Abstract
Neural reflexes support homeostasis by modulating the function of organ systems. Recent advances in neuroscience and immunology have revealed that neural reflexes also regulate the immune system. Activation of the vagus nerve modulates leukocyte cytokine production and alleviates experimental shock and autoimmune disease, and recent data have suggested that vagus nerve stimulation can improve symptoms in human rheumatoid arthritis. These discoveries have generated an increased interest in bioelectronic medicine, i.e., therapeutic delivery of electrical impulses that activate nerves to regulate immune system function. Here, we discuss the physiology and potential therapeutic implications of neural immune control.
Keywords: immunoneurology, inflammation, vagus nerve, homeostasis, immunity
a principle of mammalian physiology is that the nervous system supports homeostasis by modulating the function of organ systems. The basic organizational unit of the nervous system is the reflex arc, composed of sensory (afferent) neurons that report to the central nervous system (CNS) and motor (efferent) neurons that project regulatory signals to target tissues. CNS integration of information allows for a purposeful and rapid adaptation to changing demands on the organism through autonomic reflexes. For example, the baroreflex regulates heart rate (HR) and blood pressure to optimize organ perfusion and delivery of O2 and nutrients (58).
Although reflex physiology has been a field of extensive research, limited attention has been given to neural control of inflammation and immunity. However, recent advances in neuroscience and immunology have revealed that neural reflexes regulate immune system function in a range of species, from evolutionarily ancient animals, i.e., Caenorhabditis elegans, up to complex mammals(43, 56). These new insights have identified novel potential therapeutic targets for treatment of inflammatory disease and spawned exploration of bioelectronic medicine, i.e., therapeutic delivery of electrical impulses that activate nerves for treatment of disease (18). Applications of the knowledge on neural reflex regulation of inflammation are now evolving from experimental animal models to human clinical trials (9, 30, 43).
Nerves Sense Inflammation and Interact With Leukocytes
Inflammation is a key process in mammalian defense against pathogens and in wound healing. Molecular products of microbial invasion and tissue damage are detected by pattern recognition receptors (PRRs), which activate cells in the innate immune system, e.g. macrophages. This early response gives rise to a cascade of events aimed to clear pathogens and ultimately restore health, and includes the production of proinflammatory cytokines and recruitment of leukocytes (Fig. 1). The initial process promotes the classic symptoms of localized inflammation, including swelling, redness, and loss of function, and may progress to include systemic responses mediated by the CNS, such as fever, anorexia, and fatigue (66). Because excessive inflammation and cytokine production in itself can cause tissue damage, nonresolving inflammation, inflammatory diseases, shock, and even death (40, 61), it is essential that the inflammatory response is tightly regulated and controlled. Multiple control mechanisms that balance proinflammatory mechanisms have been described, for example, release of inhibitory cytokines and soluble cytokine receptors as well as activation of regulatory lymphocyte subsets (39). Interestingly, PRRs (e.g., Toll-like receptors and nucleotide-binding oligomerization domain receptors) and receptors for cytokines and prostaglandins are also expressed by sensory neurons (13) and provide a molecular mechanism for the CNS to acquire information on localized inflammation in the periphery. Indeed, sensory nerves can respond to the presence of microbial products independent of immune activation (12). Since sensory nerves form a dense network throughout the external surfaces of the body, it has been suggested that this innervation provides an anatomic basis for precise sensing by the CNS of pathogen invasion, tissue damage, and inflammation (13). Reciprocally, a range of immune cells express receptors for neurotransmitters, including dopamine, ACh, and norepinephrine, which can regulate leukocyte activity and differentiation (16, 29, 44, 47, 67). Autonomic nerves innervate lymphoid tissue and other organs and regulate immune responses (22, 28, 38, 55). Hence, an anatomic and molecular framework for sensing and regulating immune system activity in a reflexive manner is in place.
Fig. 1.
