Abstract
Background and Objectives:
Gestational diabetes mellitus (GDM) is a global health concern as it affects health status of both mother and fetus. In India, prevalence of GDM varies in different populations and no data is available from rural Haryana. This study was undertaken to determine the prevalence of GDM and risk factors associated with it in rural women of Haryana.
Materials and Methods:
Nine hundred and thirteen women, with estimated gestational age above 24 weeks from a rural block of Haryana who consented to participate were given a standardized 2-h 75-g oral glucose tolerance test (OGTT). Pro forma containing general information on demographic characteristics, educational level, gravida, family history of diabetes, and past history of GDM was filled-up. A World Health Organization (WHO) criterion for 2-h 75-g OGTT was used for diagnosing GDM.
Results:
GDM was diagnosed in 127/913 (13.9%) women with higher mean age as compared to non-GDM women. Majority (78.4%) of the women were housewives, rest engaged in agriculture (9.2%) and labor (5.5%). Women with gravida ≥3 and positive family history of diabetes had significantly higher prevalence of GDM. History of macrosomia (birth weight ≥4 kg) was significantly associated with prevalence of GDM (P = 0.002). On multiple logistic regression analysis, risk factors found to be significantly associated with GDM were maternal age >25 years, gravida >3, history of macrosomic baby, and family history of diabetes.
Conclusion:
The prevalence of GDM has been found quite high in rural Haryana. Appropriate interventions are required for control and risk factor modifications.
Keywords: Gestational diabetes mellitus, prevalence, rural
INTRODUCTION
Diabetes represents a spectrum of metabolic disorders, characterized by chronic hyperglycemia and disturbance in carbohydrates, fat, and protein metabolism resulting from defects in insulin secretion, insulin action, or both.[1] This disease affects 8.3% (366 million people) of the world population in 20-79 years age group.[2] Approximately, 80% of people with diabetes live in poor and developing countries without any access to basic healthcare.[3] Gestational diabetes mellitus (GDM) is a condition which affects ~7% of all pregnancies worldwide affecting 60 million women.[4] The prevalence of GDM is high in the Indian population as compared to other populations of southeast Asia.[5] In a community-based study (2005-2007) by Seshiah et al., prevalence of GDM in south India was found to be 13.0% using oral glucose tolerance test (OGTT) (World Health Organization (WHO)). The prevalence in the urban, semiurban, and rural area was 17.8, 13.8, and 9.9%, respectively.[6] In another multicentric random survey, conducted in 3,674 women all over India, prevalence of GDM was 16.2% Chennai, 17.5% Ludhiana, 15% Trivandram, 12% Bengluru, 21% Alwaye, and 18.8% Erode. Overall, prevalence of GDM was 16.55% among urban women using WHO criteria. This study documented a definite increasing trend in prevalence of GDM.[7] Still no data is available from Haryana with regard to prevalence of GDM in rural population. Therefore, we have assessed the prevalence and associated risk factors of GDM in the rural block. It is envisaged that the results of this study will provide essential information regarding the need to treat and prevent GDM in a country where there is also a challenge of combating communicable infections and other forms of malnutrition.
STUDY DESIGN AND METHODS
The study was community-based cross-sectional and carried out in 35 villages of block Beri, district Rohtak, Haryana, having a population of 152,618. After taking informed written consent, 913 pregnant women who had gestational age of ≥24 weeks, were included in the study. Sample size estimation was done by assuming prevalence of GDM in rural areas as 9.9%,[6] allowable error of 20% at level of confidence of 95%, and a sample of 900 eligible subjects was required.
Before starting the study, the help of health workers and Anganwadi workers was taken to sensitize the women regarding the need of study. Day before the study, subjects were advised to observe overnight fasting (at least 8 h) and were called at the nearest health center in the morning. Investigator explained the procedure of the study to the study participants after taking detailed history and clinical examination to exclude any systemic disease.
Anthropometric measurements including body weight (to the nearest 0.1 kg) and height (to the nearest 0.001 m) were recorded in subjects without shoes and socks. Blood pressure was recorded thrice at an interval of 5 min in seating position in non-dominant arm to the nearest 2 mmHg using a standard adult mercury sphygmomanometer. Mean of the three readings was taken as blood pressure. All participants were subjected to OGTT with 75 g anhydrous glucose powder dissolved in 250-300 ml water and consumed over 5 min, counting from the start of the drink. Fasting and 2-h post glucose capillary whole blood glucose (PGCWBG) was estimated by glucose oxidase method. Patients suffering from anemia, chronic renal, pancreatic, and other severe illness; those on steroids/nicotinic acid or other medication likely to cause dysglycemia; and women with previously diagnosed diabetes were excluded from the study.
