Abstract
Aim:
To describe the clinical profile, maternal and fetal outcomes, and the conversion rates to diabetes in women with gestational diabetes mellitus (GDM) seen at a tertiary care diabetes center in urban south India.
Materials and Methods:
Clinical case records of 898 women with GDM seen between 1991 and 2011 were extracted from the Diabetes Electronic Medical Records (DEMR) of a tertiary care diabetes center in Chennai, south India and their clinical profile was analyzed. Follow-up data of 174 GDM women was available. To determine the conversion rates to diabetes, oral glucose tolerance test (OGTT) was done in these women. Glucose tolerance status postpartum was classified based on World Health Organization (WHO) 2006 criteria.
Results:
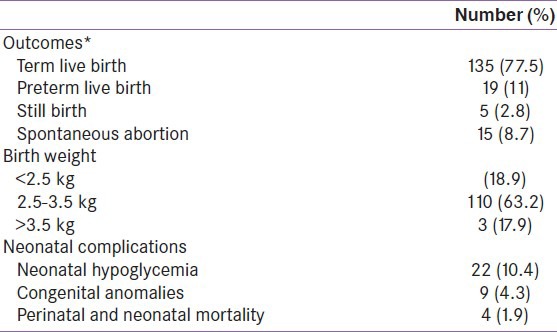
The mean maternal age of the women was 29 ± 4 years and mean age of gestation at first visit were 24 ± 8.4 weeks. Seventy percent of the women had a family history of diabetes. Seventy-eight percent of the women delivered full-term babies and 65% underwent a cesarean section. The average weight gain during pregnancy was 10.0 ± 4.2 kg. Macrosomia was present in 17.9% of the babies, hypoglycemia in 10.4%, congenital anomalies in 4.3%, and the neonatal mortality rate was 1.9%. Mean follow-up duration of the 174 women of whom outcome data was available was 4.5 years. Out of the 174, 101 women who were followed-up developed diabetes, of whom half developed diabetes within 5 years and over 90%, within 10 years of the delivery.
Conclusions:
Progression to type 2 diabetes mellitus (T2DM) in Indian women with GDM is rapid. There is an urgent need to develop standardized protocols for GDM care in India that can improve the maternal and fetal outcomes and help prevent future diabetes in women with GDM.
Keywords: American Diabetes Association, Asian Indians, gestational diabetes, progression to type 2 diabetes, WHO
INTRODUCTION
Gestational diabetes mellitus (GDM) is defined as carbohydrate intolerance of any severity identified for the first time during pregnancy.[1,2] Though this definition uniformly detected and classified GDM, its limitations have been known for many years. In 2008-2009, the International Association of Diabetes and Pregnancy Study Groups (IADPSG), an international consensus group with representatives from multiple obstetrical and diabetes organizations, including the American Diabetes Association (ADA), recommended that high-risk women found to have diabetes at their initial prenatal visit, using standard criteria, receive a diagnosis of overt, and not gestational diabetes.[3] India has the second largest number of people with diabetes in the world (62.4 million) and this number is expected to reach 100 million by the year 2030.[4,5] Rapid changes in lifestyle including unhealthy diet and physical inactivity may have contributed to this rise in prevalence of diabetes in general with a parallel increase in the rates of GDM.[6]
The prevalence of GDM is reported to vary widely from 3.8 to 21% in different parts of India depending on the geographical location and on the diagnostic criteria used.[7,8,9] GDM has been associated with neonatal morbidity and mortality, including macrosomia, shoulder dystocia, other birth injuries, and neonatal hypoglycemia, in addition to congenital anomalies and still births.[10,11] Further, the offspring are potentially at a higher risk of developing childhood obesity later in life.[12,13]
Women with GDM have higher rates of cesarean deliveries and pregnancy-induced hypertension.[14,15] Evidence suggests that a large percentage of these women are at a higher risk of developing type 2 diabetes mellitus (T2DM) in the future.[10] Despite high rates of morbidity, most women remain asymptomatic for the duration of their pregnancy and GDM goes unnoticed until a routine screening of blood sugar is carried out. Hence, it is recommended that all pregnant women undergo screening for GDM at 24-28 weeks of gestation.[16] There are several reports of GDM from India,[8,9] but few reports on outcomes or on development of diabetes postpartum.[17,18]
The objectives of this study were to describe the clinical profile, maternal and fetal outcomes, and conversion rates to diabetes in women with GDM seen at a tertiary care diabetes center in urban south India.