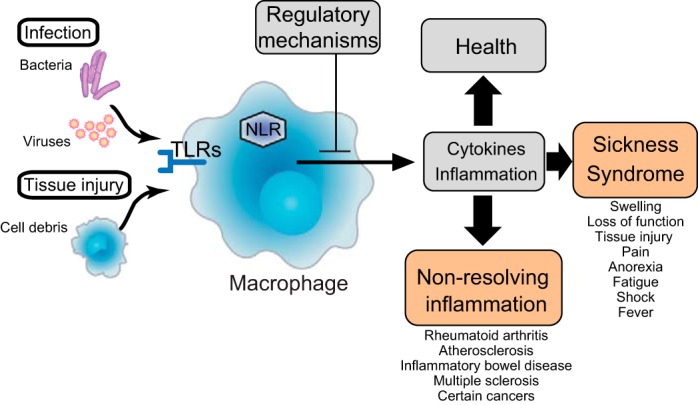
The immune response. Invasion and tissue injury are sensed by pattern recognition receptors that activate innate immune cells. This early response gives rise to a cascade of events, including the release of proinflammatory cytokines and recruitment of leukocytes, aimed to clear pathogens and ultimately restore health. In addition to the local tissue response with swelling and redness, fever, anorexia, and fatigue may develop, indicating that the inflammation is sensed by the central nervous system (CNS). It is crucially important that the immune response is regulated within narrow limits and terminated as soon as the threat has been properly addressed. Otherwise, the reaction may cause excessive tissue damage, nonresolving inflammation and inflammatory disease, and even death. A number of regulatory mechanisms are in place, including suppressor lymphocytes, inhibitory cytokines, and neural reflex circuits. Pattern recognition receptors are indicated as Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain receptors (NLRs).
The Inflammatory Reflex
Important insights into the neural control of inflammation have come from the study of a prototypical example of a neural immune-regulatory circuit termed the “inflammatory reflex.” In this reflex, afferent signals transmitted through the vagus nerve are processed in the CNS and culminate in efferent vagus nerve activity that regulates macrophage cytokine release in the spleen (60). Our current understanding of this reflex is based on a number of seminal discoveries. Watkins et al. and other research teams (10, 49, 65) found that the fever response to low doses of IL-1β injected into the intraperitoneal cavity of rats requires an intact vagus nerve, because animals with a subdiaphragmatically severed vagus nerve fail to develop a pyrexic response to the injected cytokine. Moreover, injection of IL-1β into the portal vein in rats gives rise to increased afferent activity in the vagus nerve and increased activity in the splenic nerve, but not when the hepatic branch of the vagus nerve had been ablated (41). Thus, signaling in the vagus nerve plays a role in the systemic response to cytokines. Tracey and colleagues (8) found that CNI-1493, a tetravalent guanylhydrazone with anti-inflammatory effects, is significantly more potent when given directly in the brain (intracerebroventricularly) compared with intravenous dosing and that an intact vagus nerve is required for its effect. These findings indicate that signals originating in the CNS can travel through the cholinergic vagus nerve and regulate inflammation in the periphery. Indeed, electrical stimulation of the vagus nerve significantly reduces systemic TNF levels and prevents shock in experimental endotoxemia, whereas surgical ablation of the vagus nerve has the opposite effect in this model (9).
The spleen is a major source of systemic TNF in endotoxemia, and it has been convincingly demonstrated that electrical vagus nerve stimulation (VNS) can significantly reduce cytokine production in the spleen. However, the vagus nerve does not directly innervate the spleen. It travels to the coeliac ganglion, where the adrenergic splenic nerve arises. Electrical stimulation of the splenic nerve reduces splenic cytokine production (28), and the splenic nerve is necessary for vagus nerve-mediated inhibition of TNF release from splenic macrophages (51). Furthermore, activation of cholinergic α7-nicotinic ACh receptors (α7nAChRs) is required for vagus nerve-mediated inhibition of their TNF release (64). Given that nerve fibers in the spleen lack the machinery to produce ACh, how are signals in this conduit relayed to cholinergic receptors in the spleen?
