Abstract
Introduction
The process of weaning may impose cardiopulmonary stress on ventilated patients. Heart-rate variability (HRV), a noninvasive tool to characterize autonomic function and cardiorespiratory interaction, may be a promising modality to assess patient capability during the weaning process. We aimed to evaluate the association between HRV change and weaning outcomes in critically ill patients.
Methods
This study included 101 consecutive patients recovering from acute respiratory failure. Frequency-domain analysis, including very low frequency, low frequency, high frequency, and total power of HRV was assessed during a 1-hour spontaneous breathing trial (SBT) through a T-piece and after extubation after successful SBT.
Results
Of 101 patients, 24 (24%) had SBT failure, and HRV analysis in these patients showed a significant decrease in total power (P = 0.003); 77 patients passed SBT and were extubated, but 13 (17%) of them required reintubation within 72 hours. In successfully extubated patients, very low frequency and total power from SBT to postextubation significantly increased (P = 0.003 and P = 0.004, respectively). Instead, patients with extubation failure were unable to increase HRV after extubation.
Conclusions
HRV responses differ between patients with different weaning outcomes. Measuring HRV change during the weaning process may help clinicians to predict weaning results and, in the end, to improve patient care and outcome.
Introduction
Weaning patients with respiratory failure from ventilatory support is one of the most challenging problems in intensive care. Unnecessary mechanical ventilation poses increased risk of complications to patients; however, premature liberation from mechanical ventilation may also be harmful [1]. In the past few decades, a variety of predictors have been developed to identify patients ready to breathe independently [2]. Although to date, spontaneous breathing trial (SBT) is considered the most accurate index for predicting weaning success, 15% to 20% of patients succeeding in SBT require reintubation [3,4]. The pathophysiology of weaning failure is complex and involves interaction between cardiopulmonary reserve, autonomic function, and musculoskeletal capacity [5,6]. Thus, it may be hard to assess the interplay between those factors based on a single or a few predictors.
Heart-rate variability (HRV) has been related to the balance between parasympathetic and sympathetic regulation of cardiac activity, respiration, baroreflex, and thermal regulation [7-9]. It is a noninvasive and valuable tool to characterize autonomic function and cardiorespiratory interaction [10]. The impact of mechanical ventilation on HRV has been studied in newborn babies, children with brain death, and healthy young adults placed on sedation and paralysis [11-13]. Change of HRV between different ventilator settings has also been described in a canine model [14]. Accordingly, measurement of HRV may help evaluate physiological responses to the weaning process. In a case series by Shen and associates, [15], decrease in HRV is the main finding in patients with weaning failure, and the authors suggested that change of HRV components may be a potential tool of automatically gathered parameters during ventilator weaning. However, the result has not yet been replicated in larger-scale studies. Further, change of HRV between SBT and extubation was not explored in that study.
Thus, the aim of the present study was to investigate change of HRV during the entire weaning process in patients recovering from respiratory failure. The potential predictive value of change of HRV on SBT and extubation outcomes is also evaluated.
Materials and methods
Study population and setting
This prospective observational study was conducted in the adult intensive care unit (ICU) of a university-affiliated hospital in Taiwan from July 2010 to November 2010. A respiratory-therapist-implemented weaning protocol was applied in the ICU. Patients who had been intubated and placed on mechanical ventilation for 24 or more hours, and were ready for their first SBT were screened for eligibility in the study. Patients were excluded if they had tracheostomies, had atrial or ventricular arrhythmia, took chronic antiarrhythmic medications, or were unable to follow verbal instructions. Patients who needed to resume ventilatory support within 30 minutes of SBT or were reintubated due to upper-airway obstruction were excluded from the data analysis.
This study was approved by the Research Ethics Committee of the National Taiwan University Hospital, and the need for written informed consent was waived.
Weaning protocol
The weaning protocol was modified from the statement of the Sixth International Consensus Conference on Intensive Care Medicine [16]. In brief, respiratory therapists assessed the readiness for weaning and SBT on a daily basis. During the study period, SBT was conducted on a T-piece for 60 minutes, and criteria for SBT were as follows: reliable respiratory drive, stable hemodynamics, improvement of the cause of respiratory failure, positive end-expiratory pressure 8 or less cmH2O, fraction of inspired oxygen 40% or less, and rapid shallow breathing index <200/min/L. Patients were considered to succeed in SBT if none of the following was observed at the end of SBT: anxiety, agitation, diaphoresis, thoracoabdominal dysynchrony, respiratory rate >35 per minute, arterial oxygen saturation <90%, heart rate >140 beats per minute or sustained increase or decrease of heart rate >20%, or systolic blood pressure >180 mm Hg or <90 mm Hg [17].
If SBT was successful, the patient was immediately extubated. Extubation failure was defined as reintubation within 72 hours of extubation.
Measurement of HRV
The HRV measurements were performed between 8 AM and 12 PM, and in this study, we used a 5-minute measurement of HRV. During the study period, drugs potentially affecting HRV were avoided if possible. Patients were maintained in a semirecumbent position and were asked to sit in a relaxed manner. After 20 minutes of familiarization with each setting, recordings were made during (a) pre-SBT period (patients were breathing on a mechanical ventilator); (b) SBT period (patients were breathing through a T-piece), and (c) postextubation period (if patients were extubated after successful SBT). In the present study, the Embletta system (Embla Systems, Broomfield, CO, USA) was used to record electrocardiography, and then the data were downloaded to a computer for analysis. The HRV analysis was performed in accordance with the Task Force recommendations [7]. Data were investigated based on frequency-domain analysis. Power-spectrum densities were calculated for very low frequency (VLF, <0.04 Hz), low frequency (LF, 0.04 to 0.15 Hz), high frequency (HF, 0.15 to 0.4 Hz), and total power (TP). Normalized units of the LF (LF%) or HF (HF%) were calculated as LF/(TP-VLF) or HF/(TP-VLF), multiplied by 100. Also, the ratios of LF to HF (LF/HF) were calculated.