MATERIALS AND METHODS
In this retrospective clinic-based study, clinical case records of 1,003 pregnant women seen between the year 1991 and 2011 at Dr. Mohan's Diabetes Specialties Centre (DMDSC), a tertiary diabetes center at Chennai in south India, were extracted from the Diabetes Electronic Medical Records (DEMR). The DEMR database at DMDSC connects data of 15 centers/clinics of DMDSC in different areas in southern India and has been used as an effective tool to improve diabetes care and research.[19]
Study variables collated from the DEMR for this study included demographic profile (name, age, and contact information), clinical profile (family history, age of onset of diabetes, last menstrual period, and expected date of delivery), anthropometric measurements (height, weight, body mass index (BMI)), biochemical investigations (oral glucose tolerance test (OGTT) and glycated hemoglobin (HbA1C)), and the treatment regimen (diet/exercise/oral hypoglycemic agents/insulin).
After excluding preexisting diabetes and impaired glucose tolerance cases, normal glucose tolerance and cases with incomplete data, 898 women with GDM were included in our study. Unfortunately only 174 women (19.3%) could be followed-up to determine maternal and neonatal outcomes [Figure 1]. One possible explanation for this could be that as ours being a tertiary referral center for diabetes with no obstetric unit, women tend not to follow-up with us postpartum. Another common problem in India is that women generally go back to their maternal homes for delivery which maybe in another city and hence determining pregnancy outcomes becomes difficult. Yet, we have attempted to report pregnancy outcomes in a small cohort of women, whom we were able to trace and collect, follow-up details from.
Figure 1.
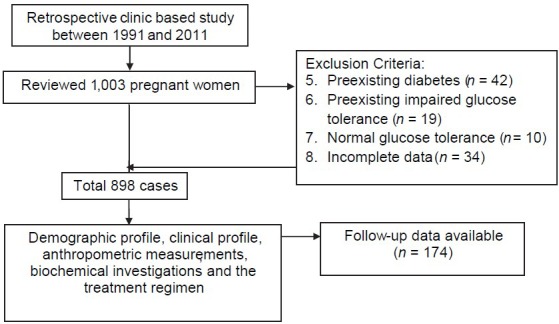
Flow chart of patient selection
Anthropometric and biochemical measurements
During the first visit, a complete medical history was elicited, including history of any past or current illnesses, dietary patterns, and family history of diabetes as well as current medications. Physical examination included height and weight measurement using standardized techniques. Height was measured in centimeters using a stadiometer and weight in kilograms using an electronic scale. BMI was calculated as weight (kg) divided by height (m) squared.
A fasting blood sample was obtained after an overnight fast of at least 8 h. Participants whose diabetes status had previously been confirmed were given a standard south Indian breakfast and a venous blood sample was drawn after 90 min for the postprandial glucose sample. In all others an OGTT using 100 g glucose load were done and were diagnosed using the earlier Carpenter and Coustan criteria adopted by the ADA.[20] HbA1C and serum fructosamine were also carried out.
Subjects in whom GDM was diagnosed were given treatment at the center. The vast majority of those not controlled with diet and exercise, received insulin. In a few cases, who were already on oral drugs (metformin) and were doing well this treatment, it was continued.
The follow-up periods varied from 6 weeks to several years and the mean follow-up duration was 4.5 years. In women who could be followed after delivery to determine progression to diabetes, an OGTT was done using 75 g glucose load.
Plasma glucose (glucose-oxidase peroxidase method) was estimated using a Hitachi–912 Autoanalyzer (Hitachi, Mannheim, Germany). HbA1c was measured by high performance liquid chromatography (HPLC) using Variant machine (Bio-Rad, Hercules, CA). The intra- and interassay coefficients of variation (CV) for the biochemical assays ranged from 3.1 to 7.6%. Serum fructosamine was measured using Beckman coulter AU480 and AU2700 analyzer. HbA1C was measured by HPLC (Bio-Rad Variant, Bio-Rad, Hercules, California, USA). All biochemical investigations were done at DMDSC laboratory, which is certified by the College of American Pathologists (CAP) and the National Accreditation Board for Testing and Calibration of Laboratories (NABL).
