Abstract
Rates of tuberculosis (TB) are increasing in most west European nations. Patients with TB can be admitted to an ICU for a variety of reasons, including respiratory failure, multiorgan failure and decreased consciousness associated with central nervous system disease. TB is a treatable disease but the mortality for patients admitted with TB to an ICU remains high. Management challenges exist in establishing a prompt diagnosis and administering effective treatment on the ICU with potentially poor gastric absorption and high rates of organ dysfunction and drug toxicity. In this review reasons for ICU admission, methods of achieving a confident diagnosis through direct and inferred methods, anti-tuberculosis treatment (including steroid and other adjuvant therapies) and specific management problems with particular relevance to the intensivist are discussed. The role of therapeutic drug monitoring, judicious use of alternative regimes in the context of toxicity or organ dysfunction and when to suspect paradoxical tuberculosis reactions are also covered. Diagnostic and therapeutic algorithms are proposed to guide ICU doctors in the management of this sometimes complicated disease.
Introduction
Tuberculosis (TB) remains a significant public health problem worldwide, with an estimated 8.7 million cases and 1.4 million deaths from it in 2011 [1]. Immigration patterns, the HIV pandemic and iatrogenic immuno-suppression have made TB a more common disease in western European nations; TB rates in the UK have increased over the past 2 years [2], with a rate of 14.4 cases/100,000 population. Patients with suspected or actual tuberculosis may be on a high dependency unit (HDU)/ICU setting for a variety of reasons. Mortality for patients admitted with active TB and respiratory failure requiring mechanical ventilation is poor, with reported in-hospital mortalities of 33 to 67% [3-5]. It is a treatable disease and a proactive approach with timely intervention is required in the treatment of critically ill TB patients, as delays to starting therapy can be associated with worse survival [6]. TB patients on ICU present special challenges, including obtaining microbiological confirmation, providing effective anti-tuberculosis treatment (ATT) with poor absorption and high rates of organ dysfunction, and apparent deterioration of TB during appropriate treatment (paradoxical reactions). This review describes the reasons patients with TB may be on a HDU/ICU and aims to discuss management with particular relevance to the intensivist and the ICU environment.
Reasons and outcome for ICU admission in tuberculosis patients
TB usually affects the lungs but may present acutely in almost any organ system and mimic other infectious or non-infectious processes [7]. Most studies of TB patients on ICU involve patients with pulmonary TB [6,8]. Common reasons for admission are acute respiratory failure [3,4], and development of multi-organ failure (MOF) [9]; high rates of acute respiratory distress syndrome (ARDS) are seen [10], although post mortem studies suggest that confluent tuberculous bronchopneumonia may mimic ARDS [3]. Neurological deterioration due to tuberculosis meningitis (TBM) is a rarer but important reason for ICU admission. Presentations of TB are myriad and more unusual reasons for ICU admission exist (some of these are listed in Table 1).
Table 1.
Other potential reasons for admission of a patient with tuberculosis to an ICU
| Presentation | Possible cause |
|---|---|
| Massive haemoptysis | Rasmussen aneurysm |
| Cardiogenic shock | Massive pericardial effusion |
| Known tuberculosis patient electively admitted to ICU | Post-thoracic surgery |
| Liver failure | Drug reaction |
| Renal failure | Drug reaction (usually rifampicin) |
| Disseminated intravascular coagulation | Miliary TB |
| Pituitary apoplexy/stroke mimic | Cerebral tuberculoma |
| Airway obstruction | Laryngeal/retropharyngeal TB |
TB, tuberculosis.
Respiratory failure due to pulmonary tuberculosis
Advanced pulmonary TB can cause respiratory failure; the incidence of respiratory failure in hospitalised pulmonary TB patients is about 1.5% [3]. The majority of patients will have abnormal chest X-rays (CXRs) including cavitatory lesions and bilateral infiltrates [8] (Figure 1). Contributing factors such as bacterial pneumonia, chronic obstructive pulmonary disease and malignancy may be present in about 72% of cases [11].
Figure 1.
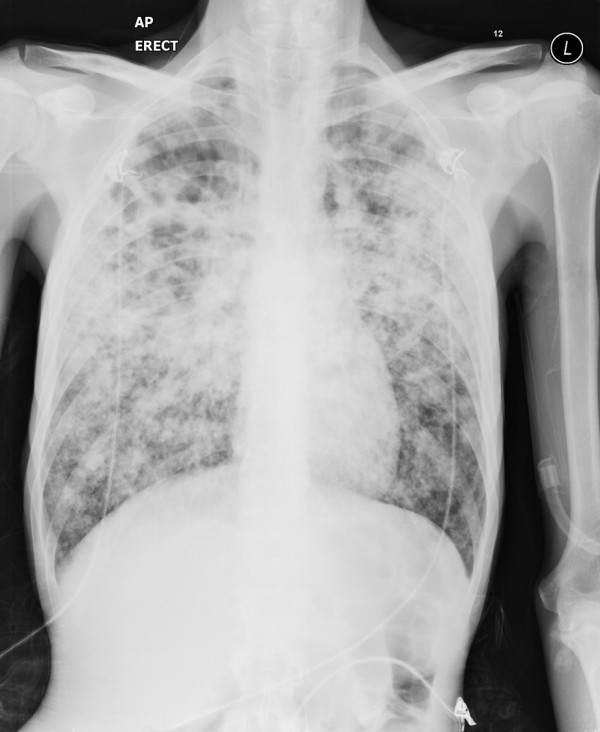
Male patient who presented with type I respiratory failure. Sputum grew fully sensitive Mycobacterium tuberculosis.
Patients with TB requiring ICU care may have high rates of co-morbidities and ICU related complications. In one German study [8] 65.5% of patients had deranged liver function, 12.1% chronic pancreatitis, 8.6% chronic renal failure and 6.9% HIV co-infection. ICU related complications were also common, with nosocomial pneumonia in 67.2% patients, pneumothorax in 13.8%, ARDS in 12.1%, acute renal failure in 12.1% and MOF in 3.4%. Rates of co-existing extra-pulmonary TB can be up to 19 to 22% [6,8].
Delay in appropriate treatment due to lack of early recognition of TB may result in progressive multi-organ involvement and ICU admission [6] and an important opportunity between hospital admission and ICU presentation may exist. Given the high ARDS rates in ventilated patients with TB, standard mechanical ventilation strategies to reduce ARDS may be appropriate, including lower tidal volumes and a conservative fluid strategy [12].