Data collection
Medical records were evaluated in detail to obtain the following information: demographic data, body mass index, acute physiology, and chronic health evaluation II score on ICU admission, comorbidities as defined in previous studies [18,19], causes of acute respiratory failure, time to first SBT, medications before the day of SBT, oxygenation status assessed by the ratio of partial pressure of oxygen in arterial blood to fraction of inspired oxygen and pre-SBT ventilator settings. Weaning parameters, including respiratory rate, minute ventilation, tidal volume, and maximal inspiratory pressure [20], were measured on the day of and before the SBT. The measured respiratory rate was then divided by the tidal volume to obtain the rapid shallow-breathing index.
Statistical analysis
Data are presented as mean ± standard deviation or number (percentage) as appropriate. Normal distribution of VLF, TP, and LF/HF was achieved by natural logarithmic transformation and confirmed by the Kolmogorov-Smirnov test. Comparisons between groups were made by using the independent samples t test for continuous variables, and the χ2 or Fisher Exact test for categoric variables. The HRV components were analyzed by using repeated-measures analysis of variance; in case of significance, a paired t test was used to compare means within a group as the post hoc analysis. Multivariate logistic regression analysis was applied to identify independent predictors of successful SBT and extubation. Age, sex, and variables with a P value of <0.1 in univariate analysis were included in the multivariate model. The HRV data were dichotomized for multivariate analysis according to the best cutoffs obtained from receiver operating characteristic (ROC) curve analysis. A two-tailed P value of <0.05 was considered statistically significant. All statistical analysis was conducted by using statistics software (SPSS 15.0; SPSS, Chicago, IL, USA).
Results
Patients
During the study period, 135 patients were screened; 32 patients were excluded for various reasons (Figure 1). Two patients who had to resume ventilatory support within 30 minutes of SBT were also excluded from the analysis because HRV measurements could not be well conducted. Of the 101 analyzed patients, 77 (76%) and 24 (24%) patients had successful and failed SBT, respectively. The demographics, baseline clinical characteristics, comorbidities, causes of acute respiratory failure, and duration of mechanical ventilation before SBT were similar between patients with successful and those with failed SBT (Table 1). Patients experiencing failure of SBT were more likely to be placed on β2 agonists and anticholinergics. Before SBT, no significant differences were found between the groups regarding weaning parameters, oxygenation, status and ventilator settings (Table 2).
Figure 1.
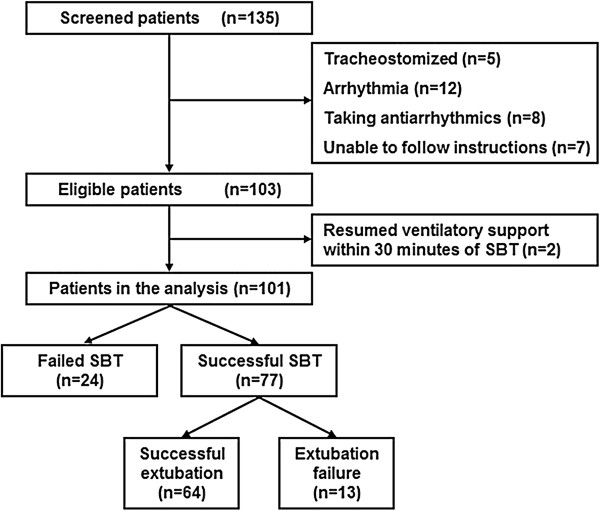
A flow diagram of the study patients and their outcomes. SBT, spontaneous breathing trial.
Table 1.
Baseline features of the study population according to the outcome of SBT
| Characteristics |
Successful SBT |
Failed SBT |
P value |
|---|---|---|---|
| ( n = 77) | ( n = 24) | ||
| Age, years |
65 ± 18 |
71 ± 16 |
0.186 |
| ≧65 years |
45 (58) |
17 (71) |
0.276 |
| Male sex |
54 (70) |
12 (50) |
0.070 |
| Body mass index |
22 ± 4 |
23 ± 4 |
0.378 |
| APACHE II |
17 ± 6 |
16 ± 7 |
0.767 |
| Comorbidities |
|
|
|
| Cerebrovascular disease |
16 (21) |
4 (17) |
0.776 |
| COPD |
19 (25) |
7 (29) |
0.660 |
| Heart failure |
3 (4) |
1 (4) |
0.999 |
| Liver cirrhosis |
4 (5) |
1 (4) |
0.999 |
| End-stage renal disease |
6 (8) |
2 (8) |
0.999 |
| Diabetes mellitus |
26 (34) |
6 (25) |
0.420 |
| Hypertension |
30 (39) |
8 (33) |
0.619 |
| Coronary artery disease |
5 (7) |
3 (13) |
0.391 |
| Cause of acute respiratory failure |
|
|
|
| Pneumonia |
34 (44) |
15 (63) |
0.116 |
| Sepsis |
15 (20) |
3 (13) |
0.551 |
| Acute exacerbation of COPD |
3 (4) |
1 (4) |
0.999 |
| Gastrointestinal bleeding |
10 (13) |
1 (4) |
0.452 |
| Lung edema |
4 (5) |
1 (4) |
0.999 |
| Neurologic disease |
6 (8) |
3 (13) |
0.440 |
| Others |
5 (7) |
0 (0) |
0.335 |
| Time to first SBT, hours |
96 ± 103 |
94 ± 71 |
0.931 |
| Medications |
|
|
|
| β2 agonist |
28 (36) |
17 (71) |
0.003 |
| Anticholinergic |
22 (29) |
16 (67) |
0.001 |
| ACEI/ARB |
8 (10) |
2 (8) |
0.999 |
| CCB |
27 (35) |
13 (54) |
0.095 |
| β blocker | 11 (14) | 3 (13) | 0.999 |
Data are presented as mean ± standard deviation or number (%).
