Abstract
Continuous glucose monitoring (CGM) systems are an emerging technology that allows frequent glucose measurements to monitor glucose trends in real time. Their use as a diagnostic tool is still developing and appears to be promising. Combining intermittent glucose self-monitoring (SGM) and CGM combines the benefits of both. Significant improvement in the treatment modalities that may prevent the progress of prediabetes to diabetes have been achieved recently and dictates screening of high risk patients for early diagnosis and management of glycemic abnormalities. The use of CGMS in the diagnosis of early dysglycemia (prediabetes) especially in high risk patients appears to be an attractive approach. In this review we searched the literature to investigate the value of using CGMS as a diagnostic tool compared to other known tools, namely oral glucose tolerance test (OGTT) and measurement of glycated hemoglobin (HbA1C) in high risk groups. Those categories of patients include adolescents and adults with obesity especially those with family history of type 2 diabetes mellitus, polycystic ovary syndrome (PCO), gestational diabetes, cystic fibrosis, thalassemia major, acute coronary syndrome (ACS), and after renal transplantation. It appears that the ability of the CGMS for frequently monitoring (every 5 min) glucose changes during real-life settings for 3 to 5 days stretches the chance to detect more glycemic abnormalities during basal and postprandial conditions compared to other short-timed methods.
Keywords: Continuous glucose monitoring system, Glycated hemoglobin (HbA1C), obesity, oral glucose tolerance test, thalassemia
INTRODUCTION
Continuous glucose monitoring (CGM) systems are an emerging technology that allows frequent glucose measurements (every 5 min) and the ability to monitor glucose trends in real time. Although these devices are currently expensive and mildly disturbing to use, there is vast potential for their use in both the research and clinical territories. Continuous glucose monitoring provides maximal information about shifting blood glucose levels throughout the day and facilitates the making of optimal treatment decisions for the diabetic patient. For the treating clinician, CGM has the potential to improve detection of hyperglycemic excursions as well as asymptomatic hypoglycemia and the data to improve management of glucose levels in diabetes patients.[1,2,3,4]
The difference between an intermittent and a continuous monitor for monitoring blood glucose is similar to that between “a regular camera and a continuous security camera” for monitoring an important situation. A regular camera takes discrete, accurate snapshots and a continuous security camera, on the other hand takes multiple frames and produces too much information for each frame to be studied carefully. Using an intermittent monitor, blood glucose can be measured accurately during selected time periods, whereas with a continuous monitor, too many data are generated to study all data points over longer periods and during real life. In addition, an intermittent blood glucose monitor requires effort to operate, whereas a continuous monitor does not. Combining intermittent and continuous monitoring and combines the benefits of both, specifically increasing the accuracy of measuring blood glucose for long periods of real life.[1,2,3,4,5] Although CGMS becomes a routine part of diabetes management, still its use as a diagnostic tool is not fully determined.
Prediabetes: Risk factors, diagnosis and possibility of early interference to prevent diabetes
Prediabetes is a condition in which blood glucose levels are higher than normal but not high enough for a diagnosis of diabetes. It is the state in which some but not all of the diagnostic criteria for diabetes are met. It is often described as the “gray area” between normal blood sugar and diabetic levels. Prediabetes includes impaired glucose tolerance (IGT) and/or impaired fasting glucose (IFG), depending on the test used to measure blood glucose levels. Having prediabetes puts one at higher risk for developing type 2 diabetes. People with prediabetes are also at increased risk for developing cardiovascular disease. However, discovering prediabetes gives the potential of early management and preventing the progress to full-diabetic state.[6,7]
Impaired fasting glycaemia or impaired fasting glucose (IFG) refers to a condition in which the fasting blood glucose is elevated above what is considered normal levels but is not high enough to be classified as diabetes mellitus. It is considered a pre-diabetic state, associated with insulin resistance and increased risk of cardiovascular pathology, although of lesser risk than impaired glucose tolerance (IGT). IFG sometimes progresses to type 2 diabetes mellitus. There is a 50% risk over 10 years of progressing to overt diabetes. Many newly identified IFG patients progress to diabetes in less than three years. IFG is also a risk factor for mortality. IGT is also a pre-diabetic state associated with insulin resistance and increased risk of cardiovascular pathology. IGT may precede type 2 diabetes mellitus by many years and is also a risk factor for mortality.[7]
World Health Organization (WHO) criteria for impaired fasting glucose differs from the (American Diabetes Association) ADA criteria, because the normal range of glucose is defined differently. Fasting glucose levels 100 mg/dL (5.5 mmol/L) and higher has been shown to increase complication rates significantly. However, WHO opted to keep its upper limit of normal at under 110 mg/dL for fear of causing too many people to be diagnosed as having impaired fasting glucose, whereas the ADA lowered the upper limit of normal to a fasting glucose under 100 mg/dL.[8,9]
Diagnosis of Prediabetes
Prediabetes is diagnosed when:
Fasting blood sugar (glucose) level of 110 to 125 mg/dL (6.1 mmol/L to 6.9 mmol/L) - WHO criteria or 100 to 125 mg/dL (5.6 mmol/L to 6.9 mmol/L) - ADA criteria and/or
Two hour glucose tolerance test after ingesting the standardized 75 gm glucose solution the blood sugar level of 140 to 199 mg/dL (7.8 to 11.0 mmol/L) and/or
Glycated hemoglobin between 5.7 and 6.4%.
