Abstract
Introduction:
Polycystic ovary disease is a common endocrine condition which is rapidly gaining epidemic proportions. No community based prevalence data is available for this syndrome in India.
Materials and Methods:
A cross-sectional community-based study was undertaken in a sampled census block of Mumbai to assess the prevalence of polycystic ovarian syndrome (PCOS) among 778 adolescents and young girls aged 15-24 years. Among them, 600 completed all clinical, ultrasonography (USG), and biochemical investigations.
Results:
The prevalence of PCOS among them was 22.5% by Rotterdam and 10.7% by Androgen Excess Society criteria. Nonobese comprised 71.8% of PCOS diagnosed by Rotterdam criteria. Mild PCOS (oligomenorrhea and polycystic ovaries on USG) was the most common phenotype (52.6%). History of oligomenorrhea had a positive predictive value of 93.3% and negative predictive value of 86.7% to detect a possible case of PCOS. Hyperinsulinemia (serum insulin >15 μlU/mL) was present among 19.2% of diagnosed PCOS cases. Obese girls with PCOS were more hirsute, hypertensive, and had significantly higher mean insulin and 2 h post 75 g glucose levels compared with nonobese PCOS.
Conclusion:
To our knowledge, this is the first urban community-based study diagnosing PCOS and phenotypes among adolescent and young girls in India. This study demonstrates that PCOS is an emerging disorder during adolescence and screening could provide opportunity to target the group for promoting healthy lifestyles and early interventions to prevent future morbidities.
Keywords: Adolescents, India, phenotypes, polycystic ovarian syndrome, prevalencte
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is one of the most common reproductive endocrinological disorders with a broad spectrum of clinical manifestations affecting about 6-8% of women of reproductive years.[1] The diverse manifestations of PCOS start at an early age when a girl is maturing into a young woman. During this pubertal transition, several features may be in evolution and thus many findings may be transitory which stabilize later during adolescence. However, it is important to make an early diagnosis in order to prevent early and late sequel of the syndrome. PCOS a diagnosis of exclusion has been a topic of debate and many consensus definitions have evolved over time. The National Institute for Health (NIH) Criteria 1990 was revised in 2003 and Rotterdam criteria[2] has been adopted world over. However, recently in 2006, Androgen Excess Society (AES) has come up with a consensus statement, defining PCOS as a hyperandrogenic state and emphasises the presence of either clinical and/or biochemical features of hyperandrogenism along with other features of PCOS for diagnosis.[3]
Globally, prevalence estimates of PCOS are highly variable, ranging from 2.2% to as high as 26%.[4,5,6,7,8] Community-based studies using Rotterdam criteria among reproductive age group women have demonstrated varied prevalence figures in few Asian countries ranging from 2% to 7.5% in China[9,10] to 6.3% in Srilanka.[11] Studies among several Caucasian populations using NIH criteria reported PCOS in the range of 5-8%.[4,5,6,7,8,9,10,11,12] An Australian retrospective birth cohort study of 728 women reported a prevalence of 11.9 ± 2.4% as per Rotterdam criteria, which increased to 17.8 ± 2.8% when imputed data were included. Under the AES recommendations, PCOS prevalence was 10.2 ± 2.2%, and 12.0 ± 2.4% with the imputed data.[13] Although there are limited studies of PCOS in India, the observational studies by endocrinologists, gynecologists, and dermatologists relate to diverse aspects of PCOS. Prevalence of obesity and diabetes mellitus in most industrialized countries including India is also on the rise owing to urbanization and change in lifestyle. Most of the young population do not visit health facilities until they have late sequel of the problem. Most prevalence studies in India are in hospital set-ups and recently a few studies among adolescents in schools report prevalence of PCOS as 9.13% to 36%.[14,15] It is appropriately pointed by Gainie and Kalra[16] that the health budget of India is unlikely to meet the costs posed to tackling the associated multiple consequences of PCOS. It is time that this warning is heeded and at national level the disease is recognized as an important noncommunicable disease.[17] Studies have demonstrated that the cost of diagnostic evaluation accounts only for a relatively minor part of the total costs of managing PCOS (approximately 2%). Hence, more widespread and liberal screening for the disorder appears to be a cost-effective strategy, benefiting earlier diagnosis and intervention and possibly the amelioration and prevention of serious sequel.[1]
The varying prevalence of PCOS in general is mainly due to using different diagnostic criteria, the heterogeneous presentation of symptoms, logistic difficulty to carry out blood or ultrasound tests, and the varied age groups and ethnic populations that have been studied. This has resulted in prevalence studies being based on convenience samples such as university employees.[4,5,6,7,8] or blood donors.[7] without any representativeness of these subgroups.