ACEI, angiotensin-converting-enzyme inhibitor; APACHE, acute physiology and chronic health evaluation; ARB, angiotensin-receptor blocker; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; SBT, spontaneous breathing trial.
Table 2.
Weaning parameters, oxygenation status, and ventilator modes before SBT based on the outcome of SBT
| Characteristics |
Successful SBT |
Failed SBT |
P value |
|---|---|---|---|
| ( n = 77) | ( n = 24) | ||
| Weaning parameters |
|
|
|
| Respiratory rate <30 per minute |
70 (91) |
21 (88) |
0.698 |
| Minute ventilation ≦10 L/minute |
63 (82) |
19 (79) |
0.770 |
| Tidal volume ≧5 ml/kg |
57 (74) |
15 (63) |
0.276 |
| RSBI <105/min/L |
71 (92) |
21 (88) |
0.440 |
| PImax ≦20 cmH2O |
73 (95) |
24 (100) |
0.570 |
| PaO2/FIO2 |
318 ± 112 |
279 ± 101 |
0.128 |
| Pre-SBT ventilator settings |
|
|
|
| PSV |
73 (95) |
21 (88) |
0.674 |
| PCV |
2 (3) |
1 (4) |
|
| SIMV |
1 (1) |
1 (4) |
|
| VCV | 1 (1) | 1 (4) |
Data are presented as mean ± standard deviation or number (%).
FIO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen in arterial blood; PCV, pressure-controlled ventilation; PImax, maximal inspiratory pressure; PSV, pressure-support ventilation; RSBI, rapid shallow-breathing index; SBT, spontaneous breathing trial; SIMV, synchronized intermittent mandatory ventilation; VCV, volume-controlled ventilation.
Frequency-domain analysis of HRV
The HRV responses to SBT in all patients are described in Table 3. Among the HRV components, significant intergroup differences were found in ln TP. Further, by comparing HRV profiles before and after SBT with the paired t test, significant decrease in ln TP was observed in patients with failed SBT (P =0.003) (Figure 2A). When change of ln TP (Δln TP) was dichotomized based on the cutoff established by ROC curve analysis, Δln TP ≤0.4 ln(ms2) was associated with an odds ratio (OR) of failed SBT of 3.1 (95% confidence interval (CI), 1.2 to 8.0). In the multivariate logistic regression model, Δln TP ≤0.4 ln(ms2) remained independently associated with failed SBT (Table 4).
Table 3.
Measurement of heart-rate variability during pre-SBT and SBT periods
| Frequency-domain measures | Pre-SBT | SBT | P value a | P value b |
|---|---|---|---|---|
| VLF [ln(ms2)] |
|
|
|
|
| Successful SBT |
6.9 (1.3) |
6.3 (1.6) |
0.220 |
|
| Failed SBT |
6.6 (1.1) |
5.5 (1.5) |
|
|
| LF% (nu) |
|
|
|
|
| Successful SBT |
38 (20) |
40 (23) |
0.687 |
|
| Failed SBT |
38 (20) |
38 (20) |
|
|
| HF% (nu) |
|
|
|
|
| Successful SBT |
45 (15) |
44 (18) |
0.775 |
|
| Failed SBT |
47 (14) |
45 (16) |
|
|
| TP [ln(ms2)] |
|
|
|
|
| Successful SBT |
7.9 (1.0) |
7.7 (1.0) |
0.036 |
0.092 |
| Failed SBT |
8.2 (0.7) |
7.4 (0.9) |
|
0.003 |
| LF/HF [ln(ratio)] |
|
|
|
|
| Successful SBT |
1.0 (0.2) |
1.0 (0.3) |
0.597 |
|
| Failed SBT | 1.0 (0.1) | 1.0 (0.2) |
aFor comparison between successful and failed spontaneous-breathing trial groups.
bFor comparison between pre-spontaneous-breathing trial and spontaneous-breathing trial periods within a group.
Data are presented as mean (standard deviation).
SBT, spontaneous breathing trial; VLF, very low frequency; HF, high frequency; LF, low frequency; ln, natural logarithm; nu, normalized unit; TP, total power.
Figure 2.
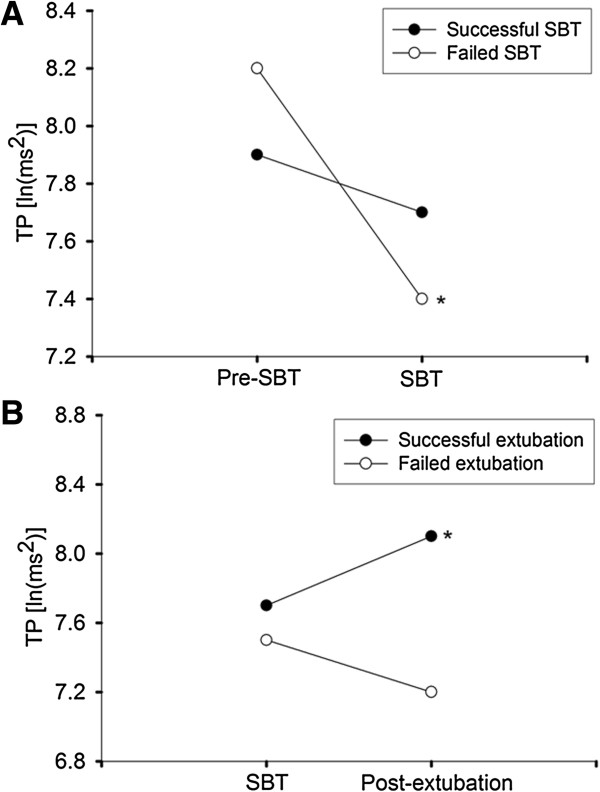
Effect of spontaneous-breathing trial (SBT) and extubation on heart-rate variability. (A) Patients who failed SBT had a significant decrease in total power (TP) at the change from pre-SBT to SBT periods. (B) Patients with successful extubation had a significant increase in TP from SBT to postextubation periods. Values are presented in means. Asterisks indicate significant changes. ln, natural logarithm.