Mortality is high for patients with active TB and respiratory failure; a Canadian study found a significantly higher in-hospital mortality of 69% for patients requiring mechanical ventilation for TB in comparison to ARDS of any cause (56%) and nontuberculous pneumonia (36%) requiring mechanical ventilation [13]. Risk factors for mortality include older age, nosocomial pneumonia, TB destroyed lung, MOF, a duration of symptoms of more than 4 weeks, and an APACHE-II score >20 [14,15].
Miliary tuberculosis
Miliary TB is a form of TB where there is haematological dissemination from focal infection into the blood, leading to seeding of multiple organs with TB bacilli. A minority of patients may present with symptoms of less than four weeks duration. An underlying predisposing condition such as diabetes or steroid use will be present in about a third of patients [16] and immunosuppression due to HIV or iatrogenic reasons may also contribute [17]. The CXR may be initially normal for the first few weeks of the disease. Mortality is about 25% overall [16] but assumed near 100% if untreated. Patients with miliary TB may be more likely to develop ARDS than patients with isolated pulmonary TB and MOF may account for most of the mortality of patients with miliary TB on ICU [10]. A retrospective study by Silva and colleagues [4], which included high rates of extrapulmonary TB (about 63%) and HIV seropositivity, described MOF in over 80% of patients.
Disseminated TB can rarely lead to a septic shock with MOF presentation, sometimes described as Landouzy septicaemia after the original report [18]. This is usually described in association with HIV infection [19], and recently associated with monoclonal antibodies used in the treatment of rheumatological disease [17], but can also occur in patients with no obvious risk factors [20]. Disseminated TB may also cause adrenal insufficiency [21], which should be considered in the context of refractory hypotension or hyponatraemia.
Tuberculosis meningitis and other central nervous system tuberculosis
Tuberculosis of the central nervous system (CNS) occurs in approximately 1% of TB cases, and includes TBM and cerebral tuberculomas. It carries a high mortality and is probably fatal if untreated. It is the reason for about 6 to 18% [4,6] of TB related ICU admissions. In a French case series of TBM admitted to ICU [22], nearly all patients were admitted due to a falling conscious level, 75% required mechanical ventilation and 33% underwent a neurosurgical procedure; 1 year mortality was 65%. Clinical symptoms range from an acute illness mimicking bacterial meningitis to a non-specific illness of fever and headache, with a smaller proportion having cranial nerve palsies [22]. The mean duration of symptoms ranges from 12 to 29 days in most series and approximately one-third will have symptoms lasting less than a week [7]. The majority of the literature on CNS TB comes from the paediatric age group. Cerebral tuberculomas can present as seizures (focal and generalised), as well as with focal neurological signs; occasionally a tuberculous brain abscess can form that may require neurosurgical intervention as well as ATT [23].
As well as a high mortality, TBM poses two additional particular challenges for the intensivist relevant to neurocritical care - hydrocephalus and hyponatraemia. Hydrocephalus is common and develops radiologically in about 77% [24] of cases. It is usually due to communicating hydrocephalus associated with tuberculous exudates in the basal cisterns, but may be non-communicating in a smaller (17 to 25%) proportion of cases - for example, due to obstruction at the outlet foramen of the fourth ventricle or the cerebral aqueduct by oedema or a tuberculoma [25]. Hydrocephalus may increase the intracranial pressure, leading to reduced cerebral perfusion and ischaemia, and in more advanced cases cause brain herniation. Non-communicating hydrocephalus usually requires a neurosurgical procedure such as a shunt procedure or an endoscopic third ventriculostomy [26]. Cases of communicating hydrocephalus should also be discussed with a neurosurgical centre. There may be a role for external ventricular drainage in patients with a low Glasgow coma score and TBM [27]. Hyponatraemia is common in TBM patients and is independently associated with a worse outcome [26]. It is multifactorial, and the syndrome of inappropriate anti-diuretic hormone and cerebral salt wasting probably both play a role [28]. The best approach to TBM-associated hyponatraemia is uncertain, and hypertonic saline, fluid restriction, fludrocortisone and demeclocycline may all have a role depending on the fluid state of the patient [26]. Other aspects of neurocritical care may be relevant for TBM. These additional interventions may include intra-cranial pressure monitoring [25], a higher transfusion threshold [29], and control of fever [30].
Diagnosis of tuberculosis
Culture confirmation of tuberculosis should be obtained where possible [31]; the World Health Organisation (WHO) recommends all patients suspected to have pulmonary TB submit at least two sputum specimens for microscopic examination [32]. Culture not only confirms the diagnosis but also provides drug susceptibility testing. In the UK, 8.4% of isolates were resistant to any first line drug, and 1.6% of isolates were multi-drug resistant TB (MDR TB) [2]; rates of MDR TB are much higher in some countries.
New liquid culture techniques should give a culture result in 2 to 4 weeks [31]. An initial targeted sample for auramine or Ziehl-Neelsen stain is important as this would help confirm TB in the appropriate clinical situation. There is little evidence specific to diagnosis in an ICU setting. TB can present acutely and a high index of suspicion should be had in the majority of ill patients with an abnormal CXR, particularly if risk factors for TB are present. An incidental finding of acid fast bacilli (AFB) in sputum in a patient not suspected to have TB may be due to non-tuberculous mycobacteria (NTM) and correlation with other diagnostic data such as radiology and immune status is advised; 35% of AFB seen in sputum was due to NTM in one study [33]. NTM may cause pulmonary disease especially in the immuno-suppressed or may not be significant. Conversely, an AFB-positive specimen in a patient with a suggestive history, clinical features and radiology should be assumed to be TB unless proved otherwise; TB PCR may have a role if there is doubt.
In patients who are expectorating, serial sputum sampling probably provides a yield similar to bronchoscopy [34]. Microbiological sampling in the non-expectorating patient may be carried out via bronchoscopy if the patient is intubated. Transbronchial biopsy may provide histology to enable rapid diagnosis and may increase the diagnostic yield [35]; this can carry a risk of complications in the mechanically ventilated patient (mainly bleeding and pneumothorax [36]) but in selected patients the risk/ benefit ratio may be favourable, particularly in patients with diffuse lung shadowing or miliary disease. Specialised procedures such as bronchoscopy through a non-invasive ventilation mask may facilitate sampling in a hypoxic non-intubated patient and this is carried out in our institution. Where facilities exist, induced sputum may have a role in some patients and has been shown to have similar or better sensitivities to bronchoscopy (73 and 87% sensitivity, respectively, in smear-negative patients [37]). No data on the utility of respiratory secretions obtained at suction for diagnosis of TB on ICU/HDU exist but transtracheal aspirates have shown a high sensitivity (88%) in smear-negative patients [38]. If there are signs and symptoms reinforced by appropriate investigations consistent with a TB diagnosis, treatment should be started without waiting for culture results, and continued even if subsequent culture results are negative [31].
