565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Benazeraf B, et al. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature. 2010;466:248–52. doi: 10.1038/nature09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Varner VD, Voronov DA, Taber LA. Mechanics of head fold formation: investigating tissue-level forces during early development. Development. 2010;137:3801–11. doi: 10.1242/dev.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Beloussov LV, Lakirev AV, Naumidi II, Novoselov VV. Effects of relaxation of mechanical tensions upon the early morphogenesis of Xenopus laevis embryos. Int J Dev Biol. 1990;34:409–19. [PubMed] [Google Scholar]
- 106.Beloussov LV, Lakirev AV, Naumidi II. The role of external tensions in differentiation of Xenopus laevis embryonic tissues. Cell Differ Dev. 1988;25:165–76. doi: 10.1016/0922-3371(88)90113-x. [DOI] [PubMed] [Google Scholar]
- 107.Beloussov LV, Dorfman JG, Cherdantzev VG. Mechanical stresses and morphological patterns in amphibian embryos. J Embryol Exp Morphol. 1975;34:559–74. [PubMed] [Google Scholar]
- 108.Sokolow A, Toyama Y, Kiehart DP, Edwards GS. Cell ingression and apical shape oscillations during dorsal closure in Drosophila. Biophys J. 2012;102:969–79. doi: 10.1016/j.bpj.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peralta XG, Toyama Y, Kiehart DP, Edwards GS. Emergent properties during dorsal closure in Drosophila morphogenesis. Phys Biol. 2008;5:15004. doi: 10.1088/1478-3975/5/1/015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Blanchard GB, et al. Tissue tectonics: morphogenetic strain rates, cell shape change and intercalation. Nat Methods. 2009;6:458–64. doi: 10.1038/nmeth.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gibson MC, Patel AB, Nagpal R, Perrimon N. The emergence of geometric order in proliferating metazoan epithelia. Nature. 2006;442:1038–41. doi: 10.1038/nature05014. [DOI] [PubMed] [Google Scholar]
- 112.Zhou J, Kim HY, Davidson LA. Actomyosin stiffens the vertebrate embryo during crucial stages of elongation and neural tube closure. Development. 2009;136:677–88. doi: 10.1242/dev.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou J, Kim HY, Wang JH, Davidson LA. Macroscopic stiffening of embryonic tissues via microtubules, RhoGEF and the assembly of contractile bundles of actomyosin. Development. 2010;137:2785–94. doi: 10.1242/dev.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wiebe C, Brodland GW. Tensile properties of embryonic epithelia measured using a novel instrument. J Biomech. 2005;38:2087–94. doi: 10.1016/j.jbiomech.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 115.Zamir EA, Taber LA. Material properties and residual stress in the stage 12 chick heart during cardiac looping. J Biomech Eng. 2004;126:823–30. doi: 10.1115/1.1824129. [DOI] [PubMed] [Google Scholar]
- 116.Foty RA, Pfleger CM, Forgacs G, Steinberg MS. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122:1611–20. doi: 10.1242/dev.122.5.1611. [DOI] [PubMed] [Google Scholar]
- 117.Forgacs G, Foty RA, Shafrir Y, Steinberg MS. Viscoelastic properties of living embryonic tissues: a quantitative study. Biophys J. 1998;74:2227–34. doi: 10.1016/S0006-3495(98)77932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Joshi SD, von Dassow M, Davidson LA. Experimental control of excitable embryonic tissues: three stimuli induce rapid epithelial contraction. Exp Cell Res. 2010;316:103–14. doi: 10.1016/j.yexcr.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Voronov DA, Taber LA. Cardiac looping in experimental conditions: effects of extraembryonic forces. Dev Dyn. 2002;224:413–21. doi: 10.1002/dvdy.10121. [DOI] [PubMed] [Google Scholar]
- 120.Zamir EA, Srinivasan V, Perucchio R, Taber LA. Mechanical asymmetry in the embryonic chick heart during looping. Ann Biomed Eng. 2003;31:1327–36. doi: 10.1114/1.1623487. [DOI] [PubMed] [Google Scholar]
- 121.Odell GM, Oster G, Alberch P, Burnside B. The mechanical basis of morphogenesis. I. Epithelial folding and invagination. Dev Biol. 1981;85:446–62. doi: 10.1016/0012-1606(81)90276-1. [DOI] [PubMed] [Google Scholar]
- 122.Brodland GW, et al. Video force microscopy reveals the mechanics of ventral furrow invagination in Drosophila. Proc Natl Acad Sci U S A. 2010;107:22111–6. doi: 10.1073/pnas.1006591107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Savin T, et al. On the growth and form of the gut. Nature. 2011;476:57–62. doi: 10.1038/nature10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brodland GW, Veldhuis JH. Assessing the mechanical energy costs of various tissue reshaping mechanisms. Biomech Model Mechanobiol. 2012;11:1137–47. doi: 10.1007/s10237-012-0411-x. [DOI] [PubMed] [Google Scholar]