Table 4.
Multivariate logistic regression model for development of SBT failure
| Variables | Odds ratio (95% CI) | P value |
|---|---|---|
| Age ≧65 years |
1.1 (0.3-3.6) |
0.917 |
| Female sex |
3.0 (1.0-9.4) |
0.054 |
| Use of β2 agonist |
4.0 (0.9-18.3) |
0.075 |
| Use of anticholinergic |
2.9 (0.6-13.0) |
0.163 |
| Use of CCB |
2.3 (0.7-6.8) |
0.149 |
| Δln TP ≤0.4 ln(ms2) | 7.2 (2.0-25.4) | 0.002 |
CCB, calcium channel blocker; CI, confidence interval; Δln TP, change of natural logarithmic transformation of total power from pre-SBT to SBT periods; SBT, spontaneous-breathing trial.
Extubated patients
Among 77 patients who passed the SBT, 13 (17%) patients required reintubation within 72 hours after extubation because of hypoxemia (four patients), respiratory acidosis (four patients), respiratory distress (three patients), or hemodynamic instability (two patients). Patients with successful extubation or extubation failure had similar baseline features (Table 5). In this study, weaning parameters did not predict the outcome of extubation (Table 6). Early change of spectral components of HRV after extubation is depicted in Table 7. Significant intergroup differences in ln VLF and ln TP were observed. In successfully extubated patients, Δln VLF and Δln TP from SBT to postextubation periods showed statistically significant increase (P = 0.003 and P = 0.004, respectively); whereas, by contrast, patients for whom extubation failed were unable to increase HRV after extubation (Figure 2B). The Δln VLF and Δln TP were dichotomized based on ROC curve analysis; Δln VLF <0.1 ln(ms2) (OR, 3.5; 95% CI, 1.0 to 12.6) and Δln TP <0.02 ln(ms2) (OR, 5.6; 95% CI, 1.4 to 22.2) were significantly associated with extubation failure. After adjustment for age and sex, Δln VLF <0.1 ln(ms2) (OR, 3.9; 95% CI, 1.1 to 14.8) and Δln TP <0.02 ln(ms2) (OR, 6.6; 95% CI, 1.6 to 27.9) remained significantly related to development of extubation failure.
Table 5.
Features of extubated patients according to the outcome of extubation
| Characteristics |
Successful extubation |
Extubation failure |
P value |
|---|---|---|---|
| ( n = 64) | ( n = 13) | ||
| Age, years |
64 ± 19 |
71 ± 15 |
0.247 |
| ≧65 years |
36 (56) |
9 (69) |
0.387 |
| Male sex |
45 (70) |
9 (70) |
0.999 |
| Body mass index |
22 ± 5 |
21 ± 3 |
0.373 |
| APACHE II |
17 ± 6 |
19 ± 7 |
0.303 |
| Comorbidities |
|
|
|
| Cerebrovascular disease |
14 (22) |
2 (15) |
0.725 |
| COPD |
16 (25) |
3 (23) |
0.999 |
| Heart failure |
3 (5) |
0 (0) |
0.999 |
| Liver cirrhosis |
3 (5) |
1 (8) |
0.530 |
| End-stage renal disease |
4 (6) |
2 (15) |
0.266 |
| Diabetes mellitus |
23 (36) |
3 (23) |
0.525 |
| Hypertension |
26 (41) |
4 (31) |
0.506 |
| Coronary artery disease |
5 (8) |
0 (0) |
0.582 |
| Cause of acute respiratory failure |
|
|
|
| Pneumonia |
29 (45) |
5 (39) |
0.650 |
| Sepsis |
13 (20) |
2 (15) |
0.999 |
| Acute exacerbation of COPD |
2 (3) |
1 (8) |
0.430 |
| Gastrointestinal bleeding |
8 (13) |
2 (15) |
0.674 |
| Lung edema |
4 (6) |
0 (0) |
0.999 |
| Neurologic disease |
4 (6) |
2 (15) |
0.266 |
| Others |
4 (6) |
1 (8) |
0.999 |
| Time to first SBT, hours |
102 ± 109 |
64 ± 55 |
0.221 |
| Medications |
|
|
|
| β2 agonist |
22 (34) |
6 (46) |
0.530 |
| Anticholinergic |
17 (27) |
5 (39) |
0.502 |
| ACEI/ARB |
7 (11) |
1 (8) |
0.999 |
| CCB |
24 (38) |
3 (23) |
0.525 |
| β blocker | 9 (14) | 2 (15) | 0.999 |
Data are presented as mean ± standard deviation or number (%).
ACEI, angiotensin-converting-enzyme inhibitor; ARB, angiotensin-receptor blocker; APACHE, Acute Physiology And Chronic Health Evaluation; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; SBT, spontaneous breathing trial.
Table 6.