Definitions
Obesity was defined as those who had a BMI ≥ 25 kg/m2 and overweight as those who had a BMI ≥ 23 kg/m2 based on World Health Organization (WHO) Asia Pacific guidelines.[21] This classification was used for nonpregnant adults, that is, during the follow-up period.
For assessing obesity during pregnancy, in 2013, the Institute of Medicine (IOM) published revised pregnancy weight gain guidelines that are based on pre-pregnancy BMI ranges recommended by the WHO. These ranges are independent of age, parity, smoking history, race, and ethnic background. The revised IOM recommendations define normal weight as a BMI of 18.5-24.9, overweight as a BMI of 25-29.9, and obesity as a BMI of 30 kg/m2 or greater.[22] This IOM criteria was used to assess obesity during pregnancy.
Gestational diabetes mellitus
Since the study was based between 1991 and 2011, the older ADA criteria were used to diagnose diabetes in which samples were drawn at 1 h (1 h PG), 2 h (2 h PG), and 3 h (3 h PG) after ingestion of the glucose load of 100 g using cut points recommended by Carpenter and Coustan and adopted by ADA.[20]
Glucose tolerance status post delivery
The WHO criteria for nonpregnant adults was used for diagnosing diabetes and prediabetes, that is, for diabetes; fasting plasma glucose (FPG) >126 mg/dl or 2 h PG >200 mg/dl and for prediabetes, impaired fasting glucose (IFG); FPG between 110 and 125 mg/dl and/or impaired glucose tolerance (IGT); and 2 h PG between 140 and 199 mg/dl.[23]
Macrosomia
The Indian consensus is that a new born weighing >3.5 kg should be considered as macrosomia.[24]
Statistical analysis
Statistical analysis was done in Statistical Package for the Social Sciences ( SPSS) software (version 15) and Microsoft Excel 2007. Percent frequencies, means, and standard deviations for quantitative variables were used wherever necessary.
RESULTS
Clinical and biochemical characteristics
The clinical profile of the GDM women is described in Table 1. The mean maternal age was 29 ± 4 years (range 19-42 years), mean gestational age at first visit was 24 ± 8.4 weeks, and mean BMI was 28.6 ± 4 kg/m2. While, 34.4% of the women were obese, 47.2% were overweight, and only 18.4% had ideal body weight as per the IOM guidelines,[22] Seventy percent of GDM women had a family history of T2DM, which included 49% with one parent (either father or mother) having diabetes and 21% with both parents having diabetes. Mean pregestational weight and gestational weight in a subset of 120 women in whom it was available were 65.7 ± 13 and 71.3 ± 10.4, respectively.
Table 1.
Baseline demographic and clinical profile of women with GDM (n=898)
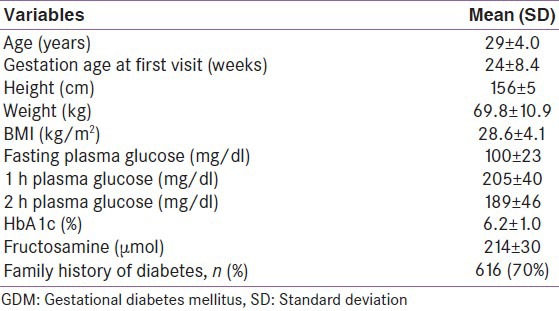
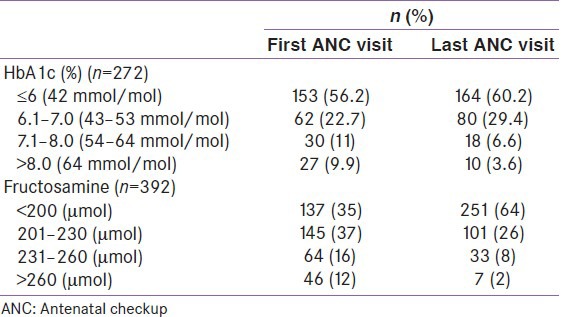
Table 2 shows the changes in HbA1C and serum fructosamine values in a subset of the population in whom the HbA1C and serum fructosamine values were available at their first and last antenatal checkup (ANC) visit before delivery. Mean HbA1c of the 272 women was found to be 6.2% at first visit which improved to 6.0% by the last ANC visit. The mean fructosamine at first visit was found to be 219 ± 37 μmol which improved to 197 ± 25 μmol at last ANC visit.
Table 2.