Non-pulmonary samples
Extrapulmonary TB is common in ICU patients with pulmonary TB and in the context of advanced immuno-suppression associated with HIV [39]; in the latter, visualised AFB may be due to NTM. Whilst culture is central to confirmation of the mycobacterium, PCR for TB may have a role in this patient population to help establish a diagnosis [40]. Most forms of extrapulmonary TB have a lower bacterial load than pulmonary disease and histology/cytology such as pleural biopsies and lymph node aspiration play a more prominent role in diagnosis. A lymphocytic pleural effusion, caseating granulomas or granulomas with Langhan's giant cells are suggestive of TB [31], although other conditions may also cause these histological changes; lymphoma is a main differential for a lymphocytic effusion. A lymphocytic effusion in an ICU setting with a high index of suspicion for TB should trigger ATT. A small proportion (6.7%) of patients, especially if the duration of illness is short, may have a predominately polymorphonuclear leucocytic pleural effusion [41]. Adenosine deaminase levels may have a role in conjuction with other pleural fluid results, although this is a controversial area [42]. Urine culture for TB may be useful in miliary disease, with positive cultures in about 25 to 69% of cases [43,44]. Urine microscopy for AFB is not usually carried out due to high rates of NTM.
Gastric aspirates may be easy to obtain on an ICU. There is little evidence for their utility in diagnosing TB in adults, and none from an ICU setting. The presence of AFB in a gastric aspirate may be due to atypical mycobacteria leading to a low specificity in some studies [45] but not others [46]. A culture rate of about 5% for Mycobacterium tuberculosis (MTB) has been described in gastric aspirates in patients with pulmonary TB [46].
The diagnosis of TBM is based on typical cerebrospinal fluid (CSF) findings (high protein, low glucose and predominately lymphocytic CSF) and confirmation if possible of TB elsewhere in the body. There may be a role for repeat lumbar puncture in 48 hours in atypical CSF findings. Culture of TB in CSF may occur in up to 80% of cases if larger volumes of CSF (>6 ml) are submitted. Repeated lumbar punctures may increase diagnostic yield [26]. TBM is probably fatal if not treated and there should be a low threshold for empirical therapy unless a clear alternative diagnosis is made.
Blood cultures for mycobacteria are particularly useful in the diagnosis of disseminated TB and NTM (especially mycobacterium avium intracellulare) in HIV affected individuals with a low CD4 count, with mycobacteraemia being proportional to the CD4 count [47]. The yield drops to zero with CD4 counts above 300 cells/μl. The utility of mycobacterial blood cultures in non-HIV immunosuppresion is unknown. Bone marrow examination yielded positive results in the majority of patients with miliary TB when it was carried out [16], although the place of bone marrow examination in the absence of haematological abnormalities requires clarification.
Interferon gamma release assays/nucleic acid amplification tests
The past decade has seen the development of interferon gamma release assays (IGRAs) in the diagnosis of latent TB. IGRAs work on the principle of measuring the cytokine interferon gamma released from T cells in response to synthetic antigens that are also found in MTB and have an approximate 90% sensitivity and 99% specificity for diagnosis of latent TB [48]. These antigens are absent from Mycobacterium bovis and most NTM so are not affected by prior Bacillus Calmette-Guérin vaccination or most NTM exposure.
IGRAs cannot distinguish between latent and active TB and therefore may be positive in an individual who has latent TB but another cause for ICU admission. It is recommended that IGRAs should not be used as a routine diagnostic tool in active TB, although there may be a role in using IGRA with other complementary tests when TB is suspected, especially if samples are difficult to obtain [49]. A negative test does not exclude active TB. ICU admission has also been associated with a false negative IGRA [50] and so the role of IGRA in intensive care patients is less clear. There is ongoing research into next generation IGRAs and T-cell-based diagnostic platforms that may overcome some of the current limitations of IGRAs [51].
Nucleic acid amplification tests (NAATs) tend not to be routinely used, and the sensitivity and specificity can be highly variable compared with culture results [40]. A negative result does not exclude TB and a positive result does not give drug sensitivities. One exception is the Xpert MTB/RIF probe, which has shown a 98.2% and 72.5% sensitivity for diagnosing TB in smear-positive and smear-negative patients, respectively [52]. The probe also identifies mutations associated with rifampicin (R) resistance and may have a role where MDR TB is suspected; for this reason WHO suggests this test as a follow on test for patients with smear-negative samples. British recommendations for NAATs currently restrict their role to the case where rapid confirmation of a TB diagnosis in a smear-positive patient would alter management (for instance, if an NTM was suspected). There are few recommendations on the role of NAATs on non-respiratory samples. Data from meta-analysis gives a 56% sensitivity and 98% specificity for CSF, which is similar to microscopy [26]. There may be a role for NAAT on CSF when ATT treatment has been started without a confirmed diagnosis, as mycobacterial DNA may remain detectable for a month after the start of treatment [26].
Radiology
Upper lobe disease on a CXR was shown to increase the odds ratio of TB by 14.6 [53]. There are fewer data specific to ICU patients; small nodular or cavitary patterns on a CXR as well as a duration of illness of more than 2 weeks may be predictive of TB in some studies [54], although other studies fail to identify radiological changes specific for TB on an ICU [55]. In HIV co-infected patients, radiological appearances can vary and cavitation becomes less common as immunosuppression advances. An American study described a normal or near normal CXR in 19% of pulmonary TB patients with a CD4 count less than 200 cells/μl [56]. Computed tomography (CT) scanning may have a role in identifying active TB and allowing differentiation from old fibrotic lesions, with centrilobular nodules and a 'tree in bud' pattern often seen in active disease. Mediastinal lymphadenopathy and cavitation may also raise the suspicion of TB (Figure 2), and miliary shadowing may be present on CT even with a normal chest X-ray [57]. CT chest may help in gathering diagnostic information in an intubated patient where TB is suspected but not confirmed, and also allow targeting of bronchoscopy.
Figure 2.