Weaning parameters, oxygenation status, and ventilator modes before SBT based on the outcome of extubation
| Characteristics |
Successful extubation |
Extubation failure |
P value |
|---|---|---|---|
| ( n = 64) | ( n = 13) | ||
| Weaning parameters |
|
|
|
| Respiratory rate <30/min |
57 (89) |
13 (100) |
0.595 |
| Minute ventilation ≦10 L/min |
51 (80) |
12 (92) |
0.442 |
| Tidal volume ≧5 ml/kg |
47 (73) |
10 (77) |
0.999 |
| RSBI <105 /min/L |
59 (92) |
12 (92) |
0.999 |
| PImax ≦20 cmH2O |
60 (94) |
13 (100) |
0.999 |
| PaO2/FIO2 |
318 ± 115 |
319 ± 100 |
0.980 |
| Ventilator settings before SBT |
|
|
|
| PSV |
61 (95) |
12 (92) |
0.592 |
| PCV |
1 (2) |
1 (8) |
|
| SIMV |
1 (2) |
0 (0) |
|
| VCV | 1 (2) | 0 (0) |
Data are presented as mean ± standard deviation or number (%).
FIO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen in arterial blood; PCV pressure-controlled ventilation; PImax, maximal inspiratory pressure; PSV, pressure-support ventilation; RSBI, rapid shallow-breathing index; SBT, spontaneous-breathing trial; SIMV, synchronized intermittent mandatory ventilation; VCV, volume-controlled ventilation.
Table 7.
Measurement of heart-rate variability during SBT and postextubation periods
| Frequency-domain measures | SBT | Postextubation | P value a | P value b |
|---|---|---|---|---|
| VLF (ln(ms2)) |
|
|
|
|
| Successful extubation |
6.4 (1.7) |
6.9 (1.2) |
0.015 |
0.003 |
| Extubation failure |
6.1 (1.4) |
5.5 (1.9) |
|
0.187 |
| LF% (nu) |
|
|
|
|
| Successful extubation |
43 (23) |
43 (19) |
0.783 |
|
| Extubation failure |
26 (12) |
27 (18) |
|
|
| HF% (nu) |
|
|
|
|
| Successful extubation |
43 (18) |
44 (15) |
0.956 |
|
| Extubation failure |
51 (14) |
52 (15) |
|
|
| TP (ln(ms2)) |
|
|
|
|
| Successful extubation |
7.7 (1.0) |
8.1 (0.8) |
0.025 |
0.004 |
| Extubation failure |
7.5 (1.1) |
7.2 (0.9) |
|
0.182 |
| LF/HF (ln(ratio)) |
|
|
|
|
| Successful extubation |
1.0 (0.3) |
1.0 (0.2) |
0.954 |
|
| Extubation failure | 0.9 (0.1) | 0.9 (0.2) |
aFor comparison between successful extubation and extubation-failure groups.
bFor comparison between spontaneous-breathing trial and postextubation periods within a group.
Data are presented as mean (standard deviation).
HF, high frequency; LF, low frequency; ln, natural logarithm; nu, normalized unit; SBT, spontaneous breathing trial; TP, total power. VLF, very low frequency.
Discussion
This is the largest study to date demonstrating the relation between change of HRV and outcomes of SBT and extubation in critically ill patients undergoing weaning from mechanical ventilation. The two major findings are that first, reduced HRV was significantly associated with SBT failure among patients with their first SBT; second, inability to increase HRV after extubation correlated with extubation failure in patients who passed SBT.
During the weaning process, the use of SBT could impose cardiopulmonary stress on certain patients. Specifically, patients for whom SBT fails are subjected to higher cardiovascular and pulmonary stress than those who succeed in the trial [21-23]. When the stress system is activated, it responds with consequent release of catecholamines due to sympathetic stimulation [24]. Decrease in HRV may reflect elevated sympathetic activity and an impairment in the physiological regulatory and adaptive mechanisms [25,26]. In line with the concepts and the findings of Shen [15], the present study showed reduced HRV in patients with failed SBT. Further, more-reduced HRV was an independent predictor of SBT failure in multivariate analysis. Continuous monitoring of electrocardiography is the routine practice in modern ICU care; thus, HRV measurements should be easily accessible and helpful in evaluating patients receiving SBT. However, real-time analysis of HRV remains challenging, because greater effective lengths of observation are required to provide better spectral resolution. Yet, in this era of rapidly advancing information technology in healthcare, we believe that this drawback will be solved in the near future, and point-of-care monitoring of HRV could be clinically feasible.
Similar to HRV, the rhythmic activity of respiration is not monotonous but is characterized by breath-to-breath variability in the tidal volume, respiratory rate, and inspiratory time of sequential breaths [27]. An inverse relation between breathing variability and respiratory loading has been observed in healthy subjects [28,29]. As expected, in patients undergoing SBT, breathing variability is greater in those successfully weaned from mechanical ventilation [30]. Breathing variability is inversely correlated with HRV in subjects free of cardiovascular and respiratory diseases [31]; however, it seems not to be the case in patients during the weaning process. Further study of simultaneously measuring breathing variability and HRV during ventilator weaning should be carried out to clarify this issue and the role it plays in predicting weaning outcomes.
One major strength in this study is that we, for the first time, assessed change of HRV after extubation. This study found significant association between increased HRV and successful extubation in patients who passed SBT. In other words, incapability to increase HRV suggested a higher probability of extubation failure. Increased HRV indicates health, fitness, and a well-functioning autonomic control mechanism [32]. Therefore, it is anticipated that patients would do better regarding the extubation outcome if they had increase in HRV after extubation. Patients requiring reintubation have a high mortality rate [1], and a direct and specific effect of extubation failure and reintubation on patient outcomes was demonstrated in a recent study [33]. Prophylactic use of noninvasive ventilation may reduce reintubation and mortality rates in patients at high risk for extubation failure [34].
However, high risk for extubation failure is difficult to define. In this study, HRV was measured shortly after extubation, and failure to increase HRV independently predicted the need for reintubation among patients being extubated after successful SBT. Early identification of at-risk patients will allow clinicians to use preventive measures such as noninvasive ventilation support, adjustment of medications, and so on, before the adverse event takes place.