With this background, a community-based study was undertaken in Mumbai, India to screen adolescents and young unmarried girls aged 15-24 years for PCOS.
MATERIALS AND METHODS
A community-based cross-sectional study, using a systematic multistage random sampling method was conducted in a sampled census block of Mumbai from July 2010 to December 2011. A sample size of 900 was calculated assuming a prevalence of 10% and precision of 2% at 95% confidence level. Considering a nonresponse rate of 10% the total sample to be enrolled was estimated to be about 1000 cases.
Girls aged 15-24 years, who had attained menarche more than 2 years before the study, who were unmarried and were willing to participate in the study were enrolled. An estimate of 10,600 eligible girls in the sampled area was derived considering that the block with a population of approximately 80,000 would have 19% female population in the age group of 15-24 years[18] and 30% of them would have been married and hence would be excluded. Using census data checklist of households in the sampled area, adolescent and young girls from every 10th household were approached. If a household had more than one girl who fit into the inclusion criteria, all were invited to participate in the study.
Operational definitions
Adolescents
Age group 10-19 years (for our study 15-19 years).
Young girls
Age group 20-24 years.
Amenorrhoea
In a girl who has been menstruating, the absence of periods for a length of time equivalent to a total of at least 3 of the previous cycle intervals or 6 months of amenorrhea.
Oligomenorrhea
Indirect marker for anovulation in absence of any hormonal evidence, but considering infrequent menstrual cycles at intervals of more than 35 days.
Body mass index[19,20]
Normal: BMI = 18-22.9 kg/m2, Underweight: BMI < 17.9 kg/m2, Overweight: BMI > 23 kg/m2, Obese: BMI > 25 kg/m2 for girls above 18 years.[18] Age matched cut offs for body mass index (BMI) for girls below 18 years.[19]
Clinical hyperandrogenism
Hirsutism was assessed by Ferriman-Gallwey.[21] score of ≥8 over 9 body parts.
Polycystic ovaries
12 or more follicles measuring 2-9 mm in diameter with or without ovarian volume >10 mL.[22]
Biochemical hyperandrogenism
Free Androgen Index level of two standard deviations above the mean level among normal controls (Formula: Total testosterone/SHbg × 100).
Probable PCOS
Signs and symptoms mimic PCOS but etiology not confirmed (without differential diagnosis of other etiologies).
Metabolic syndrome (International harmonization definition)[23]
Any three of the following criteria (i) waist circumference ≥80 cm, (ii) serum triglyceride ≥1.7 mmol/L, (iii) serum high-density lipoprotein cholesterol <1.3 mmol/L, (iv) blood pressure ≥130/85 mm Hg, and (v) fasting blood sugar of >100 g/dL.
Phenotypes
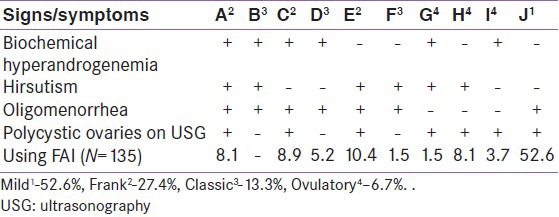
Based on signs and symptoms such as oligomenorrhea, Polycystic ovaries (PCO appearance on ultrasonography (USG), clinical and biochemical hyperandrogenism, the phenotypes are classified from A to J (3) [Table 3] and further grouped together.[24] as mild PCOS (phenotype J), Frank PCOS (phenotype A, C, and E), classic PCOS (phenotypes B, D, and F), and ovulatory PCOS (phenotypes G, H, and I).
Table 3.