Specialized T Cells Are an Integral Component of the Inflammatory Reflex
Despite the lack of splenic cholinergic innervation, the spleen is known to contain the neurotransmitter ACh (15). The source of this ACh has not been extensively studied. Pioneering work by Kawashima and colleagues (20, 21, 48) showed that some lymphocytes, including T cells, express the enzyme choline acetyltransferase (ChAT) and are capable of ACh biosynthesis. We discovered that electrical VNS fails to inhibit systemic TNF release in endotoxemia in T cell-deficient nude mice (52). Interestingly, lymphocytes are found in close apposition to adrenergic nerve endings in the spleen (19, 52), and T cell expression of adrenergic receptors is required for the integrity of the efferent arm of the inflammatory reflex (63). This proximity of nerves and ChAT+ lymphocytes in the spleen and the expression of adrenergic receptors on ChAT+ T cells (52) provide a basis for direct signals between nerves and splenic lymphocytes. Adoptive transfer of ChAT+, but not ChAT−, T lymphocytes to nude mice restores the inhibitory effect of VNS on TNF release (52). Collectively, these data support that the ACh production and release required for inhibition of TNF in the spleen is provided by ChAT+ T lymphocytes.
Macrophages in the spleen are a major source of systemic TNF in experimental endotoxemia (25, 51). Monocytes and macrophages express cholinergic receptors and respond to cholinergic agonists with reduced release of proinflammatory cytokines (4, 50). Expression of α7nAChRs is, furthermore, essential for vagus nerve-mediated inhibition of TNF release in endotoxemia and required for the macrophage response to cholinergic agonists (64). Since α7nAChRs are expressed in several organs, including the CNS and autonomic ganglia, it was conceivable that the cholinergic signal required for the integrity of the inflammatory reflex occurred outside the immune system and not in splenic macrophages. Experiments with chimeric mice created by bone marrow transfer demonstrated that α7nAChR deficiency in nonbone marrow-derived cells did not abolish vagus nerve inhibition of cytokine production in endotoxemia. In contrast, α7nAChR deficiency in bone marrow-derived cells, including leukocytes, abolished the inhibitory effect of VNS on TNF production (42). These observations indicate that signaling through α7nAChRs on macrophages in the spleen is essential for the integrity of the inflammatory reflex.
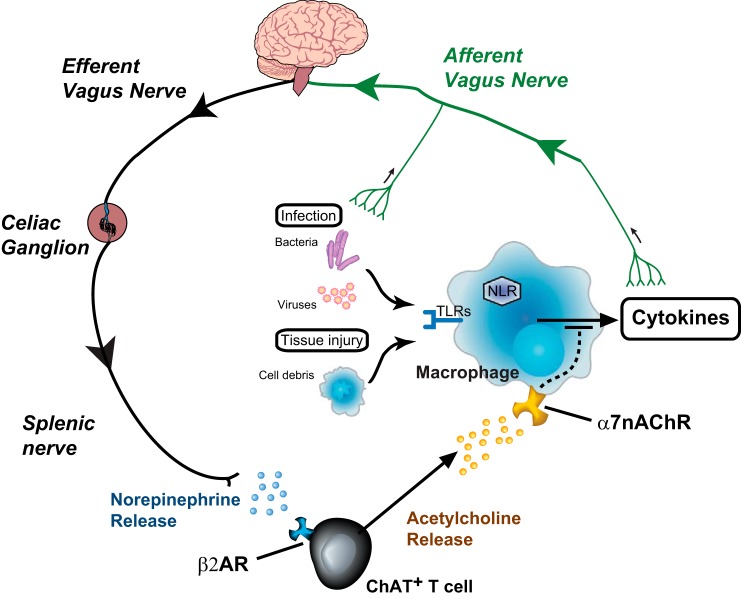
Thus, electrical signals in vagus nerve fibers that terminate in the celiac ganglion (5) activate the splenic nerve to release norepinephrine, which, in turn, activates ChAT+ T cells in the spleen to release ACh and inhibit TNF release by activating α7nAChRs on macrophages (Fig. 2).
Fig. 2.
The inflammatory reflex. The current understanding of the inflammatory reflex, an immune-regulatory vagus nerve circuit, is that sensory nerve fibers, e.g., afferent vagus nerve branches, report on localized cytokine levels and inflammation in the periphery. This information is processed in the brain stem, which generates efferent signals that travel through motor fibers in the vagus nerve and activate the adrenergic splenic nerve, which releases norepinephrine in the spleen. Specialized choline acetyltransferase-expressing T cells (ChAT+ T cells) release ACh in response to norepinephrine and inhibit macrophage cytokine production by activating their α7-nicotinic ACh receptors (α7nAChRs). β2AR, β2-adrenergic receptors.