Levels above these limits would be a diagnosis for diabetes.[10,11]
Known risk factors for prediabetes and type 2 diabetes include family history of diabetes, obesity, and the presence of a cluster of risk factors (dyslipidemia, hypertension, visceral obesity, IGT, abnormal coagulation factors, endothelial dysfunction) referred to collectively as the insulin resistance (metabolic) syndrome.[12,13]
Prediabetes: Progression to diabetes and prevention
A major goal in the treatment of diabetes in youth is in the area of prevention. Because most of the morbidity and mortality in diabetes arises from long-term complications, early detection and prevention would be expected to have a tremendous beneficial human, social, medical and economic impact. With these considerations in mind, it is logical to intervene early with measures targeted to reverse specific pathophysiological defects present in the prediabetes state and that ultimately lead to development of overt diabetes.[14,15,16,17]
Approximately 40-50% of individuals with IGT will progress to type 2 diabetes over their lifetime. In addition, investigators in the Diabetes Prevention Trial of Type 1 Diabetes (DPT-1) have detected a group of subjects with type 1 diabetes who are asymptomatic, have normal (<6.1 mmol/l) or impaired fasting glucose (6.1-<7.0 mmol/l), but have 2-h glucose values >11.1 mmol/l on their oral glucose tolerance tests (OGTT).
Therefore, treatment of high-risk individuals with IGT to prevent or delay type 2 and type 1 diabetes has important medical, economic, social and human implications. Weight loss, is effective in reducing the conversion of IGT to type 2 diabetes, but sometimes difficult to achieve and maintain. Pharmacological treatment of IGT with oral antidiabetic agents that improve insulin sensitivity and preserve β-cell function (the characteristic pathophysiological abnormalities present in IGT and type 2 diabetes) uniformly have been shown to prevent progression of IGT to type 2 diabetes. Diabetes Prevention Program (DPP) reduced the development of type 2 diabetes by 31% and has been recommended by the American Diabetes Association (ADA) for treating high-risk individuals with IGT. The glucagon-like peptide-1 analogs, which augment insulin secretion, preserve β-cell function, and promote weight loss, also would be expected to be efficacious in preventing the progression of IGT to type 2diabetes. Because individuals in the upper tertile of IGT are maximally/near-maximally insulin resistant have lost 70 to 80% of their β-cell function and have an ∼10% incidence of diabetic retinopathy, pharmacological intervention, in combination with diet plus exercise, should be instituted.[18,19]
Type 1 diabetes mellitus (T1D) is characterized by immune-mediated pancreatic β-cell destruction. Thus, the early identification of glycemic abnormalities as well as increased levels of inflammatory markers may provide an important clue.[15] Most of the studies countered the diabetes process by immuno modulation and/or enhancement of β-cell proliferation and regeneration. An initial pilot trial of a tumor necrosis factor α (TNF-α) binding agent, Entanercept, showed benefit in preserving C-peptide production in 18 young people with newly diagnosed T1D. Similarly, β-cell function was shown to be preserved in children receiving the lower of two doses of ingested human recombinant interferon-α (hrINF-α) in comparison with subjects who received placebo. A future larger trial of both of these agents will be of interest. In this review of the literature we have tried to select recent publications that offer some insight into these issues in pediatric patients with T1D.[20,21]
De Fronzo et al., studied 602 subjects with IGT, 115 with normal glucose tolerance (NGT) and 50 with IFG.[22] Insulin secretion and insulin sensitivity indices and the acute insulin response (AIR) (0-10 min) and were measured with the frequently sampled intravenous glucose tolerance test (FSIVGTT) in a subset of participants. Results strongly suggested that progressive β cell failure is the main determinant of progression of NGT to IGT. Suzuki et al., supported this view.[23] Accordingly, targeted pathophysiologic therapy based on oral OGTT-derived measures of insulin sensitivity and β-cell function can be implemented in general internal medicine and endocrine practice and is associated with marked improvement in glucose tolerance and reversion of prediabetes to normal glucose tolerance in more than 50% of patients.[24]
Accuracy of a CGMS in the detection of blood glucose during OGTT tolerance test and relation to HbA1C
Chen Z evaluated the accuracy of CGMS during OGTT in the detection of blood glucose changes in glucose in 49 out-patients with fasting plasma glucose of 3.9-11.0 mmol/L.[25] The correlation indices between CGMS values and the VBG values during the entire OGTT and in phases of stable, rapidly rising and falling glucose levels were 0.928, 0.901, 0.924 and 0.902, respectively (P < 0.001). CGMS values showed good consistency with venous blood glucose values measured during OGTT confirming the efficiency of CGMS in detection the rapidly changing blood glucose during OGTT.[25]
Zhou et al., studied the relationship between HbA1c, and 24 h mean blood glucose (MBG) from CGM (3 days) in 742 Chinese subjects with different glucose tolerance status.[26] OGTT classified the participants as non-diabetic subjects, including those with normal glucose regulation (NGR; n = 121) and impaired glucose regulation (IGR; n = 209), or newly diagnosed type 2 diabetes (n = 343). The levels of HbA1c and 24 h MBG significantly increased with presence of glucose intolerance (NGR < IGR < type 2 diabetes; both, P < 0.001). When HbA1c was 6.5%, the mean calculated 24 h MBG was 7.2 mmol/L and when HbA1c was 7.0%, the mean 24 h MBG was 7.8 mmol/L mg/dL). This study provided the reference data of the relationship between HbA1c and CGM in Chinese subjects.[26]
He et al., investigated 50 non-obese people with normal glucose tolerance (NGT, 23 to 68 years old), normal blood pressures and lipid profile using a CGMS for three days 72 h.[27] The 48 h MBG, mean amplitude of glycemic excursions (MAGE), largest amplitude of glycemic excursions (LAGE), postprandial peak glucose (PPG), postprandial glucose excursion (PPGE), mean of postprandial glucose excursion (MPPGE), and absolute means of daily differences (MODD) were measured. The CGMS values were significantly correlated with the capillary glucose measurements (r = 0.761, P < 0.005). The post-breakfast post-prandial glycemic excursions (PPGE) were lower than those of post-lunch and post-dinner (P = 0.01 and P = 0.05). In 95% of the daytime, the glucose levels fluctuated between 4.1 and 8.8 mmol/L, and 78% of the participants (n = 39) had hyperglycemia (BG > 7.8 mmol/L) and 10% (n = 5) had asymtomatic hypoglycemia (BG < 2.8 mmol/L). This study suggested that CGMS tests may be important for detecting asymptomatic hyperglycemia and hypoglycemia. The NGT people have exhibited abnormal blood glucose values in CGMS, revealing problems in people with normal range of blood glucose.
