Abstract
Aim of the study:
To evaluate semen parameters and to assess serum FSH, LH, Testosterone (T) concentrations before and 12 weeks after intravenous iron therapy (800-1200 mg elemental iron therapy - IVI) in adults with iron-deficiency anemia (IDA).
Materials and Methods:
We studied 11 eugonadal adults with IDA, aged 40 ± 5 years, due to defective intake of iron. Anemia was diagnosed when hemoglobin (Hb) was equal or below 10 g/dl. Serum iron, total iron-binding capacity (TIBC) and ferritin concentrations confirmed the diagnosis of IDA. Basal serum concentrations of FSH, LH, and T were measured. Semen parameters were evaluated before and 6-7 weeks after IVI therapy.
Results:
After IVI therapy and correction of anemia, a significant increase of Hb from 8.1 ± 1.17 g/dL to 13.1 ± 0.7 g/dL was observed and was associated with an increase of T (from 12.22 ± 1.4 nmol/L to 15.9 ± 0.96 nmol/L; P < 0.001), FSH (from 2.82 ± 0.87 to 3.82 ± 1.08 IU/L; P = 0.007), and LH (from 2.27 ± 0.9 to 3.82 ± 1.5 IU/L; P = 0.0002). Total sperm count (TSC) increased significantly from 72 ± 17.5 million/ml to 158 ± 49 million/mL (P < 0.001), rapid progressive sperm motility (RPM) increased from 22 ± 9.4 to 69 ± 30 million/ml (P < 0.001), and sperms with normal morphology (NM) increased from 33 ± 5 to 56 ± 7 million/ml (P < 0.001). Increment in Hb concentration was correlated significantly with LH, FSH, and T concentrations after IVI (r = 0.69 and r = 0.44, r = 0.75, respectively; P < 0.01). The increment in serum T was correlated significantly with increments in the TSC and total sperm motility and RPM (r = 0.66, 0.43, and 0.55, respectively; P < 0.001) but not with gonadotrophin levels.
Conclusion:
Our study proved for the first time, to our knowledge, that correction of IDA with IVI is associated with significant enhancement of sperm parameters and increased concentrations of serum LH, FSH, and T. These effects on spermatogenesis are reached by an unknown mechanism and suggest a number of pathways that need further human and/or experimental studies.
Keywords: FSH, gonadotrophins, iron-deficiency anemia, iron therapy, LH, sperm parameters, testosterone
INTRODUCTION
Iron deficiency is one of the most common disorders affecting humans, and iron-deficiency anemia (IDA) continues to represent a major public health problem worldwide. There are an estimated 3.5 billion iron-deficient people worldwide, the vast majority in developing countries.[1]
Anemia imposes a hypoxic environment in different organs and tissues including the testes. Men and adult male animals produce a great amount of sperm every day, indicating that spermatogenesis in the seminiferous tubules of the testis occurs under a high proliferation rate, which demands considerable oxygen consumption. However, blood vessels are located exclusively between the tubules, and oxygen reaches the lumen of the seminiferous tubules only by diffusion. The seminiferous epithelium was speculated to operate on the verge of hypoxia because the testicular PO2 is relatively low, oxygen extraction is highly related to the metabolic demands of spermatogenesis, oxygen diffusion distance is comparatively long, and the testis has little capacity to increase total blood flow.[2,3,4,5,6,7]
Previous studies indicated that hypoxia reduces the fertility of male rats,[2] rhesus monkeys,[3] and men[4,5] from lowland by decreasing sperm count and sperm motility in semen. Morphological studies in animals reveal that hypoxia causes damage to the germinal epithelium, folding of the basal membrane, degeneration, sloughing of spermatogenic cells in lumen of seminiferous tubule and lipid droplet deposition in Sertoli cells and spermatogonia degeneration with chromatin margination under electron microscopy.[2,3] Hypoxia also increases interstitial space of testis, which extends the oxygen diffusion distance and would impair oxygen delivery to germ cells. It makes germ cells more susceptible to damage, which is confirmed by degenerative germ cells in hypoxic rats under light and electron microscopy. These changes in testes point out to the “regeneration” of spermatogenesis to hypoxia.[6,7]
In chronic hemolytic anemia, namely thalassemia and sickle cell disease, the pituitary gonadal axis, spermatogenesis and fertility may be adversely affected by different factors including the hypoxic effect of anemia.[8,9,10,11,12,13,14]
Recently, some data showed that blood transfusion produce significant acute changes in the hormonal milieu of thalassemic patients with obvious increase in testosterone secretion and evident improvement of sperm parameters.[15,16]
In patients with anemia secondary to chronic renal failure, correction of the anemia using recombinant human erythropoietin (rHuEPO) therapy for 12 months is associated with increased T level that may affect fertility and spermatogenesis.[17] However, in all these diseases, other factors rather than the anemic hypoxia may interfere with spermatogenesis e.g. iron toxicity and effect of chelation therapy in thalassemia, vaso-occlusive attacks in sickle cell disease, and the effect of uremic toxins in CRF.[15,16,17] To the best of our knowledge, the effect of isolated IDA on spermatogenesis and fertility has not been studied.