Therapeutic Effects of Electrical VNS
Activation of signaling in the inflammatory reflex by electrical nerve stimulation or pharmacological means not only attenuates TNF production but can also treat a variety of diseases with an inflammatory component in animal models (for a review, see Ref. 2). For example, activation of the efferent arm of the inflammatory reflex reduces mortality after cecal ligation and puncture (CLP; a sepsis model), whereas disabling vagus signaling increases mortality (7, 24, 45). Central activation of the inflammatory reflex ameliorates colitis (26). VNS, as well as α7nAChR agonist administration, ameliorates a range of experimental autoimmune diseases, including collagen-induced arthritis (2, 27, 62, 68, 69). In humans, analysis of HR variability (HRV) has been used as a measure of vagus nerve activity (53). Interestingly, HRV measurements upon hospital admission have a predictive value for outcome in human sepsis (1, 11, 46). Vagus nerve activity, as measured by HRV, is also a prognostic marker for ischemic cardiovascular disease (6, 14, 31, 35) and is reduced in autoimmune diseases, including rheumatoid arthritis (17, 32, 34, 54, 57). The findings on the correlation of HRV to disease do not establish causality of vagus activity for disease development but support the notion that modulation of vagus nerve signals may treat disease. Preliminary data from a phase II clinical trial of an implantable electrical vagus nerve stimulator in patients suffering from rheumatoid arthritis were recently presented (30). In this study, the stimulator was activated by the patient for <5 min/day by passing a magnet over the implanted device. Levels of disease activity score and C-reactive protein levels in blood were significantly reduced by the treatment and returned toward baseline levels when treatment was halted. These findings are encouraging, since they suggest that symptoms of rheumatoid arthritis and inflammation may potentially be controlled and treated by VNS in humans. Clinical trials of VNS as a treatment for other inflammatory diseases, e.g., Crohn's disease, are also under way. It would be very interesting to investigate the potential of VNS for the treatment of other debilitating diseases with an inflammatory pathogenesis, including atherothrombosis (23, 33).
Other Immune-Regulatory Neural Reflexes
In addition to the vagus nerve circuit described above, a number of other neural immune control pathways have been described. Electrical stimulation of the sciatic nerve has been reported to improve outcome in experimental bacterial peritonitis (59). VNS reduces murine marginal zone B cell migration and antigen production in the spleen in response to blood-borne streptococci (37). VNS also reduces experimental inflammatory bowel disease in splenectomized animals, indicating that discrete innervation by the vagus nerve regulates the inflammatory response at different locations (36, 43). Furthermore, in experimental murine stroke, invariant natural killer T (iNKT) cell migration in the liver is arrested in response to adrenergic signals. This is a mechanism behind the increased susceptibility to fatal infections after stroke in mice, and blockade of the adrenergic nerve signaling restores iNKT migratory activity and reduces complications to infection in this model (67). In addition, Arima and colleagues (3) showed in experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis, that autoreactive T cells enter the CNS at a specific spinal cord location. In this case, CNS access for T cells depends on a reflex neural circuit originating in hindleg muscle contractions. These nervous signals promote vascular chemokine (C-C motif) ligand 20 expression, which, in turn, facilitates T cell entry into the CNS. Disease development in this model was prevented by inhibition of the afferent nerve signals originating in soleus muscle contractions that reach the spinal cord at this specific lumbar location (3). It is conceivable that some of the neural reflexes that regulate inflammation and immune cell activity can be therapeutically modulated for the treatment of clinical disease. Furthermore, monitoring of signals in the neuronal circuits that regulate immune system activity may prove to be of diagnostic value in conditions with nonresolving inflammation.
Conclusions
Neural reflex circuits regulate and optimize organ function, including immune responses, in evolutionarily ancient animals and mammals. Developments in the field of neural immune control have spawned clinical trials focused on novel therapies for inflammatory disease, for example, drugs that modulate α7nAChR signaling and implantable nerve stimulators. The emerging field of bioelectronic medicine holds promise, and it is possible that nerve stimulators will be part of the standard treatment for select inflammatory diseases in a not very distant future.
GRANTS
This work was supported by funding from Svenska Läkaresällskapet.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.S. and P.S.O. prepared figures; E.S. and P.S.O. drafted manuscript; E.S. and P.S.O. edited and revised manuscript; E.S. and P.S.O. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Ben Steinberg, Dr. Valentin A. Pavlov, and Dr. Kevin J. Tracey for helpful comments.