Glucose variability and development of diabetes complications
The deficiency in islet β cell secretion and insulin sensitivity, the two important pathophysiological mechanisms of diabetes, are responsible for glycemic disorders. Glucose variability that can be monitored by CGMS could be an independent risk factor for diabetes development and complications in addition to average glucose. Chen et al., studied four groups with different OGTT response ranging from: Normal glucose regulation (NGR, n = 47) to (IGM, n = 52) to diabetes by isolated 2-h glucose (DM2h, n = 62) to DM (n = 60).[28] Groups were monitored using the CGMS for consecutive 72 h. The multiple parameters of glycemic variability included the standard deviation of blood glucose (SD), mean of blood glucose (MBG), high blood glucose index (HBGI), mean of daily differences (MODD) and mean amplitude of glycemic excursions (MAGE). Results showed that the respective values of MBG, HGBI, CONGA1, MODD and MAGE were all increased progressively (all P < 0.05), while their oral disposition indices were decreased progressively (P < 0.05). In addition, SD, MBG, HGBI, MODD and MAGE were all negatively associated with the oral disposition index in each group (all P < 0.05).
Wang et al., studied three groups including: 53 subjects with IGR, 56 DM-2 patients and 53 NGT.[29] Using CGMS for three consecutive days, 22% of NGT and 33.9% of IGR individuals experienced blood glucose ≥11.1 mmol/l; 49.1% of NGT, 50.9% of IGR and 30.8% of DM-2 participants had hypoglycemic episodes (CGM values <3.9 mmol/l). The IGR and DM-2 groups had greater SDBG, LAGE and MAGE (P < 0.001) compared with the NGT group. Significantly greater MODD and PPGEs were found in the DM-2 groups than in the IGR and NGT groups (P < 0.001). The DM-2 patients had higher 72-MBG and glucose levels overnight than the NGT and IGR subjects (P < 0.001). These data supported the view that increased glycemic variability parameters are consistently associated with decreased oral disposition index in subjects across the range of glucose tolerance from the NGR to IGM to DM2h to DM group.
Qian et al., showed that Chinese NGT subjects with a 1-h plasma glucose > or = 11.1mmol/l are characterized by metabolic abnormalities, which may be caused by the impairment of early insulin release rather than aggravated insulin resistance.[30]
Reference values of glycemic parameters for CGM
To establish reference values of glycemic parameters for continuous glucose monitoring, Zhou et al., studied 48 individuals with normal glucose regulation using CGMS for 3 days.[31] Indexes in CGMS were analyzed, including mean level of 24 h blood glucose (BG) values (24 h MBG) and its standard deviation (SDBG), percentage of time above 7.8 mmol/L or below 3.9 mmol/L, area under the curve (AUC) of BG above 5.6 mmol/L, the largest amplitude of glycemic excursions (LAGE), mean amplitude of glycemic excursions (MAGE) and absolute means of daily differences (MODD).[31] The results are reported in the Table 1.
Table 1.
The upper limits of indexes for continuous glucose monitoring reported by Zhou et al.[31]

Kang et al., described some CGMS criteria of the deterioration of glucose regulation, the intraday and day-to-day blood glucose excursions become increasingly fluctuant.[32] The amplitude of glycemic excursion is lower in the NGT group than in the T2DM group, however, the frequency of glycemic excursion is higher in the NGT subject than in the T2DM subjects. The glucose excursion profile of the IGR subjects is between the NGR and T2DM subjects.[3] The characteristics of glucose excursion of the IGT group are similar to those of the T2DM group, and the characteristics of the IFG group are similar to those of the NGT group.[4] The loss of postprandial glycemic control precedes evident deterioration in fasting phase of IGR.
Rodbard reviewed a systematic approach to the interpretation of continuous glucose monitoring data for use by clinical researchers and clinicians to evaluate the quality of glycemic control, glucose variability including within-and between-day variability, the day-to-day stability of glycemic patterns, and changes in response to therapy.[33,34]
Collectively, these results supported that the CGMS profile can reflect the overall BG control and the feature of glycemic excursions in detail.
Use of CGMS to diagnose early glycemic abnormalities in high risk patients
Morbid obesity
Childhood obesity is epidemic in developed countries and is accompanied by an increase in the prevalence of type 2 diabetes (T2DM). In obese adolescents pancreatic beta-cells may not be able to cope with insulin resistance leading to hyperglycemia and T2DM.