The purpose of this study was to evaluate the effect of intravenous iron therapy (IVI) on the pituitary gonadal axis and sperm parameters in 11 adult males with IDA.
MATERIALS AND METHODS
Eleven adult eugonadal males with IDA, aged 40 ± 5.5 years, referred to Hematology Clinic of Al Aml Hospital, Doha (Qatar) for IDA, were studied.
All of them had iron-deficiency anemia (Hb level equal or below 10 g/dl), mainly due to defective intake of iron. IDA was diagnosed by blood tests that included complete blood count, serum ferritin, iron, total iron-binding capacity, and/or transferrin. Testing for blood in the stool was positive in 7/11 of them due to chronic gastritis (5/7) and/or peptic ulceration (2/7). The other 4/11 were not taking enough iron in their food (vegetarians, eating mainly starchy food, and soft drinks). None had bleeding piles or colonic or rectal polyps; none had other systemic disorders or malignancy. None had recent or old history of excessive bleeding or intake of non-steroidal anti-inflammatory drugs. All patients were not taking any medications that can affect their semen parameters. Stool analysis for Helicobacter pylori was positive in 5/7. Screening for mal-absorption was negative. Renal function and liver enzymes were in the normal ranges. None of our patients had other chronic or acute diseases before and during IVI therapy.
Anthropometric measurements included: Weight, height, body mass index (BMI). All patients were fully sexually developed. Sexual maturation was assessed according to Tanner et al. maturity stages,[18] and testicular volume was assessed by Prader orchidometer. Seven were married, and 5 of them had children. Four were single.
The following investigations were performed before and 12 weeks after treatment with IVI (800-1200 mg elemental iron therapy) for the first time and medical treatment for chronic gastritis and peptic ulcer disease (7/11):
Semen analysis (total count, morphology, motility) at least after 3 days of abstinence
Measurement of serum levels of FSH, LH, T and sex hormone-binding globulin (SHBG) in a fasting venous sample at 8 AM.
Conventional semen analysis was carried out using manual procedures and light microscopy, in the central hospital laboratory, according to the last WHO guidelines.[19] The semen analyzer was blinded to patients’ names and diagnosis. Two semen samples were taken from each patient 4 days apart, and the average of the two readings was calculated for different semen parameters.
The following nomenclature was used to define the semen quality:
Normozoospermia: A total number of spermatozoa, and a percentage of progressively motile and morphologically normal spermatozoa, equal to or above the lower reference limit
Oligospermia: A total sperm concentration per ejaculate below the lower reference limit (5th percentile: 33 million)
Asthenozoospermia: A percentages of total sperm motility (TPM) and sperm progressive motility below the lower reference limits (5th percentile: 38% and 31%, respectively)
Oligoasthenozoospermia was defined as a total number of spermatozoa and percentage of motile spermatozoa below the lower reference limits
Teratozoospermia: A percentage of normal morphology spermatozoa below the lower reference limit (5th percentile: 3%)
Asthenoteratozoospermia: A percentage of both progressively motile and morphologically normal spermatozoa below the lower reference limits.
The normal serum levels of T, LH, and FSH in our central lab for age subjects aged 30-45 years are: Total testosterone = 21.4 ± 5.9 nmol/L, LH = 4.5 ± 1.2 IU/L, FSH = 3.2 ± 0.9 IU/L). All hormone levels were measured by radioimmunoassay (Diagnostic Products Corporation, USA). Serum hormone levels were measured in one assay after completion of the study to avoid critical between-assay variability.
The ethical committee of Hamad Medical Center (HMC) approved the study, and informed consents were obtained from all the patients.
The results are presented as mean ± standard deviation (SD), and the paired t-test was applied to analyze the data when normally distributed, and Wilcoxon test was applied when the data were not normally distributed. Linear regression equation was used to study the relations between different variables. (P < 0.05 was chosen as the limit of significance.)