HbA1c and fructosamine values at first and last ANC visits
Treatment
Among 893 women with GDM, 585 (65.5%) were treated with insulin therapy, 280 (31.3%) were advised diet modification and exercise therapy, and 28 women (3.2%) were given oral hypoglycemic agents (metformin).
Mode of delivery
Seventy-two women (41.3%) underwent elective caesarian delivery, 42 (24.1%) had emergency cesarean delivery, 53 (30.5%) had normal vaginal delivery, and seven (4.1%) had induced vaginal delivery, which included assisted delivery.
Table 3 presents the neonatal outcomes and complications during delivery of the 174 women in whom outcome data was available.
Table 3.
Outcomes and neonatal complications (n=174)
Progression to T2DM
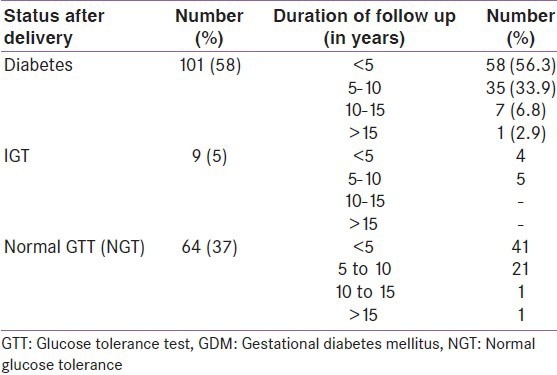
Follow up OGTT data could only be obtained in 174/898 women (19.3%). Of these 174 women, 101 (58%) developed T2DM postpartum and nine (5%) developed IGT, while 64 (37%) reverted back to normal glucose tolerance (NGT) [Table 4]. Of the 101 women who developed diabetes, 58 women (56.3%) developed diabetes within 5 years, 35 (33.9%) within 5-10 years, and eight women (9%) 10 years after delivery. Thus, 90.2% of conversion to T2DM took place within 10 years after the delivery.
Table 4.
Rate of conversion to diabetes mellitus among GDM women
DISCUSSION
This paper reports on the clinical profile, outcomes, and conversion rate to diabetes in a large sample of women with GDM seen at a tertiary diabetes care center in south India.
Several studies report a strong relationship between GDM and advancing maternal age.[25,26] Prepregnancy weight is also an established risk factor for GDM.[27] A positive correlation between maternal weight and risk of GDM was observed in studies by Seshiah et al.,[9] and Chu et al.[28] In our study, nearly half of the pregnant women were over 30 years of age and 81% were obese and 12% were overweight. Some studies have also attributed the risk of adverse outcomes associated with GDM to confounding characteristics such as obesity and advanced maternal age of women with GDM.[25,29] The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study showed that lifestyle factors and obesity contribute significantly to the increasing incidence of GDM.[12] Hence, targeting overweight women in the population with lifestyle intervention program may help prevent GDM.
There is also an important association between family history of T2DM and GDM.[9] In our study, 70% of women with GDM had family history of T2DM which is higher compared to earlier studies.
Goldman et al.,[30] reported cesarean section rates of 35.3% in women with GDM in United States with similar reports from Casey et al.[10] Along with the parallel increase in GDM, these rates have also increased over the years with 65.2% women in the present study undergoing a cesarean section.[31]
As reported in other studies,[16,32] macrosomia and perinatal mortality were the predominant adverse pregnancy outcomes. The neonatal complications are more than that reported in other studies. Higher maternal age (mean maternal age was 29 ± 5) could be hypothesized as the cause to GDM and subsequent conversion to diabetes, which is seen in our study. While macrosomic infants are at an increased risk of developing T2DM, hypertension, and obesity later in adulthood; low birth weight infants, when exposed to excess food and sedentary lifestyle later in life (catch up growth) also develop T2DM. This has been elucidated by Barker and colleagues in their thrifty phenotype hypothesis.[33,34,35] Therefore, curtailing excess weight gain during childhood is equally important to prevent diabetes.
Due to its poor sensitivity in diagnosis of GDM, very few studies have examined the role of HbA1C during pregnancy.[36,37] Our study showed that the mean HbA1C level in women with GDM at diagnosis during different trimesters was 6.2 ± 1.0%, which decreased to 6.0 ± 1% by the last ANC visit. As many women visited the clinic only in their second and third trimester there might not have been enough time for a further reduction in HbA1c to be demonstrated. Finally, some patients initially diagnosed with GDM were subsequently deemed to have type 1 diabetes which could explain higher than expected HbA1c's values.