Polycystic ovarian synrdome phenotypes
Community level enrolment
After obtaining ethical approvals from Institute Ethics Committee, 1000 girls were approached at household level and were explained about the study. About 778 consented to participate in the study. Girls were enrolled after taking an informed written consent from them and also their parents/guardians if they were minors (<18 years). At enrolment, girls were educated on PCOS using a pretested information brochure in local language. An equipped field clinic manned by a medical doctor and a laboratory assistant was established in the study area with the cooperation extended by the Municipal Corporation of Greater Mumbai. A list of all households in the area was obtained from census data. Eligible girls in the selected household were invited to participate in the study and trained investigators administered a structured questionnaire.
Clinical examination and investigations
All enrolled girls irrespective of their menstrual patterns were asked to attend the field clinic for clinical examination and investigations. Physicians involved in the study had adequate clinical experience and were guided by consultant experts for quality control. Apart from personal information, details of menstrual cycles (current and over last 2 years), any previous illness, treatment sought, were noted. Height in meters, weight in kgs, and blood pressure (mm Hg) were measured using standard methods. Signs of hyperandrogenism, galactorrhea, and thyroid disorders were noted. All the girls were then subjected to abdominal sonography performed twice a week at a center operated by an Nongovernment organization in a nearby locality, by a single trained gynaecologist and sonologist using HD6 Ultrasound Colour Doppler system. A fasting blood sample was collected between 3rd and 7th days of menstrual cycle, including for those who had cycle length of 6-8 weeks and stored at -80°C for biochemical and hormonal parameters. The laboratory was blinded to the diagnosis.
Total testosterone, insulin and SHBG levels were estimated by radioimmunoassay method using commercial kits (Diasorin, Stillwater USA and Izotop, Budapest, Hungary respectively). Enzyme-linked immunosorbent assay was used to measure follicle-stimulating hormone, lieutinizing hormone, serum prolactin (PRL) 17-hydroxy progesterone (17-OHP), thyroxine (T4), and thyroid-stimulating hormone using commercially available kits (Syntron Bioresearch, Inc., Carslbad USA). Glucose oxidase-peroxidase method was used to estimate blood glucose. Lipid profile was estimated by using enzymatic colorimetric technique. Manufacturer's instructions were followed for preparation, set-up, dilutions, adjustments, assay, and quality control procedures.
Among the screened girls, those who had normal cycles were clinically normal including BMI, no clinical hyperandrogenism and had no PCO morphology on USG were considered as non PCOS group for comparison.
Statistical analysis
Data were analyzed in the Statistical Package for the Social Sciences software (version 19.0, IBM SPSS Statistics). Frequencies and percentages were used for categorical data. Mean, median, and standard deviations were calculated for continuous variables. Pearson's Chi-square test was used to assess differences in the groups for categorical data. All hypothesis tests were two sided and P < 0.05 were considered statistically significant. Distributions were compared using “unpaired Student's t test.”
RESULTS
A total of 1000 girls in the area fulfilling the inclusion criteria were invited to participate in the study to achieve a sample size of 900. Among them, only 778 girls volunteered, got enrolled and underwent clinical examination. However, only 687 of them completed ultrasound examination (88.3%) and 600 of them completed all the biochemical and hormonal tests (77%) [Figure 1].
Figure 1.
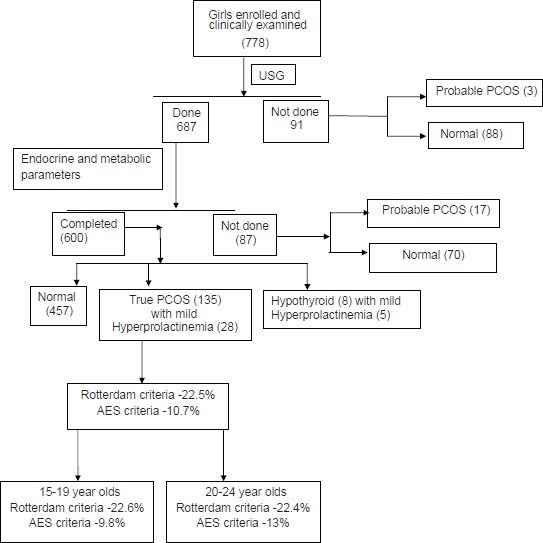
Flow of study participants
The nonparticipation rate for enrolment was 13.5% unlike the assumed 10%. Further drop out at stage two for sonography examination was 11.7% and 12.7% during the last stage, that is, for blood investigations. Participants were considered as dropouts when they failed to come for required visits even after five rounds of home-based health educative sessions with validated written information brochures. Reasons for dropout, as informed by participants, included refusal by parents, marriage and/or shift of residence, absence of clinical complaints (in about 80% of drop outs), unwillingness for investigations, and inconvenience to come fasting between 3rd and 7th days of the cycle for blood investigations due to work/educational schedule.