RESULTS
We investigated the effects of IVI on gonadotrophin-testicular axis and spermiogram in 11 adult males with IDA who had spontaneous pubertal development (Tanner's stage 5) with normal secondary sex characteristics, testicular volume (17.7 ± 4.5 ml), and spontaneous spermatogenesis.
After IVI therapy and correction of anemia, a significant increase of Hb from 8.1 ± 1.17 g/dL to 13.1 ± 0.7 g/dL was observed and was associated with increased T (from 12.22 ± 1.4 nmol/L to 15.9 ± 0.96 nmol/L; P < 0.001), FSH (from 2.82 ± 0.87 to 3.82 ± 1.08 IU/L; P = 0.007), and LH (from 2.27 ± 0.9 to 3.82 ± 1.5 IU/L; P = 0.0002). SHBG did not differ before versus after treatment (65 ± 12 versus 69 ± 24 nmol/L).
Total sperm count (TSC) increased significantly from 72 ± 17.5 million/ml to 158 ± 49 million/mL (P < 0.001), sperm volume increased from 2.3 ± 0.6 ml to 2.6 ± 0.7 ml (P = 0.045), rapid progressive sperm motility (RPM) increased from 22 ± 9.4 to 69 ± 30 million/ml (P < 0.001), and sperms with normal morphology (NM) increased from 33 ± 5 to 56 ± 7 million/ml (P < 0.001). [Table 1 and 2]
Table 1.
Hormonal data before versus after intravenous iron replacement therapy in iron-deficiency anemia
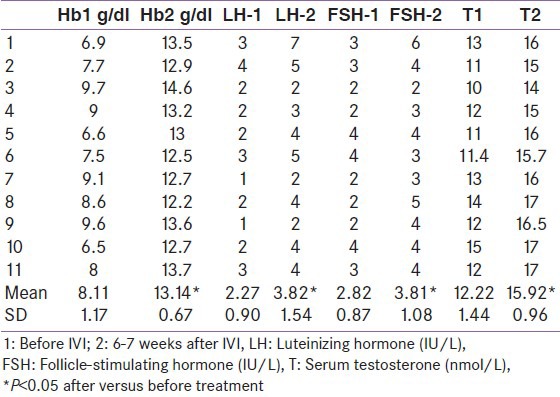
Table 2.
Sperm parameters before versus after intravenous iron replacement therapy in iron deficiency anemia
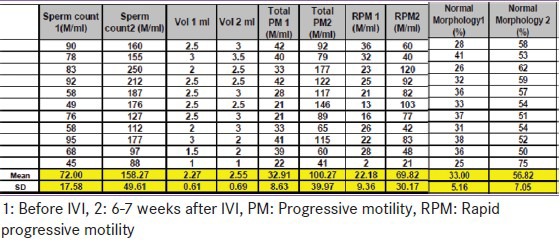
In particular, before IVI therapy, six out of 11 patients with IDA had low total sperm motility and in nine, a low progressive motility (asthenozoospermia) was also observed. All sperm parameters were normalized, except the RPM that showed significant improvement from 2% to 21%, but did not completely revert to normal (5th percentile 31%) after IVI therapy. [Table 2] One patient had a low semen volume (1 ml; the 5th percentile correspond to 1.4 ml) before and after IVI.
Increment in Hb concentration was correlated significantly with LH and FSH (r = 0.69 and r = 0.44, r = 0.22, respectively; P < 0.01) and T concentrations (r = 0.75, P < 0.001) [Figure 1], after IVI therapy. Increments in Hb were correlated significantly with increments of testosterone. The increment in serum T was correlated significantly with increments in the TSC [Figure 2] and total sperm motility and RPM (r = 0.66, 0.43, and 0.55, respectively; P < 0.001) but not with gonadotrophin levels.
Figure 1.
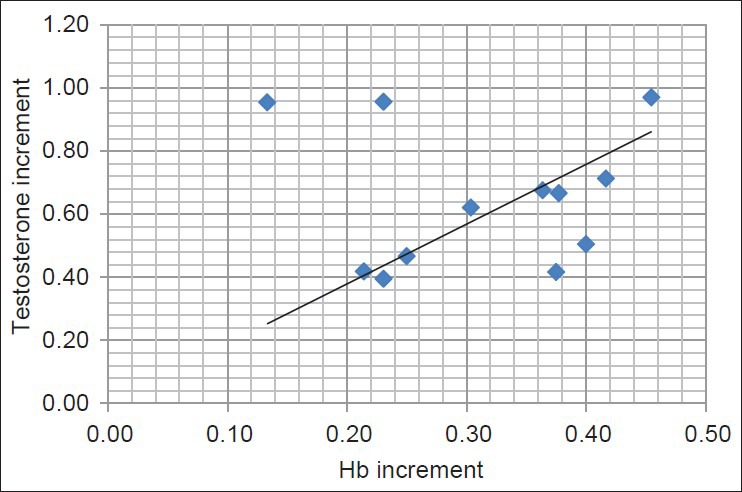
Correlation between increments of Hb and testosterone after intravenous iron replacement therapy (IVI); (r = 0.75, P < 0.001)
Figure 2.