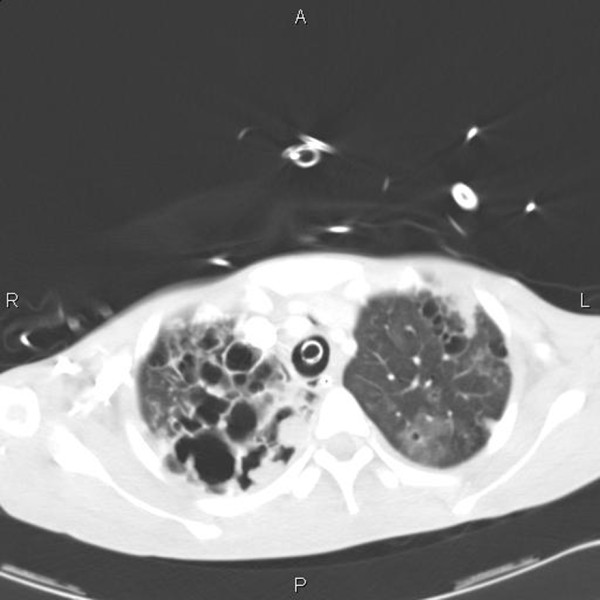
CT scan of thorax of female patient intubated due to respiratory failure. Tuberculosis subsequently cultured from bronchoscopy specimens. Note the cavitation mainly in the right upper lobe.
A proposed diagnostic algorithm for respiratory failure is presented in Figure 3.
Figure 3.
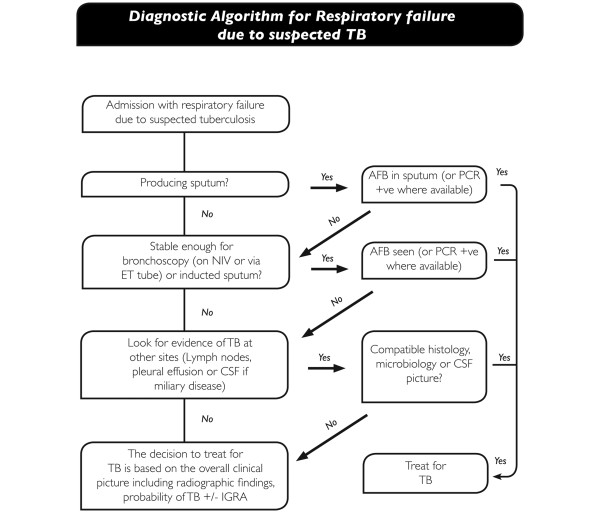
Proposed diagnostic algorithm for respiratory failure due to suspected tuberculosis (TB). AFB, acid fast bacilli; CSF, cerebrospinal fluid; ET, endotracheal; IGRA, interferon gamma release assay; NIV, non-invasive ventilation.
Anti tuberculosis treatment and adjuvant therapies (including paradoxical reactions)
The standard treatment for non-MDR TB involves combination therapy of more than three drugs; R and isoniazid (H) provide a crucial backbone to ATT regimes, allowing shorter courses of 6 to 9 months (1 year for CNS TB) to be efficacious due to their bacteriocidal action. R and H are associated with the potential serious side effects of hepatotoxicity. Two other first line ATT, pyrazinamide (Z) and ethambutol (E), are renally excreted and E may be associated with optic nerve toxicity. Other drugs used to treat TB include fluoroquinolones (for example, moxifloxacin), aminoglycosides (for example, streptomycin or amikacin) and a range of other second line anti-TB drugs such as cycloserine or prothionamide. Not all ATT is available parenterally; parenteral preparations are available for R, H, fluoroquinolones and amino-glycosides. The management of HIV co-infection is complicated and detailed discussion is beyond the scope of this article; guidelines exist for management outside of the HDU/ICU [40]. Principles of HIV/TB co-infection management include awareness of the immune reconstitution inflammatory syndrome (IRIS), and when highly active antiretroviral therapy (HAART) should be started; this depends on the CD4 count but is recommended as soon as is practical in the very immunosuppressed (CD4 count <100 cells/μl) [40].
High rates of hepatic and renal dysfunction in ICU patients with TB provide specific challenges. Patients on the ICU may have uncertain enteral absorption [58]. Subtherapeutic levels of ATT have been associated with a slow clinical response, treatment failure and drug resistance [59]. The incidence of subtherapeutic levels of anti-TB drugs in ICU patients is not known but it is reasonable to have a low threshold for therapeutic drug monitoring in TB patients on the ICU. Our practice is to prefer parenteral therapy in severely ill patients for the initial 72 hours.
Additional bacterial infection can complicate TB-related ICU admissions [8] and a low threshold for additional anti-bactericidal therapy should be present.
Hepatotoxicity
Hepatotoxicity may be associated with older age, malnutrition, alcoholism, HIV or viral hepatitis co-infection [60]. In miliary TB it is important to ensure that the cause of the deranged liver function is not due to TB itself (by imaging or consideration of liver biopsy), as the management of deranged liver function in this context would be management of the TB. International guidelines differ slightly but suggest stopping TB medication if transaminases are more than three to five times the upper limit of normal or there is a bilirubin rise [60,61]. If it is crucial to continue ATT in the short term (for instance in TBM where treatment interruptions are an independent risk factor for death [26]), a combination of relatively non-hepatotoxic drugs, such as an aminoglycoside, E and a fluoroquinolone, could be given [60]. Ethionamide and prothionamide may be an alternative to E as they penetrate the meninges well and do not have the concern of optic nerve toxicity [62].
As R and H are important for TB treatment, they are usually sequentially reintroduced once liver function tests improve with close monitoring, and standardised re-challenge protocols are provided [60,61]. In cases of severe or prolonged hepatotoxicity who have had R and H re-introduced, it may be reasonable not to re-challenge with Z and extend treatment to 9 months [60]. The management of patients with decompensated liver disease and TB is not clear, and the standard regime with close monitoring, or 18 to 24 months of E, a fluoro-quinolone and an aminoglycoside is suggested [60].
Nephrotoxicity
Dose adjustments are required with Z and E with a glomerular filtration rate of less than 30 ml/minute/1.73 m2. Aminoglycosides and cycloserine may require similar dose adjustments [63]. No data exist for patients on continuous renal replacement therapy, and collaboration between intensivists, pharmacists, TB physicians, renal physicians and monitoring of drug levels is appropriate.
