Abstract
Objectives:
This retrospective cohort study analyzed the clinical data of cancer patients conducted in a cancer hospital, Chennai to assess the correlation (if any) between use of antidiabetic agents including pioglitazone and the incidence of bladder cancer.
Materials and Methods:
Totally, 5079 cancer patients’ with and without diabetes were included and analyzed in this retrospective study.
Results:
A total of 1077 patient data were screened out of a total of 5079. A total of 20 patients were found to have bladder cancer. Out of 1077 patients, 31 were pioglitazone users on the drug for not less than 2 years. The remaining 1046 were on other drugs other than pioglitazone. It is observed that 1 out of 31 developed bladder cancer in the pioglitazone group 19 out of 1046 developed bladder cancer in the nonpioglitazone group. The result of the analysis indicates that there is no significant (P = 0.918) association between pioglitazone and bladder cancer.
Conclusion:
In this retrospective study, the number of diabetic patients on pioglitazone with bladder cancer was fewer than the diabetic patients on other medications with the disease. Further, no link could be established between any specific drug use and bladder cancer. Least number of patients with bladder cancer was on pioglitazone, suggesting that pioglitazone alone cannot be considered a cause for increased incidence of bladder cancer in diabetic patients.
Keywords: Bladder cancer, diabetes, pioglitazone
INTRODUCTION
Changes in human behavior and lifestyle over the last century have resulted in a dramatic increase in the prevalence of type 2 diabetes and in addition to “diabesity” and the “metabolic syndrome.”[1] The risk of developing type 2 diabetes mellitus (T2DM) is particularly high among South Asians, which comprise one-fifth of the total world's population.[2] Obese individuals, both diabetic and nondiabetic, are characterized by insulin resistance and compensatory hyperinsulinemia, in as a consequence of beta cell dysfunction.[3] Metformin and thiazolidinediones (TZDs) are commonly used agents in diabetes management. Metformin, a partial insulin-sensitizing agent, is the gold standard first-line treatment for type 2 diabetes.[4] This recommendation is based on data from the UKPDS 34 that showed that metformin can improve cardiovascular outcomes in overweight patients with type 2 diabetes.[5] Pioglitazone is a TZD that acts mainly via peroxisome proliferator-activated receptor gamma to improve insulin sensitivity and is licensed for use in combination with metformin in obese patients.[6,7] Combining a TZD with metformin should enable additive clinical effects to be achieved through their different mechanisms of action.[8] Recently, however, there have been contradictory reports of a probable association between pioglitazone use and bladder cancer. A study by Lewis et al.,[9] did not observe a statistically significant increase in risk of bladder cancer among patients treated with pioglitazone for <2 years. However, the analyses addressing increasing exposure to pioglitazone observed a weak increased risk with long-term therapy. In another study Tseng[10] reported that there was an insignificant 30% overall increase in bladder cancer risk among pioglitazone users. However, all bladder cancers occurred within 2 years of the start of therapy and no patients with a cumulative dose >28,000 mg developed bladder cancer, suggesting that there was no direct cause and effect relationship could be established on pioglitazone and bladder cancer. This study was undertaken to assess a link, if any, on glitazones as a cause of bladder cancer in the Indian population.
MATERIALS AND METHODS
The present study was conducted in a cancer hospital, Chennai. Totally, 5079 cancer patients’ file has analyzed for this study. From the total cancer patients’ file diabetic with cancer patients’ file has selected and gone through. Particularly patients with diabetes mellitus medications were noted.
File screening
The medical records of the patients were analyzed with the permission of the administration of the hospital. We found the total detailed files as 5079 in the hospital. Since we were focused on cancer associated with diabetes, we screened out all diabetes files out of the total files. All the details have been noted from the file like details of cancer, diabetes mellitus medications, year of diagnosis of diabetes and cancer, and these details were recorded telephonically too.
RESULTS
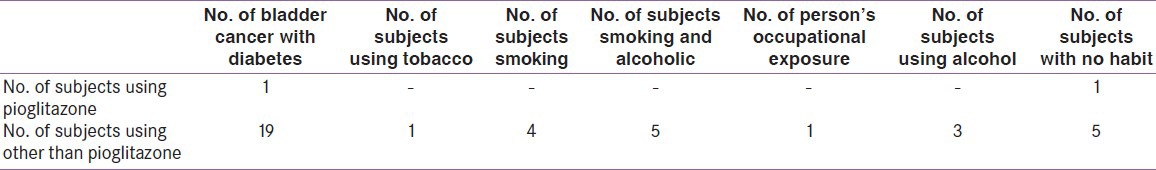
A total of 1077 diabetic patient data were screened out of a total of 5079 cancer patient. A total of 20 patients were found to have bladder cancer. Out of 1077 patients, 31 were pioglitazone users, on the drug for not less than 2 years. Pioglitazone was used for 2 or more years, at dosages of 7.5 and 15 mg. The remaining 1046 were on other drugs other than pioglitazone, namely metformin, glimepiride, combination of metformin and glimepiride, vildagliptin, acarbose, sitagliptin, glibenclamide, and iInsulin., It is observed that 1 out of 31 developed bladder cancer in the pioglitazone group 19 out of 1046 developed bladder cancer in the nonpioglitazone group. The result of the analysis indicates that there is no significant (P = 0.918) association between pioglitazone and bladder cancer [Table 1]. Out of 2.96% pioglitazone users, the occurrence of bladder cancer was very low, that is, 0.39%. The medications being taken by the subjects were recorded telephonically. Information on use of tobacco, habits of smoking and alcohol, occupational exposure were also noted [Table 2].