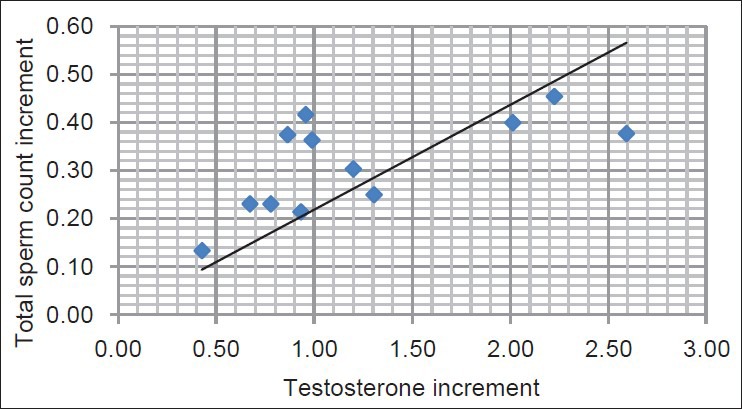
Correlations between testosterone and total sperm count increments after intravenous iron replacement therapy (IVI); (r = 0.66, P < 0.001)
DISCUSSION
Nutritional iron-deficiency anemia (IDA) is the most common disorder in the world. In addition, iron deficiency (ID) is the most prevalent single deficiency state on a worldwide basis.[19] Patients with severe IDA have significant hypoxia that affects all tissues, especially those with high metabolic activity like cardiac and skeletal muscles, liver, and testis.[20,21,22]
In this study, correction of IDA with IVI is associated with increased serum T secretion, gonadotrophins (LH and FSH) levels, and improvement of seminal parameters including total sperm count and those with normal morphology, total and rapid progressive sperm motility. These hormonal and semen changes are concomitant with correction of IDA and can be explained by the correction of anemia and/or iron deficiency.
Physiologically, the testis is considered to be poised on the brink of hypoxia, in part, because of its high metabolic requirements owing to spermatogenesis but also because of its vascular supply, in which approximately 50% of incoming arterial blood is siphoned off via arterio-venous anastomoses in the spermatic cord.[23,24,25,26,27] Therefore, factors that compromise oxygen delivery to the testis, as IDA, would be suspected to have a harmful effect on spermatogenesis. The negative impact of IDA on sperm parameters with improvements after IVI counts in men with IDA is, therefore, consistent with this belief.
Moderate hypoxia has been shown to decrease gonadotrophins’ secretion within 2 days of arrival at moderate altitude.[28] Previous studies indicated that hypoxia reduced the fertility of men from lowland by decreasing sperm count and sperm motility in semen.[29,30]
Hypobaric hypoxia markedly inhibits spermatogenesis as well as epididymal sperm parameters.[31,32,33] Testicular damage is related to overproduction of reactive oxygen species (ROS) by the hypoxic stress, with increases sperm lipo-peroxidation and sperm DNA damage.[32,33,34] In rats exposed to hypoxia, epididymal sperm count was significantly reduced at day 7 of exposure to high altitude associated with significant reduction of the time for many stages of spermatogenesis.[31,32,33,34,35,36,37] In conclusion, hypoxia due to high altitude affects spermatogenesis and negatively affects epididymal sperm count.[29,30,31,32,33,34]
In our patients, it is possible that correction of IDA, through improving oxygen supply, has favorable effects on epididymal sperm viability and progression of late spermatogenesis (helped by the increased testosterone level).
In support to our findings, recently data showed that correction of anemia in chronic hemolytic states namely, thalassemia and sickle cell disease, is associated with significant improvement in testosterone secretion as well as sperm parameters.[15,16] In addition, a study of diabetic men with low testosterone, 24% of them had anemia, which showed that lower testosterone levels were found to be independently associated with lower hemoglobin and iron levels.[38] In another study in old males, independent of age, total and bioavailable testosterone levels were linearly correlated with hemoglobin levels.[39]
Although interference with oxygen supply is an obvious mechanism via which IDA could reduce spermatogenesis, other mechanisms may also operate, including deficiency of iron-requiring enzymes. Iron is essential for many metabolic processes including DNA, RNA, and protein synthesis, the formation and maintenance of myelin, and is a co-factor of many heme and non-heme enzymes.[35,36,37]
Clinical consequences of iron deficiency involve multi-organ affection including: Central nervous system dysfunction with cognitive impairment, alterations in cellular function, growth, motor development, behavior, decreased physical capacity, impaired work performance due to chronic fatigue, reduced immunity, gastrointestinal disturbances (glossitis, stomatitis, gastritis), and impaired temperature regulation.[35,36,37,38] Deleterious effect of iron deficiency on spermatogenesis with improvement after IVI is another possibility that explains our results.