Apart from HbA1C, we also monitored serum fructosamine values during pregnancy as it is an index of control of glucose over a 2-3 week's period. Salemans et al.,[38] identified fructosamine as a more sensitive test to detect abnormal glucose tolerance in GDM than HbA1c because it is an index of shorter term diabetes control. We found that the difference in mean HbA1C between first visit and last ANC visit was only 0.2%, but that of the mean fructosamine between the visits was 22 μmol (10% decrease). During pregnancy when hormonal changes cause fluctuation in blood glucose concentrations, estimation of fructosamine levels could be a useful test to monitor diabetes control as it reflects average blood glucose levels of shorter duration (2-3 weeks).[39] Also according to Seshiah et al., (2008) serum fructosamine, though not a useful screening test, could still be used to assess short-term maternal diabetes control during pregnancy.[24] Use of this test as an alternative for HbA1c for managing GDM in our population where iron deficiency anemia is very common during pregnancy[40] needs to be investigated further.[41,42]
We found that the conversion to T2DM occurred even within 5 years post-delivery in many women, and by 10 years, most women who would convert to diabetes had already done so. It is possible that these rates are higher because those who were at higher risk only came for the follow-up checkup. Other reports have also shown that women with GDM have a 17-63% risk of T2DM within 5-16 years.[43,44] Studies have also shown that women with GDM as well as their children are at a greater risk of developing future T2DM.[45]
There are several limitations to our study. First, it is a clinic-based study, from a tertiary care diabetes center. Second our center is not attached to any OBG/GYN clinic. Thus, being a referral diabetes center, most women do not come for follow-up regularly after the initial visit. For the same reason, we could not obtain complete maternal and fetal outcomes from all our GDM patients for the cohort. However, the strength of the study are the fairly large numbers of GDM women studied, the HbA1c and fructosamine data and the follow-up data including conversion to diabetes. This study also emphasizes the need to strategize ways to follow up GDM women in India.
In conclusion, among those who came for follow-up, almost 60% women in India with GDM seen at our center progress to T2DM within 5 years and >90%, within 10 years, post-delivery. This study emphasize the need for not just prevention, but also for developing evidence based cost-effective and accessible models of care for all women with GDM. Such a model called Women in India with GDM Strategy (WINGS) is currently under evaluation at our center and is supported by the International Diabetes Federation (IDF).[46] This model plans to focus on management of GDM right from diagnosis, treating complications, ensuring a safe delivery, and preventing or delaying progression to T2DM.
ACKNOWLEDGMENTS
We thank all the participants (GDM patients) for their cooperation. We thank Ms Anitha George and Ms Tino Xavier for their help with the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Expert committee on the diagnosis and classification of diabetes mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Gestational diabetes mellitus (Position Statement) Diabetes Care. 2004;27:S88–90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 3.Diagnosis and classification of diabetes mellitus: American Diabetes Association Diabetes Care. 2013;36:4–5. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unwin N, Whiting D, Guariguata L, Ghyoot G, Gan D, editors. 5th ed. Brussels: International Diabetes Federation; 2011. International Diabetes Federation, Diabetes Atlas; pp. 11–74. [Google Scholar]
- 5.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. ICMR-INDIAB Collaborative Study Group. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical research-India Diabetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 6.Abate N, Chandalia M. Ethnicity, type 2 diabetes and migrant Asian Indians. Indian J Med Res. 2007;125:251–8. [PubMed] [Google Scholar]