Table 1.
Number of cases
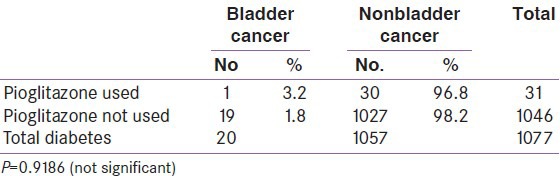
Table 2.
Analysis of bladder cancer with diabetes: A survey
DISCUSSION
This retrospective cohort study aimed to analyze the probable link between antidiabetic agents and incidence of bladder cancer in a cancer patient cohort. The study also analyzed the other potential risk factors of cancer in these patients. In this study, the number of diabetic patients on pioglitazone with bladder cancer was the lowest as compared with patients on other antidiabetic agents, including metformin, sulphonylureas, DPP 4 inhibitors, and insulin. These results are consistent with other recently published studies in which an association between pioglitazone use and bladder cancer has not been found. In the study by Song et al.,[11] a relationship between pioglitazone use and incidence of bladder cancer was not observed in Korean diabetic patients.
In another study by Wei et al.,[12] 66 and 803 new cases of bladder cancer occurred in the pioglitazone and other antidiabetic drug group respectively, suggesting that pioglitazone may not be significantly associated with an increased risk of bladder cancer in patients with type 2 diabetes.
In the light of the above results, key issues relating to bladder cancer and cancer risk associated with a medication needs to be viewed in the appropriate perspective. Bladder cancer incidence differs among different ethnicities, with men consistently showing a higher risk than their female counterparts within the same ethnicities, and a higher incidence of bladder cancer in Caucasians as compared with blacks and Asians.[13]
The incidence of bladder cancer must also be viewed keeping in mind that pioglitazone is usually a second- or third-line antidiabetic agent, and the users may be elderly, with longer diabetes duration, poorer glycemic control, and higher rates of complications and comorbidities.[14]
On the contrary, there are increasing data on benefits of pioglitazone both in diabetes prevention and in diabetes management[15] as well as in long-term cardiovascular morbidity and mortality as seen from the results of PROACTIV.[16]
Results of several studies including our own indicate that pioglitazone does not appear to raise the risk of bladder cancer in diabetic patients, any more than other antidiabetic agents. The benefits offered by pioglitazone are far higher than the alleged risk. It has been suggested that it would be ideal to use pioglitazone where indicated, when indicated, as per guidelines, preferably at lower doses of 7.5-15 mg. In our study too, patients were on this dosage.[14]
CONCLUSION
In this retrospective study, the number of diabetic patients on pioglitazone with bladder cancer was fewer than the diabetic patients on other medications with the disease. Further, no link could be established between any specific drug use and bladder cancer. Least number of patients with bladder cancer was on pioglitazone, suggesting that pioglitazone alone cannot be considered a cause for increased incidence of bladder cancer in diabetic patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Bakker LE, Sleddering MA, Schoones JW, Meinders AE, Jazet IM. Pathogenesis of type 2 diabetes in South Asians. Eur J Endocrinol. 2013;169:R99–114. doi: 10.1530/EJE-13-0307. [DOI] [PubMed] [Google Scholar]
- 3.Seshiah V, editor. A Hand Book on Diabetes Mellitus. 6th ed. Chennai: All India Publishers and distributors; 2013. Aetiopathogenesis of diabetes mellitus; pp. 29–53. [Google Scholar]
- 4.Goodarzi MO, Bryer-Ash M. Metformin revisited: Re-evaluation of its properties and role in the pharmacopoeia of modern antidiabetic agents. Diabetes Obes Metab. 2005;7:654–65. doi: 10.1111/j.1463-1326.2004.00448.x. [DOI] [PubMed] [Google Scholar]
- 5.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 6.Avandia Summary of Product Characteristics. 2005 [Google Scholar]
- 7.ACTOS Summary of Product Characteristics. 2005 [Google Scholar]
- 8.Bailey CJ, Feher MD. Birmingham: Sherborne Gibbs Limited; 2004. Therapies for diabetes including oral agents and insulins. [Google Scholar]
- 9.Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, Jr, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: Interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916–22. doi: 10.2337/dc10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng CH. Pioglitazone and bladder cancer: A population-based study of Taiwanese. Diabetes Care. 2012;35:278–80. doi: 10.2337/dc11-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song SO, Kim KJ, Lee BW, Kang ES, Cha BS, Lee HC. The risk of bladder cancer in korean diabetic subjects treated with pioglitazone. Diabetes Metab J. 2012;36:371–8. doi: 10.4093/dmj.2012.36.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei L, MacDonald TM, Mackenzie IS. Pioglitazone and bladder cancer: A propensity score matched cohort study. Br J Clin Pharmacol. 2012;75:254–9. doi: 10.1111/j.1365-2125.2012.04325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tseng CH. Pioglitazone and bladder cancer in human studies: Is it diabetes itself, diabetes drugs, flawed analyses or different ethnicities? J Formos Med Assoc. 2012;111:123–31. doi: 10.1016/j.jfma.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Kalra S, Dhamija P, Sahay R. Pioglitazone: A prudent prescription. Indian J Endocrinol Metab. 2013;17:370–2. doi: 10.4103/2230-8210.111594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: A 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23:1605–11. doi: 10.2337/diacare.23.11.1605. [DOI] [PubMed] [Google Scholar]
- 16.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (prospective pioglitazone clinical trial in macrovascular events): A randomized controlled trial. Lancet. 2005;366:1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]