Physiological level of iron is required for normal spermatozoa production. In general, the semen contains a certain amount of Fe, and the Fe content of seminal plasma is important for the preservation of sperm motility and viability after ejaculation, and its presence in the seminal plasma helps spermatozoa to maintain their functions.[39,40,41] In our patients, correction of IDA improved markedly both the number of sperms with normal morphology and rapid progressive sperm motility.
Hormones act on all phases of spermatogenesis. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) act directly on the testes to stimulate somatic cell function in support of spermatogenesis. Three phases of spermatogenesis that are regulated by gonadotrophins in men are: i) The maturation of type A spermatogonia to type B spermatogonia, ii) meiosis, and iii) spermiation, both acutely and chronically. Both FSH and testosterone actions are important for the progression of meiosis, perhaps by the regulation of the survival via the intrinsic and the extrinsic apoptotic machineries, and also spermiation in both rodents and humans.
Data show that FSH predominantly regulates spermatogonial development, while testosterone regulates the latter phase of spermiogenesis, while both FSH and testosterone seem to be equally important in supporting spermatocyte development.[41,42,43,44]
Testosterone is important for the conversion of step 7 round spermatids into step 8 spermatids by regulating the adhesion between Sertoli cells and round/elongating/elongated spermatids at the apical ectoplasmic specialization adherent junctions.[44,45,46,47]
In our study, increments in Hb concentration after IVI are correlated significantly with LH, FSH, and T concentrations. In addition, testosterone increments after correction of IDA are associated and correlated significantly with increments in the total sperm count, total sperm motility, and rapid sperm motility. Similarly, decreased testosterone secretion and low gonadotrophins levels associated with decreased spermatogenesis have been reported in male rats exposed to hypobaric hypoxia.[32]
The opioid system is known to operate as a multi-messenger system that participates in the regulation of reproductive physiology at multiple levels, for example, at the levels of the central nervous system, at the testes level, and at sperm level.[48,49]
Studies have shown that an important mechanism of adaptation to the hypoxic conditions acts through activating the endogenous opioid system. This provides increased tolerance of the heart and nervous system to the harmful effects of hypoxia. The increased opioid secretion/action, secondary to hypoxia due to IDA, may explain the decreased secretion of LH, testosterone, and defective spermatogenesis.[50,51,52]
A comprehensive high quality semen analysis is an essential first-line investigation for male fertility. Semen quality is conventionally determined according to the number, motility, and morphology of spermatozoa in an ejaculate. Of all semen parameters, sperm morphology turns out to be the best predictor of a man's fertilizing ability. Besides, motility is an important fertility-relevant quality parameter.[53,54] A link has been established between sperm morphological characteristics and infertility by many investigators. However, total number of sperm per ejaculate is an important measure for clinicians to provide advice to patient couples.[54,55] In this study, significant improvement of sperm count, morphology, and motility occurred after 12 weeks of treatment with iron and correction of anemia [Table 2] and suggest a positive effect of correction of anemia on fertility.
Limitation of the study: The small number of patients (n = 11) may partially limit the significant findings of this pilot study. Larger studies are required to enforce these original results.
In conclusion: In men with IDA, the correction of anemia with IVI is associated with significant enhancement of sperm parameters coupled with increased serum concentrations of testosterone, LH, and FSH. These positive changes may improve the reproductive ability of these patients. These effects on spermatogenesis are reached by an unknown mechanism and suggest a number of pathways that need further human and/or experimental studies.