Brufani et al., screened 510 overweight/obese (8 to 13 years) children and adolescents. Using OGTT, IGT was the most frequent alteration (11.2%), with a higher prevalence in adolescents than in children.[35] Silent T2DM was identified in two adolescents (0.4%). HOMA-IR and glucose-stimulated insulin levels were higher in patients with IGT than individuals with normal glucose tolerance. Multivariate analysis showed that age, fasting glucose, and insulin resistance influenced independently plasma glucose at 120 min of OGTT. Morandi et al., screened 817 obese children and adolescents (8-18.4 years) and reported 39 children (4.7%) with IGT.[36] Cambuli et al., during 1-year observational study conducted on 736 (535 overweight/obese and 201 normal weight) diagnosed IFG in (7.66%), IGT in 3.18% and T2D in 0.18%.[37] These reports supported high prevalence of glucose metabolism alterations among children and adolescents with overweight. Elawwa et al., assessed, 72-h CGM, OGT and calculated homeostatic model assessment (HOMA), and the quantitative insulin sensitivity check index (QUICKI) in 13 adolescents with simple obesity (BMI SDS = 4 ± 1.06). OGTT revealed 3 cases (23%) with IFG (FG > 5.6 mmol/L), 4 cases (30%) with (IGT: 2 h blood glucose >7.8 < 11 mmol/L), and none with diabetes.[38] Using CGMS, IFG was detected in 4 cases, the maximum serum blood glucose 2h or more after meal) was >7.8 and <11.1 mmol/L (IGT) in 9 children (69%) and >11.1 mmol/L (diabetes) in one case (7.6%). No glycemic abnormality was detected using HbA1C (5.7 ± 0.3%). 11/13 patients had HOMA values >2.6 and QUICKI values <0.35 denoting insulin resistance. Beta cell mass percent (B%) and insulin sensitivity values (IS) denoted insulin resistance with hyper-insulinaemia and preserved beta cell mass.
First-degree relatives of individuals with type 2diabetes and obesity are at increased risk of developing hyperglycemia. Jensen et al., investigated 531 first-degree relatives with no known history of diabetes (aged 44.1 ± 0.7 years; BMI 29.0 ± 0.3 kg/m2).[39] They identified diabetes in 19% (n = 100), and impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) in 36% (n = 191). Thus, only 45% (n = 240) had normal glucose tolerance (NGT). Both insulin resistance and impaired beta-cell function are associated with impaired glucose metabolism in all ethnic groups.
Madhu et al., investigated glycemic profiles by CGMS in 20 obese first-degree relatives of type 2 diabetes mellitus patients and found 3 (15%) NGT, 7 (35%) IFG, and 8 (40%) IGT subjects showed excursions in the diabetes range, whereas 18 (90%) NGT and 17 (85%) pure IFG subjects showed excursions in the IGT range.[40]
These studies denoted that in obese children and adolescents, CGMS is superior to OGTT and HbA1C in detecting early glycemic abnormalities.
Polycystic ovary syndrome
Tao et al., investigated 20 PCOS women with normal glucose tolerance and 20 age-matched healthy women with normal menstruation using OGTT and CGMS.[41] Results showed the followings: (A) The 1-hour and 3-hour plasma glucose levels during OGTT of the PCOS group were higher than those of the control group. The fasting insulin level and insulin levels 30, 60, 120 and 180 min after the glucose uptake during OGTT of the PCOS group were all significantly higher than those of the control group. (B) The daily MBG, SDBG, and MAGE of the PCOS group were all similar to those of the control group. (C) The peaking time of post-breakfast plasma glucose level of the PCOS group was significantly longer than that of the control group. CGMS diagnosed an abnormal mode of daily glucose change characterized by a delayed peak of post-breakfast plasma glucose level.
Cystic fibrosis
A long pre-diabetic phase of abnormal glucose tolerance is described in subjects with cystic fibrosis (CF) since childhood. Under certain circumstances, OGTT screening, used to diagnose CF-related diabetes (CFRD), fails to reveal early glucose tolerance abnormalities. In this situation, CGM could be a useful tool for evaluating early abnormalities of glucose tolerance in CF patients. Seventeen children with CF and 14 controls were investigated (mean age 13.3 years). All subjects underwent OGTT and CGMS monitoring for 3 days. On the basis of OGTT, children were classified as NGT, IGT, IGT plus at least one glucose value above 200 mg/dl at intermediate OGTT points (IGTC 200) and CFRD. HbA1c, glucose area under the curve, insulin sensitivity, and insulinogenic and disposition indexes were also measured. Subjects with CF underwent another OGTT after 2.5 years. Baseline OGTT revealed 3/17 (7.6%) children with CF with at least one glucose value above 200 mg/dl, while CGMS revealed (35.3%) with glucose excursions above 200 mg/dl. None of the controls showed glucose over 200 mg/dl either at OGTT or at CGMS. At the 2.5-year follow-up OGTT, all the six subjects who had diabetic glucose excursion (i.e. O200 mg/dl) at baseline CGMS presented IGTC200 or CFRD. In logistic regression analysis, CGMS diabetic excursion was the strongest predictor of IGTC200 and CFRD. This study suggested that CGMS is a useful tool to predict glucose metabolism derangements in children affected by CF.[42]
Thirty eight children with CF >10 years, with normal OGTT were grouped into 2 groups according to the max CGM glucose value obtained during 3-days CGMS monitoring. Group 1 < 11 mmol/l (n = 26) and Group 2 (n = 12) ≥11 mmol/l. Group 2 patients exhibited a significant impairment in lung function: FEV1, 68.2 ± 25.6% vs. 87.3 ± 17%, P = 0.01 and FVC, 86.1 ± 19.4% vs. 99.3 ± 13.4%, P = 0.021, as well as a higher rate of colonization by P. aeruginosa: 83.3% vs. 44%, P = 0.024.[43] It appears that CGM may aid the early diagnosis of CFRD when considered in conjunction with the OGTT.[44]