Corticosteroids as adjuvant therapy
Corticosteroids inhibit release of inflammatory cytokines, which may help lessen tissue damage and constitutional symptoms. There have been many studies of corticosteroids in TB, including advanced pulmonary TB. The only indications for steroids recognised in most international guidelines are a) TBM, where corticosteroids help reduce risk of death or disability [26,31] and b) tuberculous pericardial effusion where corticosteroids decrease the amount and rate of re-accumulation of tuberculous pericardial effusion [31,64]. The largest trial of steroids in TBM used a reducing dose of dexamethasone, initially given intravenously at a dose of 0.3 to 0.4 mg/kg/day (depending on the severity of the meningitis) for a total of 6 to 8 weeks [65]; British guidelines suggest prednisolone (or equivalent) 20 to 40 mg/day with gradual withdrawal. There are no direct comparisons of the dose or type of steroid in TBM. For tuberculous pleural effusion there is some evidence for adjuvant corticosteroids resulting in a faster resolution of pleural effusion at 4 weeks, but no difference in residual fluid at 8 weeks or death rates between corticosteroid and non-corticosteroid groups [66]. Many trials of corticosteroids for pulmonary TB were carried out in the 1950s to 1960s and suggested a more rapid clinical and radiological improvement compared to control patients, particularly in severe disease [67], but an absence of longer term effects on survival or risk of long-term lung damage. The implications of this for patients with advanced TB and respiratory failure on an ICU are unclear. A recent meta-analysis suggests a non-significant trend towards benefit of steroids in pulmonary TB [68], and a 2008 study [14] suggested a lower mortality rate in patients with pulmonary TB who received corticosteroids but firm conclusions cannot be drawn due to the retrospective nature of the study.
Paradoxical reactions/immune reconstitution inflammatory syndrome
A paradoxical reaction in TB is defined as a clinical or radiological worsening of pre-existing tuberculous lesions or the development of new lesions in patients receiving ATT in the absence of an alternative explanation such as drug resistance, ineffective drug delivery or a secondary diagnosis [69]. Patients with HIV are more likely to develop these reactions, which are referred to in the context of HIV co-infection as IRIS [40]. The aetiology of these reactions is unknown, but in HIV may relate to HAART causing a reconstitution of immunity leading to an immune response to dead bacilli. The incidence in HIV-negative patients is probably between 2 and 23% [70], and about 32 and 36% in HIV-positive patients, and may be more in patients with advanced TB. Paradoxical reactions are usually mild and may manifest as recurrent fever, deterioration in radiological appearances or lymph node inflammation [70] but may cause significant morbidity, including airway obstruction, splenic rupture, or worsening neurology due to new or enlarging intracranial tuberculomas [69,71]. The median time of onset is about 26 days after treatment [70], but can occur months into treatment. Risk factors for IRIS include a low baseline CD4 count, rapid recovery in CD4 numbers and HAART started within 2 months of diagnosis [40]. There are no firm recommendations on how to treat paradoxical reactions but a tapering dose of corticosteroids is reasonable [69]. Thalidomide may have a role in severe CNS TB paradoxical reactions unresponsive to corticosteroids [72], and montelukast has been used in IRIS. An algorithm for treatment of pulmonary TB on the ICU based on the above review is presented in Figure 4.
Figure 4.
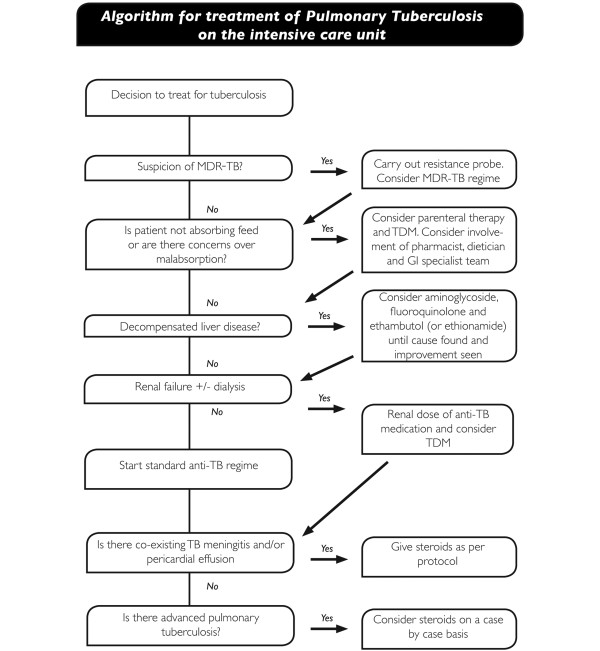
Proposed algorithm for treatment of suspected or actual pulmonary tuberculosis (TB) on the ICU. MDR-TB, multi-drug resistant tuberculosis; TDM, therapeutic drug monitoring.
Conclusion
Respiratory failure and miliary TB are common reasons for admission of patients with TB to an ICU. The evidence base for diagnosis and management of TB specific to an ICU setting is sparse and what may be true for stable TB patients may not translate to an ICU. These patients have a high mortality and high rates of organ dysfunction. Diagnosis of TB if not confirmed prior to ICU admission may be challenging but culture of mycobacterium TB should be attempted, with radiology, histology and possibly IGRAs contributing to the clinical picture. Treatment is complicated by drug toxicity, erratic absorption and organ dysfunction, and therapeutic drug monitoring and judicious use of alternative regimes may help this problem. Corticosteroids have a role in TBM and pericardial TB and may have a role in far advanced pulmonary TB on a case by case basis. The clinician should be alert to paradoxical reactions as a cause of apparent treatment failure or disease progression.
Abbreviations
AFB: acid fast bacilli; ARDS: acute respiratory distress syndrome; ATT: anti-tuberculosis treatment; CNS: central nervous system; CSF: cerebrospinal fluid; CT: computed tomography; CXR: chest X-ray; E: ethambutol; H: isoniazid; HAART: highly active antiretroviral therapy; HDU: high dependency unit; IGRA: interferon gamma release assay; IRIS: immune reconstitution inflammatory syndrome I; MDR TB: multi-drug resistant tuberculosis; NAAT: nucleic acid amplification test; NTM: non-tuberculous mycobacterium; PCR: polymerase chain reaction; R: rifampicin; TB: multi-drug resistant tuberculosis; MOF: multiorgan failure; TB: tuberculosis; TBM: tuberculosis meningitis; WHO: World Health Organisation; Z: pyrazinamide.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Guy Hagan, Email: guy.hagan@nhs.net.
Nazim Nathani, Email: n.nathani@nhs.net.