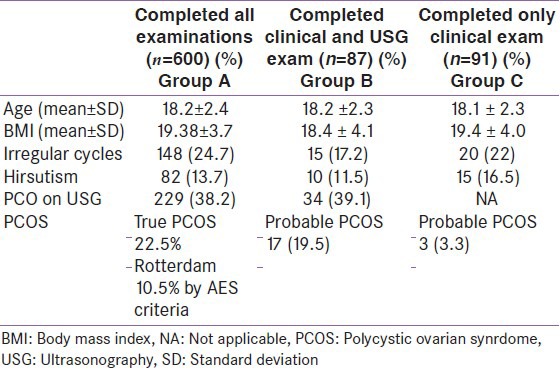
The mean age of the participants was 18.15 (±2.4) years. Majority of the participants (73.2%) were adolescents (15-19 years) from lower socioeconomic strata. The mean age at menarche was 13.22 (±1.3) years. We divided the enrolled girls into three groups as shown in Table 1. Group A comprised of girls who completed all desired investigations (n = 600), Group B were girls who completed only clinical and ultrasonography examinations (n = 87), and Group C who underwent only clinical examination (n = 91). As seen in the Table 1, all three groups had similar mean ages. All groups had mean BMI within normal range. History of irregular cycles was more among girls in Group A and feature of huirsuitism was more in Group C. Groups A and C had almost same number of girls with PCO picture on USG.
Table 1.
Differences in the three groups of enrolled girls
Prevalence of PCOS and their phenotypes
Among those who underwent clinical and sonographic assessment, probable PCOS was diagnosed among 22.85% using Rotterdam criteria (Group A: 23.3% and B: 19.5%). Since all essential investigation parameters were available for only Group A, further details of this group is presented. Excluding five cases of subclinical hypothyroidism the prevalence of PCOS among 600 adolescent and young girls (15-24 years) was 22.5% by Rotterdam and 10.7% by the AES criteria [Figure 1].
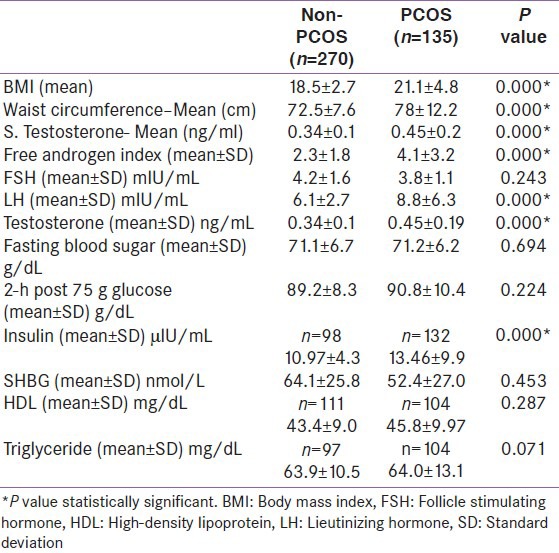
Among those diagnosed with PCOS, 71.8% were nonobese, 7.5% cases were overweight, and 20.7% were obese. PCOS cases had statistically significant higher mean values with respect to waist circumference, BMI, and serum testosterone and FAI levels compared with the non-PCOS (control) group [Table 2].
Table 2.