Thalassemia major
Both insulin deficiency and resistance are reported in patients with β-thalassemia major (BTM). The use of continuous blood glucose monitoring (CGM), among the different methods for early detection of glycemic abnormalities.[45,46,47]
Rimondi et al., investigated the value of using CGMS in six TM patients with abnormal glucose homeostasis after an oral glucose tolerance test (OGTT).[45] Two-hour OGTT glucose values and CGMS fluctuations were classified as normal if <7.8 mmol/l, impaired if 7.8 to 11.1 mmol/l, diabetic if >11.1 mmol/l. The TM patients spent from 1 to 23% of the time with a blood glucose level from 7.8 to 11.1 mmol/l. In five patients the CGMS confirmed the impaired glucose tolerance diagnosis and in one patient the CGMS excluded the diagnosis of diabetes. Similarly, Soliman et al., studied 16 adolescents with TM (19.75 ± 3 years) were investigated using OGTT and CGMS for 3 days.[46] Using OGTT 25% had IFG, 12.5% had IGT and one of them had diabetes. Using CGMS the maximum (postprandial) 25% had diabetes and 56% had IGT. Ferritin concentrations were correlated significantly with the fasting BG and the 2-h blood glucose levels in the OGTT as well as with the average BG recorded by CGM. Collectively, these results demonstrate that the CGMS is a useful method to detect the variability of glucose fluctuations and offers the opportunity for better assessment of glucose homeostasis in TM patients. Proper and early iron chelation or the use of intensive iron chelation in those with high iron load the new oral chelators has been shown to decrease or reverse these glycemic abnormalities.[47]
Gestational diabetes
Bühling et al., investigated 8 non-pregnant (NP) and 56 pregnant women (17 dietary-treated gestational diabetics (GDM), 15 women with impaired glucose tolerance (IGT), and 24 non-diabetic pregnant women (NDP) using a 72-h measurement with the CGMS.[48] Self-monitored blood glucose measurements (SMGS), performed 30 min before and 120 min after each meal, were compared to the duration of hyperglycemia monitored by the continuous glucose monitoring system. Using the SGMS 88% of the NP and 54% of the NDP had no measurements above 6.7 mmol/l. The CGMS detected more frequent and longer durations of hyperglycemia in GDM compared to NDP women than the SMBG and women with an IGT exhibited higher glucose levels than patients with gestational diabetes.
Acute coronary syndrome
Intensive monitoring for hyperglycemia is essential during care for ACS. Radermecker et al., studied the occurrence and the distribution of glucose excursions >7.8 mmol/l by CGMS in 21 non-diabetic patients admitted with acute coronary syndrome (ACS) without baseline hyperglycaemia.[49] CGMS data disclosed time spent >7.8 mmol/l in 17 patients, whereas only seven of them showed at least one capillary blood glucose test value above the threshold for the same time period. Glucose excursions were detectable earlier from CGMS data. Hyperglycaemia was detected most frequently in the morning, more than 2 h after breakfast. CGM disclosed early and frequent hyperglycaemia in non-diabetic patients with ACS compared to SGMS.
After renal transplantation
New onset of diabetes after transplantation (NODAT) and IGT are well-known complications of immunosuppressive therapy after transplantation being a risk factor for cardiovascular disease affecting patient and graft survival. Therefore, early identification and treatment are of high importance. Pasti et al., examined the glycemic homeostasis of 20 renal-transplanted children using routine laboratory tests and CGMS. Six patients (30%) had IGT, and one patient had NODAT (5%).[50] CGMS analysis showed that IGT patients had higher “lowest glucose” level, and the incidence of hypoglycemic episodes was significantly lower compared with patients with normal OGTT result. In IGT patients, glucose variability tended to be lower. Furthermore, in the whole patient cohort, glucose variability significantly decreased with time after transplantation.
Wojtusciszyn et al., assessed blood glucose (BG) levels immediately following kidney transplantation in non-diabetic subjects to explore their relationship to later graft outcomes and occurrence.[51] They reported that early hyperglycemia detected by CGMS or SGMS is frequent and may herald post-transplantation diabetes mellitus and graft failure.
In critically ill and in perioperative, intraoperative and postoperative periods
Given the demonstrated benefit of euglycemia in critically ill patients as well as the risk for hypoglycemia during their management. Piper et al., used CGMS in comparison with laboratory venous glucose values in 20 children up to 36 m of age who were undergoing cardiac bypass surgery.[52] They reported effective glucose monitoring by the CGMS and a mean absolute relative deviation of 17.6% for all comparisons. In addition, the sensor performance was not affected by body temperature, inotrope dose, or body-wall edema. Poljakova et al., explored the feasibility of subcutaneous continuous glucose monitoring (CGM) in 20 perioperative settings and to evaluate the perioperative development of glycaemia in persons with diabetes mellitus or impaired glucose tolerance by means of CGM.[53] The Wilcoxon signed-rank test revealed no significant difference between sensor and laboratory analyser values. They reported that subcutaneous CGM is safe and offered detailed insight into glucose homeostasis in the dynamic perioperative intraoperative and first postoperative day [Figure 1].
Figure 1.