The interview schedule included information on sociodemographic profile, relevant medical and obstetric history, previous history of gestational diabetes, family history of diabetes mellitus, and awareness about gestational diabetes. WHO criteria were used to categorize the subjects with GDM and normoglycemia.[1]
Data analysis
Analysis was carried out using Statistical Package for Social Studies (SPSS) for Windows version 18.0. Categorical data are presented as percentage (%). Pearson's Chi-square test was used to evaluate differences between groups for categorized variables. In case, expected cell count was less than five in >20% cells, Fisher's exact test was used. Normally, distributed data are presented as means and standard deviation or 95% confidence interval (CI). Student's t-test for independent samples was used for comparison between gestational diabetic group and non-GDM group. Binary logistic regression analysis (stepwise method) was used to evaluate the independent associations of various factors with prevalence of GDM.
RESULTS
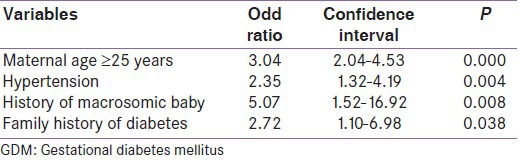
Mean age of the study population was 22.74 years (standard deviation (SD) =3.0). Prevalence of GDM was found to be 13.9% (95% CI = 11.8-16.3) using 2 h OGTT (WHO criteria). Majority of study participants (68.8%) belonged to age group 20-24 years. 78.4% study subjects were housewives, followed by 9.2% engaged in agriculture, while 5.5% were laborers. Mean height and weight of study participants were 155.6 cm (±6.7) and 54.2 kg (±8.3), respectively. Mean systolic and diastolic blood pressure were 114 mmHg (±11.9) and 74.4 mmHg (±9.4), whereas mean 2-h PGCWBG was 127 mg/dl (±24.1). Mean age of GDM women was (24.0 ± 3.1 years), significantly higher than non-GDM women (22.5 ± 2.9 years, P = 0.000). There was no significant difference between mean weight of GDM women (54.0 ± 7.6 kg) and non-GDM women (54.2 ± 8.4 kg, P = 0.796). Systolic (117 ± 13 mmHg) and diastolic blood pressure (76 ± 10 mmHg) was also higher in GDM women than non-GDM women (P = 0.009 and 0.152, respectively). The mean 2-h PGCWBG (169 ± 25 mg/dl) of GDM women was significantly higher than non-GDM women (120 ± 15.6 mg/dl, P = 0.000) [Table 1]. Significantly high prevalence of gestational diabetes was found in women with height ≤150 cm. The prevalence of GDM was higher in pregnant women with gestational period ≥30 weeks (15.8%) than women with gestational period less than 30 weeks (11.2%, P = 0.051). Women with gravida ≥3 had significantly higher prevalence of GDM (18.2%) compared to gravida <3 (11.9%, P = 0.010). History of macrosomia (birth weight ≥4,000 g) and family history of diabetes were significantly associated with prevalence of GDM (P = 0.002 and 0.03, respectively). In our study population when stepwise logistic regression analysis was performed; maternal age ≥25 years, hypertension, history of macrosomic baby, and family history of diabetes were found to have independent association with prevalence of GDM [Table 2]. Other variables like education, socioeconomic status, past history of abortion, stillbirth, birth of baby with congenital anomaly, history of previous GDM, and cesarean section were not significantly related to GDM.
Table 1.
Baseline characteristics of study population
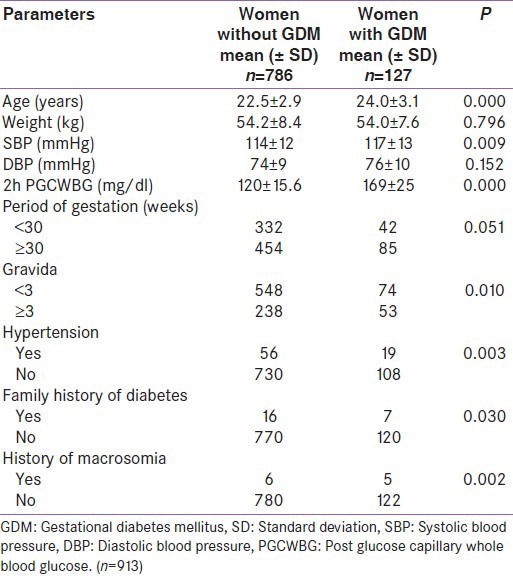
Table 2.