Acknowledgements
The authors are grateful to Mark McBreen, Head Graphic Designer, Department of Medical Illustration, City Hospital Birmingham, for assistance with layout of the diagnostic algorithms.
References
- WHO. Global tuberculosis report 2012. http://www.who.int/tb/publications/global_report/en
- Pedrazzoli D, Fulton N, Anderson L, Lalor M, Abubakar A, Zenna D. Tuberculosis in the UK: 2012 report. http://www.hpa.org.uk/Publications/InfectiousDiseases/Tuberculosis/1206TBintheUK2012report/
- Levy H, Kallenbach JM, Feldman C, Thorburn JR, Abramowitz JA. Acute respiratory failure in active tuberculosis. Crit Care Med. 1987;17:221–225. doi: 10.1097/00003246-198703000-00008. [DOI] [PubMed] [Google Scholar]
- Silva DR, Menegotto DM, Schulz LF, Gazzana MB, Dalcin PT. Mortality among patients with tuberculosis requiring intensive care:a retrospective cohort study. BMC Infect Dis. 2010;17:54. doi: 10.1186/1471-2334-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SM, Wang TY, Liu WT. Predictive factors for mortality among non-HIV-infected patients with pulmonary tuberculosis and respiratory failure. Int J Tuberc Lung Dis. 2009;17:335–340. [PubMed] [Google Scholar]
- Zahar JR, Azoulay E, Klement E, De Lassence, Lucet JC, Regnier B, Schlemmer B, Bedos JP. Delayed treatment contributes to mortality in ICU patients with severe active pulmonary tuberculosis and acute respiratory failure. Intensive Care Med. 2001;17:513–520. doi: 10.1007/s001340000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob JT, Mehta AK, Leonard MK. Acute forms of tuberculosis in adults. Am J Med. 2009;17:12–17. doi: 10.1016/j.amjmed.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Erbes R, Oettel K, Raffenberg M, Mauch H, Schmidt-Ioanas, Lode H. Characteristics and outcome of patients with active pulmonary tuberculosis requiring intensive care. Eur Resp J. 2006;17:1223–1228. doi: 10.1183/09031936.06.00088105. [DOI] [PubMed] [Google Scholar]
- Sydow M, Schauer A, Crozier TA, Burchardi H. Multiple organ failure in generalised disseminated tuberculosis. Respir Med. 1992;17:517–519. doi: 10.1016/S0954-6111(96)80014-X. [DOI] [PubMed] [Google Scholar]
- Piqueras AR, Marruecos L, Artigas A, Rodriguez C. Miliary tuberculosis and adult respiratory distress syndrome. Intensive Care Med. 1987;17:175–182. doi: 10.1007/BF00254701. [DOI] [PubMed] [Google Scholar]
- Frame RN, Johnson MC, Eichenhorn MS, Bower GC, Popovich J. Active tuberculosis in the medical intensive care unit:a 15-year retrospective analysis. Crit Care Med. 1987;17:1012–1014. doi: 10.1097/00003246-198711000-00005. [DOI] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R. Surviving Sepsis Campaign:International guidelines for management of severe sepsis and septic shock:2012. Crit Care Med. 2013;17:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- Penner C, Roberts D, Kunimoto D, Manfreda J, Long R. Tuberculosis as a primary cause of respiratory failure requiring mechanical ventilation. Am J Respir Crit Care Med. 1995;17:867–872. doi: 10.1164/ajrccm/151.3_Pt_1.867. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Pack KM, Jeong E, Na JO, Oh YM, Lee SD, Kim WS, Kim DS, Kim WD, Shim TS. Pulmonary tuberculosis with acute respiratory failure. Eur Resp J. 2008;17:1625–1630. doi: 10.1183/09031936.00070907. [DOI] [PubMed] [Google Scholar]
- Ryu YJ, Koh WJ, Kang EH, Suh GY, Chung MP, Kim H, Kwon OJ. Prognostic factors in pulmonary tuberculosis requiring mechanical ventilation for acute respiratory failure. Respirology. 2007;17:406–411. doi: 10.1111/j.1440-1843.2006.01007.x. [DOI] [PubMed] [Google Scholar]
- Maartens G, Willcox PA, Benatar SR. Miliary tuberculosis: rapid diagnosis, haematologic abnormalities, and outcome in 109 treated adults. Am J Med. 1990;17:291–296. doi: 10.1016/0002-9343(90)90340-J. [DOI] [PubMed] [Google Scholar]
- Hess S, Hospach T, Nossal R, Dannecker G, Magdorf K, Uhlemann F. Life-threatening disseminated tuberculosis as a complication of TNF-α blockade in an adolescent. Eur J Pediatr. 2011;17:1337–1342. doi: 10.1007/s00431-011-1501-y. [DOI] [PubMed] [Google Scholar]
- Landouzy L. A note of la typho-bacillose. Lancet. 1908;17:1440–1441. doi: 10.1016/S0140-6736(01)47390-9. [DOI] [Google Scholar]
- Barber TW, Craven DE, McCabe WR. Bacteremia due to mycobacterium tuberculosis in patients with human immunodeficiency virus infection, A report of 9 cases and a review of the literature. Medicine (Baltimore) 1990;17:375–383. doi: 10.1097/00005792-199011000-00005. [DOI] [PubMed] [Google Scholar]
- Lim KH, Chong KL. Multiple organ failure and septic shock in disseminated tuberculosis. Singapore Med J. 1999;17:176–178. [PubMed] [Google Scholar]
- Al-Mamari A, Balkhair A, Gujjar A, Ben Abid F, Al-Farqani A, Al-Hamadani A, Jain R. A case of disseminated tuberculosis with adrenal insufficiency. Sultant Qaboos Univ Med J. 2009;17:324–327. [PMC free article] [PubMed] [Google Scholar]
- Verdon R, Chevret S, Laissy JP, Wolff M. Tuberculous meningitis in adults: review of 48 cases. Clin Infect Dis. 1996;17:982–988. doi: 10.1093/clinids/22.6.982. [DOI] [PubMed] [Google Scholar]
- Kumar R, Pandey CK, Bose N, Sahay S. Tuberculous brain abscess:clinical presentation, pathophysiology and treatment (in children) Childs Nerv Syst. 2002;17:118–123. doi: 10.1007/s00381-002-0575-2. [DOI] [PubMed] [Google Scholar]