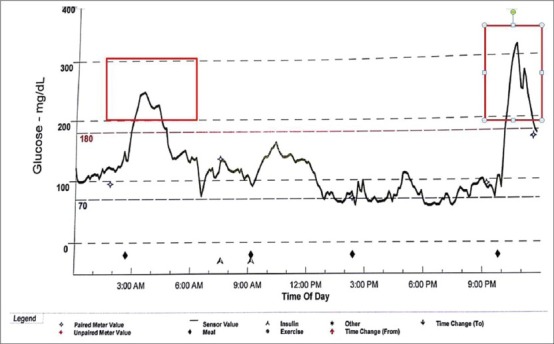
A CGM tracing one thalassemic patient with normal glucose tolerance (0 h = 98 mg/dl, 2h = 124 mg/dl) and twice abnormal (diabetic) postprandial blood glucose (red rectangles)
CONCLUSIONS
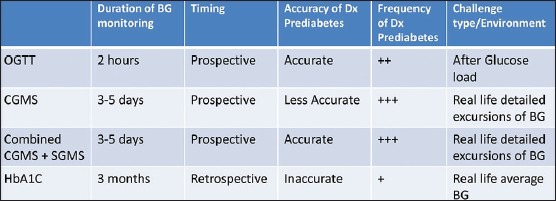
Those categories of patients include adolescents and adults with: Obesity with family history of type 2 diabetes mellitus, polycystic ovary syndrome, cystic fibrosis, thalassemia major, gestational diabetes, acute coronary syndrome and after renal transplantation. The use of CGMS in the diagnosis of early dysglycemia (prediabetes) especially in high risk patients appears to promising and in many occasions superior to other known diagnostic modalities namely oral glucose tolerance test and measurement of HbA1C. Its use in combination with intermittent glucose monitoring adds to its accuracy and reliability [Table 2].
Table 2.
Comparison of different diagnostic methods for detecting (Dx) glycemic abnormalities
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Gross TM, Bode BW, Einhorn D, Kayne DM, Reed JH, White NH, et al. Performance evaluation of the MiniMed continuous glucose monitoring system during patient home use. Diabetes Technol Ther. 2000;2:49–56. doi: 10.1089/152091500316737. [DOI] [PubMed] [Google Scholar]
- 2.Potts RO, Tamada JA, Tierney MJ. Glucose monitoring by reverse iontophoresis. Diabetes Metab Res Rev. 2002;18(Suppl 1):S49–53. doi: 10.1002/dmrr.210. [DOI] [PubMed] [Google Scholar]
- 3.Bode B, Gross K, Rikalo N, Schwartz S, Wahl T, Page C, et al. Alarms based on real-time sensor glucose values alert patients to hypo- and hyperglycemia: The guardian continuous monitoring system. Diabetes Technol Ther. 2004;6:105–13. doi: 10.1089/152091504773731285. [DOI] [PubMed] [Google Scholar]
- 4.Høi-Hansen T, Pedersen-Bjergaard U, Thorsteinsson B. Reproducibility and reliability of hypoglycaemic episodes recorded with Continuous Glucose Monitoring System (CGMS) in daily life. Diabet Med. 2005;22:858–62. doi: 10.1111/j.1464-5491.2005.01552.x. [DOI] [PubMed] [Google Scholar]
- 5.Klonoff DC. Continuous Glucose Monitoring Roadmap for 21st century diabetes therapy. Diabetes Care. 2005;28:1231–9. doi: 10.2337/diacare.28.5.1231. [DOI] [PubMed] [Google Scholar]
- 6.Nichols GA, Hillier TA, Brown JB. Progression from newly acquired impaired fasting glusose to type 2 diabetes. Diabetes Care. 2007;30:228–33. doi: 10.2337/dc06-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab) Circulation. 2007;116:151–7. doi: 10.1161/CIRCULATIONAHA.106.685628. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO Consultation. Part 1. Diagnosis and classification of diabetes mellitus. [Retrieved on 2007 May 29]; [Google Scholar]
- 9.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28(Suppl 1):S37–42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 10.Jellinger PS. What You Need to Know about Prediabetes. Power of Prevention, American College of Endocrinology, May. 2009. [Last Accessed on 2014 Feb 02]. Available from: http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf .
- 11.American Association of Clinical Endocrinologists Board of Directors and American College of Endocrinologists Board of Trustees. American Association of Clinical Endocrinologists/American College of Endocrinology statement on the use of hemoglobin A1c for the diagnosis of diabetes. Endocr Pract. 2010;16:155–6. doi: 10.4158/EP.16.2.155. [DOI] [PubMed] [Google Scholar]
- 12.De Fronzo RA, Abdul-Ghani M. Type 2 diabetes can be prevented with early pharmacological intervention. Diabetes Care. 2011;34(Suppl 2):S202–9. doi: 10.2337/dc11-s221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabet Med. 2007;24:137–44. doi: 10.1111/j.1464-5491.2007.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The American College of Endocrinology (ACE) and the American Association of Clinical Endocrinologists (AACE) have developed lifestyle intervention guidelines for preventing the onset of type 2 diabetes. Endocr Pract. 2011;17(Suppl 2):S1–10. [Google Scholar]
- 15.National Diabetes Information Clearinghouse (NDIC) Available from: http://diabetes.niddk.nih.gov/dm/pubs/preventionprogram22-2-2014 .