Footnotes
Source of Support: Funding is only done by Hamad Medical Center, Doha, Qatar
Conflict of Interest: None declared
REFERENCES
- 1.Stoltzfus R. Defining iron-deficiency anemia in public health terms: A time for reflection. J Nutr. 2001;131:565–7. doi: 10.1093/jn/131.2.565S. [DOI] [PubMed] [Google Scholar]
- 2.Gosney JR. Effects of hypobaric hypoxia on the Leydig cell population of the testis of the rat. J Endocrinol. 1984;103:59–62. doi: 10.1677/joe.0.1030059. [DOI] [PubMed] [Google Scholar]
- 3.Saxena DK. Effect of hypoxia by intermittent altitude exposure on semen characteristics and testicular morphology of male rhesus monkeys. Int J Biometerol. 1995;38:137–40. doi: 10.1007/BF01208490. [DOI] [PubMed] [Google Scholar]
- 4.Donayre J, Guerra-García R, Moncloa F, Sobrevilla LA. Endocrine studies at high altitude. IV. Changes in the semen of men. J Reprod Fertil. 1968;16:55–8. doi: 10.1530/jrf.0.0160055. [DOI] [PubMed] [Google Scholar]
- 5.Verratti V, Berardinelli F, Di Giulio C, Bosco G, Cacchio M, Pellicciotta M, et al. Evidence that chronic hypoxia causes reversible impairment on male fertility. Asian J Androl. 2008;10:602–6. doi: 10.1111/j.1745-7262.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- 6.Farias J, Bustos-Obregón E, Orellana R, Bucarey J, Quiroz E, Reyes JG. Effects of chronic hypobaric hypoxia on testis histology and round spermatid oxidative metabolism. Andrologia. 2005;37:47–52. doi: 10.1111/j.1439-0272.2004.00651.x. [DOI] [PubMed] [Google Scholar]
- 7.Lysiak JJ, Nguyen QA, Turner TT. Fluctuations in rat testicular interstitial oxygen tensions are linked to testicular vasomotion: Persistence after repair of torsion. Biol Reprod. 2000;63:1383–9. doi: 10.1095/biolreprod63.5.1383. [DOI] [PubMed] [Google Scholar]
- 8.De Sanctis V, Borsari G, Brachi S, Govoni M, Carandina G. Spermatogenesis in young adult patients with beta-thalassaemia major long-term treated with desferrioxamine. Georgian Med News. 2008;156:74–7. [PubMed] [Google Scholar]
- 9.De Sanctis V, Katz M, Wonke B, Hoffbrand V, Di Palma A, Mazzotta D, et al. Semen parameters in patients with homozygous β-thalassemia. Infertility. 1989;12:167–74. [Google Scholar]
- 10.Allegra A, Capra M, Cuccia L, Pulejo ML, Raineri L, Corselli F, et al. Hypogonadism in beta-thalassemic adolescents: A characteristic pituitary-gonadal impairment. The ineffectiveness of long-term iron chelating therapy. Gynecol Endocrinol. 1990;4:181–91. doi: 10.3109/09513599009009805. [DOI] [PubMed] [Google Scholar]
- 11.Modebe O, Ezeh UO. Effect of age on testicular function in adult males with sickle cell anemia. Fertil Steril. 1995;63:907–12. doi: 10.1016/s0015-0282(16)57500-1. [DOI] [PubMed] [Google Scholar]
- 12.Parshad O, Stevens MC, Preece MA, Thomas PW, Serjeant GR. The mechanism of low testosterone levels in homozygous sickle-cell disease. West Indian Med J. 1994;43:12–4. [PubMed] [Google Scholar]
- 13.Agbaraji VO, Scott RB, Leto S, Kingslow LW. Fertility studies in sickle cell disease: Semen analysis in adult male patients. Int J Fertil. 1988;33:347–52. [PubMed] [Google Scholar]
- 14.Osegbe DN, Akinyanju O, Amaku EO. Fertility in males with sickle cell disease. Lancet. 1981;2:275–6. doi: 10.1016/s0140-6736(81)90525-0. [DOI] [PubMed] [Google Scholar]
- 15.Soliman A, Yasin M, El-Awwa A, Osman M, De Sanctis V. Acute effects of blood transfusion on pituitary gonadal axis and sperm parameters in adolescents and young men with thalassemia major: A pilot study. Fertil Steril. 2012;98:638–43. doi: 10.1016/j.fertnstert.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Yassin MA, Soliman AT, Elawwa AS, El-Ayoubi HR, De Sanctis V. Acute effects of blood transfusion on pituitary gonadal axis and sperm parameters in young males with sickle cell disease (SCD). A Pilot Study. Blood (ASH Annual Meeting Abstracts) 2012;120:3209. [Google Scholar]
- 17.Trembecki J, Kokot F, Wiecek A, Marcinkowski W, Rudka R. Influence of long-term erythropoietin (rHuEPO) therapy on the function of the pituitary-gonadal axis in hemodialyzed male patients with end stage renal failure. Pol Arch Med Wewn. 1995;94:144–52. [PubMed] [Google Scholar]