- Thwaites GE, Macmullen-Price J, Tran TH, Pham PM, Nguyen TD, Simmons CP, White NJ, Tran TH, Summers D, Farrar JJ. Serial MRI to determine the effect of dexamethasone on the cerebral pathology of tuberculous meningitis: an observational study. Lancet Neurol. 2007;17:230–236. doi: 10.1016/S1474-4422(07)70034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figaji AA, Fieggen AG. The neurosurgical and acute care management of tuberculous meningitis: Evidence and current practice. Tuberculosis. 2010;17:393–400. doi: 10.1016/j.tube.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J. British Infection Society guidelies for the diagnosis and treatment of tuberculosis of the central nervous system in adults and childen. J Infection. 2009;17:167–187. doi: 10.1016/j.jinf.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Mathew JM, Rajshekhar V, Chandy MJ. Shunt surgery in poor grade patients with tuberculous meningitis and hydrocephalus:effects of response to external ventricular drainage and other variables on long term outcome. J Neurol Neurosurg Psychiatry. 1998;17:115–118. doi: 10.1136/jnnp.65.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller K, Larsen FS, Bie P, Skinhoj P. The syndrome of inappropriate secretion of antidiuretic hormone and fluid restriction in meningitis-how strong is the evidence? Scand J Infect Dis. 2001;17:13–26. doi: 10.1080/003655401750064022. [DOI] [PubMed] [Google Scholar]
- Figaji AA, Zwane E, Kogels M, Fieggen AG, Argent AC, Le Roux PD, Peter JC. The effect of blood transfusion on brain oxygenation in children with severe traumatic brain injury. Pediatr Crit Care Med. 2010;17:325–331. doi: 10.1097/PCC.0b013e3181b80a8e. [DOI] [PubMed] [Google Scholar]
- Axelrod YK, Diringer MN. Temperature management in acute neurologic disorders. Neurol Clin. 2008;17:585–603. doi: 10.1016/j.ncl.2008.02.005. [DOI] [PubMed] [Google Scholar]
- National Institue for Health and Clinical Excellence. Tuberculosis: Clinical diagnosis and management of tuberculosis, and measures for its prevention and control. http://guidance.nice.org.uk/CG117 [PubMed]
- World Health Organisation. Treatment of Tuberculosis: guidelines for national programmes; WHO/HTM/TB/2009.420. http://www.who.int/tb/publications/tb_treatmentguidelines/en/index.html
- Shu CC, Lee CH, Wang JY, Jerng JS, Yu CJ, Hsueh PR, Lee LN, Yang PC. Nontuberculous mycobacteria pulmonary infection in medical intensive care unit: the incidence, patient characteristics and clinical significance. Intensive Care Med. 2008;17:2194–2201. doi: 10.1007/s00134-008-1221-6. [DOI] [PubMed] [Google Scholar]
- Mase S, Ramsay A, Ng N, Henry M, Hopewell PC, Cunningham J, Urbanczik R, Perkins M, Aziz MA, Pai M. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis a systematic review. Int J Tuberc Lung Dis. 2007;17:485–495. [PubMed] [Google Scholar]
- Jacomelli M, Silva PR, Rodrigues AJ, Demarzo SE, Seicento M, Figueiredo VR. Bronchoscopy for the diagnosis of pulmonary tuberculosis in patients with negative sputum smear microscopy results. J Bras Pneumol. 2012;17:167–173. doi: 10.1590/s1806-37132012000200004. [DOI] [PubMed] [Google Scholar]
- O'Brien JD, Ettinger NA, Shevlin D, Kollef MH. Safety and yield of transbronchial biopsy in mechanically ventilated patients. Crit Care Med. 1997;17:440–446. doi: 10.1097/00003246-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Anderson C, Inhaber N, Menzies D. Comparison of sputum induction with fibre-optic bronchoscopy in the diagnosis of tuberculosis. Am J Resp Crit Care Med. 1995;17:1570–1574. doi: 10.1164/ajrccm.152.5.7582296. [DOI] [PubMed] [Google Scholar]
- Thadepalli H, Rambhatla K, Niden AH. Transtracheal aspiration in diagnosis of sputum-smear negative tuberculosis. JAMA. 1977;17:1037–1040. doi: 10.1001/jama.1977.03280110041019. [DOI] [PubMed] [Google Scholar]
- Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;17:1292–1297. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- Pozniak AL, Coyne KM, Miller RF, Lipman MC, Freedman AR, Omerod LP, Johnson MA, Collins S, Lucas SB. British HIV Association guidelines for the treatment of TB/HIV coinfection. HIV Med. 2011;17:517–524. doi: 10.1111/j.1468-1293.2011.00954.x. [DOI] [PubMed] [Google Scholar]
- Light RW. Update on pleural effusion. Respirology. 2010;17:451–458. doi: 10.1111/j.1440-1843.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- McGrath EE, Anderson PB. Diagnostic tests for tuberculous pleural effusion. Eur J Clin Microbiol Infect Dis. 2010;17:1187–1193. doi: 10.1007/s10096-010-0986-z. [DOI] [PubMed] [Google Scholar]
- Alsoub H, Al Alousi FS. Miliary tuberculosis in Qatar: a review of 32 adult cases. Ann Saudi Med. 2001;17:16–20. doi: 10.5144/0256-4947.2001.16. [DOI] [PubMed] [Google Scholar]
- Kim JH, Langston AA, Gallis HA. Miliary tuberculosis: epidemiology, clinical manifestations, diagnosis and outcome. Rev Infect Dis. 1990;17:583–590. doi: 10.1093/clinids/12.4.583. [DOI] [PubMed] [Google Scholar]
- Chierakul N, Anantasetagoon T, Chaiprasert A, Tingtoy N. Diagnostic value of gastric aspirate smear and polymerase chain reaction in smear-negative pulmonary tuberculosis. Respirology. 2003;17:492–496. doi: 10.1046/j.1440-1843.2003.00503.x. [DOI] [PubMed] [Google Scholar]
- Bahammam A, Choudhri S, Long R. The validity of acid-fast smears of gastric aspirates as an indicator of pulmonary tuberculosis. Int J Tuberc Lung Dis. 1999;17:62–67. [PubMed] [Google Scholar]
- Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;17:1292–1297. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- Pai M, Zwerling A, Menzies D. Systemic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;17:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Protection Agency. Health Protection Agency position statement on the use of Interferon Gamma Release Assay (IGRA) tests for Tuberculosis (TB) http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1204186168242