In the present study, dynamic change of TP was the only recognized HRV component associated with both SBT and extubation outcomes. The TP is a net effect of all possible physiological mechanisms contributing to HRV and thus represents the overall variability of the cardiac autonomic nervous system. Of the two major components of power-spectral analysis, HF reflects modulation of parasympathetic activity; and LF indicates sympathetic modulation on the heart, although it may be also associated with parasympathetic contribution [7]. Usually, change of TP is accompanied by corresponding change in either or both of HF and LF, but we did not find such a result. This could be explained by the complex pathophysiologic process of ventilator weaning. This study enrolled a heterogeneous group of critically ill patients, and they should respond differently to the weaning process and have a variety of causes of weaning failure. Thus, the change of certain HRV components may be obscured. Further studies aiming to explore the association between HRV change and mechanisms of weaning failure will make clear the puzzle.
Other than TP, which is a reflection of total HRV, we found that change of VLF was useful in risk stratification for predicting extubation outcome. The VLF contains rhythms from several physiological variables like hormones, temperature, and vasomotion, and depends on parasympathetic outflow [10,35]. Evidence suggests that the power in this range is substantially influenced by physical activity, reflecting the activity of both parasympathetic and renin-angiotensin systems [36]. Recently, the value of VLF measurements has also been proven in risk stratification in patients with multiple organ dysfunction syndrome, because it may represent a viable interorgan communication [37]. In that study, lower VLF was significantly associated with a worse outcome. Accordingly, it is not surprising that increased VLF served as a useful index of patient well-being after extubation in the present study.
Weaning parameters have been held as a holy grail for ICU clinicians to predict weaning outcomes. However, recent studies argued against their utility in general ICU patients, neurocritical care patients, and patients with prolonged mechanical ventilation [38-40]. Similarly, weaning parameters were not associated with SBT or extubation outcomes in our work. Weaning failure is usually multifactorial; thus, weaning parameters that assess a single physiological function may have limited predictive accuracy [16,20]. Several studies have demonstrated poor specificity of these parameters for predicting weaning success [41,42]. Instead, HRV probably represents the complex interplay of multiple physiological inputs to the heart, and it would intuitively be better to predict weaning outcomes, as shown in this study.
HRV can be significantly influenced by various categories of medications; thus, the impact should be considered while interpreting HRV. The use of β2 agonists and anticholinergics were known to affect HRV and were more commonly seen in our patients with failed SBT [43,44]. Because all the measures in our study were completed within hours, and we compared change of HRV between study periods by using a paired t test, the influence of drug effects on HRV could be minimized or even eliminated. Moreover, to reduce this source of error, we tried not to administer possible confounding drugs during HRV measurements. In addition, multivariate analysis in the present study did not show an independent role of these medications on the prediction of SBT outcomes.
Certain limitations of the study must be considered. First, the results obtained must be interpreted with caution, as external factors other than SBT or extubation could have influenced the HRV values. This might hinder appropriate interpretation of the data, even though we tried hard to eliminate these intervening factors. Second, our study was conducted in a single center, and the findings may not apply similarly to other institutions, although incidence rates of SBT and extubation failure were within previously reported ranges [16]. Future multicenter studies would be helpful in this regard. Third, for the accurate measurement of HRV, we excluded patients with arrhythmia or taking antiarrhythmics, and these patients may have different HRV responses to the weaning process. More-sophisticated methods for dealing with arrhythmia during HRV analysis are needed to allow investigation of clinical implications of HRV in this specific patient population. Fourth, the HRV thresholds derived have not been prospectively validated, although the findings were pathophysiologically reasonable and predictable. Undoubtedly, validation with independent data sets will consolidate our results.
Conclusions
Our results provide the first evidence that HRV increased after extubation in patients who succeeded in 1-hour SBT and were successfully extubated. Also, we demonstrated that patients with SBT failure had reduced HRV during the SBT period. This novel analysis of HRV data and outcomes yields a simple, noninvasive method for predicting weaning results. With prospective confirmation of these findings, this analytic method could be easily integrated into current ICU monitoring systems to help clinicians in decision making and to improve patient care. Future research could serve to explore the association of HRV change and mechanisms of weaning failure in critically ill patients.
Key messages
• Heart-rate variability responses to the weaning process vary between patients with different weaning outcomes.
• Measurements of heart-rate variability may be of some practical value in taking care of patients weaning from mechanical ventilation.
Abbreviations
CI: confidence interval; HF: high frequency; HRV: heart-rate variability; ICU: intensive care unit; LF: low frequency; OR: odds ratio; ROC: receiver operating characteristic; SBT: spontaneous breathing trial; TP: total power; VLF: very low frequency
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CTH, YJT, and CJY conceived the study and participated in its design and coordination. CTH, JWL, SYR, and HDW participated in the data analysis and interpretation. CTH drafted the manuscript. CTH, YJT, JWL, SYR, HDW, and CJY participated in the revision of the manuscript. All authors read and approved the final manuscript.
Please see related letter by Van de Louw:http://ccforum.com/content/18/2/426
Contributor Information
Chun-Ta Huang, Email: huangct@ntu.edu.tw.
Yi-Ju Tsai, Email: med0056@mail.fju.edu.tw.
Jou-Wei Lin, Email: jouweilin@gmail.com.
Sheng-Yuan Ruan, Email: syruan@ntu.edu.tw.
Huey-Dong Wu, Email: hdwuntuh@ntu.edu.tw.
Chong-Jen Yu, Email: jefferycjyu@ntu.edu.tw.