Odds ratio for risk factors found to be associated with GDM (based on logistic regression analysis, n=913)
DISCUSSION
Increasing trend in prevalence of GDM has been shown in various studies conducted in different regions of the country. Zargar et al., reported prevalence of GDM as 3.8% among Kashmiri women, while Verma et al., in a cross-sectional study in rural areas of Jammu reported that the prevalence of GDM was 6.7%.[8,9] In another community-based study Seshiah et al., found prevalence of GDM in urban, semiurban, and rural area of south India as 17.8, 13.8, and 9.9, respectively.[6] In our study, prevalence of gestational diabetes was 13.9% (WHO criteria) and 9.7% (American Diabetes Association (ADA) criteria) in rural population of Haryana, India. Use of different criteria for diagnosis of GDM may be responsible for different prevalence rates such as lower thresholds of Carpenter-Coustan criteria led to 50% increase in prevalence than the previously followed National Diabetes Data Group (NDDG) thresholds based on the rationale that plasma glucose levels lower than the NDDG thresholds were also associated with some increase in risk of perinatal complications.[10] Community-based studies used WHO criteria (single step 75-g OGTT) in comparison to more cumbersome ADA criteria as it serves both as screening and diagnostic test for GDM, is simple, economic, and feasible. Pregnant women need not be fasting and cause least disturbance in their routine activities, which is very important for a study carried out in community. However, the Brazilian Gestational Diabetes Study evaluated the ADA and WHO diagnostic criteria against pregnancy outcomes in an observational study of nearly 5,000 women, and found that although WHO criteria identified more cases of GDM (7.2% vs 2.4%), but both ADA and WHO criteria are valid options for the diagnosis of GDM and the prediction of adverse pregnancy outcomes.[11] The high prevalence of GDM in vast rural background, spread over 35 villages, is because of proximity of study area to National Capital Region (NCR) of Delhi and villages in small and prosperous state of Haryana, enjoy almost all urban amenities—means they have vehicles for daily movements and various electric appliances for farming and day-to-day work, leading to more sedentary lifestyle. Proximity to NCR has influenced the rural lifestyle, leading to an increase in prevalence of GDM in the area. High prevalence of GDM in Indian population has been supported by studies conducted in Australia, Europe, and the United States. Dornhorst et al., reported a higher relative risk of 7.6 (4.1-14.1) in southeast Asian women and 11.3 (6.8-11.8) in Indian women than Anglo-Celtic women living in London.[12] Yue et al., in an Australian study, reported a higher prevalence in Indian women (17.0%) compared to 3.0, 10.0, and 15.0% in Anglo-Celtic, Aboriginal, and Chinese participants, respectively.[13] Apart from ethnicity, the high prevalence of GDM in Indian population as compared to western countries can be due to trend toward older maternal age, decrease in physical activity and adoption of modern lifestyles, and increasing prevalence of obesity and diabetes.[14]
GDM showed an association with increasing age, high gravida, history of macrosomic baby, and family history of diabetes and hypertension in various studies.[6,8,9,15] In our study, prevalence of GDM was found to be significantly associated with maternal age ≥25 years, gravida ≥3, maternal height ≤150 cm, hypertension, history of macrosomic baby, and family history of diabetes on bivariate analysis; but on multiple logistic regression analysis maternal age ≥25 years, hypertension, history of macrosomic baby, and family history of diabetes were found as independent predictors of GDM.