REFERENCES
- 1.Ahmad S, Tejuja A, Newman KD, Zarychanski R, Seely AJ. Clinical review: a review and analysis of heart rate variability and the diagnosis and prognosis of infection. Crit Care 13: 232, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol 30: 313–335, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arima Y, Harada M, Kamimura D, Park JH, Kawano F, Yull FE, Kawamoto T, Iwakura Y, Betz UA, Marquez G, Blackwell TS, Ohira Y, Hirano T, Murakami M. Regional neural activation defines a gateway for autoreactive T cells to cross the blood-brain barrier. Cell 148: 447–457, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Bencherif M, Lippiello PM, Lucas R, Marrero MB. Alpha7 nicotinic receptors as novel therapeutic targets for inflammation-based diseases. Cell Mol Life Sci 68: 931–949, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthoud HR, Powley TL. Interaction between parasympathetic and sympathetic nerves in prevertebral ganglia: morphological evidence for vagal efferent innervation of ganglion cells in the rat. Microsc Res Tech 35: 80–86, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation 88: 927–934, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Boland C, Collet V, Laterre E, Lecuivre C, Wittebole X, Laterre PF. Electrical vagus nerve stimulation and nicotine effects in peritonitis-induced acute lung injury in rats. Inflammation 34: 29–35, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Borovikova LV, Ivanova S, Nardi D, Zhang M, Yang H, Ombrellino M, Tracey KJ. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci 85: 141–147, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Bret-Dibat JL, Bluthe RM, Kent S, Kelley KW, Dantzer R. Lipopolysaccharide and interleukin-1 depress food-motivated behavior in mice by a vagal-mediated mechanism. Brain Behav Immun 9: 242–246, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Chen WL, Kuo CD. Characteristics of heart rate variability can predict impending septic shock in emergency department patients with sepsis. Acad Emerg Med 14: 392–397, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, Bubeck Wardenburg J, Hwang SW, Carroll MC, Woolf CJ. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501: 52–57, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci 15: 1063–1067, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coviello I, Pinnacchio G, Laurito M, Stazi A, Battipaglia I, Barone L, Mollo R, Russo G, Villano A, Sestito A, Lanza GA, Crea F. Prognostic role of heart rate variability in patients with ST-segment elevation acute myocardial infarction treated by primary angioplasty. Cardiology 124: 63–70, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Dale HH, Dudley HW. The presence of histamine and acetylcholine in the spleen of the ox and the horse. J Physiol 68: 97–123, 1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52: 595–638, 2000. [PubMed] [Google Scholar]
- 17.Evrengul H, Dursunoglu D, Cobankara V, Polat B, Seleci D, Kabukcu S, Kaftan A, Semiz E, Kilic M. Heart rate variability in patients with rheumatoid arthritis. Rheumatol Int 24: 198–202, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. Drug discovery: a jump-start for electroceuticals. Nature 496: 159–161, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Felten SY, Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J Neurosci Res 18: 37–48, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Fujii T, Tsuchiya T, Yamada S, Fujimoto K, Suzuki T, Kasahara T, Kawashima K. Localization and synthesis of acetylcholine in human leukemic T cell lines. J Neurosci Res 44: 66–72, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Fujii T, Yamada S, Watanabe Y, Misawa H, Tajima S, Fujimoto K, Kasahara T, Kawashima K. Induction of choline acetyltransferase mRNA in human mononuclear leukocytes stimulated by phytohemagglutinin, a T-cell activator. J Neuroimmunol 82: 101–107, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Grebe KM, Hickman HD, Irvine KR, Takeda K, Bennink JR, Yewdell JW. Sympathetic nervous system control of anti-influenza CD8+ T cell responses. Proc Natl Acad Sci USA 106: 5300–5305, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352: 1685–1695, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Hofer S, Eisenbach C, Lukic IK, Schneider L, Bode K, Brueckmann M, Mautner S, Wente MN, Encke J, Werner J, Dalpke AH, Stremmel W, Nawroth PP, Martin E, Krammer PH, Bierhaus A, Weigand MA. Pharmacologic cholinesterase inhibition improves survival in experimental sepsis. Crit Care Med 36: 404–408, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med 203: 1623–1628, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol 7: 335–347, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji H, Rabbi MF, Labis B, Pavlov VA, Tracey KJ, Ghia JE. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol 7: 335–347, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kees MG, Pongratz G, Kees F, Scholmerich J, Straub RH. Via β-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J Neuroimmunol 145: 77–85, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Kipnis J, Cardon M, Avidan H, Lewitus GM, Mordechay S, Rolls A, Shani Y, Schwartz M. Dopamine, through the extracellular signal-regulated kinase pathway, downregulates CD4+CD25+ regulatory T-cell activity: implications for neurodegeneration. J Neurosci 24: 6133–6143, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koopman FA, Miljko S, Grazio S, Sokolovic S, Tracey KJ, Levine YA, Zitnik RJ, Tak PP. Pilot study of stimulation of the cholinergic anti-inflammatory pathway with an implantable vagus nerve stimulation device in patients with rheumatoid arthritis (Abstract). Arthritis Rheum 64: S195, 2012. [Google Scholar]
- 31.Lanza GA, Guido V, Galeazzi MM, Mustilli M, Natali R, Ierardi C, Milici C, Burzotta F, Pasceri V, Tomassini F, Lupi A, Maseri A. Prognostic role of heart rate variability in patients with a recent acute myocardial infarction. Am J Cardiol 82: 1323–1328, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Laversuch CJ, Seo H, Modarres H, Collins DA, McKenna W, Bourke BE. Reduction in heart rate variability in patients with systemic lupus erythematosus. J Rheumatol 24: 1540–1544, 1997. [PubMed] [Google Scholar]
- 33.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 473: 317–325, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Lindgren S, Stewenius J, Sjolund K, Lilja B, Sundkvist G. Autonomic vagal nerve dysfunction in patients with ulcerative colitis. Scand J Gastroenterol 28: 638–642, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Makikallio TH, Huikuri HV, Makikallio A, Sourander LB, Mitrani RD, Castellanos A, Myerburg RJ. Prediction of sudden cardiac death by fractal analysis of heart rate variability in elderly subjects. J Am Coll Cardiol 37: 1395–1402, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, Michel K, Tracey KJ, Schemann M, Boesmans W, Vanden Berghe P, Boeckxstaens GE. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut; 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 37.Mina-Osorio P, Rosas-Ballina M, Valdes-Ferrer SI, Al-Abed Y, Tracey KJ, Diamond B. Neural signaling in the spleen controls B-cell responses to blood-borne antigen. Mol Med 18: 618–627, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miura T, Kudo T, Matsuki A, Sekikawa K, Tagawa Y, Iwakura Y, Nakane A. Effect of 6-hydroxydopamine on host resistance against Listeria monocytogenes infection. Infect Immun 69: 7234–7241, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathan C. Points of control in inflammation. Nature 420: 846–852, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Nathan C, Ding A. Nonresolving inflammation. Cell 140: 871–882, 2010. [DOI] [PubMed] [Google Scholar]
- 41.Niijima A. The afferent discharges from sensors for interleukin 1 beta in the hepatoportal system in the anesthetized rat. J Auton Nerv Syst 61: 287–291, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Olofsson PS, Katz DA, Rosas-Ballina M, Levine YA, Ochani M, Valdes-Ferrer SI, Pavlov VA, Tracey KJ, Chavan SS. α7 Nicotinic acetylcholine receptor (α7nAChR) expression in bone marrow-derived non-T cells is required for the inflammatory reflex. Mol Med 18: 539–543, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olofsson PS, Rosas-Ballina M, Levine YA, Tracey KJ. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev 248: 188–204, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pachero R, Contreras F, Prado C. Cells, molecules, and mechanisms involved in the neuro-immune interaction. In: Cell Interaction, edited by Gowder S. Rijeka, Croatia: InTech, 2012, chapt. 6; http://www.intechopen.com/books/cell-interaction/cells-molecules-and-mechanisms-involved-in-the-neuro-immune-interaction. [Google Scholar]