- 16.Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP. Preventing type 2 diabetes mellitus: Room for residual risk reduction after lifestyle changes? Curr Pharm Des. 2010;16:3939–47. doi: 10.2174/138161210794455085. [DOI] [PubMed] [Google Scholar]
- 17.Davies MJ, Tringham JR, Troughton J, Khunti KK. Prevention of Type 2 diabetes mellitus. A review of the evidence and its application in a UK setting. Diabet Med. 2004;21:403–14. doi: 10.1111/j.1464-5491.2004.01176.x. [DOI] [PubMed] [Google Scholar]
- 18.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenbaum CJ, Cuthbertson D, Krischer JP Disease Prevention Trial of Type I Diabetes Study Group. Type I diabetes manifested solely by 2-h oral glucose tolerance test criteria. Diabetes. 2001;50:470–6. doi: 10.2337/diabetes.50.2.470. [DOI] [PubMed] [Google Scholar]
- 20.Shalitin S, Peter Chase H. Diabetes technology and treatments in the paediatric age group. Int J Clin Pract Suppl. 2011;170:76–82. doi: 10.1111/j.1742-1241.2010.02582.x. [DOI] [PubMed] [Google Scholar]
- 21.De Fronzo RA, Abdul-Ghani MA. Preservation of β-cell function: The key to diabetes prevention. J Clin Endocrinol Metab. 2011;96:2354–66. doi: 10.1210/jc.2011-0246. [DOI] [PubMed] [Google Scholar]
- 22.De Fronzo RA, Banerji MA, Bray GA, Buchanan TA, Clement S, Henry RR, et al. ACT NOW Study Group. Determinants of glucose tolerance in impaired glucose tolerance at baseline in the Actos Now for Prevention of Diabetes (ACT NOW) study. Diabetologia. 2010;53:435–45. doi: 10.1007/s00125-009-1614-2. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, Fukushima M, Usami M, Ikeda M, Taniguchi A, Nakai Y, et al. Factors responsible for development from normal glucose tolerance to isolated post challenge hyperglycemia. Diabetes Care. 2003;26:1211–5. doi: 10.2337/diacare.26.4.1211. [DOI] [PubMed] [Google Scholar]
- 24.Armato J, De Fronzo RA, Abdul-Ghani M, Ruby R. Successful treatment of prediabetes in clinical practice: Targeting insulin resistance and β-cell dysfunction. Endocr Pract. 2012;18:342–50. doi: 10.4158/EP11194.OR. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Shen J, Xu LL, Fu XJ, Li JM, Ma YY. Accuracy of a continuous glucose monitoring system in detection of blood glucose during oral glucose tolerance test. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31:1256–8. [PubMed] [Google Scholar]
- 26.Zhou J, Mo Y, Li H, Ran X, Yang W, Li Q, et al. Relationship between HbA1c and continuous glucose monitoring in Chinese population: A multicenter study. PLoS One. 2013;8:e83827. doi: 10.1371/journal.pone.0083827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He LP, Wang C, Zhong L, Yang YZ, Long Y, Zhang XX, et al. Glycemic excursions in people with normal glucose tolerance in Chengdu. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:704–7. [PubMed] [Google Scholar]
- 28.Chen T, Xu F, Su JB, Wang XQ, Chen JF, Wu G, et al. Glycemic variability in relation to oral disposition index in the subjects with different stages of glucose tolerance. Diabetol Metab Syndr. 2013;23:38. doi: 10.1186/1758-5996-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Lv L, Yang Y, Chen D, Liu G, Chen L, et al. Glucose fluctuations in subjects with normal glucose tolerance, impaired glucose regulation and newly diagnosed type 2 diabetes mellitus. Clin Endocrinol (Oxf) 2012;76:810–5. doi: 10.1111/j.1365-2265.2011.04205.x. [DOI] [PubMed] [Google Scholar]
- 30.Qian L, Fu X, Xu L, Zheng S, Zhou W, Wang X, et al. Metabolic characteristics of subjects with normal glucose tolerance and 1-h hyperglycaemia. Clin Endocrinol (Oxf) 2008;69:575–9. doi: 10.1111/j.1365-2265.2008.03209.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Jia WP, Yu M, Yu HY, Bao YQ, Ma XJ, et al. The reference values of glycemic parameters for continuous glucose monitoring and its clinical application. Zhonghua Nei Ke Za Zhi. 2007;46:189–92. [PubMed] [Google Scholar]
- 32.Kang Y, Lu JM, Sun JF, Li CL, Wang XL, Zhang XQ, et al. Characteristics of glycemic excursion in different subtypes of impaired glucose intolerance. Zhonghua Yi Xue Za Zhi. 2009;89:669–72. [PubMed] [Google Scholar]
- 33.Rodbard D. Interpretation of continuous glucose monitoring data: Glycemic variability and quality of glycemic control. Diabetes Technol Ther. 2009;11(Suppl 1):S55–67. doi: 10.1089/dia.2008.0132. [DOI] [PubMed] [Google Scholar]
- 34.Rodbard D. New and improved methods to characterize glycemic variability using continuous glucose monitoring. Diabetes Technol Ther. 2009;11:551–65. doi: 10.1089/dia.2009.0015. [DOI] [PubMed] [Google Scholar]
- 35.Brufani C, Ciampalini P, Grossi A, Fiori R, Fintini D, Tozzi A, et al. Glucose tolerance status in 510 children and adolescents attending an obesity clinic in Central Italy. Pediatr Diabetes. 2010;11:47–54. doi: 10.1111/j.1399-5448.2009.00527.x. [DOI] [PubMed] [Google Scholar]
- 36.Morandi A, Maschio M, Marigliano M, Miraglia Del Giudice E, Moro B, Peverelli P, et al. Screening for impaired glucose tolerance in obese children and adolescents: A validation and implementation study. Pediatr Obes. 2014;9:17–25. doi: 10.1111/j.2047-6310.2012.00136.x. [DOI] [PubMed] [Google Scholar]