- 18.Tanner JM. Auxology. In: Kappy MS, Blizzard RM, Migeon CJ, editors. The diagnosis and treatment of endocrine disorders in childhood and adolescence. 4th ed. Springfield, IL: Charles C Thomas; 1995. pp. 137–92. [Google Scholar]
- 19.De Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993-2005, WHO Global database on anemia. 2008:1–48. [Google Scholar]
- 20.Hegde N, Rich MW, Gayomali C. The cardiomyopathy of iron deficiency. Tex Heart Inst J. 2006;33:340–4. [PMC free article] [PubMed] [Google Scholar]
- 21.Macdonald VW, Charache S, Hathaway PJ. Iron deficiency anemia: Mitochondrial alpha-glycerophosphate dehydrogenase in guinea pig skeletal muscle. J Lab Clin Med. 1985;105:11–8. [PubMed] [Google Scholar]
- 22.Oski FA. The non-hematologic manifestations of iron deficiency. Am J Dis Child. 1979;133:315–22. doi: 10.1001/archpedi.1979.02130030091017. [DOI] [PubMed] [Google Scholar]
- 23.Reyes JG, Farias JG, Henríquez-Olavarrieta S, Madrid E, Parraga M, Zepeda AB, et al. The hypoxic testicle: Physiology and pathophysiology. Oxid Med Cell Longev 2012. 2012 doi: 10.1155/2012/929285. 929285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1697–712. doi: 10.1098/rstb.2009.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maddocks S, Hargreave TB, Reddie K, Fraser HM, Kerr JB, Sharpe RM. Intratesticular hormone levels and the route of secretion of hormones from the testis of the rat, guinea pig, monkey and human. Int J Androl. 1993;16:272–8. doi: 10.1111/j.1365-2605.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 26.Piner J, Sutherland M, Millar M, Turner K, Newall D, Sharpe RM. Changes in vascular dynamics of the adult rat testis leading to transient accumulation of seminiferous tubule fluid after administration of a novel 5-hydroxytryptamine (5-HT) agonist. Reprod Toxicol. 2002;16:141–50. doi: 10.1016/s0890-6238(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 27.5th edition. Geneva: World Health Organization; 2010. World Health Organisation. WHO Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 28.Okumura A, Fuse H, Kawauchi Y, Mizuno I, Akashi T. Changes in male reproductive function after high altitude mountaineering. High Alt Med Biol. 2003;4:349–53. doi: 10.1089/152702903769192304. [DOI] [PubMed] [Google Scholar]
- 29.Bomhard EM, Gelbke HP. Hypoxaemia affects male reproduction: A case study of how to differentiate between primary and secondary hypoxic testicular toxicity due to chemical exposure. Arch Toxicol. 2013;87:1201–18. doi: 10.1007/s00204-013-1024-6. [DOI] [PubMed] [Google Scholar]
- 30.Liao W, Cai M, Chen J, Huang J, Liu F, Jiang C, et al. Hypobaric hypoxia causes deleterious effects on spermatogenesis in rats. Reproduction. 2010;139:1031–8. doi: 10.1530/REP-09-0557. [DOI] [PubMed] [Google Scholar]
- 31.Yu LK, Gui JH, Feng J, Hu B, Huang GX, Wang Z, et al. Comparison of sperm parameters between male adults at different altitudes. Zhonghua Nan Ke Xue. 2007;13:122–4. [PubMed] [Google Scholar]
- 32.Vargas A, Bustos-Obregón E, Hartley R. Effects of hypoxia on epididymal sperm parameters and protective role of ibuprofen and melatonin. Biol Res. 2011;44:161–7. [PubMed] [Google Scholar]
- 33.Cikutovic M, Fuentes N, Bustos-Obregón E. Effect of intermittent hypoxia on the reproduction of rats exposed to high altitude in the Chilean Altiplano. High Alt Med Biol. 2009;10:357–63. doi: 10.1089/ham.2009.1035. [DOI] [PubMed] [Google Scholar]
- 34.Tunc O, Tremellen K. Oxidative DNA damage impairs global sperm DNA methylation in infertile men. J Assist Reprod Genet. 2009;26:537–44. doi: 10.1007/s10815-009-9346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umbreit J. Iron deficiency: A concise review. Am J Hematol. 2005;78:225–31. doi: 10.1002/ajh.20249. [DOI] [PubMed] [Google Scholar]
- 36.Fretham SJ, Carlson ES, Georgieff MK. The role of iron in learning and memory. Adv Nutr. 2011;2:112–21. doi: 10.3945/an.110.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotze MJ, van Velden DP, van Rensburg SJ, Erasmus R. Pathogenic mechanisms underlying iron deficiency and iron overload: New insights for clinical application. IFCC. 2013. Available from: http://www.ifcc.org/ifcc-communications-publications-division-(cpd)/ifcc-publications/ejifcc-(journal)/e-journal-volumes/vol 20, no 2 September 23 . [PMC free article] [PubMed]