- 45.Pavlov VA, Ochani M, Yang LH, Gallowitsch-Puerta M, Ochani K, Lin X, Levi J, Parrish WR, Rosas-Ballina M, Czura CJ, Larosa GJ, Miller EJ, Tracey KJ, Al-Abed Y. Selective α7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit Care Med 35: 1139–1144, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Pontet J, Contreras P, Curbelo A, Medina J, Noveri S, Bentancourt S, Migliaro ER. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. J Crit Care 18: 156–163, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Prado C, Contreras F, Gonzalez H, Diaz P, Elgueta D, Barrientos M, Herrada AA, Lladser A, Bernales S, Pacheco R. Stimulation of dopamine receptor D5 expressed on dendritic cells potentiates Th17-mediated immunity. J Immunol 188: 3062–3070, 2012. [DOI] [PubMed] [Google Scholar]
- 48.Rinner I, Kawashima K, Schauenstein K. Rat lymphocytes produce and secrete acetylcholine in dependence of differentiation and activation. J Neuroimmunol 81: 31–37, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol Regul Integr Comp Physiol 273: R407–R413, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, Chavan S, Al-Abed Y, Yang H, Tracey KJ. The selective α7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med 15: 195–202, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosas-Ballina M, Ochani M, Parrish WR, Ochani K, Harris YT, Huston JM, Chavan S, Tracey KJ. Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc Natl Acad Med USA 105: 11008–11013, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosas-Ballina M, Olofsson PS, Ochani M, Valdes-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334: 98–101, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: the CARDIA study. Mol Med 13: 178–184, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein KS, McFarlane IC, Goldberg N, Ginzler EM. Heart rate variability in patients with systemic lupus erythematosus. Lupus 5: 44–48, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Straub RH, Rauch L, Fassold A, Lowin T, Pongratz G. Neuronally released sympathetic neurotransmitters stimulate splenic interferon-gamma secretion from T cells in early type II collagen-induced arthritis. Arthritis Rheum 58: 3450–3460, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Sun J, Singh V, Kajino-Sakamoto R, Aballay A. Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science 332: 729–732, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thayer JF. Vagal tone and the inflammatory reflex. Cleve Clin J Med 76, Suppl 2: S23–S26, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Thomas GD. Neural control of the circulation. Adv Physiol Educ 35: 28–32, 2011. [DOI] [PubMed] [Google Scholar]
- 59.Torres-Rosas R, Yehia G, Pena G, Mishra P, Del Rocio Thompson-Bonilla M, Moreno-Eutimio MA, Arriaga-Pizano LA, Isibasi A, Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat Med 20: 291–295, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tracey KJ. The inflammatory reflex. Nature 420: 853–859, 2002. [DOI] [PubMed] [Google Scholar]
- 61.Tracey KJ, Beutler B, Lowry SF, Merryweather J, Wolpe S, Milsark IW, Hariri RJ, Fahey TJ, 3rd, Zentella A, Albert JD, et al. Shock and tissue injury induced by recombinant human cachectin. Science 234: 470–474, 1986. [DOI] [PubMed] [Google Scholar]
- 62.van Maanen MA, Lebre MC, van der Poll T, LaRosa GJ, Elbaum D, Vervoordeldonk MJ, Tak PP. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum 60: 114–122, 2009. [DOI] [PubMed] [Google Scholar]
- 63.Vida G, Pena G, Kanashiro A, Del Rocio Thompson-Bonilla M, Palange D, Deitch EA, Ulloa L. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J 25: 4476–4485, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421: 384–388, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Watkins LR, Goehler LE, Relton JK, Tartaglia N, Silbert L, Martin D, Maier SF. Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neurosci Lett 183: 27–31, 1995. [DOI] [PubMed] [Google Scholar]
- 66.Watkins LR, Maier SF. Implications of immune-to-brain communication for sickness and pain. Proc Natl Acad Sci USA 96: 7710–7713, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 334: 101–105, 2011. [DOI] [PubMed] [Google Scholar]
- 68.Zhang P, Han D, Tang T, Zhang X, Dai K. Inhibition of the development of collagen-induced arthritis in Wistar rats through vagus nerve suspension: a 3-month observation. Inflamm Res 57: 322–328, 2008. [DOI] [PubMed] [Google Scholar]
- 69.Zitnik RJ. Treatment of chronic inflammatory diseases with implantable medical devices. Cleve Clin J Med 78, Suppl 1: S30–S34, 2011. [DOI] [PubMed] [Google Scholar]