Difference in endocrine and metabolic profiles between polycystic ovarian synrdome and nonpolycystic ovarian synrdome
All four signs/symptoms of PCOS were present in only 8.1% of the cases, 28.9% had three and majority (63%) had two symptoms/signs. Mild PCOS was the most common variety with predominant J phenotype (52.6%) followed by 27.4% frank PCOS. Presumptive ovulatory (based on regular cycles of 26-35 days) PCOS was found among 13.3% and classic PCOS was present in 6.7% of cases [Table 3].
Using both Rotterdam and AES criteria symptom of oligomenorrhea had good positive predictive value (PPV) (86.7%, 71.9%) and negative predictive value (NPV) (93.3%, 81%), respectively, followed by appearance of PCO on USG to detect PCOS (PPV 93.3%, 85.9% and NPV 77.8% and 67.5%).
Biochemical and hormonal parameters among PCOS
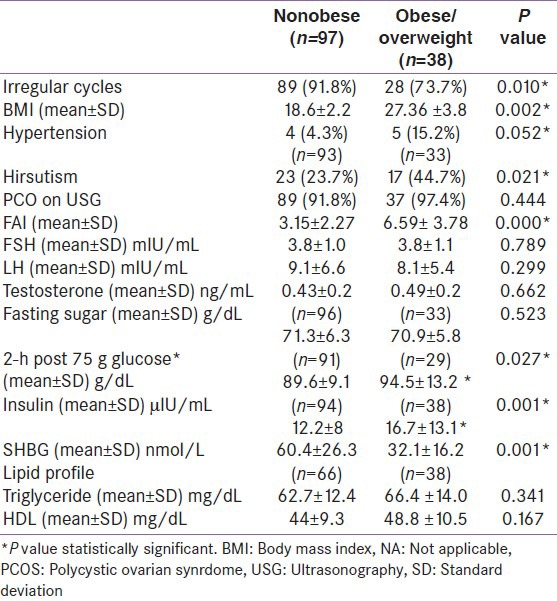
PCOS cases had statistically significant higher mean values of LH, testosterone, and insulin compared with non-PCOS cases Table 2. Overall PCOS cases had higher percentages of girls who had all biochemical and hormonal parameters more than the 80th percentile of normal. The mean plasma glucose levels were normal in all PCOS cases except one, where 2 h post 75 g plasma glucose value was above 140 g/dL. Hyperinsulinemia was present among 19.2% cases and 6.6% had serum triglycerides above normal range. Statistically, significant differences were observed between obese and nonobese PCOS girls with respect to symptoms, clinical features, and biochemical and hormonal parameters [Table 4]. Irregular cycles were reported by 92% of nonobese girls compared to only 47% of obese girls with PCOS. Obese girls were more hirsute and hypertensive and had higher mean values of post 75 g glucose and insulin and lower levels of SHBG compared with nonobese and the differences were statistically significant. No differences were found in the lipid profile of these two groups. Only one PCOS case was diagnosed with metabolic syndrome.
Table 4.
Differences in clinical, biochemical, and hormonal features between obese and nonobese polycystic ovarian synrdome (n=135)
DISCUSSION
PCOS among adolescents is an emerging problem that needs careful assessment, timely intervention, and appropriate treatment. To our knowledge, this is the first urban community based study in India, estimating the prevalence of PCOS among relatively younger population with a much lower mean age unlike reported earlier.[25] As Rotterdam criteria is much more broad-based and does not necessarily require evidence of hyperandrogenism, the prevalence by Rotterdam criteria (22.5%) was almost double compared to that using AES criteria (10.7%). If all the enrolled girls would have participated in the study, the prevalence could further reduce as most girls who did not come for further investigations were symptom free. The prevalence figures among the older age groups is slightly high especially considering the AES criteria, probably indicating increase in hyperandrogenism with age and hence diagnosis and timely intervention could possibly reduce the morbidities in older age groups. This prevalence is relatively higher than that reported by most studies, mainly due to use of different diagnostic criteria, study settings, age groups of the sample studied and hence cannot be compared.[4,5,6,7,8,9,10,11,12,13,14]
Puberty is a period when there is physiological hyperandrogenism and hyperinsulinemia which mimics some features of PCOS from Tanner stages I to III with return to prepubertal stage by Tanner stage V.[26] Hence, we enrolled girls 2 years postmenarche when H-P-O axis settles down to normal. Due to this transitory appearance of symptoms and signs of PCOS during adolescence, care must be taken to avoid premature labelling of a case as PCOS to avoid over treatment and psychological stress. Presence of oligomenorrhea among adolescent girls, 2 years postmenarche, can be a good screening indicator to diagnose a probable case of PCOS as reported earlier.[27,28] A diagnosis is confirmed if all three signs/symptoms of PCOS are present.[29] which was observed in 27.4% of PCOS cases in the study. The diagnosis may be considered but not confirmed among those who have two signs/symptoms.[29] and this phenotype in our study was 63% which is being followed-up for emergence of confirmed PCOS or settling down to normal hypothalamopituitary ovarian axis. Free testosterone assays are not reliable and the accurate ones are complicated, expensive, and labor intensive.[30] Hence, FAI is a better marker of free testosterone.[31], but as observed in our study it is more correlated with the degree of obesity.[32] Adolescent obesity and PCOS individually and together have emerged as important public health issues in India. There is an urgent need to organize preventive strategy to thwart the oncoming epidemics of obesity and obesity associated PCOS in India.[33]