- 38.Grossmann M, Panagiotopolous S, Sharpe K, MacIsaac RJ, Clarke S, Zajac JD, et al. Low Testosterone and Anemia in Men with Type 2 Diabetes. Clin Endocrinol. 2009;70:547–53. doi: 10.1111/j.1365-2265.2008.03357.x. [DOI] [PubMed] [Google Scholar]
- 39.Ferrucci L, Maggio M, Bandinelli S, Basaria S, Lauretani F, Ble A, et al. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006;166:1380–8. doi: 10.1001/archinte.166.13.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humpeler E, Skraba F, Bartsch G. Influence of exposure to moderate altitude on the plasma concentration of cortisol, aldosterone, renin, testosterone, and gonadotropins. Eur J Appl Physiol. 1980;45:167–76. doi: 10.1007/BF00421324. [DOI] [PubMed] [Google Scholar]
- 41.Gamčík P, Kozumplík J, Mesároš P, Schwarz F, Vlček Z, Zibrín M, et al. Bratislava. Príroda; 1992. Príroda, Bratislava, 1992. Andrology and artificial insemination of farm animals (in Slovak) p. 299 s. [Google Scholar]
- 42.Love CC. Relationship between sperm motility, morphology and the fertility of stallions. Theriogenology. 2011;76:547–57. doi: 10.1016/j.theriogenology.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 43.McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, et al. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–79. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- 44.Ruwanpura SM, McLachlan RI, Meachem SJ. Hormonal regulation of male germ cell development. J Endocrinol. 2010;205:117–31. doi: 10.1677/JOE-10-0025. [DOI] [PubMed] [Google Scholar]
- 45.Holdcraft RW, Braun RE. Hormonal regulation of spermatogenesis. Int J Androl. 2004;27:335–42. doi: 10.1111/j.1365-2605.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 46.Walker WH. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis. 2011;1:116–20. doi: 10.4161/spmg.1.2.16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLachlan RI, Wreford NG, Robertson DM, De Kretser DM. Hormonal control of spermatogenesis. Trends Endocrinol Metab. 1995;6:95–101. doi: 10.1016/1043-2760(94)00215-p. [DOI] [PubMed] [Google Scholar]
- 48.Subirán N, Casis L, Irazusta J. Regulation of male fertility by the opioid system. Mol Med. 2011;17:846–53. doi: 10.2119/molmed.2010.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fabbri A, Jannini EA, Gnessi L, Ulisse S, Moretti C, Isidori A. Neuroendocrine control of male reproductive function: The opioid system as a model of control at multiple sites. J Steroid Biochem. 1989;32:145–50. doi: 10.1016/0022-4731(89)90155-6. [DOI] [PubMed] [Google Scholar]
- 50.Sun YM, Hoang T, Neubauer JA, Walters AS. Opioids protect against substantia nigra cell degeneration under conditions of iron deprivation: A mechanism of possible relevance to the Restless Legs Syndrome (RLS) and Parkinson's disease. J Neurol Sci. 2011;304:93–101. doi: 10.1016/j.jns.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Blank MS, Fabbri A, Catt KJ, Dufau ML. Direct inhibition of gonadotroph function by opiates. Trans Assoc Am Physicians. 1985;98:1–9. [PubMed] [Google Scholar]
- 52.Vuong C, Van Uum SH, O’Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31:98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sofikitis N, Giotitsas N, Tsounapi P, Baltogiannis D, Giannakis D, Pardalidis N. Hormonal regulation of spermatogenesis and spermiogenesis. J Steroid Biochem Mol Biol. 2008;109:323–30. doi: 10.1016/j.jsbmb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Cassuto NG, Bouret D, Plouchart JM, Jellad S, Vanderzwalmen P, Balet R, et al. A new real-time morphology classification for human spermatozoa: A link for fertilization and improved embryo quality. Fertil Steril. 2009;92:1616–25. doi: 10.1016/j.fertnstert.2008.08.088. [DOI] [PubMed] [Google Scholar]
- 55.Elia J, Imbrogno N, Delfino M, Mazzilli R, Rossi T, Mazzilli F. The importance of the sperm motility classes-future directions. Open Androl J. 2010;2:42–3. [Google Scholar]