- Cho K, Cho E, Kwon S, Im S, Sohn I, Song S, Kim H, Kim S. Factors associated with indeterminate and false negative results of QuantiFERON-TB Gold In-Tube Test in active tuberculosis. Tuberc Respir Dis (Seoul) 2012;17:416–425. doi: 10.4046/trd.2012.72.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abubakar I, Stagg HR, Whitworth H, Lalvani A. How should I interpret an interferon gamma release assay result for tuberculosis infection? Thorax. 2013;17:298–301. doi: 10.1136/thoraxjnl-2013-203247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O'Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. Rapid molecular detection of tuberculosis and rifampicin resistance. N Engl J Med. 2010;17:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnivesky JP, Kaplan J, Henschke C, McGinn TG, Crystal RG. Evaluation of clinical parameters to predict mycobacterium tuberculosis in inpatients. Arch Int Med. 2000;17:2471–2476. doi: 10.1001/archinte.160.16.2471. [DOI] [PubMed] [Google Scholar]
- Hui C, Wu CL, Chan MC, Kuo IT, Chiang CD. Features of severe pneumonia in patients with undiagnosed pulmonary tuberculosis in an intensive care unit. J Formos Med Assoc. 2003;17:563–569. [PubMed] [Google Scholar]
- Wu JY, Ku SC, Shu CC, Fan JY, Chen HY, Chen YC, Yu CJ. The role of chest radiography in the suspicion for and diagnosis of pulmonary tuberculosis in intensive care units. Int J Tuberc Lung Dis. 2009;17:1380–1386. [PubMed] [Google Scholar]
- Greenberg SD, Frager D, Suster B, Walker S, Stavropoulos C, Rothpearl A. Active pulmonary tuberculosis in patients with AIDS: spectrum of radiographic findings (including a normal appearance) Radiology. 1994;17:115–119. doi: 10.1148/radiology.193.1.7916467. [DOI] [PubMed] [Google Scholar]
- Lee KS, Im JG. CT in adults with tuberculosis of the chest: characteristic findings and role in management. Am J Roentgenol. 1995;17:1361–1367. doi: 10.2214/ajr.164.6.7754873. [DOI] [PubMed] [Google Scholar]
- Thompson JS. The intestinal response to critical illness. Am J Gastroenterol. 1995;17:190–200. [PubMed] [Google Scholar]
- Kimberling ME, Phillips P, Patterson P, Hall M, Robinson CA, Dunlap NE. Low serum antimycobacterial drug levels in non-HIV-infected tuberculosis patients. Chest. 1998;17:1178–1183. doi: 10.1378/chest.113.5.1178. [DOI] [PubMed] [Google Scholar]
- Saukkonon JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, Peloquin CA, Gordin FM, Nunes D, Stader DB, Bernardo J, Venkataramanan R, Sterling TR. An Official ATS Statement: Hepatotoxicity of Anti-tuberculosis Therapy. Am J Resp Crit Care Med. 2006;17:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- Joint Tuberculosis Committee of the British Thoracic Society. Chemotherapy and management of tuberculosis in the United Kingdom:recommendations. Thorax. 1998;17:536–548. [PMC free article] [PubMed] [Google Scholar]
- Donald PR, Seifart HI. Cerebrospinal fluid concentrations of ethionamide in children with tuberculous meningitis. J Pediatr. 1989;17:483–486. doi: 10.1016/S0022-3476(89)80862-5. [DOI] [PubMed] [Google Scholar]
- Milburn H, Ashman N, Davies P, Doffman S, Drobniewski F, Khoo S, Ormerod P, Ostermann M, Snelson C. Guidelines for the prevention and management of Mycobacterium tuberculosis infection and disease in adult patients with chronic kidney disease. Thorax. 2010;17:559–570. doi: 10.1136/thx.2009.133173. [DOI] [PubMed] [Google Scholar]
- Strang JI, Kakaza HH, Gibson DG, Girling DJ, Nunn AJ, Fox W. Controlled trial of prednisolone as adjuvant in treatment of tuberculous constrictive pericarditis in Transke. Lancet. 1987;17:1418–1422. doi: 10.1016/s0140-6736(87)91127-5. [DOI] [PubMed] [Google Scholar]
- Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, Nguyen QH, Nguyen TT, Nguyen NH, Nguyen TN, Nguyen NL, Nguyen HD, Vu NT, Cao HH, Tran TH, Pram PM, Nguyen TD, Stepniewska K, White NJ, Tran TH, Farrar JJ. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;17:1741–1751. doi: 10.1056/NEJMoa040573. [DOI] [PubMed] [Google Scholar]
- Engel ME, Matchaba PT, Volmink J. Corticosteroids for tuberculous pleurisy. Cochrane Database of Syst Rev. 2007. p. CD001876. [DOI] [PubMed]
- Dooley DP, Carpenter JL, Rademacher S. Adjunctive corticosteroid therapy for tuberculosis: a critical reappraisal of the literature. Clin Infect Dis. 1997;17:872–887. doi: 10.1086/515543. [DOI] [PubMed] [Google Scholar]
- Critchley JA, Young F, Orton L, Garner P. Corticosteroids for prevention of mortality in people with tuberculosis:a systemic review and meta-analysis. Lancet Infect Dis. 2013;17:223–237. doi: 10.1016/S1473-3099(12)70321-3. [DOI] [PubMed] [Google Scholar]
- Bloch S, Wickremasinghe M, Wright A, Rice A, Thompson M, Kon OM. Paradoxical reactions in non-HIV tuberculosis presenting as endobronchial obstruction. Eur Resp J. 2009;17:295–299. doi: 10.1183/09059180.00003709. [DOI] [PubMed] [Google Scholar]
- Cheng SL, Wang HC, Yang PC. Paradoxical response during anti-tuberculosis treatment in HIV negative patients with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2007;17:1290–1295. [PubMed] [Google Scholar]
- Nicolls DJ, King M, Holland D, Bala J, del Rio C. Intracranial tuberculomas developing while on therapy for pulmonary tuberculosis. Lancet Infect Dis. 2005;17:795–801. doi: 10.1016/S1473-3099(05)70299-1. [DOI] [PubMed] [Google Scholar]
- Schoeman JF, Fieggen G, Seller N, Mandelson M, Hartzenberg B. Intractable intracranial tuberculous infection responsive to thalidomide:report of four cases. J Child Neurol. 2006;17:301–308. doi: 10.1177/08830738060210040801. [DOI] [PubMed] [Google Scholar]