Community-based screening among Asian population found prevalence of PCOS among 6.3% of reproductive age women aged 15-45 years in Srilanka[11] and 5.6% in China[10] using Rotterdam criteria unlike the higher prevalence observed in our study among younger population aged 15-24 year olds or probably due to smaller group of girls who got enrolled due to more symptoms.
Classic phenotype was commonly observed among 86% of Greek Caucasian women aged 17-45 years[5] and other retrospective and prospective studies in smaller number of patients with varying age profiles.[34,35] However in Asian context as that in China, the most common phenotype was ovulatory PCOS unlike the mild phenotype observed in Sri Lanka and our study. Lean PCOS with milder phenotypes as per Rotterdam criteria are known to have less severe hyperinsulinemia and insulin resistance.[27] and long-term health risks in these groups are not well-documented.[36] Hyperinsulinemia in our study group was present among both obese and nonobese PCOS as reported in literature.[36,37] However, the prevalence was much less compared with other studies in Indian context.[26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] probably due to overall less number of obese cases and younger population from lower socioeconomic strata in the study. Our study demonstrated that PCOS cases were on the higher scale of the normal range for biochemical parameters, especially obese PCOS had higher mean values compared with their lean counterparts suggesting pertinent danger to jump off the thin line and turn into metabolic syndrome with increasing age as is already known.[41,42] Hence in overweight/obese cases, lifestyle and dietary changes along with exercises for weight management must be recommended, coupled with medical management to treat clinical symptoms and hyperinsulinemia.
All the investigations were carried out by a single laboratory technician in a single laboratory which enhanced accuracy and consistency in the reports. Even the ultrasound examinations were carried out by a single-trained gynaecologist on a single machine. A retention rate of 77.2% in the study highlights the need to create awareness among general population about this disorder and its sequele. One of the limitations of the study was a poor representation of various socioeconomic strata; hence, the findings cannot be extrapolated to higher socioeconomic group with more westernised lifestyle and intake of junk food.
The feasibility of conducting such community-based study justifies the need to upscale this effort to get an overall estimate of the disorder in a diverse sociocultural and economic background, providing an opportunity for early detection and prevention of morbidities among adolescents and young women in India.
ACKNOWLEDGMENT
We acknowledge the support extended by ICMR for the conduct of the study (NIRRH/M/101/13). The project medical doctors Parul Salunke and Dr. Saloni Balgi, Health educator Neelawanti Gaikwad, Social workers Varsha Tryambake, project field workers Kirti Bodke and Manisha Waghmare, laboratory technician Samiksha Chauhan and Statistical Asst. Samruddhi Panchal have taken great efforts and contributed to the conduct of the study. Cancer Patient AIDS association have been very co-operative in providing diagnostic facilities at an affordable cost. Last but not the least we acknowledge the support extended by Municipal Corporation of Greater Mumbai for permitting use of their premises for conduct of the study. The parents and participants of the study who participated whole heartedly in this important endeavour need a special acknowledgement. We also would like to acknowledge Dr. Kamal Hazari for editing the manuscript.