References
- Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112:186–192. doi: 10.1378/chest.112.1.186. [DOI] [PubMed] [Google Scholar]
- Meade M, Guyatt G, Cook D, Griffith L, Sinuff T, Kergl C, Mancebo J, Esteban A, Epstein S. Predicting success in weaning from mechanical ventilation. Chest. 2001;120:400S–424S. doi: 10.1378/chest.120.6_suppl.400S. [DOI] [PubMed] [Google Scholar]
- MacIntyre NR, Cook DJ, Ely EW Jr, Epstein SK, Fink JB, Heffner JE, Hess D, Hubmayer RD, Scheinhorn DJ. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120:375S–395S. doi: 10.1378/chest.120.6_suppl.375S. [DOI] [PubMed] [Google Scholar]
- Esteban A, Ferguson ND, Meade MO, Frutos-Vivar F, Apezteguia C, Brochard L, Raymondos K, Nin N, Hurtado J, Tomicic V. et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med. 2008;177:170–177. doi: 10.1164/rccm.200706-893OC. [DOI] [PubMed] [Google Scholar]
- Vassilakopoulos T, Zakynthinos S, Roussos C. Respiratory muscles and weaning failure. Eur Respir J. 1996;9:2383–2400. doi: 10.1183/09031936.96.09112383. [DOI] [PubMed] [Google Scholar]
- Vassilakopoulos T, Zakynthinos S, Roussos C. The tension-time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am J Respir Crit Care Med. 1998;158:378–385. doi: 10.1164/ajrccm.158.2.9710084. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- Perini R, Veicsteinas A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur J Appl Physiol. 2003;90:317–325. doi: 10.1007/s00421-003-0953-9. [DOI] [PubMed] [Google Scholar]
- Malpas SC. Neural influences on cardiovascular variability: possibilities and pitfalls. Am J Physiol Heart Circ Physiol. 2002;282:H6–H20. doi: 10.1152/ajpheart.2002.282.1.H6. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Muller-Werdan U, Hoffmann T, Francis DP, Piepoli MF, Rauchhaus M, Prondzinsky R, Loppnow H, Buerke M, Hoyer D, Werdan K. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit Care Med. 2005;33:1994–2002. doi: 10.1097/01.CCM.0000178181.91250.99. [DOI] [PubMed] [Google Scholar]
- Kero P, Antila K, Ylitalo V, Valimaki I. Decreased heart rate variation in decerebration syndrome: quantitative clinical criterion of brain death? Pediatrics. 1978;62:307–311. [PubMed] [Google Scholar]
- Aarimaa T, Oja R, Antila K, Valimaki I. Interaction of heart rate and respiration in newborn babies. Pediatr Res. 1988;24:745–750. doi: 10.1203/00006450-198812000-00019. [DOI] [PubMed] [Google Scholar]
- Koh J, Brown TE, Beightol LA, Eckberg DL. Contributions of tidal lung inflation to human R-R interval and arterial pressure fluctuations. J Auton Nerv Syst. 1998;68:89–95. doi: 10.1016/S0165-1838(97)00114-8. [DOI] [PubMed] [Google Scholar]
- Frazier SK, Moser DK, Stone KS. Heart rate variability and hemodynamic alterations in canines with normal cardiac function during exposure to pressure support, continuous positive airway pressure, and a combination of pressure support and continuous positive airway pressure. Biol Res Nurs. 2001;2:167–174. doi: 10.1177/109980040100200302. [DOI] [PubMed] [Google Scholar]
- Shen HN, Lin LY, Chen KY, Kuo PH, Yu CJ, Wu HD, Yang PC. Changes of heart rate variability during ventilator weaning. Chest. 2003;123:1222–1228. doi: 10.1378/chest.123.4.1222. [DOI] [PubMed] [Google Scholar]
- Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, Fernandez R, de la Cal MA, Benito S, Tomas R. et al. A comparison of four methods of weaning patients from mechanical ventilation: Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332:345–350. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- Esteban A, Alia I, Gordo F, Fernandez R, Solsona JF, Vallverdu I, Macias S, Allegue JM, Blanco J, Carriedo D. et al. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation: The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1997;156:459–465. doi: 10.1164/ajrccm.156.2.9610109. [DOI] [PubMed] [Google Scholar]
- Esteban A, Alia I, Tobin MJ, Gil A, Gordo F, Vallverdu I, Blanch L, Bonet A, Vazquez A, de Pablo R. et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation: Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1999;159:512–518. doi: 10.1164/ajrccm.159.2.9803106. [DOI] [PubMed] [Google Scholar]
- MacIntyre N. Discontinuing mechanical ventilatory support. Chest. 2007;132:1049–1056. doi: 10.1378/chest.06-2862. [DOI] [PubMed] [Google Scholar]
- Jubran A, Tobin MJ. Pathophysiologic basis of acute respiratory distress in patients who fail a trial of weaning from mechanical ventilation. Am J Respir Crit Care Med. 1997;155:906–915. doi: 10.1164/ajrccm.155.3.9117025. [DOI] [PubMed] [Google Scholar]
- Jubran A, Mathru M, Dries D, Tobin MJ. Continuous recordings of mixed venous oxygen saturation during weaning from mechanical ventilation and the ramifications thereof. Am J Respir Crit Care Med. 1998;158:1763–1769. doi: 10.1164/ajrccm.158.6.9804056. [DOI] [PubMed] [Google Scholar]