- 37.Cambuli VM, Incani M, Pilia S, Congiu T, Cavallo MG, Cossu E, et al. Oral glucose tolerance test in Italian overweight/obese children and adolescents results in a very high prevalence of impaired fasting glycaemia, but not of diabetes. Diabetes Metab Res Rev. 2009;25:528–34. doi: 10.1002/dmrr.980. [DOI] [PubMed] [Google Scholar]
- 38.El Awwa A, Soliman A, Al-Ali M, Yassin M, De Sanctis V. Continuous glucose monitoring, oral glucose tolerance, and insulin-glucose parameters in adolescents with simple obesity. Georgian Med News. 2012;210:47–53. [PubMed] [Google Scholar]
- 39.Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE American Diabetes Association GENNID Study Group. Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes. 2002;51:2170–8. doi: 10.2337/diabetes.51.7.2170. [DOI] [PubMed] [Google Scholar]
- 40.Madhu SV, Muduli SK, Avasthi R. Abnormal glycemic profiles by CGMS in obese first-degree relatives of type 2 diabetes mellitus patients. Diabetes Technol Ther. 2013;15:461–5. doi: 10.1089/dia.2012.0333. [DOI] [PubMed] [Google Scholar]
- 41.Tao MF, Zhu JP, Zhou J, Lu W, Qin W, Teng YC, et al. Insulin release and daily glucose change in polycystic ovary syndrome women with normal glucose tolerance. Zhonghua Yi Xue Za Zhi. 2009;89:659–63. [PubMed] [Google Scholar]
- 42.Schiaffini R, Brufani C, Russo B, Fintini D, Migliaccio A, Pecorelli L, et al. Abnormal glucose tolerance in children with cystic fibrosis: The predictive role of continuous glucose monitoring system. Eur J Endocrinol. 2010;162:705–10. doi: 10.1530/EJE-09-1020. [DOI] [PubMed] [Google Scholar]
- 43.Leclercq A, Gauthier B, Rosner V, Weiss L, Moreau F, Constantinescu AA, et al. Early assessment of glucose abnormalities during continuous glucose monitoring associated with lung function impairment in cystic fibrosis patients. J Cyst Fibros. 2013;pii:S1569–1993. doi: 10.1016/j.jcf.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 44.ISPAD Clinical Practice Consensus Guidelines 2009 Compendium. Management of cystic fibrosis-related diabetes in children and adolescents. Pediatr Diabetes. 2009;10(Suppl 12):S43–50. [Google Scholar]
- 45.Rimondi F, Banin P, Gamberini MR, De Sanctis V. The continuous glucose monitoring system (CGMS) in patients with beta-thalassemia major and impaired glucose homeostasis: Preliminary results. Pediatr Endocrinol Rev. 2008;6(Suppl 1):S190–2. [PubMed] [Google Scholar]
- 46.Soliman AT, Yasin M, El-Awwa A, De Sanctis V. Detection of glycemic abnormalities in adolescents with beta thalassemia using continuous glucose monitoring and oral glucose tolerance in adolescents and young adults with β-thalassemia major: Pilot study. Indian J Endocrinol Metab. 2013;17:490–5. doi: 10.4103/2230-8210.111647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farmaki K, Tzoumari I, Pappa C, Chouliaras G, Berdoukas V. Normalisation of total body iron load with very intensive combined chelation reverses cardiac and endocrine complications of thalassaemia major. Br J Haematol. 2010;148:466–75. doi: 10.1111/j.1365-2141.2009.07970.x. [DOI] [PubMed] [Google Scholar]
- 48.Bühling KJ, Kurzidim B, Wolf C, Wohlfarth K, Mahmoudi M, Wäscher C, et al. Introductory experience with the continuous glucose monitoring system (CGMS; Medtronic Minimed) in detecting hyperglycemia by comparing the self-monitoring of blood glucose (SMBG) in non-pregnant women and in pregnant women with impaired glucose tolerance and gestational diabetes. Exp Clin Endocrinol Diabetes. 2004;112:556–60. doi: 10.1055/s-2004-830399. [DOI] [PubMed] [Google Scholar]
- 49.Radermecker RP, Sultan A, Piot C, Remy AS, Avignon A, Renard E. Continuous glucose monitoring as a tool to identify hyperglycaemia in non-diabetic patients with acute coronary syndromes. Diabet Med. 2009;26:167–70. doi: 10.1111/j.1464-5491.2008.02643.x. [DOI] [PubMed] [Google Scholar]
- 50.Pasti K, Szabo AJ, Prokai A, Meszaros K, Peko N, Solyom R, et al. Continuous glucose monitoring system (CGMS) in kidney-transplanted children. Pediatr Transplant. 2013;17:454–60. doi: 10.1111/petr.12106. [DOI] [PubMed] [Google Scholar]
- 51.Wojtusciszyn A, Mourad G, Bringer J, Renard E. Continuous glucose monitoring after kidney transplantation in non-diabetic patients: Early hyperglycaemia is frequent and may herald post-transplantation diabetes mellitus and graft failure. Diabetes Metab. 2013;39:404–10. doi: 10.1016/j.diabet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Poljakova I, Elsikova E, Chlup R, Kalabus S, Hasala P, Zapletalova J. Glucose sensing module-is it time to integrate it into real-time perioperative monitoring? An observational pilot study with subcutaneous sensors. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2013;157:346–57. doi: 10.5507/bp.2013.049. [DOI] [PubMed] [Google Scholar]
- 53.Piper HG, Alexander JL, Shukla A, Pigula F, Costello JM, Laussen PC, et al. Real-time continuous glucose monitoring in pediatric patients during and after cardiac surgery. Pediatrics. 2006;118:1176–84. doi: 10.1542/peds.2006-0347. [DOI] [PubMed] [Google Scholar]