- 7.Zargar AH, Sheikh MI, Bashir MI, Masoodi SR, Laway BA, Wani AI, et al. Prevalence of gestational diabetes mellitus in Kashmiri women form the Indian subcontinent. Diabetes Res Clin Pract. 2004;66:139–45. doi: 10.1016/j.diabres.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 8.Seshiah V, Balaji V, Balaji MS, Panneerselvam A, Kapur A. Pregnancy and diabetes scenario around the world. Int J Gynaecol Obstet. 2009;104:S35–8. doi: 10.1016/j.ijgo.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 9.Seshiah V, Balaji V, Balaji MS, Panneerselvam A, Arthi T, Thamizharasi M, et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu)--A community based study. J Assoc Physicians India. 2008;56:329–33. [PubMed] [Google Scholar]
- 10.Casey BM, Lucas MJ, McIntire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90:869–73. doi: 10.1016/s0029-7844(97)00542-5. [DOI] [PubMed] [Google Scholar]
- 11.Dang K, Homko C, Reece EA. Factors associated with fetal macrosomia in offspring of gestational diabetic women. J Matern Fetal Med. 2000;9:114–7. doi: 10.1002/(SICI)1520-6661(200003/04)9:2<114::AID-MFM5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 12.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 13.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: The ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–92. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 14.Naylor CD, Sermer M, Chen E, Sykora K. Cesarean delivery in relation to birth weight and gestational glucose tolerance: Pathophysiology or practice style. Toronto Trihospital Gestational Diabetes Investigators? JAMA. 1996;275:1165–70. [PubMed] [Google Scholar]
- 15.Yogev Y, Ben-Haroush A, Chen R, Glickman H, Kaplan B, Hod M. Active induction management of labor for diabetic pregnancies at term; Mode of delivery and fetal outcome--a single center experience. Eur J Obstet Gynecol Reprod Biol. 2004;114:166–70. doi: 10.1016/j.ejogrb.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Seshiah V, Das AK, Balaji V, Joshi SR, Parikh MN, Gupta S Diabetes in Pregnancy Study Group. Gestational diabetes mellitus--guidelines. J Assoc Physicians India. 2006;54:622–8. [PubMed] [Google Scholar]
- 17.Balaji V, Balaji M, Anjalakshi C, Cynthia A, Arthi T, Seshiah V. Diagnosis of gestational diabetes mellitus in Asian-Indian women. Indian J Endocrinol Metab. 2011;15:187–90. doi: 10.4103/2230-8210.83403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxena P, Tyagi S, Prakash A, Nigam A, Trivedi SS. Pregnancy outcome of women with gestational diabetes in a tertiary level hospital of north India. Indian J Community Med. 2011;36:120–3. doi: 10.4103/0970-0218.84130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pradeepa R, Prabu AV, Jebarani S, Subhashini S, Mohan V. Use of a large diabetes electronic medical record system in India: Clinical and research applications. J Diabetes Sci Technol. 2011;5:543–52. doi: 10.1177/193229681100500309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–73. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 21.Melbourne, Australia: International Obesity Task Force; 2000. [Last accessed on March 2014]. World Health Organization, Western Pacific Region The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. WHO/IASO/IOTF. Available from: http://www.wpro.who.int/nutrition/documents/docs/Redefiningobesity.pdf . [Google Scholar]
- 22.Weight Gain during pregnancy. Committee Opinion No. 549. American college of obsteticians and gynecologists. Obstet Gynecol. 2013;121:120–2. doi: 10.1097/01.aog.0000425668.87506.4c. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organisation (WHO); 2006. [Last accessed on Jan 2014]. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: Report of a WHO/IDF consultation. Available from: http://whqlibdoc.who.int/publications/2006/9241594934_eng.pdf . [Google Scholar]
- 24.Seshiah V, Balaji V. Diabetes and pregnancy (Gestational Diabetes) In: Tripathy BB, Chandalia HB, Das AK, Rao PV, Madhu SV, Mohan V, editors. RSSDI Textbook of Diabetes Mellitus. 2nd ed. RSSDI; 2008. pp. 1060–1079. [Google Scholar]
- 25.Xiong X, Saunders LD, Wang FL, Demianczuk NN. Gestational diabetes mellitus: Prevalence, risk factors, maternal and infant outcomes. Int J Gynaecol Obstet. 2001;75:221–8. doi: 10.1016/s0020-7292(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 26.Makgoba M, Savvidou MD, Steer PJ. An analysis of the interrelationship between maternal age, body mass index and racial origin in the development of gestational diabetes mellitus. BJOG. 2012;119:276–82. doi: 10.1111/j.1471-0528.2011.03156.x. [DOI] [PubMed] [Google Scholar]