Footnotes
Source of Support: Indian Council of Medical Research
Conflict of Interest: None declared
REFERENCES
- 1.Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90:4650–8. doi: 10.1210/jc.2005-0628. [DOI] [PubMed] [Google Scholar]
- 2.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Androgen Excess Society. Position statement: Criteria for defining pcos as a predominantly hyperandrogenic syndrome: An Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–45. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 4.Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: A prospective study. J Clin Endocrinol Metab. 1998;83:3078–82. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- 5.Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: Hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–11. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 6.Michelmore KF, Balen AH, Dunger DB, Vessey MP. Polycystic ovaries and associated clinical and biochemical features in young women. Clin Endocrinol (Oxf) 1999;51:779–86. doi: 10.1046/j.1365-2265.1999.00886.x. [DOI] [PubMed] [Google Scholar]
- 7.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–8. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 8.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Yang D, Mo Y, Li L, Chen Y, Huang Y. Prevalence of polycystic ovary syndrome in unselected women from southern China. Eur J Obstet Gynecol Reprod Biol. 2008;139:59–64. doi: 10.1016/j.ejogrb.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, et al. Prevalence of polycystic ovary syndrome in women in China: A large community based study. Hum Reprod. 2013;28:2562–9. doi: 10.1093/humrep/det262. [DOI] [PubMed] [Google Scholar]
- 11.Kumarapeli V, Seneviratne R de A, Wijeyaratne CN, Yapa RM, Dodampahala SH. A simple screening approach for assessing community prevalence and phenotypes of polycystic ovary syndrome in semiurban population in Srilanka. Am J Epidemiol. 2008;168:321–7. doi: 10.1093/aje/kwn137. [DOI] [PubMed] [Google Scholar]
- 12.Escobar-Morreale HF, San Millan JL. Abdominal adiposity and the polycystic ovary syndrome. Trends Endocrinol Metab. 2007;18:266–72. doi: 10.1016/j.tem.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 13.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–51. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 14.Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Prevalence of polycystic ovarian syndrome in Indian adolescents. J Pediatr Adolesc Gynecol. 2011;24:223–7. doi: 10.1016/j.jpag.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Nair MK, Pappachan P, Balakrishnan S, Leena ML, George B, Russell PS. Menstrual irregularity and poly cystic ovarian syndrome among adolescent girls: A two year follow-up study. Indian J Pediatr. 2012;79(Suppl 1):S69–73. doi: 10.1007/s12098-011-0432-y. [DOI] [PubMed] [Google Scholar]
- 16.Gainie MA, Kalra S. Polycystic ovary syndrome A metabolic malady, the mother of all lifestyle disorders in women Can Indian health budget tackle it in future? Indian J Endocrinol Metab. 2011;15:239–41. doi: 10.4103/2230-8210.85571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaidya RA. Polycystic Ovarian Syndrome, a Public Health Issue: Indian perspective oration delivered at the national institute for research in reproductive health ICMR- on the occasion of NIRRH 24 th Foundation Day [Google Scholar]
- 18.Mumbai: IIPS; [Last accessed on 2014 Jan 1]. International Institute for Population Sciences (IIPS) and Macro International. 2007. National Family Health Survey (NFHS-3), 2005-06: India: Volume I. Available from: http://www.nfhsindia.org/NFHS-3%20Data/NFHS-3%20NKF/Report.pdf . [Google Scholar]
- 19.Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- 20.Khadilkar VV, Khadilkar AV, Borade AB, Chiplonkar SA. Body mass index cut-offs for screening for childhood overweight and obesity in Indian children. Indian Pediatr. 2012;49:29–34. doi: 10.1007/s13312-012-0011-y. [DOI] [PubMed] [Google Scholar]