- Lemaire F, Teboul JL, Cinotti L, Giotto G, Abrouk F, Steg G, Macquin-Mavier I, Zapol WM. Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology. 1988;69:171–179. doi: 10.1097/00000542-198808000-00004. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Pavlatou M. Exercise as a stress model and the interplay between the hypothalamus-pituitary-adrenal and the hypothalamus-pituitary-thyroid axes. Hormone Metabol Res. 2005;37:577–584. doi: 10.1055/s-2005-870426. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C. et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–570. doi: 10.1161/01.CIR.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- Freeman JV, Dewey FE, Hadley DM, Myers J, Froelicher VF. Autonomic nervous system interaction with the cardiovascular system during exercise. Prog Cardiovasc Dis. 2006;48:342–362. doi: 10.1016/j.pcad.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Tobin MJ, Mador MJ, Guenther SM, Lodato RF, Sackner MA. Variability of resting respiratory drive and timing in healthy subjects. J Appl Physiol. 1988;65:309–317. doi: 10.1152/jappl.1988.65.1.309. [DOI] [PubMed] [Google Scholar]
- Brack T, Jubran A, Tobin MJ. Effect of elastic loading on variational activity of breathing. Am J Respir Crit Care Med. 1997;155:1341–1348. doi: 10.1164/ajrccm.155.4.9105077. [DOI] [PubMed] [Google Scholar]
- Brack T, Jubran A, Tobin MJ. Effect of resistive loading on variational activity of breathing. Am J Respir Crit Care Med. 1998;157:1756–1763. doi: 10.1164/ajrccm.157.6.9704114. [DOI] [PubMed] [Google Scholar]
- Wysocki M, Cracco C, Teixeira A, Mercat A, Diehl JL, Lefort Y, Derenne JP, Similowski T. Reduced breathing variability as a predictor of unsuccessful patient separation from mechanical ventilation. Crit Care Med. 2006;34:2076–2083. doi: 10.1097/01.CCM.0000227175.83575.E9. [DOI] [PubMed] [Google Scholar]
- Anderson DE, McNeely JD, Chesney MA, Windham BG. Breathing variability at rest is positively associated with 24-h blood pressure level. Am J Hypertens. 2008;21:1324–1329. doi: 10.1038/ajh.2008.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wecht JM, Marsico R, Weir JP, Spungen AM, Bauman WA, De Meersman RE. Autonomic recovery from peak arm exercise in fit and unfit individuals with paraplegia. Med Sci Sports Exerc. 2006;38:1223–1228. doi: 10.1249/01.mss.0000227306.34149.ba. [DOI] [PubMed] [Google Scholar]
- Thille AW, Harrois A, Schortgen F, Brun-Buisson C, Brochard L. Outcomes of extubation failure in medical intensive care unit patients. Crit Care Med. 2011;39:2612–2618. doi: 10.1097/CCM.0b013e3182282a5a. [DOI] [PubMed] [Google Scholar]
- Ferrer M, Valencia M, Nicolas JM, Bernadich O, Badia JR, Torres A. Early noninvasive ventilation averts extubation failure in patients at risk: a randomized trial. Am J Respir Crit Care Med. 2006;173:164–170. doi: 10.1164/rccm.200505-718OC. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.CIR.98.6.547. [DOI] [PubMed] [Google Scholar]
- Casadei B, Cochrane S, Johnston J, Conway J, Sleight P. Pitfalls in the interpretation of spectral analysis of the heart rate variability during exercise in humans. Acta Physiol Scand. 1995;153:125–131. doi: 10.1111/j.1748-1716.1995.tb09843.x. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Hoyer D, Hennen R, Heinroth K, Rauchhaus M, Prondzinsky R, Hottenrott K, Buerke M, Muller-Werdan U, Werdan K. Autonomic dysfunction predicts both 1- and 2-month mortality in middle-aged patients with multiple organ dysfunction syndrome. Crit Care Med. 2008;36:967–970. doi: 10.1097/CCM.0B013E3181653263. [DOI] [PubMed] [Google Scholar]
- Ko R, Ramos L, Chalela JA. Conventional weaning parameters do not predict extubation failure in neurocritical care patients. Neurocrit Care. 2009;10:269–273. doi: 10.1007/s12028-008-9181-9. [DOI] [PubMed] [Google Scholar]
- Tanios MA, Nevins ML, Hendra KP, Cardinal P, Allan JE, Naumova EN, Epstein SK. A randomized, controlled trial of the role of weaning predictors in clinical decision making. Crit Care Med. 2006;34:2530–2535. doi: 10.1097/01.CCM.0000236546.98861.25. [DOI] [PubMed] [Google Scholar]
- Huang CT, Yu CJ. Conventional weaning parameters do not predict extubation outcome in intubated subjects requiring prolonged mechanical ventilation. Respir Care. 2013;58:1307–1314. doi: 10.4187/respcare.01773. [DOI] [PubMed] [Google Scholar]
- Jacob B, Chatila W, Manthous CA. The unassisted respiratory rate/tidal volume ratio accurately predicts weaning outcome in postoperative patients. Crit Care Med. 1997;25:253–257. doi: 10.1097/00003246-199702000-00010. [DOI] [PubMed] [Google Scholar]
- Chatila W, Jacob B, Guaglionone D, Manthous CA. The unassisted respiratory rate-tidal volume ratio accurately predicts weaning outcome. Am J Med. 1996;101:61–67. doi: 10.1016/S0002-9343(96)00064-2. [DOI] [PubMed] [Google Scholar]
- Penttila J, Kuusela T, Scheinin H. Analysis of rapid heart rate variability in the assessment of anticholinergic drug effects in humans. Eur J Clin Pharmacol. 2005;61:559–565. doi: 10.1007/s00228-005-0953-2. [DOI] [PubMed] [Google Scholar]
- Coumel P, Hermida JS, Wennerblom B, Leenhardt A, Maison-Blanche P, Cauchemez B. Heart rate variability in left ventricular hypertrophy and heart failure, and the effects of beta-blockade: a non-spectral analysis of heart rate variability in the frequency domain and in the time domain. Eur Heart J. 1991;12:412–422. doi: 10.1093/oxfordjournals.eurheartj.a059910. [DOI] [PubMed] [Google Scholar]