- 27.Torloni MR, Betrán AP, Horta BL, Nakamura MU, Atallah AN, Moron AF, et al. Prepregnancy BMI and the risk of gestational diabetes: A systematic review of the literature with meta-analysis. Obes Rev. 2009;10:194–203. doi: 10.1111/j.1467-789X.2008.00541.x. [DOI] [PubMed] [Google Scholar]
- 28.Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care. 2007;30:2070–6. doi: 10.2337/dc06-2559a. [DOI] [PubMed] [Google Scholar]
- 29.Spellacy WN, Miller S, Winegar A, Peterson PQ. Macrosomia--maternal characteristics and infant complications. Obstet Gynecol. 1985;66:158–61. [PubMed] [Google Scholar]
- 30.Goldman M, Kitzmiller JL, Abrams B, Cowan RM, Laros RK., Jr Obstetric complications with GDM. Effects of maternal weight. Diabetes. 1991;40:79–82. doi: 10.2337/diab.40.2.s79. [DOI] [PubMed] [Google Scholar]
- 31.Moses RG, Knights SJ, Lucas EM, Moses M, Russell KG, Coleman KJ, et al. Gestational diabetes: Is a higher cesarean section rate inevitable? Diabetes Care. 2000;23:15–7. doi: 10.2337/diacare.23.1.15. [DOI] [PubMed] [Google Scholar]
- 32.Mitanchez D. Foetal and neonatal complications in gestational diabetes: Perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab. 2010;36:617–27. doi: 10.1016/j.diabet.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 33.McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH. Birth weight and non-insulin dependent diabetes: Thrifty genotype, thrifty phenotype, or surviving baby genotype? BMJ. 1994;308:942–5. doi: 10.1136/bmj.308.6934.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsons TJ, Power C, Logan S, Summerbell CD. Childhood predictors of adult obesity: A systematic review. Int J Obes Relat Metab Disord. 1999;23:S1–107. [PubMed] [Google Scholar]
- 35.Tian JY, Cheng Q, Song XM, Li G, Jiang GX, Gu YY, et al. Birth weight and risk of type 2 diabetes, abdominal obesity and hypertension among Chinese adults. Eur J Endocrinol. 2006;155:601–7. doi: 10.1530/eje.1.02265. [DOI] [PubMed] [Google Scholar]
- 36.Cousins L, Dattel BJ, Hollingsworth DR, Zettner A. Glycosylated hemoglobin as a screening test for carbohydrate intolerance in pregnancy. Am J Obstet Gynecol. 1984;150:455–60. doi: 10.1016/s0002-9378(84)90420-4. [DOI] [PubMed] [Google Scholar]
- 37.Rajput R, Yadav Y, Rajput M, Nanda S. Utility of HbA1c for diagnosis of gestational diabetes mellitus. Diabetes Res Clin Pract. 2012;98:104–7. doi: 10.1016/j.diabres.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Salemans TH, van Dieijen-Visser MP, Brombacher PJ. The value of HbA1c and fructosamine in predicting impaired glucose tolerance--an alternative to OGTT to detect diabetes mellitus or gestational diabetes. Ann Clin Biochem. 1987;24:447–52. doi: 10.1177/000456328702400504. [DOI] [PubMed] [Google Scholar]
- 39.Swami KK, Choudhary PR. Role of serum fructosamine assay in screening of gestational diabetes mellitus. Indian J Appl Basic Med Sci. 2012;4A(1B):469–471. [Google Scholar]
- 40.Unnikrishnan R, Mohan V. Challenges in estimation of glycated hemoglobin in India. Diabetes Technol Ther. 2013;15:897–9. doi: 10.1089/dia.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nasrat HA, Ajabnoor MA, Ardawi MS. Fructosamine as a screening-test for gestational diabetes mellitus: A reappraisal. Int J Gynaecol Obstet. 1991;34:27–33. doi: 10.1016/0020-7292(91)90534-c. [DOI] [PubMed] [Google Scholar]
- 42.Lao TT, Ho LF. Impact of iron deficiency anemia on prevalence of gestational diabetes mellitus. Diabetes Care. 2004;27:650–6. doi: 10.2337/diacare.27.3.650. [DOI] [PubMed] [Google Scholar]
- 43.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: A systematic review. Diabetes Care. 2002;25:1862–8. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 44.Hanna FW, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351–8. doi: 10.1046/j.1464-5491.2002.00684.x. [DOI] [PubMed] [Google Scholar]
- 45.Fletcher B, Gulanick M, Lamendola C. Risk factors for type 2 diabetes mellitus. J Cardiovasc Nurs. 2002;16:17–23. doi: 10.1097/00005082-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 46. [Last accessed on 2013 Sept 26]. Available from: http://www.idf.org/women-india-gdm-strategy-wings .