- 21.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–7. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 22.Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: International consensus definitions. Hum Reprod Update. 2003;9:505–14. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- 23.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donator KA, et al. American Heart Association, World Heart Federation, International Atherosclerosis Society, International Association for the Study of Obesity. Harmonizing the Metabolic Syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; And International Association for the Study Of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 24.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–97. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 25.Gainie MA, Khurana ML, Eunice M, Gupta N, Diwivedi SN, Gulati MS, et al. Prevalence of glucose intolerance among adolescent and young women with polycystic ovary syndrome in India. Indian J Endocrinol Metab. 2004 [Google Scholar]
- 26.Goran M, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50:2444–50. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 27.Franks S, White DM. Prevalence of and etiological factors in polycystic ovarian syndrome. Ann N Y Acad Sci. 1993;687:112–4. doi: 10.1111/j.1749-6632.1993.tb43859.x. [DOI] [PubMed] [Google Scholar]
- 28.Taponen S, Martikainen H, Jarvilin MR, Laiatinen J, Pouta A, Hartikainen AL, et al. Hormonal profile of women with self-reported symptoms of oligomenorhea and/or hirsutism: Northern Finland birth cohort 1966 study. J Clin Endocrinol Metab. 2003;88:141–7. doi: 10.1210/jc.2002-020982. [DOI] [PubMed] [Google Scholar]
- 29.Carmina E, Oberfield SE, Lobo RA. The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol. 2010:201–4. doi: 10.1016/j.ajog.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfield RL. Clinical practice. Hirsutism. N Engl J Med. 2005;353:2578–88. doi: 10.1056/NEJMcp033496. [DOI] [PubMed] [Google Scholar]
- 31.Gungor O, Erden G, Bal C, Uguz N, Sezer S, Ozdemir S, et al. The comparison of free androgen index and serum free testosterone levels in women with hirsutism or polycystic ovary syndrome. J Clin Exp Invest. 2011;2:152–6. [Google Scholar]
- 32.Li X, Lin JF. Clinical features, hormonal profile, and metabolic abnormalities of obese women with obese polycystic ovary syndrome. Zhonghua Yi Xue Za Zhi. 2005;85:3266–71. [PubMed] [Google Scholar]
- 33.Vaidya RA, Joshi B. Adolescent Obesity and PCOS: A dual emergence during childhood and/or pubertal transition. Manuscript accepted for book on Obesity in children and adolescents, An IJCP. In press. [Google Scholar]
- 34.Carmina E, Rosato F, Janni A, Rizzo M, Longo RA. Extensive clinical experience: Relative prevalence of different androgen excess disorders in 950 women referred because of clinical hyperandrogenism. J Clin Endocrinol Metab. 2006;91:2–6. doi: 10.1210/jc.2005-1457. [DOI] [PubMed] [Google Scholar]
- 35.Belosi C, Selvaggi L, Apa R, Guido M, Romualdi D, Fulghesu AM, et al. Is the PCOS diagnosis solved by ESHRE/ASRM 2003 consensus or could it include ultrasound examination of the ovarian stroma? Hum Reprod. 2006;21:3108–15. doi: 10.1093/humrep/del306. [DOI] [PubMed] [Google Scholar]
- 36.Chang RJ, Nakamura RM, Judd HL, Kaplan SA. Insulin resistance in nonobese patients with polycystic ovarian disease. J Clin Endocrinol Metab. 1983;57:356–9. doi: 10.1210/jcem-57-2-356. [DOI] [PubMed] [Google Scholar]
- 37.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in the polycystic ovary syndrome. Diabetes. 1989;38:1165–74. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 38.Shringi M, Vaidya RA, Vaidya AB. Insulin resistance in polycystic ovarian syndrome: A study of 90 patients. J Endocrinol Metab. 2003;1:19–23. [Google Scholar]
- 39.Meherji P. From National Institute of Research in Reproductive Health-ICMR. Personal communication [Google Scholar]
- 40.Katra A, Nair S, Rai L. Association of obesity and insulin resistance with dyslipidemia in India women with polycystic ovarian syndrome. Indian J Med Sci. 2006;60:447–53. [PubMed] [Google Scholar]
- 41.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929–35. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 42.Majumdar A, Singh TA. Comparison of clinical features and health manifestations in lean vs. obese Indian women with polycystic ovarian syndrome. J Hum Reprod Sci. 2009;2:12–7. doi: 10.4103/0974-1208.51336. [DOI] [PMC free article] [PubMed] [Google Scholar]