In the age group ≥25 years, prevalence of GDM (26.3%) was significantly higher and logistic regression analysis showed that GDM is three times more likely to occur in this age group (odds ratio (OR)-3.04; 95% CI = 2.04-4.53; P < 0.001). Various other authors also reported similar findings from other parts of India.[8,15,16,17] Seshiah et al., reported significantly higher prevalence in age group ≥25 years (OR-2.10, CI = 1.87-2.37, P < 0.001).[6] Swami et al., reported maternal age as important risk factor for GDM (OR-1.32, CI = 1.20-1.55, P < 0.001).[17]
In our study, a significantly higher prevalence of GDM was observed in women with history of birth to a macrosomic baby (45.5%) as compared to those with no such history (13.5%). This is an important predicator of GDM with odds ratio of 5.07 (OR-5.07, 95% CI 1.52-16.2, P < 0.01). Bhat et al., reported that history of macrosomic baby increases the chance of developing GDM by four times (OR-4).[5] Similar findings were reported by Wagaarachchi et al., Zargar et al., and Soheilykhah et al.[8,18,19]
Family history of diabetes mellitus has been reported to be associated with higher chances of developing GDM.[8,18,20,21] In our study also, a significantly higher percentage of women with GDM had positive family history of diabetes mellitus. Logistic regression analysis revealed that family history of diabetes is a strong predicator of gestational diabetes (OR-2.72, 95% CI = 1.10-6.98, P < 0.05). In a community-based study, Seshiah et al., reported that family history of diabetes was an independent predictor of GDM (OR-1.58, 95% CI = 1.39-1.79).[6] Bhat et al., in a case-control study revealed a significant relationship between GDM and family history (OR-4.5).[5]
Highest prevalence of gestational diabetes (19.8%) was found in women having height ≤150 cm and it was statistically significant (P < 0.05). In accordance with our study, Ogonowski et al., reported that Caucasian women with GDM were significantly shorter than women without GDM (163.8 ± 6.6 vs 165.7 ± 5.6 cm; P < 0.001).[22] Moses et al., reported that GDM women were a mean of 2.8 cm shorter than non-GDM women.[23] Yang et al., and Rudra et al., also revealed similar observations.[24,25]
Zargar et al., and Berner et al., reported that women with hypertension had a higher prevalence of GDM.[8,21] In our study, mean systolic and mean diastolic blood pressure was higher in GDM women than non-GDM women. On logistic regression analysis, GDM is 2.3 times more likely to be predicted in hypertensive women (OR-2.35; CI: 1.32-4.19, P < 0.01).
To conclude, high prevalence of GDM in the present study may be because of the proximity of the study area to NCR, but it further highlights the importance of carrying out various community-based prevalence studies in different geographical regions of India to delineate the exact prevalence of GDM in the country.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Geneva: WHO; 1999. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Part 1: Diagnosis and classification of diabetes mellitus. WHO/NCD/NCS/99. [Google Scholar]
- 2.5th ed. Brussels: International Diabetes Federation; 2011. International Diabetes Federation. A summary of the figures and key findings. The IDF Diabetes Atlas. [Google Scholar]
- 3.Sadikot SM. The Epidemiology of diabetes in women and the looming epidemic of GDM in the third world. In: Tsatsoulis A, Wyckoff JA, Brown FM, editors. Diabetes in women. New York: Springer; 2009. pp. 221–36. [Google Scholar]
- 4.Seshiah V, Das AK, Balaji V, Joshi SR, Parikh MN, Gupta S Diabetes in pregnancy study group. Gestational diabetes mellitus--guidelines. For diabetes in pregnancy study group India (DIPSI) J Assoc Physicians India. 2006;54:622–8. [PubMed] [Google Scholar]
- 5.Bhat M, Ramesha KN, Sarma SP, Menon S, Sowmini CV, Kumar SG. Determinants of gestational diabetes mellitus: A case control study in a district tertiary care hospital in South India. Int J Diabetes Dev Ctries. 2010;30:91–6. doi: 10.4103/0973-3930.62599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seshiah V, Balaji V, Balaji MS, Paneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu)--A community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 7.Seshiah V, Balaji V, Balaji MS, Sanjeevi CB, Green A. Gestational diabetes mellitus in India. J Assoc Physicians India. 2004;52:707–11. [PubMed] [Google Scholar]
- 8.Zargar AH, Sheikh MI, Bashir MI, Masoodi SR, Laway BA, Wani AI, et al. Prevalence of gestational diabetes mellitus in Kashmiri women from the Indian subcontinent. Diabetes Res Clin Pract. 2004;66:139–45. doi: 10.1016/j.diabres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Verma AK, Singh B, Mengi V. Gestational diabetes in rural women of Jammu. Indian J Community Med. 2008;33:54–5. doi: 10.4103/0970-0218.39247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara A, Hedderson MM, Quesenberry CP, Selby JV. Prevalence of gestational diabetes mellitus detected by the national diabetes data group or the carpenter and coustan plasma glucose threshold. Diabetes Care. 2002;25:1625–30. doi: 10.2337/diacare.25.9.1625. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt MI, Ducan BB, Reichelt AJ, Branchtein L, Matos MC, Costa e Forti A, et al. Brazilian Gestational Diabetes Study Group. Gestational diabetes mellitus diagnosed with a 2-h 75 gm oral glucose tolerance test and adverse pregnancy outcomes. Diabetes Care. 2001;24:1151–5. doi: 10.2337/diacare.24.7.1151. [DOI] [PubMed] [Google Scholar]
- 12.Dornhorst A, Paterson CM, Nicholls JS, Wadsworth J, Chiu DC, Elkeles RS, et al. High prevalence of gestational diabetes in women from ethnic minority groups. Diabet Med. 1992;9:820–5. doi: 10.1111/j.1464-5491.1992.tb01900.x. [DOI] [PubMed] [Google Scholar]
- 13.Yue DK, Molyneaux LM, Ross GP, Constantino MI, Child AG, Turtle JR. Why does ethnicity affect prevalence of gestational diabetes? The underwater volcano theory. Diabet Med. 1996;13:748–52. doi: 10.1002/(SICI)1096-9136(199608)13:8<748::AID-DIA164>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara A, Kahn HS, Quesenberry CP, Reley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991-2000. Obstet Gynecol. 2004;103:526–33. doi: 10.1097/01.AOG.0000113623.18286.20. [DOI] [PubMed] [Google Scholar]
- 15.Kale SD, Kulkarni SR, Lubree HD, Meenakumari K, Deshpande VU, Rege SS, et al. Characteristics of gestational diabetic mothers and their babies in an Indian diabetes clinic. J Assoc Physicians India. 2005;53:857–63. [PubMed] [Google Scholar]
- 16.Seshiah V, Balaji V, Balaji MS, Sekar A, Sanjeevi CB, Green A. One step procedure for screening and diagnosis of gestational diabetes mellitus. J Obstet Gynecol India. 2005;55:525–9. [Google Scholar]
- 17.Swami SR, Mehetre R, Shivane V, Bandgar TR, Menon PS, Shan NS. Prevalence of carbohydrate intolerance of varying degrees in pregnant females in western India (Maharashtra)--a hospital- based study. J Indian Med Assoc. 2008;106:712–5. [PubMed] [Google Scholar]
- 18.Wagaarachchi PT, Fernando L, Premachadra P, Fernando DJ. Screening based on risk factors for gestational diabetes in an Asian population. J Obstet Gynaecol. 2001;21:32–4. doi: 10.1080/01443610020022087. [DOI] [PubMed] [Google Scholar]
- 19.Soheilykhah S, Mogibian M, Saghand SR, Rashidi M, Piroz M. Incidence of gestational diabetes mellitus in pregnant women. Iran J Reprod Med. 2010;8:24–8. [Google Scholar]
- 20.Garshasbi A, Faghihzadeh S, Naghizadeh MM, Ghavam M. Prevalence and risk factors for gestational diabetes mellitus in Tehran. J Family Reprod Health. 2002;2:75–80. [Google Scholar]
- 21.Bener A, Saleh NM, Al- Hamaq A. Prevalence of gestational diabetes and associated maternal and neonatal complications in a fast developing community: Global comparisons. Int J Womens Health. 2011;3:367–73. doi: 10.2147/IJWH.S26094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogonowski J, Miazgowski T. Are short women at risk for gestational diabetes mellitus? Eur J Endocrinol. 2010;162:491–7. doi: 10.1530/EJE-09-0992. [DOI] [PubMed] [Google Scholar]
- 23.Moses RG, Mackay MT. Gestational diabetes: Is there a relationship between leg length and glucose tolerance? Diabetes Care. 2004;27:1033–5. doi: 10.2337/diacare.27.5.1033. [DOI] [PubMed] [Google Scholar]
- 24.Yang X, Hsu- Hage B, Zhang H, Yu L, Dong L, Li J, et al. Gestational diabetes mellitus in women of single gravidity in Tainjin City, China. Diabetes Care. 2002;25:847–51. doi: 10.2337/diacare.25.5.847. [DOI] [PubMed] [Google Scholar]
- 25.Rudra CB, Sorensen TK, Leisenring WM, Dashow E, Williams MA. Weight characteristics and height in relation to risk of gestational diabetes mellitus. Am J Epidemiol. 2007;165:302–8. doi: 10.1093/aje/kwk007. [DOI] [PubMed] [Google Scholar]


